Abstract
Protection in the murine model of Helicobacter pylori infection may be mediated by CD4+ T cells, but the mechanism remains unclear. To better understand how protection occurs in this model, we generated and characterized H. pylori urease-specific CD4+ T cells from BALB/c mice immunized with Salmonella enterica serovar Typhimurium expressing H. pylori urease (subunits A and B). The CD4+ T cells were found to be specific for subunit A (UreA). Upon antigen-specific stimulation, expression of interleukin 4 (IL-4), IL-10, gamma interferon (IFN-γ), and tumor necrosis factor alpha was induced. Immunocytochemical analysis showed that the majority of cells produced IFN-γ and IL-10. Adoptive transfer of the UreA-specific CD4+ T cells into naive syngeneic recipients led to a threefold reduction in the number of bacteria in the recipient group when compared to that in the nonrecipient group. Stomach colonization was also reduced significantly after transfer of these cells into patently infected mice. Adoptive transfer of UreA-specific CD4+ T cells into IL-4 receptor α chain-deficient BALB/c mice indicated that IL-4 and IL-13 were not critical in the control of bacterial load. In addition, synthetic peptides were used to identify three functional T-cell epitopes present in subunit A which were recognized by the UreA-specific T cells. Analysis of H. pylori-specific cellular immune responses in recipient challenged and nonrecipient infected mice indicated a strong local restriction of the response in infected animals. The implications of these findings for the mechanism of protection and the development of peptide-based vaccination are discussed.
Helicobacter pylori is a gram-negative spiral bacterium which colonizes the stomach of humans and is associated with the development of chronic gastritis, peptic ulcer, gastric adenocarcinoma, and gastric lymphoma (17, 33, 42). Vaccination against H. pylori is a highly desirable alternative to antibiotic treatment (2). Successful vaccination trials in animal models have been performed using whole-cell sonicate, native or recombinant proteins from Helicobacter, and mucosal adjuvant (6, 8, 9, 13–15, 19, 20, 22, 25). Protection was dependent on major histocompatibility complex (MHC) class II-restricted, cell-mediated mechanisms (12, 32). Mohammadi et al. (26) showed production of interleukin 4 (IL-4) when immunized mice challenged with Helicobacter felis were treated with an anti-gamma interferon (IFN-γ) monoclonal antibody (MAb). This was not the case for nonimmunized infected mice, indicating that immunization did induce a Th2 response. The results of adoptive transfers of Helicobacter-specific Th2 cell lines generated from protected mice also suggested that Th2 cell-mediated immune responses play a protective role in this system (27). In addition, Saldinger et al. (36) and Ikewaki et al. (18) reported that repeated therapeutic mucosal immunization with recombinant urease B (UreB) and cholera toxin or H. pylori whole-cell lysate induced a Th2 response correlating with elimination of H. pylori. Together the data suggest that a type 2 response is correlated with protection, although the effector mechanism remains unclear.
Studies in this laboratory (16) and by Corthésy-Theulaz et al. (7) demonstrated that attenuated recombinant Salmonella enterica serovar Typhimurium expressing urease from H. pylori also induced high levels of protection in mice against H. pylori infection. Salmonella replicates directly in the Peyer's patches and disseminates via the mesenteric lymph nodes to systemic sites (spleen and liver) (21). It induces CD4+ and CD8+ T-cell responses as well as humoral and secretory antibody responses (40). However, the delivery of foreign antigen by live recombinant Salmonella preferentially induces the development of the Th1 subset of CD4+ T cells (4, 40, 43).
Here we investigated the role of CD4+ T cells induced by vaccination with live recombinant Salmonella in the murine model of H. pylori infection. CD4+ T cells specific for UreA of H. pylori were generated and characterized. The epitope specificity was determined by screening against synthetic peptides. The ability of these UreA-specific CD4+ T cells to reduce bacterial load in mouse stomachs prior to infection (prophylactic experiment) or after infection with H. pylori (therapeutic experiment) was assessed by adoptive transfers. Furthermore, we analyzed the cellular response in recipient and nonrecipient mice at the local and systemic levels. Finally, we addressed the question of the potential role of IL-4 and/or IL-13 in protection by transferring the cells to IL-4Rα chain-deficient mice.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free female BALB/c mice (6 to 8 weeks old) were obtained from the Federal Institute for Health Protection of Consumers and Veterinary Medicine (Berlin, Germany). Male and female BALB/c IL-4Rα−/− mice (31) were obtained from the Max-Planck-Institut für Immunobiologie (Freiburg, Germany) and were maintained in our animal facility. Mice were kept under specific-pathogen-free conditions, and the experiments were conducted according to the German animal protection law.
Bacterial strains.
Serovar Typhimurium SL3261 aroA mutant was used to express UreA and UreB from the plasmid pYZ97 as described earlier (16). Wild-type H. pylori P1 strain, urease-negative H. pylori P11, and mouse-adapted H. pylori P49 and P76 were described previously (16). H. pylori strains were grown on GC (gonococci) agar plates (Gibco, Eggenstein, Germany) containing 10% horse serum (serum plates; Gibco) or in brain heart infusion broth (Difco, Becton Dickinson, Sparks, Md.) containing 10% fetal calf serum (FCS) (Gibco) supplemented with 400 μg of streptomycin/ml when appropriate.
Immunization of mice and H. pylori infection.
Immunizations were performed as described in reference 16. For H. pylori infection, a primary broth culture with an optical density at 590 nm (OD590) of 0.1 was incubated for 24 h at 37°C under microaerophilic conditions with shaking. A secondary culture was set up under the same conditions. Bacteria were harvested by centrifugation, washed twice in phosphate-buffered saline (PBS), and resuspended in PBS to a final OD590 of 4.0/ml. An aliquot was taken to determine the number of CFU by replating on serum plates. Mice were infected with 100 μl of the suspension at an OD590 of 4.0/ml (corresponding to 1.0 × 108 to 1.0 × 109 CFU/mouse) by gastric intubation.
Assessment of H. pylori colonization.
Stomachs were processed as described earlier (16) and were cut into three tissue fragments. Each fragment then contained cardia, body, and antrum. One-third was submitted to a rapid urease test (Jatrox test; Procter and Gamble, Weiterstadt, Germany) (16). One-third of the stomach was placed in an Eppendorf tube containing brain heart infusion medium (150 μl), weighed, and vortexed for 1 min to resuspend H. pylori. Dilutions of the suspension were plated on serum plates containing streptomycin. The average number of CFU was expressed as log10 CFU/gram of tissue ± standard deviation.
Preparation of H. pylori antigens.
