Abstract
Although systemic treatment for hepatocellular carcinoma has advanced after the development of tyrosine kinase inhibitors such as sorafenib and lenvatinib, the effectiveness of a single tyrosine kinase inhibitor in survival extension of unresectable hepatocellular carcinoma is limited to a few months. Therefore, novel treatment options are required for unresectable hepatocellular carcinomas, including those with multiple lung metastases. This case report describes a hepatocellular carcinoma patient with a recurrence of multiple lung metastases, which was successfully treated with conversion pneumonectomy after treatment with tyrosine kinase inhibitors. A 79-year-old man underwent right hepatectomy for hepatocellular carcinoma, along with removal of the tumor thrombus in the inferior vena cava. Multiple lung metastases were detected 4 months after hepatectomy. Treatment with tyrosine kinase inhibitors, mainly lenvatinib, resulted in complete remission of the lung metastases, except for one lesion in segment 3 of the right lung which gradually enlarged. Twenty-three months after hepatectomy, partial resection of the right lung was performed using video-assisted thoracic surgery for this residual lesion in the right lung. The patient remained disease-free for 11 months after conversion pneumonectomy, without any adjuvant therapies. This is the first case report of multiple lung metastases originating from hepatocellular carcinoma which were successfully treated with conversion pneumonectomy after treatment with tyrosine kinase inhibitors. Conversion pneumonectomy after systemic therapy with tyrosine kinase inhibitors should be considered as a treatment strategy for patients with unresectable multiple lung metastases from hepatocellular carcinomas.
Keywords: Hepatocellular carcinoma, Lung metastasis, Tyrosine kinase inhibitors, Lenvatinib, Conversion surgery
Introduction
The development of tyrosine kinase inhibitors (TKIs) has improved the treatment outcomes of unresectable hepatocellular carcinoma (HCC), including HCC with unresectable extrahepatic metastasis [1]. However, the effectiveness of single-TKI therapy is limited to improving overall survival (OS) to approximately 3 months [2], necessitating the development of novel treatment strategies for unresectable HCC with extrahepatic metastasis.
The lung is the most frequent site of extrahepatic metastasis originating from HCC, accounting for 30–50% of such lesions [3–5]. The prognosis of lung metastasis originating from HCC (LM-HCC) in the absence of any anti-cancer therapies is poor, with a median survival time of 6–10 months [5, 6]. Moreover, the complete remission rate, < 1%, with systemic therapy for HCC is low [1]. Therefore, surgery is considered the only treatment for LM-HCC that can yield long-term survival, with a 5-year OS rate of 27.5–66.9% [7–12]. However, surgical resection for LM-HCC is reportedly effective only for cases with localized lung metastasis. Hence, novel treatment strategies are required for the treatment of unresectable LM-HCC.
Conversion surgery has become routine practice for some malignancies, including colorectal cancer and gastric cancer with unresectable metastases [13–17]. However, few studies have investigated the utility of conversion surgery for unresectable HCC [18–24]. Herein, we report the first case of successful removal of unresectable LM-HCC using conversion surgery, after oral TKI administration.
Case report
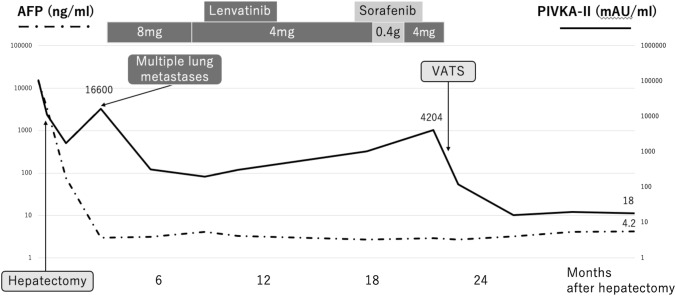
