Abstract
Bowen’s disease (BD) is a form of intraepidermal squamous cell carcinoma (SCC), and it occasionally occurs on the perianal site. BD is often treated with surgical excision; however, sometimes surgical excision for perianal BD cannot preserve anal function. We report the case of a 72-year-old man presenting with perianal pain and BD. He was treated with Radiotherapy (RT) and preserved his normal anal sphincter function without any recurrence or late adverse event. Moreover, we observed the unique skin reaction called ‘tumoritis’, which is characterized by mucosal inflammation. Tumoritis indicates the true extent of the tumor and evaluating the tumor or lesion size based on the extent of tumoritis when performing RT is important.
Keywords: Bowen’s disease, Radiotherapy, Anal, Preserving anal function, Tumoritis
Introduction
Bowen’s disease (BD) is a form of intraepidermal squamous cell carcinoma (SCC) that was first described in 1912 [1]. BD commonly occurs on sites exposed to the sun such as the head and neck. In rare cases, the perianal site can be affected [2]. The preferred treatment for BD is wide local surgical excision; however, excision of the perianal site is problematic in terms of anal function preservation. Radiotherapy (RT) is an alternative treatment for BD that preserves organ function [3]. We encountered a case of perianal BD treated with RT that preserved the anal function, where the patient developed a unique skin reaction known as “tumoritis” during RT.
Case report
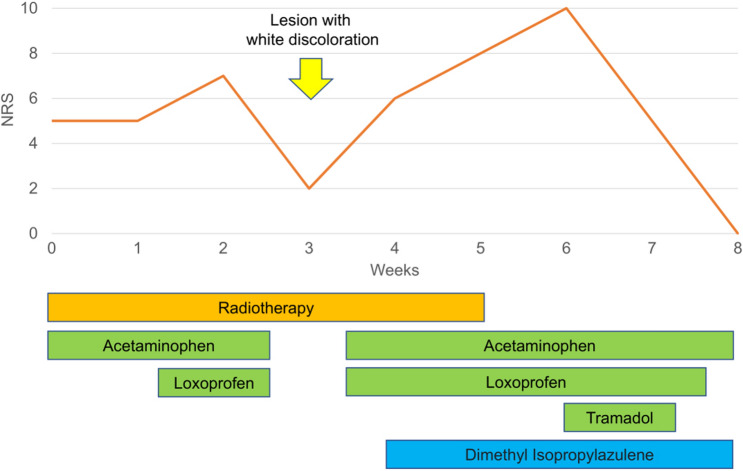
The patient was a 72-year-old man who presented with perianal erosion and pain. A black perianal lesion, measuring approximately 2 × 1 cm, was found on rectal examination, and the biopsy revealed BD. The lesion extended to the anal canal, hence, surgical excision was too invasive because it did not preserve anal function. Thus, non-surgical treatments were initially considered. The patient was treated with imiquimod cream (5%) and multiple cryotherapy sessions for 3 years. However, he was not completely cured, and the erosions gradually expanded (Fig. 1). He experienced persistent pain that felt like a needle prick; when it worsened, other treatment options were considered. The biopsy was still consistent with BD, and he was advised to undergo surgical excision. This required a stoma since anal canal preservation was not feasible. However, he wished for anal function preservation, and hence, decided to undergo RT. Before RT, digital rectal examination, contrast-enhanced magnetic resonance imaging (MRI), and 18F-flurodeoxyglucose-positron emission tomography (FDG-PET) were performed. No mass was identified by digital rectal examination. Diffusion-weighted imaging showed a high signal area 2.5 cm in diameter, which is considered a lesion (Fig. 2). Contrast-enhanced MRI revealed an enhancement around the lesion measuring approximately 5 cm in diameter, which is considered a microscopic lesion or inflammation, reaching the anal mucosa. FDG-PET showed FDG uptake in the lesion with no evidence of metastasis. RT was performed with a total dose of 50 Gy, delivered in 25 fractions, using 10 MV photons over 5 weeks (Fig. 3). Two weeks after the initiation of RT, only the visible lesion and its surrounding area flared up with some white discoloration. The pain then worsened during the treatment period with the numeric rating scale (NRS) increasing from 5 to 7. Three weeks after RT initiation, the NRS temporarily decreased to 2, and the lesion exhibited complete white discoloration (Fig. 4). Four weeks after RT initiation, the patient developed radiation dermatitis in all the irradiated sites, and the NRS increased to 6. After completing RT, the NRS increased to 10, but it gradually decreased to 0 within 3 weeks (Fig. 5). One week after completing RT, white discoloration of the lesion started to disappear, and the irradiated region became epithelialized within 3 weeks. FDG-PET was performed 3 months and 1 year after RT, and there was no significant FDG accumulation, suggestive of a recurrence or metastasis. One year and a half after RT, the patient became disease-free without developing late adverse events or pain that had bothered him for over 3 years. Moreover, his anal sphincter control was intact.
