Abstract
The phenolic substances, antioxidant capacity, and enzyme inhibitory activity of germinated Fagopyrum tataricum (Tartary buckwheat) under different microwave and l-phenylalanine (l-Phe) were investigated for the potential of enriching polyphenols. With the germination of seeds, the contents of total phenolics and total flavonoids increased, the antioxidant capacity and enzyme inhibitory activity were enhanced. The highest contents of total phenolics and total flavonoids in Tartary buckwheat sprouts were 17.41 mg GAE/g and 6.26 g RE/100 g DW (7 days), respectively. Correlation analysis and principal component analysis indicated that T3 (microwave 250 W, 90 s; l-Phe 2.9 mmol/L) could effectively improve the content of polyphenols, enzyme inhibition activity and antioxidant capacity of Tartary buckwheat sprouts obviously. This study hopes to provide some new ideas for enriching phenolics and improving antioxidation of Tartary buckwheat sprouts.
Keywords: Microwave, l-phenylalanine, Tartary buckwheat, Flavonoids, Antioxidant ability
Introduction
With the development of health food, the research and development of sprout food has attracted wide attention in food. Tartary buckwheat is a kind of grain rich in flavonoids. After germination, its flavonoids content and antioxidant capacity are significantly increased, and its nutritional value is greatly enhanced (Abdel-Aty et al., 2021).
In recent years, as a non-thermal processing technology, microwave has once again aroused the interest of researchers in the field of plant seed germination. Microwave can change the charge density of the cell membrane surface and the voltage inside and outside the membrane, and then affect the switch of ion channels and membrane permeability, thus triggering a series of biochemical reactions in the plant system (Vian et al., 2016). And suitable microwave power and time can regulate plant growth and metabolism and improve the speed of seed germination. Wang et al. (2018) irradiated Tartary buckwheat with different power of microwave and found that the germination rate of seeds treated with 600 W microwave for 10 s was the highest, the content of flavonoids increased and the scavenging ability on DPPH free radicals was enhanced.
l-Phe is not only the intermediate of plant phenylpropane metabolism, but also the precursor of flavonoid biosynthesis. Adding exogenous l-Phe can effectively enrich flavonoids, increase the content of polyphenols and other nutrients, and enhance the antioxidant capacity (Ma et al., 2019). The researchers used different concentrations of l-Phe to treat Tartary buckwheat to germinate, and found that the content of rutin and chlorogenic acid reacetylcholinesterased the highest when the concentration of l-Phe was 5 mmol/L, and in a certain concentration range, the content of polyphenols increased with the increase of l-Phe concentration (Seo et al., 2015).
Enzyme inhibition is one of effective methods to treat diseases such as Alzheimer's disease, dermatosis and diabetes (Balkan et al., 2018). Studies have found that inhibitors such as acetylcholinesterase play a good role in the control and treatment of Alzheimer's disease (Su et al., 2014). Tyrosinase inhibitors effectively improve and treat skin diseases such as melanoma, freckles, and age spots by inhibiting the activity of tyrosinase (the rate-limiting enzyme in melanin synthesis) (Tian et al., 2019). Flavonoids and polyphenols have been reported to have inhibitory effects on acetylcholinesterase activity (Szwajgier 2014). In addition, flavonoids also have a strong inhibitory effect on tyrosinase. Tian et al. (2019) isolated nine flavonoids from the root bark of Broussonetia papyrifera, among which compound 3 (newly discovered flavonoids) had the strongest tyrosinase inhibitory activity, with an IC50 value of 9.29 μmol/L, which was significantly lower than that of the positive control. However, although synthase inhibitors can be used in the treatment of corresponding diseases, there are some side effects such as gastrointestinal diseases (Zheng et al., 2012). Natural enzyme inhibitors produced by plants are gradually favored because of their high inhibition ability, sustainability, biocompatibility and safety (Lee et al., 2016).
In recent years, researchers have interfered with the germination of Tartary Buckwheat by various physical and chemical means, promoted the synthesis of flavonoids and other functional components, and enriched flavonoids. Bao et al. (2015) used microwave and exogenous hormone to treat Cyclobalanopsis glaucoides seeds, and found that this treatment could effectively promote seed germination and seedling growth. There are many studies on the germination of Tartary buckwheat treated with microwave and l-Phe alone, but the effect of microwave combined with exogenous l-Phe treatment on stimulating germination of Tartary buckwheat is rarely reported. In this paper, we used microwave combined with l-Phe method to treat Tartary buckwheat, to explore its effects on polyphenol content, antioxidant capacity, and acetylcholinesterase, and tyrosinase inhibitory activity of Tartary buckwheat sprouts. In addition, we analyzed the relationship between the content of phenolics and antioxidant capacity and two enzyme inhibitory activities. At the same time, the results of PCA were used to obtain the best conditions for flavonoids enrichment among 9 treatments.