A 79-year-old man in good general condition was referred to our department for HCC treatment. Despite a prior history of hepatitis B virus infection, after seroconversion, the patient had good liver function with Child–Pugh class A. Alpha-fetoprotein (AFP) was 14,626.7 ng/mL, and protein induced by vitamin K absence/antagonist-II (PIVKA-II) was 104,990 mAU/mL. Dynamic contrast-enhanced computed tomography (CT) revealed an HCC in segment 8 (S8) of the liver (maximum dimension: 10.8 cm) with a tumor thrombus in the inferior vena cava (Fig. 1a–c). Right hepatectomy was performed at our department. The tumor thrombus in the inferior vena cava was removed through a small incision in this vessel, after securing the right hepatic vein with a rubber vessel loop and half-clamping the inferior vena cava (Fig. 1e). The pathological section showed that the HCC was mainly moderately differentiated with a mixture of well and poorly differentiated. There was no intrahepatic metastasis. Tumor thrombi were present in the inferior vena cava and right hepatic vein. Union for International Cancer Control (UICC) tumor, nodes, metastasis (TNM) classification was pT4N0M0 pStage IIIB. The postoperative clinical course was good. The patient was discharged from our hospital on post-hepatectomy day 13. The serum AFP and PIVKA-II levels (Fig. 2) decreased to 76.4 ng/mL and 1745 mAU/mL, respectively, but did not return to the normal range 1 month after hepatectomy. AFP declined to 3 ng/mL (i.e., within the normal range) 4 months post-hepatectomy. However, PIVKA-II was re-elevated to 16,600 mAU/mL, and multiple lung metastases were detected on CT (Figs. 2, 3a). Systemic therapy was initiated with lenvatinib at a dose of 8 mg per day, 5 months after hepatectomy. The patient’s body weight at the time of systemic therapy initiation was 61.2 kg. However, lenvatinib was reduced from 8 mg per day to 4 mg per day due to the appearance of grade 3 proteinuria 8 months after hepatectomy. Thereafter, all lung metastases decreased gradually and complete remission was achieved, except for one lung metastasis in segment 3 (S3) of the right lung, which was identified 18 months after hepatectomy. The metastatic lesion in S3 of the right lung had enlarged to 2.3 cm (Fig. 3b). Lenvatinib was replaced with sorafenib 400 mg per day, 18 months after hepatectomy. However, lenvatinib therapy was reinstated since the patient developed grade 2 proteinuria 20 months after hepatectomy. CT detected an enlargement of the residual lung metastasis to 4.2 cm, 22 months after hepatectomy (Fig. 3c). However, the other lung metastases continued to show complete response. Since the remaining lung metastasis was the only evaluable lesion, partial resection of the right lung was performed with video-assisted thoracic surgery (VATS) at another hospital, 23 months after hepatectomy (Fig. 4a). Oral administration of lenvatinib was withdrawn 19 days before VATS. The pathological findings of the excised lung tumor corresponded to metastatic lung tumor derived from HCC and showed that the lung tumor contained poorly, moderately, and well-differentiated components and did not differ from the primary tumor. There were no signs indicating the effectiveness of systemic therapy (Fig. 4b). The patient was followed up at the outpatient clinic of our department without adjuvant systemic therapies.
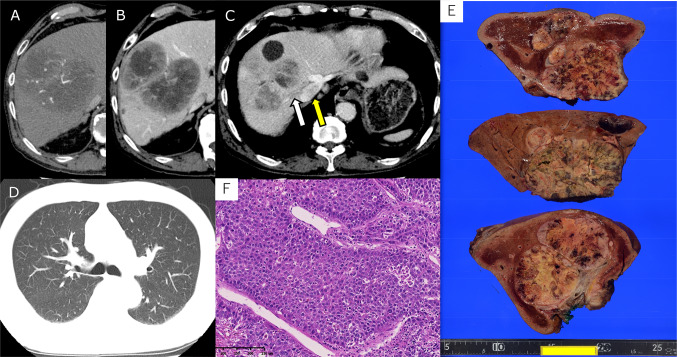
Fig. 1.
a Computed tomography (CT) during the arterial phase showed a liver tumor measuring 10.8 cm that exhibited enhancement in segment 8 (S8) of the liver. b CT during the delayed phase depicted a liver tumor measuring 10.8 cm, which did not exhibit enhancement, in S8 of the liver. c CT during the delayed phase showed a tumor thrombus was observed extending from the right hepatic vein to the inferior vena cava. The white arrow points to the tumor thrombus in right hepatic vein, and the tumor thrombus in the inferior vena cava is indicated by the yellow arrow showing a low-density area in the inferior vena cava. d CT of the lung field showed no pulmonary metastasis before hepatectomy. e Macroscopic appearance of the liver tumor. The cut surface of the resected specimen showed a white, solid and simple nodular mass with extranodular growth. The greatest dimension of the mass measured 10.0 cm. A tumor thrombus was observed in right hepatic vein. f Histological appearance of the tumor. The tumor cells had abundant eosinophilic cytoplasm and hyperchromatic nuclei, and showed a macrotrabecular growth pattern. Pseudoglandular and solid growth patterns, and necrosis were also observed (not shown here). H–E: hematoxylin–eosin, scale bar: 250 μm
Fig. 2.
