Abstract
Background
Chronic wounds are of many etiologies and difficult to treat. Many commercial products to manage such wounds are available, which claim to have good outcomes. Aim of this study was to compare the efficacy of Ionic Silver Solution and Super Oxidized Solution in the management of chronic wounds.
Methods
Patients with chronic wounds were randomly placed in two groups-Group A (Ionic Silver Solution) and Group B (Super Oxidized Solution) with 30 patients each. The dressings were continued until the wound healed completely or the wound was ready for a definitive procedure. Wound parameters were recorded as per Bates Jensen Wound Assessment Tool (BJWAT) Score.
Results
FIfty patients completed the study. The scores were compared at the initiation and endpoint of treatment. The pretreatment total for BJWAT was 916 and 924 in group A and group B respectively, which was not statistically significant. Post-treatment improvement was noticed in both the groups and the score decreased to 510 and 675 in group A and group B respectively (p = 0.001). Ionic Silver Solution and Super Oxidized Solution both were found to be effective in improving the overall wound condition. However, Ionic Silver Solution was found to be more effective than Super Oxidized Solution in the healing of chronic wounds. Complete healing was noticed in a small number (6%) of patients. These agents can therefore best prepare the wounds for early surgical intervention.
Conclusion
Both the agents were found to be safe and useful in the management of chronic wounds. However, Ionic Silver Solution was found to be more effective than the super oxidized solution in this study.
Keywords: Chronic wounds, Ionic Silver Solution, Super Oxidized Solution, Biofilm
Introduction
A chronic wound is a wound that has a slow healing phase, or where healing is delayed, interrupted, or nonhealing due to intrinsic and extrinsic factors.1 These are difficult to manage wounds. Common chronic wounds in practice are diabetic foot ulcers, pressure sores, venous ulcers, arterial insufficiency ulcers, and nonhealing ulcers due to burns and infections. Various local agents are used for dressings to manage these wounds. These agents are meant for preventing infection or reducing bacterial load and promoting granulation thus helping in wound healing. These wounds take a longer time to heal with conventional dressings and are a big drain on resources. Delayed healing may be due to the presence of a biofilm. There are many commercially available products that offer improvement in these wound conditions and overall healing by acting on biofilm. In this study, the role and efficacy of two such agents, Ionic Silver Solution and Super Oxidized Solution in the management of chronic wounds were compared.
Materials and methods
The study was conducted in a tertiary-level hospital over a period of 12 months. The prospective study included patients presenting to the hospital with chronic wounds irrespective of the etiology. Institutional Ethical Committee clearance was obtained before the start of the study. As our study compared two agents that were not studied earlier so a reference study could not be applied. A pilot study with ten patients in each arm revealed good response to both agents. An observational analytical study was designed to enroll all the eligible patients with chronic wounds. An average of sixty patients with chronic wounds sought consultation every year at this center; hence, a sample size of sixty cases was taken. They were divided into two groups – Group A (Ionic Silver Solution) and Group B (Super Oxidized Solution), by a randomization coding system derived from a computer-generated randomization table. Group A and Group B had 30 patients each. This study included all willing patients with chronic wounds in the age group of 12–65. Exclusion Criteria: Patients with pregnancy, lactation, allergic reactions to silver or superoxide solution, chronic renal dysfunction, acute lung infection, clinically manifested arrhythmia, epilepsy or taking chemotherapy, immunosuppressant, or corticosteroids.
Informed consent was obtained from each patient. Demographic parameters were recorded. A detailed history pertaining to the duration, etiologic factors, evidence of any systemic disease was obtained. Wound parameters were recorded as per the Bates Jensen Wound Assessment Tool Score (BJWAT). Wound parameters were recorded first at the initiation of intervention, and then at weekly intervals by another independent experienced observer, blinded to the treatment received by the patient.