Soluble extracts of H. pylori P49 and P11 were prepared in PBS by sonicating five times with a Sonifier (Branson, Danbury, Conn.) at 5-s intervals (35% pulses) for 45 s. This suspension was centrifuged at 10,000 × g for 10 min at 4°C to remove cell debris. The protein content of the supernatant was determined by Bio-Rad protein assay (Bio-Rad Laboratories, München, Germany). This supernatant was used to coat plates for enzyme-linked immunosorbent assay (Nunc, Wiesbaden, Germany). Alternatively it was detoxified by addition of NaOH to a final concentration of 0.25 M and was incubated at 37°C for 3 h, followed by the addition of HCl in the presence of phenol red for neutralization (41). The preparation was diluted in culture medium, sterile filtered, and used in cell proliferation assays. UreA was purified from the sonicate by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After staining with Coomassie brillant blue (Serva; Boehringer, Ingelheim, Germany) the band corresponding to UreA (at 30 kDa) was cut and placed in 10 ml of 50 mM Tris-HCl (pH 8.0) for 30 min under shaking. The buffer was changed until the pH reached 8.0. The gel was cut into small pieces and was incubated overnight at room temperature with shaking in 6 ml of 50 mM Tris-HCl (pH 8.0) to elute proteins from the gel. The eluate was then filtered and used in proliferation assays.
UreB from H. pylori was expressed as a recombinant protein in Escherichia coli and was purified under denaturing conditions in 8 M urea by affinity chromatography on Ni2+-nitrilotriacetic acid resin (Qiagen, Hilden, Germany). Elution was performed with imidazole (200 mM), and the eluate was dialyzed overnight against l-arginine, glutathione, and 100 mM Tris-HCl (pH 7.8) and was dialyzed twice for 4 h against 10 mM Tris-HCl and 100 mM NaCl (pH 7.8). The concentrations of soluble proteins were quantified by Bradford assay.
Peptides from UreA were designed after predicted T-cell epitopes. All Iad-restricted epitopes that are known to activate T cells compiled in the MHCPEP database of MHC-binding peptides (http://wehih.wehi.edu.au/mhcpep) (5) were identified, and redundant and overlapping epitopes were disregarded. Seventy-nine unique epitopes were aligned using anchor position 1 with a large hydrophobic residue (I, L, V, F, Y, W). Amino acid residue frequencies for positions −3 to 14 were determined, and the resulting matrix was used to scan the H. pylori UreA gene (sequence expressed in the vaccine strain SL3261[pYZ97]) for potential T-cell epitopes using an algorithm developed by Davenport et al. (10). The 50 epitopes with the highest score were analyzed for clustering along the UreA sequence, which could be indicative of regions containing promising candidates (23). Based on this analysis, 10 17-mers were chosen (i.e., amino acids, 24 to 40, 28 to 44, 32 to 48, 35 to 51, 42 to 58, 74 to 90, 90 to 106, 170 to 186, 180 to 196, 209 to 225) and were synthesized.
Lymphocyte proliferation.
Single-cell suspensions were prepared from spleens as described earlier (29), resuspended in the antibody cocktail (anti-CD45/B220 [RA3-6B2], anti-CD8 [53.6], anti-CD11b [M1/70]), and incubated for 20 min on ice. Magnetic goat anti-rat immunoglobulin G (IgG) beads were used to deplete cells as described in the manufacturer's instructions (Vario-MACS; Miltenyi Biotec, Bergisch-Gladbach, Germany). The resulting population contained over 95% CD4+ T cells as determined by cytofluorimetry. Magnetic cell sorting-enriched CD4+ T cells were resuspended in complete Dulbecco minimal essential medium (DMEM) (29). The CD4+ cell suspension was adjusted to 4 × 105 cells/well in 96-well round-bottomed cell culture plates (Nunc) together with 2 × 105 syngeneic irradiated (2,500 rads) spleen cells from naive mice in a final volume of 200 μl. Gastric lymph nodes were harvested, and single-cell suspensions were prepared by forcing the nodes through nylon cell strainers. Cells were centrifuged for 10 min at 80 × g; the pellet was resuspended in buffered salt solution-FCS (29); and large particles were sedimented. The supernatant containing the cells was centrifuged at 80 × g for 10 min; the pellet was resuspended in complete DMEM. Cell suspensions were dispensed at 4 × 105 cells/well in 96-well round-bottomed cell culture plates (Nunc) in a final volume of 200 μl.
H. pylori P49 lysates or H. pylori P11 lysates detoxified by NaOH treatment were added at 2.5 μg/ml. UreA was added at 25 μl/well, and UreA peptides were added at 10, 1, 0.1, and 0.01 μl/ml. Concanavalin A (Con A; Sigma-Aldrich Chemicals, Deisenhofen, Germany) was used at 2 μg/ml. Cells were incubated at 37°C and 5% CO2 for 2 days (Con A) and for 5 days (antigens) and were pulsed with 0.5 μCi of [3H]thymidine (Amersham Pharmacia Biotech, Freiburg, Germany) for 16 h. Incorporated radioactivity was measured in a liquid scintillation counter. Values are expressed in counts per minute. Supernatants (100 μl) were taken for cytokine measurement.
Derivation of H. pylori urease-specific CD4+ T cells.
CD4+ T cells isolated from the spleens of two SL3261[pYZ97]-immunized BALB/c mice 21 weeks after immunization were restimulated with detoxified H. pylori P49 lysates (2.5 μg/ml) in the presence of irradiated, syngeneic spleen cells (106 cells/ml) in complete DMEM. Five days later, the medium was replaced by complete DMEM containing mouse IL-2 at 20 U/ml. Cells were stimulated every 2 weeks by using the same procedure and were tested periodically for specificity by measurement of thymidine uptake after antigen stimulation. Similarly, CD4+ T cells were generated against ovalbumin from ovalbumin-immunized mice and were used as a control.
Adoptive transfer protocol.
The CD4+ T cells (4 × 106/mouse) were administered intravenously to naive mice or mice infected 10 weeks earlier with H. pylori P76. Recipient naive mice were challenged the following day with H. pylori P76 as described above. Mice were sacrificed at various time points after infection (prophylactic experiment) or transfer (therapeutic experiment) and were evaluated for H. pylori colonization, cellular proliferation, cytokine production, and serum antibodies.
Cytokine measurement.
IL-4 and IFN-γ levels were determined by bioassay as described previously (30, 35). Briefly, WEHI-279 cells were used as indicator cells for the presence of IFN-γ. CT4-S cells were used to determine IL-4 levels. Serial dilutions of culture supernatants taken between 48 and 72 h after stimulation were performed, and 2 × 105 WEHI-279 cells/ml or 1.6 × 105 CT4-S indicator cells/ml were added to each well. Plates were incubated for 72 h (WEHI-279 cells) or 24 h (CT4-S cells) at 37°C in a CO2 incubator, and 10 μl of WST-1 (Boehringer GmbH, Mannheim, Germany) was added per well. Absorbance was measured at 450 nm using a microtiter plate reader (Titertek Multiskan MCC/340).