Fig. 1.
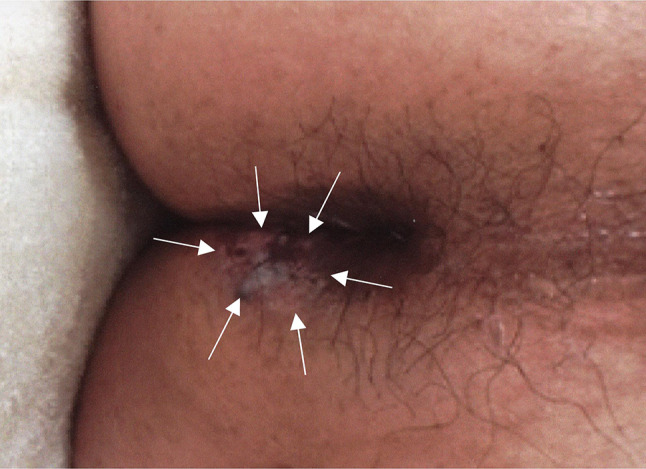
Perianal Bowen’s disease before radiotherapy (arrows)
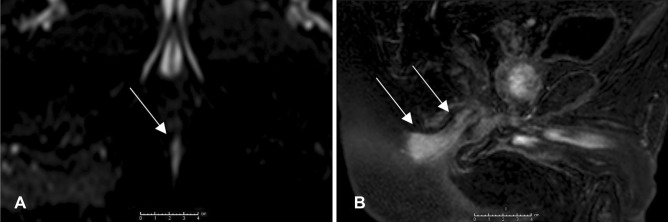
Fig. 2.
A Axial diffusion-weighted imaging showing a high signal area as a lesion (arrows). B Enhanced sagittal T1 weighted imaging showing an enhancement around the lesion reaching the anal mucosa (arrows)
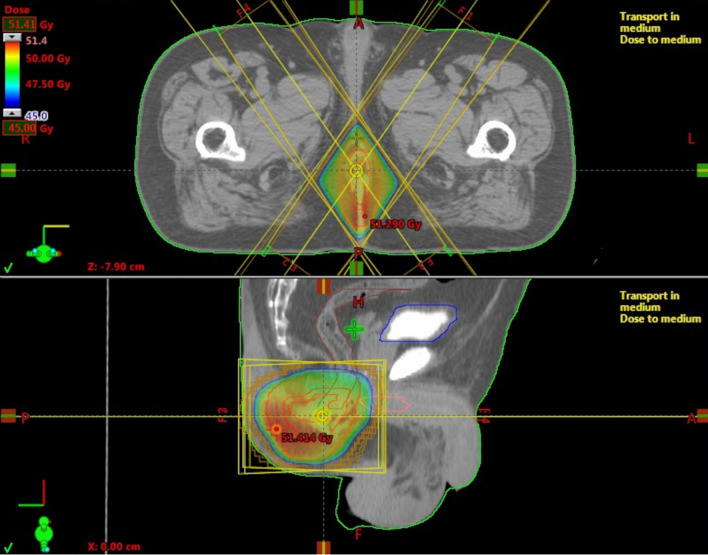
Fig. 3.
Irradiation field for perianal Bowen’s disease
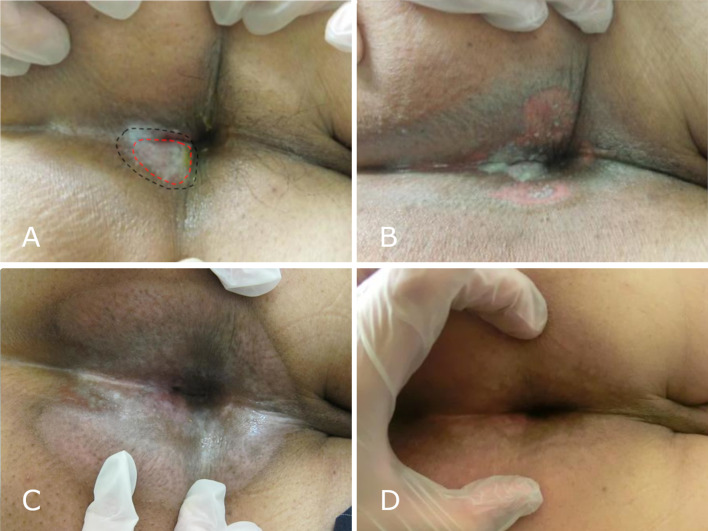
Fig. 4.