In group A, Ionic Silver Solution containing 0.01% ionic silver, menthol 0.05%, and 10%V/V glycerol was employed. It was administered by irrigating the solution directly from the bottle or via a syringe with a 22G needle. After cleansing the wound, solution-soaked gauze was applied on or into the wound to provide a physical barrier against outside bacterial contamination. Dressing change was done on alternate days. The dosage used was 1 ml/cm2 of the wound, half the dose was used to irrigate the wound, and another half to cover the wound with the dressing soaked in the solution. It was covered with semi-occlusive dressings.
In Group B, Super Oxidized Solution was used. It was applied as a spray on the wound for 5 min followed by keeping the soaked gauze in the solution for 10 min. The dosage used was 2 ml/cm2 of the wound, half the dose was used to irrigate the wound, and another half to cover the wound with the dressing soaked in the solution. It was covered with semi-occlusive dressings. The dressing change was done on alternate days. Patients were kept under observation for the next 30 min for immediate complications like pain, erythema, or any other local reactions. The dressings continued until the wound healed completely or the wound was ready for a definitive procedure. Once the wound healing was complete, the patients were followed up after one month.
Statistical analysis for the change in size was presented as percentages, using the paired student t-test. The Bates-Jensen wound assessment tool was compared before and after treatment. All statistical analyses were carried out using XLSTAT software for Windows. A “p” value of <0.05 was considered significant. Analysis of differences between age, sex, duration of scar, site of wounds, between the groups was done using the Chi-square test.
Results
An initial number of 60 patients were considered for the study. They were divided into two groups - Group A (Ionic Silver Solution) and Group B (Super Oxidized Solution), with 30 patients in each group. Ten patients, five patients from each group, were lost to the follow-up. Therefore, the final analysis is based on the data of 50 patients, 25 each from both the groups, who were followed up for the entire study.
A total of 50, 25 from each group, completed the study. There were 29 males and 21 females with male to female ratio of 1.27:1 and1.5:1 in Group A and B, respectively. Mean age was 42.4 years and 43.2 years in group A and group B respectively (Table 1). The commonest comorbidity in this study was diabetes mellitus that was present in 18 patients (36%). This was followed by chronic venous insufficiency in 05 patients (10%), paraplegia in 5 patients (10%), and peripheral arterial disease in 4 patients (8%). There was no comorbidity in the rest of the 18 patients (36%) (Table 2). Lower limbs (66%) were the most common site of chronic wounds followed by chest, upper limbs, and sacrum (Table 3). The etiology of wounds in both groups was comparable. Chi-square test was applied to analyze and compare the location of scars in various body areas and found no statistically significant difference in the etiology of wounds in both groups. The number of dressing changes varies from two to fourteen (Table 4). Maximum response (70%) was noticed after three to four dressings in 18 and 17 patients in group A and group B respectively.
Table 1.
Demographic data of cases.
| Group A | Group B | Statistical analysis | |
|---|---|---|---|
| Mean age (in years) | 42.4 | 43.2 | ANOVA- p > 0.05 |
| Gender | Male-14 | Male 15 | Chi-Square P > 0.05 |
| Female-11 | Female 10 | ||
| Gender ratio (M:F) | 1.27:1 | 1.5:1 |
Table 2.
Comorbidity of cases.
| Comorbidity | Group A | Group B |
|---|---|---|
| Diabetes mellitus | 08 | 10 |
| Varicose veins/venous insufficiency | 03 | 02 |
| Paraplegia | 02 | 03 |
| Arterial disease/ischemia | 02 | 02 |
| No comorbidity | 10 | 08 |
| Total | 25 | 25 |
Table 3.
Location of the wounds.
| Location | Group A | Group B |
|---|---|---|
| Lower limbs | 17 | 16 |
| Chest | 04 | 03 |
| Upper limbs | 02 | 03 |
| Sacrum | 02 | 03 |
Table 4.