Expression of IL-10 and TNF-α mRNA was determined by semiquantitative reverse transcriptase (RT)-PCR as previously described (34). Briefly, total RNA from UreA-specific CD4+ T cells stimulated with urease-positive H. pylori lysate or from unstimulated control cells was extracted using a RNeasy Kit (Qiagen) and was reverse transcribed by SuperScript II RT (Gibco) using random hexamer primers (Promega, Mannheim, Germany). PCR was performed in the presence of a polycompetitor DNA construct containing IL-10, tumor necrosis factor alpha (TNF-α), and hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene segments (34), generously provided by Richard Locksley (University of California at San Francisco). The cDNA samples were first normalized on the basis of the levels of the constitutively expressed HPRT by using 10-fold dilutions of the cDNA. Tenfold dilutions of the equalized cDNA were then used in the presence of a fixed concentration of the polycompetitor DNA to determine the relative amounts of IL-10 and TNF-α mRNA by PCR. The point of equivalence in intensity of the competitor and cDNA sample allows comparison of cytokine mRNA levels between stimulated and unstimulated cells. Primer sequences and PCR conditions were as described by Reiner et al. (34). Amplification products were resolved on 2% agarose gels (FCM Bioproduct, Rockland, Maine) and were stained with ethidium bromide (Fluka Chemicals, Seelze, Germany).
For detection of intracellular cytokines, resting UreA-specific CD4+ T cells were stimulated for 6 h at 37°C on plate-bound anti-CD3 MAbs (clone 145-2C11) in the presence of brefeldin A (Sigma) at 12 μg/ml. Cells were fixed for 20 min in 2% paraformaldehyde–100 mM HEPES (pH 7.0), washed twice in PBS, and incubated for 20 min at room temperature in PBS containing 2% FCS and 0.1% saponin (Fluka Chemicals, Seelze, Germany) (buffer 1). Cells were incubated for 20 min with one of the following antibodies: anti-IFN-γ (XMG 1.2), anti-IL-4 (11B11), or rat IgG1 isotype control. They were washed twice in buffer 1 and fluorescein isothiocyanate-labeled F(ab′)2 goat anti-rat IgG (Biozol, Eching, Germany), which was added for 20 min at room temperature. After two washing steps, labeled cells were analyzed by flow cytometry (FACScan; Becton Dickinson) using Cell Quest software. Immunocytochemical detection of intracellular cytokines was performed according to the standard protocol as recommended by Pharmingen. Resting UreA-specific CD4+ T cells were stimulated overnight with anti-CD3 MAb. Eight-well microscope slides were coated with poly-l-lysine and washed in PBS, and cells were added for 10 to 20 min before fixation with 2% paraformaldehyde–100 mM HEPES (pH 7.0). Permeabilization of cells was performed with 0.1% saponin in PBS containing 0.5% bovine serum albumin. Anti-IL-10 MAb (JES5-16E3), anti-IL-4 MAb (11B11) or anti-IFN-γ MAb (clone XMG 1.2) was added for 30 min, and washed and biotinylated anti-rat IgG antibodies in 2% rabbit serum were added for 10 min. After washing, slides were incubated with avidin-biotin complex (Vector Laboratories, Burlingame, Calif.) for 30 min and were washed again. Labeling was developed with diaminobenzidine tetrahydrochloride.
Serum antibodies.
Serum antibody titers specific for urease were determined by enzyme-linked immunosorbent assay as previously described (16). Microtiter plates were coated with a soluble extract of H. pylori P49 (urease positive) (50 μg/ml) or H. pylori P11 (urease negative) (50 μg/ml). Bound specific antibodies were detected with goat anti-mouse IgG2a or goat anti-mouse IgG1 conjugated to horseradish peroxidase, used at a dilution of 1/5,000 (Nordic Immunological Laboratories, Tilburg, The Netherlands).
Statistics.
The statistical significance of results was determined using the GraphPad Prism program (version 3.0; GraphPad Software, San Diego, Calif.). An unpaired one-tailed t test and an unpaired two-tailed t test were used to determine the P values.
RESULTS
Characterization of the CD4+ T cells generated against UreA from H. pylori.
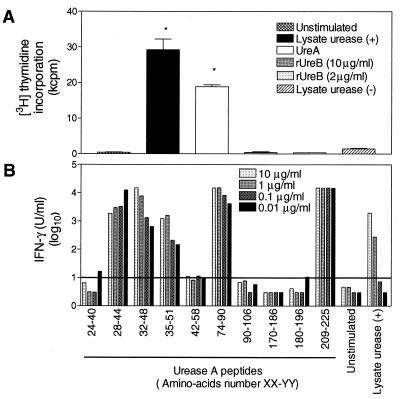
CD4+ T cells were derived from spleen cells of mice vaccinated with Salmonella expressing urease (subunits A and B) from H. pylori. By flow cytometry analysis, the cells were CD3+ CD4+ (>98%) (data not shown). Proliferation assays were performed to determine antigen specificity. As shown in Fig. 1A, almost no proliferation was observed when cells were stimulated by the urease-negative H. pylori P11 lysate. A significant response was obtained with urease-positive H. pylori P49 lysate and with purified UreA. No proliferation was obtained with recombinant UreB. To confirm that the response was specific for UreA, we also investigated whether the T cells recognized peptides of UreA predicted to contain T-helper cell epitopes. Different concentrations of peptides were used to stimulate the T cells, and IFN-γ production was measured in culture supernatants. Peptides corresponding to amino acids 28 to 44, 32 to 48, 35 to 51, 74 to 90, and 209 to 225 induced IFN-γ production (Fig. 1B). These data indicated that the CD4+ T cells were specific for UreA from H. pylori and defined at least three independent epitopes in H-2d recognized by corresponding T cells.
FIG. 1.
The CD4+ T cells isolated from the spleens of vaccinated mice are specific for subunit A of urease from H. pylori. (A) Cells were restimulated with a lysate from H. pylori containing (+) or not containing (−) urease (2.5 μg/ml), with purified UreA (25 μl/ml), or with recombinant UreB (rUreB). Values represent the average of three wells ± standard error of the mean. Statistically significant differences were calculated in comparison with unstimulated cells (P < 0.01, two-tailed t test) (∗). (B) Cells were restimulated with 10 synthetic UreA peptides at various concentrations (10 to 0.01 μg/ml) or with urease-positive H. pylori lysate (5 to 0.005 μg/ml). IFN-γ was measured in culture supernatant as an indication of T-cell activation. The horizontal line represents the mean concentration of IFN-γ detected in supernatants of unstimulated cells plus 3 standard deviations. The detection limit of the assay was 0.03 U/ml.