A Perianal region with the white lesion 3 weeks after radiotherapy (RT) initiation (red dashed line shows the visible tumor lesion before radiotherapy, the black line shows a region of flair and white discoloration at 28 Gy). B Perianal region 5 weeks after RT initiation, dermatitis in the radiation field (at 48 Gy). C Perianal region 8 weeks after RT initiation. D Perianal region 9 months after RT initiation
Fig. 5.
Neurologic rating scale scoring throughout the treatment course
Discussion
The various therapeutic regimens for BD include excision, cryotherapy, photodynamic therapy, imiquimod cream, 5-FU cream, and RT [2]. L. Lukas reported a series of 44 BD patients treated with RT, and the crude local control rate was 93% [3]. Despite the adequate control rate of RT for BD, surgical excision is more commonly performed, even for recurrent cases, due to the disadvantages of RT in terms of cost and inconvenience [2–4]. Especially, only a few studies reported on perianal BD treatment using RT [5, 6]. Conversely, RT with or without chemotherapy is the standard treatment for SCC of the anal canal or margin [7, 8]. Although BD is different from anal canal SCC, and RT is more invasive than other treatments such as cryotherapy or imiquimod cream, RT is an efficacious and safe treatment for anal lesions. As seen in this case, BD was adequately controlled in the patient, and the persistent pain was alleviated, although a longer follow-up was necessary. Moreover, RT preserves the anal canal. This positively affects the patient’s quality of life (QOL) because a stoma is no longer required [9]. RT is a viable treatment option for perianal BD that is refractory to less invasive treatment options. RT provides a favorable QOL for the patient.
The prescription dose for BD is unestablished. A previous report showed that a total dose of 10–52.5 Gy was suitable for BD, and some studies reported that local control seemed to be equal in patients treated with high- and low-dose regimens [3, 10]. In addition, because BD can occur in various sites in the body, the optimal dose might differ depending on the site. For example, in a study by Dupree et al., all patients with lower extremity BD treated with 50 Gy in 2.5–3.5-Gy fractions had nonhealing ulcers probably due to its poor vascularity [10]. We used 50 Gy in 25 fractions since our disease was refractory, and this dose is commonly used for anal canal cancer. The dosing was considered adequate; however, a large number of cases were needed to pursue optimal radiation dose for BD.
In this case, only the visible lesion and surrounding area flared up with some white discoloration before the appearance of radiation dermatitis. This unique skin reaction is considered as “tumoritis”, which is characterized by mucosal inflammation and pseudomembrane formation, indicating the true extent of the tumor or lesion. Tumoritis has been known to be secondary to prompt apoptosis resulting in rapid desquamation of the tumor site itself with relative sparing of the normal surrounding area because of the higher radiation sensitivity of tumor cells [11]. It sometimes develops in patients receiving up to 20 Gy of radiation [12, 13]. Tumoritis is a well-known reaction in head and neck SCC among radiation oncologists. Although the epidermis was different from the mucous membrane, tumoritis of skin lesions was only reported in mycosis fungoides [11]. In addition, to the best of our knowledge, this was the first report of tumoritis occurring in BD. Considering the mechanism of tumoritis, its occurrence in the skin lesion would be expected; however, the reports of tumoritis in the skin lesion are rare. This might be explained by the smaller number of opportunities to irradiate skin cancer than head and neck SCC. Also, our case had erosion so that the broken barrier structure of the skin might be affected, giving rise to tumoritis. It is difficult to distinguish tumoritis from radiation dermatitis, however, the change in NRS also probable supported the white discoloration, which indicated tumoritis. That is, the NRS for the patient’s pain increased at 10–20 Gy while white discoloration of the lesion was observed. The NRS decreased at approximately 30 Gy, although the pain severity steadily increased during RT in typical cases of radiation dermatitis or mucositis [14]. The increase in NRS probably reflected the ongoing inflammation secondary to tumoritis. Meanwhile, the decrease in NRS probably reflected that the erosion was covered by the pseudomembrane, resulting in less irritation or reduced tumor cells and inflammation. Tumoritis status is used to determine the presence of tumors in the radiation field. The occurrence of tumoritis on the radiation field edge implies that the tumor is larger than its estimated size, based on the initial physical examination. In these cases, subsequent field expansion is required. In the present case, the radiation field was not modified because the tumoritis was within the planned field. It is important to evaluate the extent of tumoritis in relation to the radiation field for BD and head and neck SCC cases.