Number of dressings required for wounds to be healed/fit for surgical intervention.
| Number of dressings | Group A | Group B |
|---|---|---|
| Two | 03 | 01 |
| Three | 11 | 09 |
| Four | 07 | 08 |
| More than four | 04 | 07 |
| Total | 25 | 25 |
Comparison of Bates Jensen Wound Assessment Tool was done (Table 5). In Group A Score decreased to 510 from the pretreatment score of 916. In Group B Score decreased to 675 from the pretreatment score of 924. This shows that mean volume decreased in both the groups. When we compared both the groups, the mean pretreatment score was not significant (p value = 0.90). At the final assessment both the groups showed a decrease in BJWAT score, which was significant (in each group). The final mean BJWAT Score was compared between the groups and the difference was found to be significant. Thus, the results obtained for the change in mean volume were better in Group A. To further clarify the results, the percentage change in volume for both the groups was calculated and compared using descriptive parametric analyses test – ANOVA. Only three wounds (6%) healed completely. 40 wounds (80%) were ready for surgical intervention for which split skin grafting or flap cover was done. Four patients did not consent for surgery and three wounds did not show marked improvement in the wound condition; hence, surgical debridement was carried out after the fourth dressing. Photographs of representative cases are shown in Fig. 1, Fig. 2, Fig. 3, Fig. 4.
Table 5.
Comparison of Bates Jensen wound assessment tool.
| Group A | Group B | |
|---|---|---|
| Total pretreatment score | 916 | 924 |
| Total post-treatment score | 510 | 675 |
| Mean pretreatment score | 36.64 | 36.96 |
| Mean post-treatment score | 20.4 | 27 |
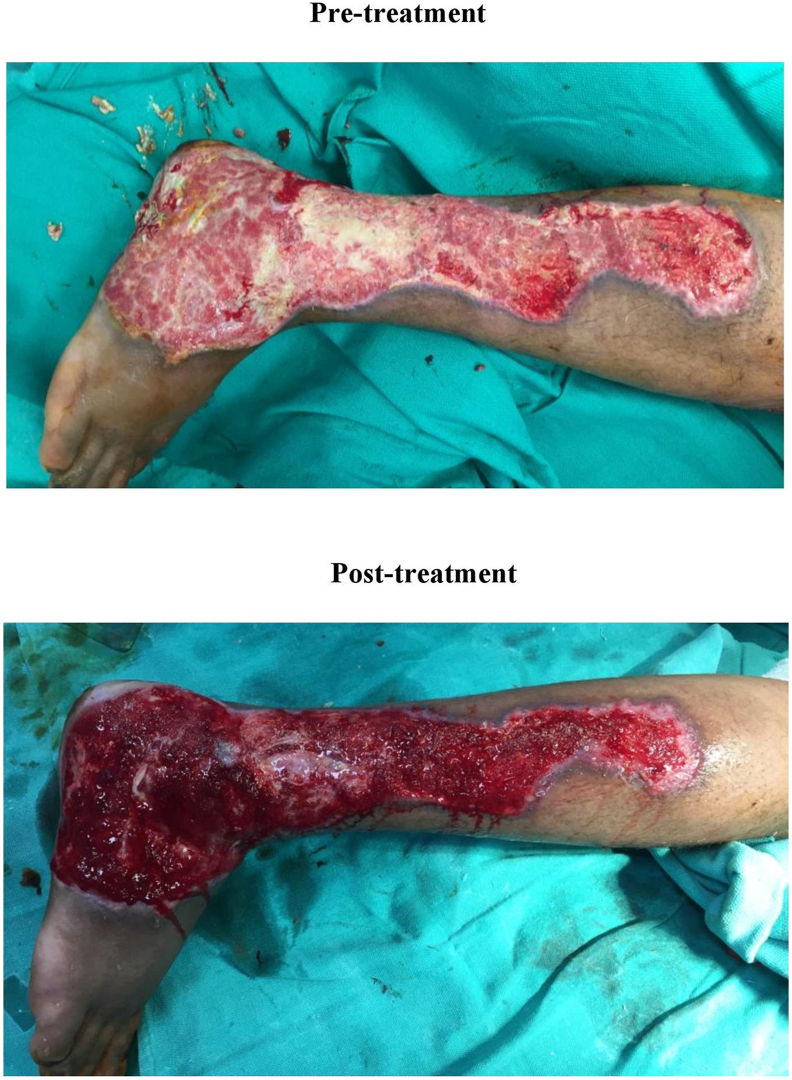
Fig. 1.