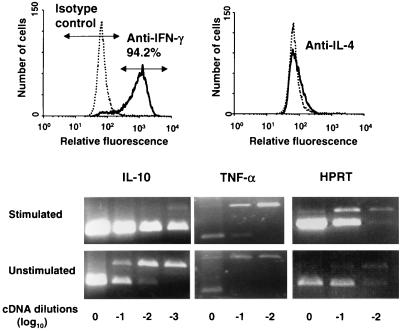
To further characterize the cells, cytokine secretion was analyzed. IFN-γ (72.5 to 98.5 U/ml) and IL-4 (27.7 to 158 U/ml) were detected in culture supernatants taken after restimulation with urease-positive H. pylori lysate or UreA but not in culture supernatants of unstimulated cells. Intracellular staining for IFN-γ and IL-4 showed that most of the cells (94.2%) produced IFN-γ (Fig. 2, top). Only a slight shift of the population was observed when cells were stained with an anti-IL-4 MAb (Fig. 2, top). Immunocytochemical detection of IFN-γ, IL-10, and IL-4 showed that most of the cells produced IFN-γ and IL-10, whereas IL-4 was detectable in only a minority of cells (data not shown). Analysis of IL-10 mRNA expression by RT-PCR confirmed that stimulation of cells by urease-positive H. pylori lysate induces IL-10 production. Expression of IL-10 was increased 100 times when compared to that in unstimulated cells (Fig. 2, bottom). Stimulated cells were also found to contain 10 times more TNF-α transcript than did nonstimulated cells (Fig. 2, bottom).
FIG. 2.
UreA-specific CD4+ T cells produce mainly IFN-γ and IL-10 and lower amounts of IL-4 and TNF-α. Fluorescence-activated cell sorter analysis (top) of intracellular staining for IFN-γ and IL-4 of cells after restimulation with plate-bound anti-CD3 MAb was carried out, as was detection of IL-10, TNF-α, and HPRT transcripts (bottom) by RT-PCR in RNA isolated from cells restimulated with H. pylori lysate containing urease or in control unstimulated cells. PCR products were separated on 2% agarose gels and were visualized after ethidium bromide staining. Upper bands correspond to products obtained for the competitor construct; lower bands correspond to products obtained for the cDNA isolated from stimulated cells. Expression of IL-10 was also confirmed by immunocytochemistry (data not shown).
In conclusion, we generated CD3+ CD4+ T cells which were specific for the subunit A of urease and produced mainly IFN-γ and IL-10 in vitro.
Adoptive transfer of UreA-specific CD4+ T cells into naive mice reduces gastric colonization by H. pylori.
In order to investigate whether the UreA-specific CD4+ T cells were able to induce protection against gastric colonization by H. pylori P76, adoptive transfers were performed into naive mice (prophylactic experiment, 4 × 106 cells/mouse). Control mice received CD4+ T cells generated against ovalbumin. A group of mice immunized with Salmonella expressing UreA and UreB was included in the experiment for comparison. All recipients were challenged with H. pylori P76 the day following the transfer. Vaccinated mice and a group of naive mice were challenged at the same time.
Mice from each group except the recipients of the ovalbumin T cells were sacrificed on days 7, 21, and 42 after infection. Mice which received ovalbumin-specific T cells were sacrificed on day 42.
The number of H. pylori CFU recovered from stomachs of mice receiving UreA-specific CD4+ T cells was reduced by a factor of 3 to 4 (P < 0.05, one- and two-tailed t tests) for all time points tested when compared to the number of CFU recovered from infected mice (Table 1). The reduction was observed as early as 7 days after infection (4.68 ± 0.26 CFU/g for mice which received UreA-specific T cells; 5.28 ± 0.27 CFU/g for infected mice [Table 1]) and persisted through at least day 42 after infection (5.00 ± 0.81 CFU/g for recipient mice; 5.50 ± 0.59 CFU/g for infected mice [Table 1]). Mice which received the ovalbumin-specific CD4+ T cells did not display any decrease in the number of CFU (Table 1). As expected, mice vaccinated with Salmonella expressing urease (subunits A and B) from H. pylori showed a strong reduction in the colonization of the stomach (to less than 10% of infected mice) for all time points (Table 1) (16).
TABLE 1.
Adoptive transfer of UreA-specific CD4+ T cells to naive mice reduces gastric colonization by H. pylori as early as 7 days after infection
| Daysb after infection | No. of CFUa recovered from the stomachs of:
|
|||
|---|---|---|---|---|
| Infected mice | SL3261[pYZ97]-vaccinated, challenged mice | Challenged mice receiving UreA- specific CD4+ T cells | Challenged mice receiving ovalbumin-specific CD4+ T cells | |
| 7 | 5.28 ± 0.27 | 4.23 ± 0.47 | 4.68 ± 0.26 | Not done |
| 21 | 5.53 ± 0.18 | 4.52 ± 0.39 | 5.01 ± 0.28 | Not done |
| 42 | 5.50 ± 0.59c | 3.85 ± 0.91c | 5.00 ± 0.81c | 5.77 ± 0.31d |
Values are expressed as log10 CFU/gram tissue ± standard deviation.
Values for days 7 and 21 are from one experiment (five to seven mice per group).
Values are the average of three experiments (20 mice in all).
This experiment was done once.
Adoptive transfer of UreA-specific CD4+ T cells is therapeutically effective.
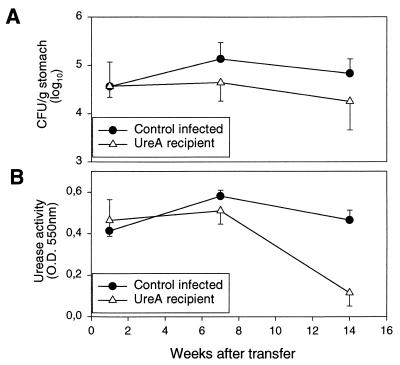
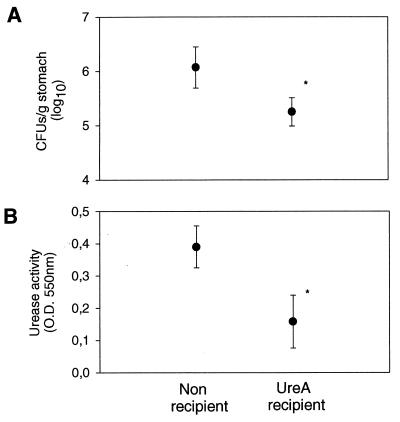
Mice which were infected for 10 weeks with H. pylori P76 were treated by intravenous injection of 4 × 106 UreA-specific CD4+ T cells. Recipient mice and nonrecipient mice were sacrificed 1, 7, and 14 weeks after transfer. Figure 3 shows that the gastric colonization in mice receiving UreA-specific CD4+ T cells was reduced in a time-dependent manner. One week after transfer, no differences in the numbers of CFU were observed between recipient and nonrecipient mice (Fig. 3A). On weeks 7 and 14 after transfer, however, the level of colonization in recipient mice was significantly reduced (P < 0.05, one-tailed t test), by factors of 3 and 3.8, respectively, when compared to the level in nonrecipient groups (Fig. 3A). This was also reflected in the levels of the urease activity measured (Fig. 3B).