Serum levels of the tumor markers, AFP, and PIVKA-II, during the clinical course. AFP decreased markedly and returned to the normal range after the first hepatectomy. However, PIVKA-II remained high. PIVKA-II decreased and turned to the normal range after VATS. AFP: alpha-fetoprotein, PIVKA-II: protein induced by vitamin K absence/antagonist-II, VATS: video-assisted thoracic surgery
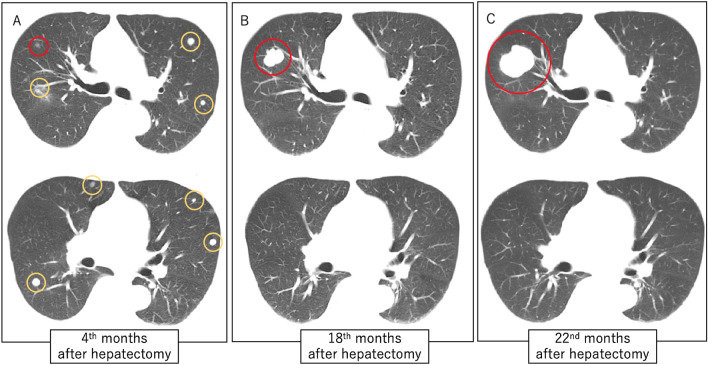
Fig. 3.
a CT of the lungs 3 months after hepatectomy showed multiple lung metastases. The lung metastases in S3 of the right lung are circled in red; other lesions are circled in yellow. b CT of the lungs 18 months after hepatectomy showed all lung metastases had disappeared, except for the one in S3 of the right lung. c CT of the lungs 22 months after hepatectomy showed that the size of the residual lesion in S3 of the right lung had increased to 4.2 cm. CT: computed tomography
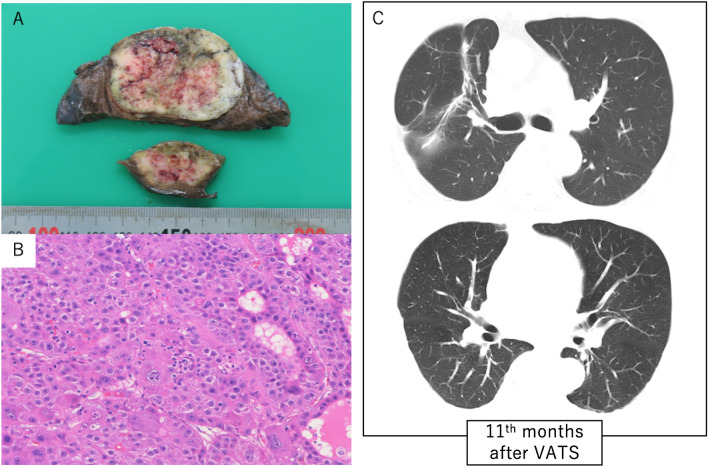
Fig. 4.
a Macroscopic appearance of the lung tumor. The cut surface of the resected specimen showed a white, substantial proliferative mass with necrosis and hemorrhage at the center of the tumor. The greatest dimension of the mass measured 5.5 cm. b Histological appearance of the tumor. The nuclei of the tumor cells were enlarged, and the cells showed partial pseudoglandular structures with cord-like structures similar to hepatocellular carcinoma. There was no finding indicating the effect of lenvatinib (hematoxylin and eosin stain, scale bar: 250 μm). c CT of the lungs 11 months after VATS showed no recurrence of lung metastasis. CT: computed tomography, VATS: video-assisted thoracic surgery
Discussion
This case report described HCC that recurred as unresectable multiple lung metastases after hepatectomy. Complete response was achieved for 13 months, mainly with lenvatinib, except for one lesion in S3 of the right lung. The only evaluable residual metastasis in the right lung was resistant to lenvatinib, resulting in focal progression, necessitating conversion surgery with VATS. Complete remission of the evaluable lesion was achieved via conversion pneumonectomy, and the patient remained disease-free for 11 months without any adjuvant treatments. Systemic therapy with TKIs succeeded in downstaging the unresectable LM-HCC to resectable LM-HCC, to achieve disease-free status via conversion surgery.