Pretreatment – Chronic wound on the day of presentation to the hospital. Post-treatment – Chronic wound on day 7 (after 3 dressings) post wound management with Ionic Silver solution.
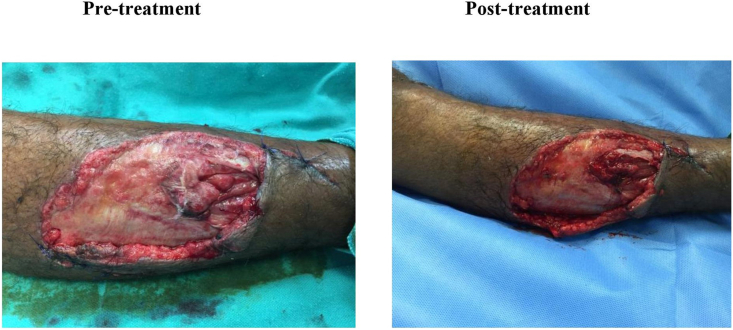
Fig. 2.
Pretreatment – Chronic wound on the day of presentation to the hospital. Post-treatment – Chronic wound on day 3 (after one dressing) after wound management with Ionic Silver solution.
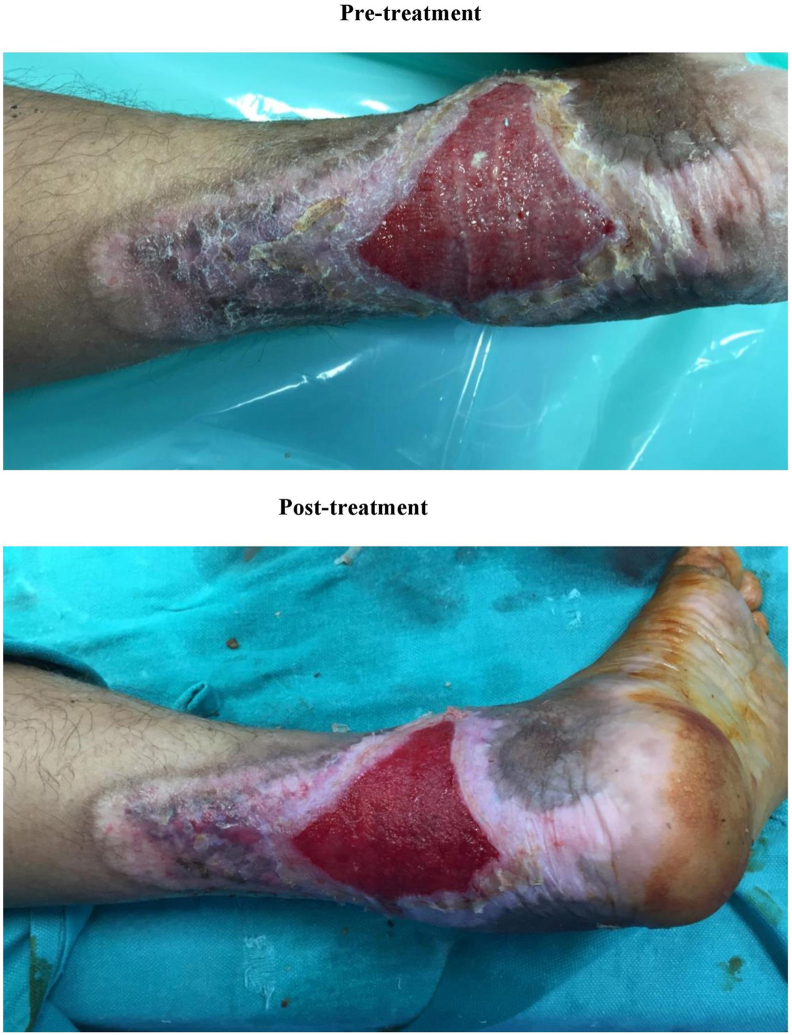
Fig. 3.