FIG. 3.
Adoptive transfer of UreA-specific CD4+ T cells is therapeutically effective. T cells were transferred to recipients (UreA recipient) 10 weeks postinfection. Levels of colonization were assessed by quantitative culture (A) and by urease activity (B) at different time points after transfer. Results are expressed as the mean of five mice ± standard deviation. Statistically significant differences were calculated for nonrecipient and UreA recipient mice for each time point. The numbers of CFU were significantly different at weeks 7 (P < 0.05, one-tailed t test; P = 0.06, two-tailed t test) and 14 (P < 0.05, one-tailed t test; P = 0.08, two-tailed t test) posttransfer. Urease activities were significantly different (P < 0.05, one- and two-tailed t tests) at week 14.
Only UreA-specific CD4+ T cells are recovered from spleens of recipient mice early after challenge infection.
CD4+ T cells were isolated from spleens of recipient and nonrecipient infected mice. Cells were restimulated with urease-positive H. pylori lysate (2.5 μg/ml), purified UreA (25 μl/ml), and urease-negative H. pylori lysate (2.5 μg/ml) in the presence of syngeneic stimulator cells. Similarly, gastric lymph node cells were isolated from recipient and control infected mice and were restimulated with either urease-positive H. pylori lysate, purified UreA, or urease-negative H. pylori lysate.
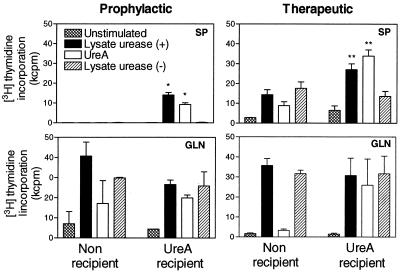
Six weeks postchallenge, proliferation of splenic CD4+ T cells in response to urease-positive H. pylori lysate and purified UreA was observed only with cells from recipient mice (Fig. 4, prophylactic experiment, SP). These cells did not proliferate in response to urease-negative H. pylori lysate, indicating that the response is solely due to the transferred cells. This is supported by the fact that at 6 weeks postchallenge, CD4+ T cells isolated from the spleens of infected mice did not respond to any of the tested antigens (Fig. 4, prophylactic experiment, SP). They proliferated, however, in response to Con A in a magnitude similar to that found in recipient mice (data not shown). CD4+ T cells isolated from spleens of naive mice did not respond to any of the tested antigens (data not shown). In contrast, cells isolated from gastric lymph nodes of recipient and nonrecipient mice proliferated when stimulated with urease-positive or urease-negative H. pylori lysates to a similar degree, indicating that the response is specific mostly for H. pylori antigens other than urease (Fig. 4, prophylactic experiment, GLN).
FIG. 4.
Only UreA-specific CD4+ T cells are recovered from spleens of recipient mice early after challenge. Proliferation of CD4+ T cells isolated from five to seven spleens (SP) or proliferation of total cells isolated from gastric lymph nodes (GLN) after prophylactic (6 weeks after infection) and therapeutic (24 weeks after infection, 14 weeks after prophylactic transfer) transfer was measured. Values represent the average of three wells ± standard error of the mean. Data are representative of three experiments for prophylactic transfer and one experiment for therapeutic transfer. Statistically significant differences were calculated on comparison with findings for unstimulated cells (P < 0.01, two-tailed t test) (∗) or for cells stimulated with lysate urease (−) (P < 0.05, two-tailed t test) (∗∗).
Twenty-four weeks after infection and 14 weeks after therapeutic transfer of UreA-specific CD4+ T cells, splenic CD4+ T cells isolated from recipient mice proliferated specifically to urease-positive H. pylori lysate and purified UreA, indicating a specific response to urease in the spleens of recipient animals (Fig. 4, therapeutic experiment, SP). Splenic CD4+ T cells from control mice infected for the same 24 weeks proliferated in response to urease-positive as well as to urease-negative lysates, showing that a systemic response to Helicobacter antigens is developing in long-term-infected mice (Fig. 4, therapeutic experiment, SP). Cells isolated from gastric lymph nodes of recipient and nonrecipient mice clearly responded to urease-positive and -negative H. pylori lysates, showing no specific response to urease, as observed for cells isolated from animals 6 weeks postinfection (Fig. 4, therapeutic experiment, GLN; prophylactic experiment, GLN).
Urease-specific antibody titers in recipient mice.
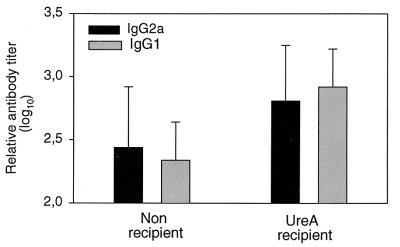
The level of anti-urease IgG2a and IgG1 antibodies in serum was evaluated 6 weeks after infection of mice receiving or not receiving UreA-specific CD4+ T cells. Both subclasses IgG2a and IgG1 were induced to a higher level in recipient mice than in controls (Fig. 5), indicating functional T-cell help by the transferred T cells. The average increase in antibody titers in recipient animals from those in nonrecipient animals was 2.3-fold for IgG2a (P > 0.1, two-tailed t test) but was 4-fold for IgG1 (P < 0.01, two-tailed t test). The tendency to have higher antibody titers for IgG1 than for IgG2a in recipient mice compared to control infected animals was noted in all experiments (n = 4).
FIG. 5.
Mice which received UreA-specific CD4+ T cells mounted IgG2a and IgG1 antibody responses against urease. Sera were taken 6 weeks after infection. Results are expressed as mean ± standard deviation. Groups contained five to seven mice. Statistically significant differences (two-tailed t test) between recipient and nonrecipient mice were calculated for IgG2a (P > 0.1) and IgG1 (P < 0.01).
Adoptive transfer of UreA-specific CD4+ T cells into naive IL-4Rα−/− mice reduces gastric colonization by H. pylori.
The increased IgG1 response in recipient mice could indicate a significant functional role for IL-4 produced by the transferred T cells in vivo. This may also be true for the protective effect exerted by these cells. To test this hypothesis, UreA-specific CD4+ T cells were adoptively transferred into naive BALB/c IL-4Rα−/− mice. These mice have a disrupted IL-4Rα gene and are sensitive neither to IL-4 nor to IL-13 (28, 31). Recipient and nonrecipient mice were infected the day following transfer with H. pylori P76, and stomach colonization was determined 34 days later (Fig. 6).