Materials and methods
Materials and reagents
The Sichuan buckwheat No. 1 variety of Tartary buckwheat seed was grown in Liangshan, Sichuan Province. It was stored in the refrigerator at 4 °C after purchase. tyrosinase and acetylcholinesterase were purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). DPPH and ABTS were purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). l-Phe, 6-Hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid (Trolox), 2, 4, 6-Tris-(2-pyridyl)-s-triazine, 3, 4-dihydroxy-l-phenylalanine (L-DOPA), 5, 5'-Dithio bis-(2-nitrobenzoic acid) (DTNB), and acetylcholine iodide (ATCI) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Potassium persulfate, L-tyrosine, Huperzine A (HupA), and ethanol were purchased from National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China).
Conditions for the germination of Tartary buckwheat
Tartary buckwheat seeds were treated with nine methods of T1~T9 (Table 1) to study the effects of microwave combined with l-Phe treatment on the contents of total flavonoids and total phenolics, antioxidant capacity and enzyme inhibitory activity of germinated Tartary buckwheat in vitro. Firstly, all Tartary buckwheat seeds were soaked in potassium permanganate solution of 1 g/L for 5 min, then soaked in pure water for 4 h, washed, and irradiated by microwave. Lastly, the seeds were cultured in a seed incubator 25 °C, 75 ± 5% RH for 7 days (l-Phe was placed in the germination plate as a nutrient solution, changing the l-Phe solution every 24 h). During this period, the indexes of Tartary buckwheat sprouts at 3, 5, and 7 days were determined.
Table 1.
Treatment conditions of Tartary buckwheat germination
| Treatment methods | Treatment conditions | |
|---|---|---|
| Microwave (time/s, power/W) | l-Phe (mmol/L) | |
| CK | 0 s, 0 W | 0 |
| T1 | 90 s, 250 W | 0 |
| T2 | 90 s, 250 W | 1.0 |
| T3 | 90 s, 250 W | 2.9 |
| T4 | 90 s, 250 W | 5.0 |
| T5 | 60 s, 250 W | 2.9 |
| T6 | 120 s, 250 W | 2.9 |
| T7 | 90 s, 150 W | 2.9 |
| T8 | 90 s, 350 W | 2.9 |
| T9 | 0 s, 0 W | 2.9 |
Extraction of Tartary buckwheat sprouts
Tartary buckwheat sprouts were freeze-dried and ground into powder. 0.2 g ‘powder’ sample was weighed and put in a centrifugal tube. In each group of Tartary buckwheat powder samples, 4 mL 70% ethanol solution was added in ultrasonic water bath to extract 30 min (60 °C). Finally, it was centrifuged using an centrifuge (Changsha ordinary instrument Co., Ltd., Sichuan, China) at 12,000×g for 10 min, and the supernatant was prepared.
Determination of total flavonoids
Using sodium nitrite-aluminum nitrate colorimetric method (Ji et al., 2016), the absorbance value was determined at the wavelength of 502 nm with no sample solution as the blank control. Total flavonoids content of Tartary buckwheat sprouts were expressed as the rutin equivalent per 100 g of the sprouts (g RE/100 g).
Determination of total phenolics
According to the Folin-phenol colorimetric method (Kim et al., 2004), the absorbance of the sample solution at 765 nm was determined, and the standard curve was drawn with gallic acid standard. The results were expressed as gallic acid equivalent per gram of Tartary buckwheat sprout (mg GAE/g).
Determination of antioxidant capacity
The total reducing force was determined by ferric reducing antioxidant power (FRAP) method (Gao 2011). The absorbance was measured at 593 nm, and distilled water was used instead of the sample as a blank control. The scavenging ability on DPPH free radicals was determined by absorbance at 517 nm according to the method of Lee et al. (2020). The scavenging ability on ABTS free radicals was determined according to the method of Lee and Kim (2022). The absorbance was measured at 734 nm, and ethanol was used as blank control. The above results were expressed in terms of trolox equivalent per gram sprout (μmol TE/g).