Surgical resection and systemic therapy can be considered treatment options for LM-HCC. Some retrospective cohort studies recommend surgical excision for resectable LM-HCC, since it is practically the only modality that can be expected to prolong long-term survival (5-year OS: 27.5–66.9%) [7–12]. In addition, the safety and feasibility of lung resection combined with lenvatinib administration should be considered, as both approaches may cause deterioration of respiratory function, warranting further investigation. Although a phase-3 clinical trial investigating the effectiveness of lenvatinib on HCC [1] and thyroid cancer [25] did not report interstitial pneumonia, 4 case reports described the occurrence of interstitial pneumonia during lenvatinib administration [26–29]. Our patient did not show signs of interstitial pneumonia during treatment with TKIs. His preoperative examination of pulmonary function was within the normal range. Hence, we performed lung resection for our patient.
Characteristics of the residual lesion in S3 of the right lung and the other metastatic lesions that disappeared were considered different, given the different responses to lenvatinib. These differences in the characteristics may be attributed to the differences between the original primary HCC component of the S3 lesion and the other lung metastases, which resulted from the intra-tumoral HCC heterogeneity [30]. Since lung metastases with complete response could not be identified, this aspect of our case could not be evaluated by pathological examination. However, the lung tumors that disappeared were assumed to be metastatic lesions from HCC, given the history of PIVKA-II increase with the appearance of the lesions and decrease in PIVKA-II levels with lenvatinib initiation. Focal progression during systemic therapy has also been reported for other neoplasms, such as a gastrointestinal stromal tumor and non-small cell lung cancer during imatinib administration [31] and gefitinib administration [32], respectively. Retrospective cohort studies reported the survival benefits of local therapy, including surgery and ablation therapy, for residual evaluable lesions that underwent focal progression during TKI therapy for these aforementioned malignancies [31, 33].
Conversion surgery has become a widespread clinical modality for other malignancies, such as colorectal and gastric cancer [13–17]. Although the efficacy of conversion surgery post-TKI administration remains to be established for HCC, favorable postoperative outcomes have been reported for 9 cases of conversion surgery after TKI administration for HCC (Table 1) [18–24]. A recent, large cohort study indicated that the rate of conversion surgery for unresectable HCC after administration of lenvatinib was 8.4% [34], but conversion surgery for LM-HCC has not been reported previously and the appropriate timing for conversion surgery is unknown. The partial response rate by modified Response Evaluation Criteria in Solid Tumor (mRECIST) of lenvatinib for unresectable HCC was 22.8% [1], and the presence of LM-HCC could be a good indication for conversion VATS if the LM-HCC responds to lenvatinib and is resectable. The rate of conversion surgery for lung metastases is expected to be lower than the partial response rate of lenvatinib, based on a previous report that the rate of conversion surgery for unresectable HCC [34]. In this case, VATS was performed because it was difficult to continue TKI at a sufficient dose due to adverse events and lung metastases, except for the S3 lesion of the right lung, which had been in complete remission for 5 months, a relatively long period. Moreover, there is no unified consensus to determine whether TKI administration should be continued or withdrawn after conversion hepatectomy. Objective and scientifically sound decisions cannot be derived from prior studies, necessitating further evaluation. Adjuvant systemic therapy with lenvatinib was not performed for any possible residual lesions after conversion pneumonectomy, per the patient’s will. The patient remained disease-free for 11 months without any adjuvant therapies.
Table 1.