FIG. 6.
Adoptive transfer of UreA-specific CD4+ T cells to male IL-4Rα−/− mice reduces gastric colonization by H. pylori. Levels of colonization were assessed by quantitative culture (A) and by urease activity (B). Results are expressed as mean ± standard deviation. Groups contained six mice. The difference between values for recipient and nonrecipient mice was statistically significant (P < 0.01, one- and two-tailed t tests) for both CFU (A, ∗) and urease (B, ∗) activity. A significant reduction (P < 0.05, one- and two-tailed t tests) in number of CFU between recipient and nonrecipient female IL-4Rα−/− mice was also observed in two other independent experiments.
IL-4Rα−/− mice which received UreA-specific CD4+ T cells had a significantly reduced number of H. pylori in the stomach (5.25 ± 0.26 CFU/g, P < 0.05 for one- and two-tailed t tests) when compared to nonrecipient control IL-4Rα−/− mice (6.07 ± 0.39 CFU/g) (see also Fig. 6, top). The protective effect of UreA-specific CD4+ T cells was confirmed by a significant reduction in urease activity (Fig. 6, bottom).
The bacterial load in nonrecipient control IL-4Rα−/− mice was not significantly higher than in nonrecipient control wild-type mice.
H. pylori specific cellular and antibody responses in IL-4Rα−/− mice receiving UreA-specific CD4+ T cells.
When CD4+ T cells isolated from spleens of recipient IL-4Rα−/− mice were restimulated with H. pylori lysates in the presence of syngeneic stimulator cells, specific proliferation to urease-positive lysate was observed (Fig. 7A). As observed for wild-type BALB/c mice, no such response was detected with CD4+ T cells isolated from spleens of infected IL-4Rα−/− animals. CD4+ T cells from both recipient and nonrecipient mice proliferated in response to Con A (data not shown). The analysis of serum antibody responses showed that both recipient and control infected IL-4Rα−/− mice were able to mount a specific IgG2a antibody response (Fig. 7B). Recipient animals had a titer significantly higher than that in control infected mice. IgG1 antibodies were not detectable in any group.
FIG. 7.
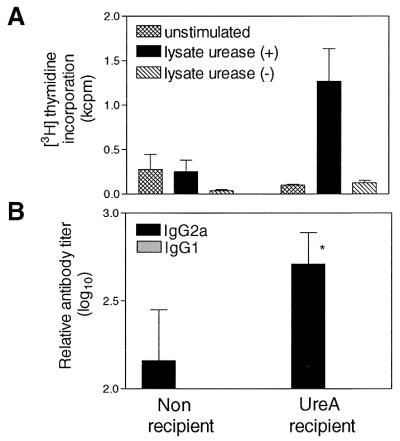
Induction of specific immune responses in IL-4Rα−/− mice receiving UreA-specific CD4+ T cells. (A) Proliferation of splenic CD4+ T cells isolated from recipient challenged or nonrecipient infected mice. Results are expressed as mean ± standard error of the mean. (B) Analysis of IgG2a and IgG1 antibody responses in serum. Results are expressed as the mean ± standard deviation. IgG2a titers in recipient mice were significantly higher than in nonrecipient mice (∗) (P < 0.05, two-tailed t test). IgG1 was not detectable in either group.
DISCUSSION
Several groups recently identified CD4+ T cells as mediators of protection against Helicobacter by immunizing gene knockout mice with urease or Helicobacter lysate in combination with mucosal adjuvant (3, 12, 32). Here we demonstrate that immunization with recombinant UreA- and UreB-expressing Salmonella also induces protective CD4+ T cells. Transfer of Helicobacter-specific CD4+ T cells into naive wild-type recipient mice strongly reduced H. pylori colonization, both prophylactically and therapeutically. The therapeutic effect in patently infected mice became detectable only weeks after the transfer. This suggests either that the mechanism of clearance is slow or that it is inhibited by the immune response first generated against the infection.
The immune response against H. pylori infection in mice and humans is ineffective in controlling the bacteria and appears to be biased towards a Th1 response (1, 11, 26, 38, 39, 44). In contrast, the results of several studies in mice suggest that type 2 responses characterized by IL-4 secretion are important in immunity against this pathogen (18, 26, 27, 36, 38), but their significance has never been tested under stringent conditions. Mohammadi et al. (27) reported that adoptive transfer of a Th2 cell line but not of a Th1 cell line reduced the magnitude of infection by a factor of 3 to 4 (as assessed by histological evaluation). These lines were not characterized with respect to antigen(s) recognized within H. felis sonicate. We generated CD4+ T cells which have a defined specificity. Adoptive transfer of these cells also reduced stomach colonization by a factor of 3 to 4. Moreover, the CD4+ T cells generated here produced IFN-γ and TNF-α as well as IL-10 and IL-4, indicating a mixed Th1-Th2 phenotype. Most cells produced IFN-γ and IL-10 upon specific restimulation. Coproduction of these two cytokines by murine CD4+ T cells has been shown in mice immunized with Salmonella (7, 40). Interestingly, the UreA-specific CD4+ T cells supported a bias towards Helicobacter-specific IgG1 formation after transfer in vivo. This suggested that IL-4 produced by these cells is functional in vivo and may play a significant role in reduction of colonization in recipient mice. To clarify the contribution of IL-4 in control of bacterial load, we transferred the UreA-specific CD4+ T cells into IL-4Rα−/− mice and found that H. pylori colonization was reduced to the same extent as in wild-type animals. We propose therefore that IL-4 and IL-13 play no role in protection mediated by urease-specific CD4+ T cells. It is also unlikely that IFN-γ plays a key role in protection induced by vaccination, because immunization in IFN-γ-deficient mice was reported to be as efficient as in wild-type animals (37). We are led to conclude that IL-10, TNF-α, or other as-yet-uninvestigated products of CD4+ T cells are responsible for protection.
The two chains of urease were the first H. pylori antigens whose vaccine potential was tested (6, 13, 19, 22, 25). Ferrero et al. (13) found that recombinant UreB but not UreA alone did protect mice against H. felis infection. The protective cells that we generated, however, are specific for UreA. MHC-restricted presentation of UreA may explain why Ferrero and colleagues, using Swiss Webster mice, did not classify this antigen as protective (13), since Michetti and colleagues (25), using BALB/c mice, demonstrated protection with UreA. We also predicted epitopes within the UreA sequence that should be presented by H-2d. At least three of these were indeed recognized by the UreA-specific CD4+ T cells. They constitute a first set of epitopes defined for an H. pylori antigen and open the attractive avenue of peptide immunization against this pathogen.