In conclusion, RT was an effective treatment option for perianal BD, and it preserved anal function. It is necessary to evaluate the tumor size based on the extent of tumoritis when performing RT.
Acknowledgements
We would like to thank Hiroshi Kosaka and Hisateru Yasui for the patient treatment.
Abbreviations
- BD
Bowen’s disease
- SCC
Squamous cell carcinoma
- RT
Radiotherapy
- MRI
Magnetic resonance imaging
- FDG-PET
18F-flurodeoxyglucose-positron emission tomography
- FDG
18F-flurodeoxyglucose
- NRS
Numeric rating scale
- QOL
Quality of life
Author contributions
TI performed the patient treatment and wrote the final manuscript. TI, SO, RA, TM, TM, YT, TN and MK contributed to patient treatment and reviewed the manuscript. DY contributed to pathological diagnosis. All authors read and approved the final manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Research involving human participants and/or animals
This article does not contain any studies with human participants performed by any of the authors.
Ethics approval and consent to participate
The study was approved by the ethics committee of Kobe City Medical Center General Hospital. Informed consent was obtained from participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bowen JT. Precancerous drematoses: a study of two cases of chronic atypical epithelial proliferation. J Cutan Dis Syph. 1912;30(7):241–255. [PubMed] [Google Scholar]
- 2.Morton CA, Birnie AJ, Eedy DJ. British Association of Dermatologists’ guidelines for the management of squamous cell carcinoma in situ (Bowen’s disease) 2014. Br J Dermatol. 2014;170(2):245–260. doi: 10.1111/bjd.12766. [DOI] [PubMed] [Google Scholar]
- 3.Lukas VanderSpek LA, Pond GR, Wells W, Tsang RW. Radiation therapy for Bowen’s disease of the skin. Int J Radiat Oncol Biol Phys. 2005;63(2):505–510. doi: 10.1016/j.ijrobp.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Sahai A, Kodner IJ. Premalignant neoplasms and squamous cell carcinoma of the anal margin. Clin Colon Rectal Surg. 2006;19(2):88–93. doi: 10.1055/s-2006-942349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troicki F, Pappas A, Noone R, DeNittis A. Radiation therapy of recurrent anal squamous cell carcinoma in-situ: a case report. J Med Case Rep. 2010;4:2–4. doi: 10.1186/1752-1947-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhan W, Liao X, Tian T, Zhang RY, Li P, Wu XP, Yang Q. Perianal multiple Bowen’s disease: a case report. Int J Clin Exp Pathol. 2015;8(11):15039–15041. [PMC free article] [PubMed] [Google Scholar]
- 7.Anal Carcinoma V1 (2022) National Comprehensive Cancer Network. Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/anal.pdf
- 8.UKCCCR Anal Cancer Trial Working Party Epidermoid anal cancer Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. Lancet. 1996;348(9034):1049–1054. doi: 10.1016/S0140-6736(96)03409-5. [DOI] [PubMed] [Google Scholar]
- 9.Näsvall P, Dahlstrand U, Löwenmark T, Rutegård J, Gunnarsson U, Strigård K. Quality of life in patients with a permanent stoma after rectal cancer surgery. Qual Life Res. 2017;26(1):55–64. doi: 10.1007/s11136-016-1367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupree MT, Kiteley RA, Weismantle K, Panos R, Johnstone PAS. Radiation therapy for Bowen’s disease: lessons for lesions of the lower extremity. J Am Acad Dermatol. 2001;45(3):401–404. doi: 10.1067/mjd.2001.116581. [DOI] [PubMed] [Google Scholar]
- 11.Tward JD, Anker CJ, Gaffney DK, Bowen GM. Radiation therapy and skin cancer. Mod Pract Radiat Ther. 2012 doi: 10.5772/35843. [DOI] [Google Scholar]
- 12.Wang CC. Radiation therapy for head and neck neoplasms. New York: Wiley-Liss; 1997. [Google Scholar]
- 13.Kaanders JHAM, Kian Ang K. Early reactions as dose-limiting factors in radiotherapy. Semin Radiat Oncol. 1994;4(2):55–67. doi: 10.1016/S1053-4296(05)80033-5. [DOI] [PubMed] [Google Scholar]
- 14.Spijkervet FKL, van Saene HKF, Panders AK, Vermey A, Mehta DM. Scoring irradiation mucositis in head and neck cancer patients. J Oral Pathol Med. 1989;18(3):167–171. doi: 10.1111/j.1600-0714.1989.tb00756.x. [DOI] [PubMed] [Google Scholar]