Pretreatment – Chronic wound on the day of presentation to the hospital. Post-treatment – chronic wound on day 7 (after 3 dressings) after wound management with superoxidized solution.
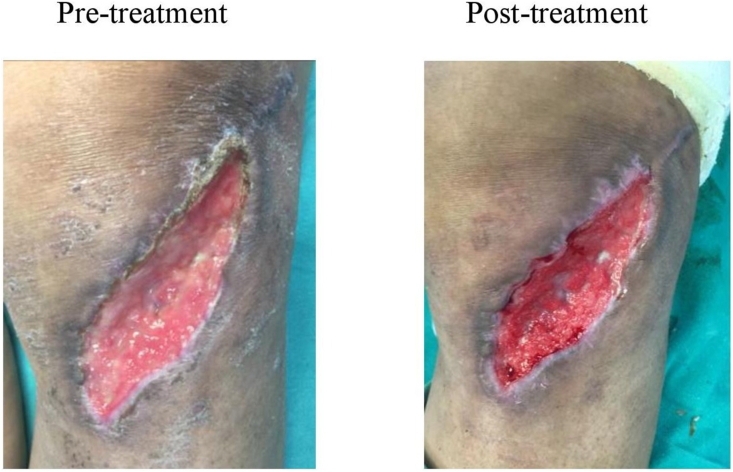
Fig. 4.
Pretreatment – Chronic wound on the day of presentation to the hospital. Post-treatment – chronic wound on day 3 (after one dressing) after wound management with superoxidized solution.
No adverse or allergic reaction was noticed while using both the agents. The application of both the drugs was largely pain-free and soothing. The patient’s perception of pain was recorded in terms of the VAS – Visual Analogue Scale (for a range of 1–10) and analyzed. Pain was not a major complaint after the application of these agents. Statistical analysis was done and both pretreatment and post-treatment difference was not found to be significant.
Discussion
Chronic wounds may have prolonged phases of wound healing. The depth of these wounds may vary.2 BJWAT is a commonly used score to assess progress of chronic wounds. There are 13 parameters that are size, depth, edges, undermining, necrotic tissue type, necrotic tissue amount, exudate type, exudate amount, skin color surrounding the wound, peripheral tissue edema, peripheral tissue induration, granulation tissue, and epithelialization. Each parameter is assessed on a scale of 1–5 by picking the response that best describes the wound. The total score is determined by adding together the thirteen parameter scores. It is used for overall wound assessment; a higher total score indicates more severe wounds while a decline in score suggests improvement. In chronic wounds, a biofilm is formed.3, 4, 5 It contains microorganisms such as bacteria and fungi, they secrete a variety of protective compounds which decrease the drug penetration and delay wound healing.6,7 Better understanding of the role of biofilm has shifted the attention to address biofilm to improve healing in chronic wounds.8 Traditionally chronic wounds have been managed by local application of agents most commonly being Povidone Iodine.
Ionic Silver Solution: This solution is commercially available. It contains 0.01% Silver nitrate that is a broad-spectrum antimicrobial; 0.05% Menthol that increases cell migration and gives a soothing sensation to the wounds; Sorbitan monolaurate (Tween-20) is a surfactant, which helps to disrupt the biofilm.9,10 This leads to a multipronged attack and optimizes the wound condition and shifts the balance toward healing. This combination has been shown to disrupt the mature biofilm and also prevents biofilm formation.11,12
Super Oxidized Solution: This solution is processed electrochemically, which produces reactive species of oxygen and chlorine are formed. It acts on the cell wall and leads to lipid peroxidation hence effective against various types of microorganisms.13,14 It penetrates and disrupts the biofilm. It selectively targets unicellular organisms thus host human cells are not a target for action. It has been shown to improve healing in the infected diabetic foot ulcer.15 Ionic Silver Solution and Super Oxidized Solution both were found to be effective in improving the overall wound condition. In this study, usage of Ionic Silver Solution and Super Oxidized Solution was found to hasten the wound healing with good control of local infection and pain and preparing the wound bed for early surgical intervention. In this study, Ionic Silver Solution has been found to be more effective than Super Oxidized Solution in the management of chronic wounds. After four sittings of dressing, if wound did not improve, surgical debridement was done.