Detoxified lysates of urease-positive and -negative H. pylori strains enabled us to trace UreA- or UreB-specific T-cell responses in spleens and gastric lymph nodes of recipient, recombinant Salmonella-vaccinated, or H. pylori-infected control mice. This revealed that in the early phase of the infection (up to 6 weeks), no proliferation of the spleen cells isolated from infected mice was detectable in response to any H. pylori antigens. Splenocytes isolated from UreA-specific CD4+ T-cell recipients or from vaccinated mice also did not proliferate to H. pylori antigens other than urease. By contrast, gastric lymph node cells isolated from all groups of animals proliferated when stimulated with H. pylori lysates with or without urease. The fact that gastric lymph node cells from recipient mice did not show a significant increase in proliferation in the presence of urease suggests that the transferred cells were not homing preferentially to this lymphoid organ. It is reasonable to assume, however, that they homed not only to the spleen but also to the gastric mucosa to mediate protection. Homing may depend on expression of α4β7 integrin, as Michetti et al. (24) recently reported that T cells homing to the stomach mucosa preferentially expressed this integrin. Preliminary analysis indicates that the UreA-specific CD4+ T cells do express both integrin β1 and β7 chains as well as the α4 integrin chain (data not shown).
Interestingly, splenocytes from infected animals did not respond to any H. pylori antigens in the early phase of infection, but a response was detectable in the long-term-infected (14 weeks) mice, indicating that a systemic response against H. pylori develops only with time. Since reactive cells are, however, present in the gastric lymph nodes early after infection, the immune response to this pathogen appears to be locally constrained. The stomach may thus be considered an immunologically contained system as recently suggested (24). To our knowledge this is the first report where immune response in gastric lymph nodes was investigated in the Helicobacter model, and it appears that this organ is likely to be the site of induction of the response to H. pylori infection. A thorough analysis of the local response prompted by the observations reported here will help to understand why this immune response is ineffective in clearing the bacteria.
In conclusion, we generated CD4+ T cells specific for UreA from recombinant Salmonella-immunized mice, which protect subjects from stomach colonization by H. pylori. Although the UreA-specific CD4+ T cells had a mixed Th1-Th2 profile, IL-4 and IL-13 were not necessary for protection. These T cells allowed the identification of three independent epitopes that can be used to explore peptide vaccination. In addition, these T cells will be valuable tools for investigating homing sites and in situ behavior in order to study the mechanism that leads to clearance of the bacteria.
ACKNOWLEDGMENTS
This work was supported by the Fonds der Chemischen Industrie (T. F. Meyer). B. Lucas was a recipient of grant no. ERBBIO4CT 965114 from the European Community.
We are grateful to F. Brombacher for providing the IL-4Rα−/− mice. We thank R. Hurwitz for purification of urease B, C. Lattemann and V. Spehr for helpful advice, and A. Dietrich and U. Sack for technical assistance.
REFERENCES
- 1.Bamford K B, Fan X, Crowe S E, Leary J F, Gourley W K, Luthra G K, Brooks E G, Graham D Y, Reyes V E, Ernst P B. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard T G, Czinn S J, Nedrud J G. Host response and vaccine development to Helicobacter pylori infection. Curr Top Microbiol Immunol. 1999;241:181–213. doi: 10.1007/978-3-642-60013-5_10. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard T G, Czinn S J, Redline R W, Sigmund N, Harriman G, Nedrud J G. Antibody-independent protective mucosal immunity to gastric Helicobacter infection in mice. Cell Immunol. 1999;191:74–80. doi: 10.1006/cimm.1998.1421. [DOI] [PubMed] [Google Scholar]
- 4.Brett S J, Dunlop L, Liew F Y, Tite J P. Influence of the antigen delivery system on immunoglobulin isotype selection and cytokine production in response to influenza A nucleoprotein. Immunology. 1993;80:306–312. [PMC free article] [PubMed] [Google Scholar]
- 5.Brusic V, Rudy G, Kyne A P, Harrison L C. MHCPEP, a database of MHC-binding peptides: update 1997. Nucleic Acids Res. 1998;26:368–371. doi: 10.1093/nar/26.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corthesy-Theulaz I, Porta N, Glauser M, Saraga E, Vaney A C, Haas R, Kraehenbuhl J P, Blum A L, Michetti P. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology. 1995;109:115–121. doi: 10.1016/0016-5085(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 7.Corthésy-Theulaz I E, Hopkins S, Bachmann D, Saldinger P F, Porta N, Haas R, Zheng-Xin Y, Meyer T, Bouzourène H, Blum A L, Kraehenbuhl J-P. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect Immun. 1998;66:581–586. doi: 10.1128/iai.66.2.581-586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuenca R, Blanchard T G, Czinn S J, Nedrud J G, Monath T P, Lee C K, Redline R W. Therapeutic immunization against Helicobacter mustelae in naturally infected ferrets. Gastroenterology. 1996;110:1770–1775. doi: 10.1053/gast.1996.v110.pm8964402. [DOI] [PubMed] [Google Scholar]
- 9.Czinn S J, Cai A, Nedrud J G. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine. 1993;11:637–642. doi: 10.1016/0264-410x(93)90309-l. [DOI] [PubMed] [Google Scholar]
- 10.Davenport M P, Ho Shon I A, Hill A V. An empirical method for the prediction of T-cell epitopes. Immunogenetics. 1995;42:392–397. doi: 10.1007/BF00179401. [DOI] [PubMed] [Google Scholar]
- 11.D'Elios M M, Manghetti M, Almerigogna F, Amedei A, Costa F, Burroni D, Baldari C T, Romagnani S, Telford J L, Del Prete G. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. Eur J Immunol. 1997;27:1751–1755. doi: 10.1002/eji.1830270723. [DOI] [PubMed] [Google Scholar]
- 12.Ermak T H, Giannasca P J, Nichols R, Myers G A, Nedrud J, Weltzin R, Lee C K, Kleanthous H, Monath T P. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277–2288. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrero R L, Thiberge J M, Huerre M, Labigne A. Recombinant antigens prepared from the urease subunits of Helicobacter spp.: evidence of protection in a mouse model of gastric infection. Infect Immun. 1994;62:4981–4989. doi: 10.1128/iai.62.11.4981-4989.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrero R L, Thiberge J M, Kansau I, Wuscher N, Huerre M, Labigne A. The groES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499–6503. doi: 10.1073/pnas.92.14.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghiara P, Rossi M, Marchetti M, Di Tommaso A, Vindigni C, Ciampolini F, Covacci A, Telford J L, De Magistris M T, Pizza M, Rappuoli R, Del Giudice G. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect Immun. 1997;65:4996–5002. doi: 10.1128/iai.65.12.4996-5002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez-Duarte O G, Lucas B, Yan Z X, Panthel K, Haas R, Meyer T F. Protection of mice against gastric colonization by Helicobacter pylori by single oral dose immunization with attenuated Salmonella typhimurium producing urease subunits A and B. Vaccine. 1998;16:460–471. doi: 10.1016/s0264-410x(97)00247-8. [DOI] [PubMed] [Google Scholar]