Case reports on protocols with lenvatinib followed by conversion surgery for hepatocellular carcinoma
| Ref. | Age/sex | Reason for unresectivity | Effect of lenvatinib | Type of hepatectomy | R0 resection | Prognosis | Postoperative drug therapy |
|---|---|---|---|---|---|---|---|
| [18] | 59/M | PVTT | reduction of PVTT | Left hepatectomy + PVTT removal | (+) | 8 m alive with no recurrence | (−) |
| [19] | 69/M | Growth rate | reduction of tumor | Hepatectomy of S7 segment | (−) | 1 y alive | (+) |
| [20] | 69/M | Number | PR | Partial hepatectomy | (+) | 6 m alive with no recurrence | (−) |
| 72 /F | Number | PR | Left hepatectomy | (+) | 3 m alive with no recurrence | (−) | |
| 73/M | Number | PR | MWA | – | 6 m alive with no recurrence | (+) | |
| [21] | 58/M | Large size, PVTT, lung metastasis | Disappearance of metastasis | Extended right hepatectomy | – | 9 m alive with no recurrence | (+) |
| [22] | 82/F | Lung metastasis | Disappearance of metastasis | Extended posterior sectionectomy | (+) | 5 m alive with no recurrence | (−) |
| [23] | 66/F | Large size | PR | Extended right hepatectomy | (+) | 3 m alive with no recurrence | – |
| [24] | 82/F | Lung metastasis | – | Extended posterior segmentectomy | (+) | 5 m alive with no recurrence | – |
| This case report | 79/M | Lung metastasis | Disappearance of all but one metastasis | Partial pneumonectomy | (+) | 7 m alive with no recurrence | (−) |
In conclusion, this is the first reported case of unresectable multiple LM-HCC, successfully treated with lenvatinib and consecutive conversion surgery with VATS for the only remaining lung metastasis in the right lung. Conversion surgery after systemic therapy with lenvatinib should be considered as a treatment strategy, even for cases with unresectable multiple lung metastases derived from hepatocellular carcinoma.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all the individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Abbas A, Medvedev S, Shores N, et al. Epidemiology of metastatic hepatocellular carcinoma, a nationwide perspective. Dig Dis Sci. 2014;59:2813–2820. doi: 10.1007/s10620-014-3229-9. [DOI] [PubMed] [Google Scholar]
- 4.Uchino K, Tateishi R, Shiina S, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117:4475–4483. doi: 10.1002/cncr.25960. [DOI] [PubMed] [Google Scholar]
- 5.Kojima Y, Ueno H, Okusaka T, et al. 6610 Hepatocellular carcinoma presenting with lung metastasis: clinical characteristics and prognostic factors. Eur J Cancer Suppl. 2009;7:393. doi: 10.1016/S1359-6349(09)71331-X. [DOI] [Google Scholar]
- 6.Zhang S-M, Zeng Z-C, Tang Z-Y, et al. Prognostic analysis of pulmonary metastases from hepatocellular carcinoma. Hepatol Int. 2008;2:237–243. doi: 10.1007/s12072-008-9052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo S-W, Chang Y-L, Huang P-M, et al. Prognostic factors for pulmonary metastasectomy in hepatocellular carcinoma. Ann Surg Oncol. 2007;14:992–997. doi: 10.1245/s10434-006-9217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han KN, Kim YT, Yoon J-H, et al. Role of surgical resection for pulmonary metastasis of hepatocellular carcinoma. Lung Cancer. 2010;70:295–300. doi: 10.1016/j.lungcan.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Lee CY, Bae MK, Park IK, et al. Surgical resection for pulmonary metastasis from hepatocellular carcinoma: analysis of prognosis in relation to primary control. J Surg Oncol. 2010;101:239–243. doi: 10.1002/jso.21487. [DOI] [PubMed] [Google Scholar]
- 10.Yoon YS, Kim HK, Kim J, et al. Long-term survival and prognostic factors after pulmonary metastasectomy in hepatocellular carcinoma. Ann Surg Oncol. 2010;17:2795–2801. doi: 10.1245/s10434-010-1073-5. [DOI] [PubMed] [Google Scholar]
- 11.Kitano K, Murayama T, Sakamoto M, et al. Outcome and survival analysis of pulmonary metastasectomy for hepatocellular carcinoma. Eur J Cardiothorac Surg. 2012;41:376–382. doi: 10.1016/j.ejcts.2011.05.052. [DOI] [PubMed] [Google Scholar]
- 12.Kow AWC, Kwon CHD, Song S, et al. Clinicopathological factors and long-term outcome comparing between lung and peritoneal metastasectomy after hepatectomy for hepatocellular carcinoma in a tertiary institution. Surgery. 2015;157:645–653. doi: 10.1016/j.surg.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Maeda Y, Shinohara T, Nagatsu A, et al. Long-term outcomes of conversion hepatectomy for initially unresectable colorectal liver metastases. Ann Surg Oncol. 2016;23:S242–S248. doi: 10.1245/s10434-015-4460-0. [DOI] [PubMed] [Google Scholar]
- 14.Folprecht G, Grothey A, Alberts S, et al. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol. 2005;16:1311–1319. doi: 10.1093/annonc/mdi246. [DOI] [PubMed] [Google Scholar]