In conclusion, this study has tried to achieve the objective to compare the efficacy of Ionic Silver Solution and Super Oxidized Solution. Both the agents were found to be safe and useful in the management of chronic wounds. However, Ionic Silver Solution was found to be more effective than the super oxidized solution in this study.
Limitation of present study This study comprised of a small sample size and thus cannot be said to be conclusive for general population.
Disclosure of competing interest
The authors have none to declare.
Acknowledgments
This paper is based on Armed Forces Medical Research Committee Project No. 4957/2017 granted and funded by the office of the Directorate General Armed Forces Medical Services and Defence Research Development Organization, Government of India.
References
- 1.International Wound Infection Institute (IWII) Wounds International; 2016. Wound Infection in Clinical Practice. [Google Scholar]
- 2.Crovetti Giovanni, Martinelli Giovanna, Issi Marwan, et al. Angelo. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci. 2004;30(2):145–151. doi: 10.1016/j.transci.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Bjarnsholt T., Kirketerp-Moller K., Jensen P.O., et al. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen. 2008;16(1):2–10. doi: 10.1111/j.1524-475X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 4.James G.A., Swogger E., Wolcott R., et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16(1):37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 5.Taylor J.E., Laity P.R., Hicks J., et al. Extent of iron pick-up in deforoxamine-coupled polyurethane materials for therapy of chronic wounds. Biomaterials. 2005 Oct;26(30):6024–6033. doi: 10.1016/j.biomaterials.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Taylor Jennifer E., Laity Peter R., Hicks John, et al. Extent of iron pick-up in deforoxamine-coupled polyurethane materials for therapy of chronic wounds. Biomaterials. 2005;26(30):6024–6033. doi: 10.1016/j.biomaterials.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Edwards J., Howley P., Cohen I.K. In vitro inhibition of human neutrophil elastase by oleic acid albumin formulations from derivatized cotton wound dressings. Int J Pharm. 2004;284(1–2):1–12. doi: 10.1016/j.ijpharm.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Swanson T., Keast D.H., Cooper R., et al. Ten top tips: identification of wound infection in a chronic wound. Wounds Middle East. 2015;2(1):20–25. [Google Scholar]
- 9.Bellingeri A., Falciani F., Traspedini P., et al. Effect of wound cleansing solution on wound bed preparation and inflammation in chronic wounds: a single-blind RCT. J Wound Care. 2016;25(3) doi: 10.12968/jowc.2016.25.3.160. [DOI] [PubMed] [Google Scholar]
- 10.Yang Q., Larose C., Porta A.D., Della Porta A.C., Schultz G.S., Gibson D.J. A surfactant-based wound dressing can reduce bacterial biofilms in a porcine skin explant model. Int Wound J. 2016 doi: 10.1111/iwj.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowler P., Parsons D. Combatting wound biofilm and recalcitrance with novel anti-biofilm hydrofiber wound dressing. Wound Med. 2016;14:6–11. [Google Scholar]
- 12.Metcalf D., Parsons D., Bowler P. A next-generation antimicrobial wound dressing: a real-life clinical evaluation in the UK and Ireland. J Wound Care. 2016;25(3):132–138. doi: 10.12968/jowc.2016.25.3.132. [DOI] [PubMed] [Google Scholar]
- 13.Mustoe Thomas. Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. Am J Surg. 2004;187(5):S65. doi: 10.1016/S0002-9610(03)00306-4. [DOI] [PubMed] [Google Scholar]
- 14.Edwards-Jones V., Flanagan M., Wolcott R. Technological advancements in the fight against antimicrobial resistance. Wounds Int. 2015;6(2):47–51. [Google Scholar]
- 15.Piaggesi A., Goretti C. A randomized controlled trial to examine the efficacy of a new super oxidized solution for the management of wide postsurgical lesions of the diabetic foot. Int J Low Extrem Wounds. 2010;9(1):1. doi: 10.1177/1534734610361945. [DOI] [PubMed] [Google Scholar]