- 17.Hussell T, Isaacson P G, Crabtree J E, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet. 1993;342:571–574. doi: 10.1016/0140-6736(93)91408-e. [DOI] [PubMed] [Google Scholar]
- 18.Ikewaki J, Nishizono A, Goto T, Fujioka T, Mifune K. Therapeutic oral vaccination induces mucosal immune response sufficient to eliminate long-term Helicobacter pylori infection. Microbiol Immunol. 2000;44:29–39. doi: 10.1111/j.1348-0421.2000.tb01243.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee C K, Weltzin R, Thomas W D J, Kleanthous H, Ermak T H, Soman G, Hill J E, Ackerman S K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–172. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 20.Lee C K, Soike K, Hill J, Georgakopoulos K, Tibbitts T, Ingrassia J, Gray H, Boden J, Kleanthous H, Giannasca P, Ermak T, Weltzin R, Blanchard J, Monath T P. Immunization with recombinant Helicobacter pylori urease decreases colonization levels following experimental infection of rhesus monkeys. Vaccine. 1999;17:1493–1505. doi: 10.1016/s0264-410x(98)00365-x. [DOI] [PubMed] [Google Scholar]
- 21.Mäkelä P H, Hoemaeche C E. Immunity to Salmonella. In: Kaufmann S H E, editor. Host response to intracellular pathogens. Austin, Tex: Medical Intelligence Unit, R. G. Landes Co.; 1997. pp. 143–166. [Google Scholar]
- 22.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 23.Meister G E, Roberts C G, Berzofsky J A, De Groot A S. Two novel T cell epitope prediction algorithms based on MHC-binding motifs; comparison of predicted and published epitopes from Mycobacterium tuberculosis and HIV protein sequences. Vaccine. 1995;13:581–591. doi: 10.1016/0264-410x(94)00014-e. [DOI] [PubMed] [Google Scholar]
- 24.Michetti M, Kelly C P, Kraehenbuhl J P, Bouzourene H, Michetti P. Gastric mucosal alpha(4)beta(7)-integrin-positive CD4 T lymphocytes and immune protection against Helicobacter infection in mice. Gastroenterology. 2000;119:109–118. doi: 10.1053/gast.2000.8548. [DOI] [PubMed] [Google Scholar]
- 25.Michetti P, Corthesy-Theulaz I, Davin C, Haas R, Vaney A C, Heitz M, Bille J, Kraehenbuhl J P, Sagara E, Blum A L. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology. 1994;107:1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 26.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 27.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn S J. Murine CD4 T-cell response to Helicobacter infection: Th1 cells enhance gastritis and Th2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 28.Mohrs M, Ledermann B, Kohler G, Dorfmuller A, Gessner A, Brombacher F. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol. 1999;162:7302–7308. [PubMed] [Google Scholar]
- 29.Morris L, Aebischer T, Handman E, Kelso A. Resistance of BALB/c mice to Leishmania major infection is associated with a decrease in the precursor frequency of antigen-specific CD4+ cells secreting interleukin-4. Int Immunol. 1993;5:761–767. doi: 10.1093/intimm/5.7.761. [DOI] [PubMed] [Google Scholar]
- 30.Morris L, Troutt A B, Handman E, Kelso A. Changes in the precursor frequencies of IL-4 and IFN-gamma secreting CD4+ cells correlate with resolution of lesions in murine cutaneous leishmaniasis. J Immunol. 1992;149:2715–2721. [PubMed] [Google Scholar]
- 31.Noben-Trauth N, Shultz L D, Brombacher F, Urban J F, Jr, Gu H, Paul W E. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc Natl Acad Sci USA. 1997;94:10838–10843. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pappo J, Torrey D, Castriotta L, Savinainen A, Kabok Z, Ibraghimov A. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect Immun. 1999;67:337–341. doi: 10.1128/iai.67.1.337-341.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 34.Reiner S L, Zheng S, Corry D B, Locksley R M. Constructing polycompetitor cDNAs for quantitative PCR. J Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. . (Errata, 173:33 and 175:275, 1994.) [DOI] [PubMed] [Google Scholar]
- 35.Reynolds D S, Boom W H, Abbas A K. Inhibition of B lymphocyte activation by interferon-gamma. J Immunol. 1987;139:767–773. [PubMed] [Google Scholar]
- 36.Saldinger P F, Porta N, Launois P, Louis J A, Waanders G A, Bouzourene H, Michetti P, Blum A L, Corthesy-Theulaz I E. Immunization of BALB/c mice with Helicobacter urease B induces a T helper 2 response absent in Helicobacter infection. Gastroenterology. 1998;115:891–897. doi: 10.1016/s0016-5085(98)70261-6. [DOI] [PubMed] [Google Scholar]
- 37.Sawai N, Kita M, Kodama T, Tanahashi T, Yamaoka Y, Tagawa Y, Iwakura Y, Imanishi J. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect Immun. 1999;67:279–285. doi: 10.1128/iai.67.1.279-285.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smythies L E, Waites K B, Lindsey J R, Harris P R, Ghiara P, Smith P D. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol. 2000;165:1022–1029. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 39.Sommer F, Faller G, Konturek P, Kirchner T, Hahn E G, Zeus J, Röllinghoff M, Lohoff M. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect Immun. 1998;66:5543–5546. doi: 10.1128/iai.66.11.5543-5546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Cott J L, Staats H F, Pascual D W, Roberts M, Chatfield S N, Yamamoto M, Coste M, Carter P B, Kiyono H, McGhee J R. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]
- 41.Villarreal B, Mastroeni P, de Hormaeche R D, Hormaeche C E. Proliferative and T-cell specific interleukin (IL-2/IL-4) production responses in spleen cells from mice vaccinated with aroA live attenuated Salmonella vaccines. Microb Pathog. 1992;13:305–315. doi: 10.1016/0882-4010(92)90040-u. [DOI] [PubMed] [Google Scholar]
- 42.Wotherspoon A C, Doglioni C, Diss T C, Pan L, Moschini A, de Boni M, Isaacson P G. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 43.Yang D M, Fairweather N, Button L L, McMaster W R, Kahl L P, Liew F Y. Oral Salmonella typhimurium (aroA-) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990;145:2281–2285. [PubMed] [Google Scholar]
- 44.Zevering Y, Jacob L, Meyer T F. Naturally acquired human immune responses against Helicobacter pylori and implications for vaccine development. Gut. 1999;45:465–474. doi: 10.1136/gut.45.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]