- 15.Tomasello G, Petrelli F, Ghidini M, et al. FOLFOXIRI plus bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: a systematic review and pooled analysis. JAMA Oncol. 2017;3:e170278. doi: 10.1001/jamaoncol.2017.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinoshita J, Yamaguchi T, Moriyama H, et al. Current status of conversion surgery for stage IV gastric cancer. Surg Today. 2021;51:1736–1754. doi: 10.1007/s00595-020-02222-0. [DOI] [PubMed] [Google Scholar]
- 17.Morgagni P, Solaini L, Framarini M, et al. Conversion surgery for gastric cancer: a cohort study from a western center. Int J Surg. 2018;53:360–365. doi: 10.1016/j.ijsu.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Kim J, Takahashi A, et al. Conversion hepatectomy for hepatocellular carcinoma with main portal vein tumour thrombus after lenvatinib treatment: a case report. World J Hepatol. 2021;13:384–392. doi: 10.4254/wjh.v13.i3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokoo H, Takahashi H, Hagiwara M, et al. Successful hepatic resection for recurrent hepatocellular carcinoma after lenvatinib treatment: a case report. World J Hepatol. 2020;12:1349–1357. doi: 10.4254/wjh.v12.i12.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomonari T, Sato Y, Tanaka H, et al. Conversion therapy for unresectable hepatocellular carcinoma after lenvatinib: three case reports. Medicine (Baltimore) 2020;99:e22782. doi: 10.1097/MD.0000000000022782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohya Y, Hayashida S, Tsuji A, et al. Conversion hepatectomy for advanced hepatocellular carcinoma after right portal vein transection and lenvatinib therapy. Surg Case Rep. 2020;6:318. doi: 10.1186/s40792-020-01078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuki R, Kawai K, Suzuki Y, et al. Pathological complete response in conversion hepatectomy induced by lenvatinib for advanced hepatocellular carcinoma. Liver Cancer. 2020;9:358–360. doi: 10.1159/000506202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato N, Beppu T, Kinoshita K, et al. Conversion hepatectomy for huge hepatocellular carcinoma with arterioportal shunt after chemoembolization and lenvatinib therapy. Anticancer Res. 2019;39:5695–5701. doi: 10.21873/anticanres.13768. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi A, Moriguchi M, Seko Y, et al. Impact of relative dose intensity of early-phase lenvatinib treatment on therapeutic response in hepatocellular carcinoma. Anticancer Res. 2019;39:5149–5156. doi: 10.21873/anticanres.13710. [DOI] [PubMed] [Google Scholar]
- 25.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 26.Liu SQ, Zhang XJ, Xue Y, et al. A case of lenvatinib therapy-induced unilateral interstitial pneumonia and cavity formation in advanced liver cancer (in Chinese) Zhonghua Gan Zang Bing Za Zhi. 2021;29:1109–1110. doi: 10.3760/cma.j.cn501113-20201208-00646. [DOI] [PubMed] [Google Scholar]
- 27.Kotani K, Enomoto M, Okada M, et al. Interstitial pneumonia suspected during regorafenib administration and exacerbated by subsequent therapy with lenvatinib for unresectable hepatocellular carcinoma. Clin J Gastroenterol. 2019;12:355–360. doi: 10.1007/s12328-019-00983-x. [DOI] [PubMed] [Google Scholar]
- 28.Imakura T, Sato S, Tomonari T, et al. Lenvatinib-induced interstitial pneumonia in a patient with hepatocellular carcinoma. Intern Med. 2022;61:1211–1217. doi: 10.2169/internalmedicine.7300-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura-Tsuchiya R, Sasaki E, Nakamura I, et al. A case of squamous cell carcinoma of unknown primary that responded to the multi-tyrosine kinase inhibitor lenvatinib. Case Rep Oncol. 2018;11:75–80. doi: 10.1159/000486569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng H, Pomyen Y, Hernandez MO, et al. Single-cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology. 2018;68:127–140. doi: 10.1002/hep.29778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Batran S-E, Hartmann JT, Heidel F, et al. Focal progression in patients with gastrointestinal stromal tumors after initial response to imatinib mesylate: a three-center-based study of 38 patients. Gastric Cancer. 2007;10:145–152. doi: 10.1007/s10120-007-0425-8. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 33.Jackman DM, Holmes AJ, Lindeman N, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006;24:4517–4520. doi: 10.1200/JCO.2006.06.6126. [DOI] [PubMed] [Google Scholar]
- 34.Shindoh J, Kawamura Y, Kobayashi Y, et al. Prognostic Impact of Surgical Intervention After Lenvatinib Treatment for Advanced Hepatocellular Carcinoma. Ann Surg Oncol. 2021;28:7663–7672. doi: 10.1245/s10434-021-09974-0. [DOI] [PubMed] [Google Scholar]