Determination of inhibitory activity on tyrosinase and acetylcholinesterase
The detection of tyrosinase monophenolase inhibitory activity was slightly modified according to the method of Wang et al. (2019). The specific operations were as follows: sample group (A1): 50 μL sample solution, 50 μL tyrosinase (147 U/mL) and 100 μL phosphate buffered solution (PBS) (50 mmol/L pH 6.8) were added to 96-well enzyme plate, and incubated at room temperature for 5 min, then 50 μL L-tyrosine (2.5 mmol/L) was mixed, 25 min was incubated at room temperature and the absorbance was measured at 490 nm. Sample control group (A2): 50 μL tyrosinase was replaced by 50 μL PBS. Blank group (A3): 50 μL sample solution was replaced by 50 μL PBS. Blank control group (A4): 50 μL sample solution was replaced by 50 μL PBS, and 50 μL tyrosinase was replaced with 50 μL PBS. The inhibition rate is calculated as shown in Eq. 1 (same below):
| 1 |
The detection of tyrosinase diphenolase inhibitory activity was slightly modified according to Jo et al. (2012). The specific operations were as follows: A1: 50 μL tyrosinase (147 U/mL), 50 μL sample solution and 100 μL PBS (50 mmol/L, pH 6.8) were added to the 96-well enzyme plate, and incubated at room temperature for 5 min, then 50 μL L-DOPA (2.5 mmol/L) was mixed. 25 min was incubated at room temperature and the absorbance was measured at 475 nm. A2, A3, and A4 were treated the same as described above.
The acetylcholinesterase inhibitory activity test was slightly modified according to the Ellman method (Ingkaninan et al., 2003), and the sample acetylcholinesterase inhibitory activity test was carried out on the 96-well enzyme plate. The specific operations were as follows: A1: 140 μL PBS (0.1 mol/L, pH 7.6), 20 μL acetylcholinesterase (0.5 U/mL) and 20 μL sample solution were added to the plate and incubated at room temperature for 20 min, then 20 μL DTNB (10 mmol/L) and ATCI (10 mmol/L) were added to incubate for 30 min at 37 °C. The absorbance was measured at 405 nm. A2: 20 μL acetylcholinesterase was replaced by 20 μL PBS. A3: 20 μL sample solution was replaced by 20 μL PBS. A4: 20 μL 0.1 mg/mL HupA was used instead of 20 μL sample solution.
Correlation between antioxidant capacity, enzyme inhibitory activity, and the content of bioactive substances
The contents of total phenolics and total flavonoids in Tartary buckwheat sprouts were determined, and then the results of antioxidant capacity and inhibitory activity to tyrosinase and acetylcholinesterase were analyzed by correlation analysis and PCA.
Statistical analysis
The experiment was repeated for 3 times, and the results were expressed in the form of mean ± standard deviation. SPSS 22.0 was used for significance analysis and correlation analysis, and Duncan's multiple comparison method was used for variance analysis. The drawing was made by SigmaPlot 10.0 software.
Results and discussion
Changes of total flavonoids and total phenolics
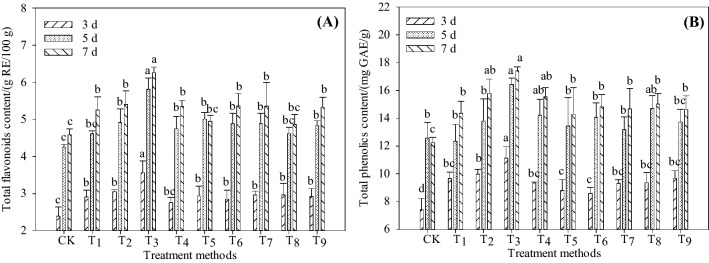
It can be seen from Fig. 1 that the contents of total flavonoids and total phenolics in Tartary buckwheat sprouts increased significantly after germination, and the content of flavonoids in sprouts increased significantly in 0–5 days after germination, and the trend was smooth in 5–7 days. It can be seen from Fig. 1 A that the content of flavonoids in sprouts germinated for 7 days increased by 14.40% and 16.14% respectively compared with CK under T1 and T9 treatments. Under the condition of T3, the highest flavonoids content was 6.26 ± 0.15 g RE/100 g DW (7 days), which increased by 36.38%, 19.22%, and 17.43% compared with CK, T1, and T9, respectively. The results of Fig. 1 B showed that the total phenolics content of T3 was significantly higher than CK, and increased by 49.98%, 30.77%, and 42.33% compared with CK at 3, 5, and 7 days, respectively. It showed that microwave and l-Phe treatment were helpful to the enrichment of polyphenols in Tartary buckwheat and had a certain synergistic effect. Seo et al. (2015) showed that low concentration of l-Phe can promote the accumulation of phenolics such as rutin in buckwheat sprouts, which is similar to the results of this study.
Fig. 1.
Contents of total flavonoids and total phenolics of Tartary buckwheat sprouts germinated at various conditions with microwave and l-Phe Note Different lowercase letters a, b, c, d on the same day indicate significant differences (P < 0.05). CK: 0 s, 0 W, 0 mmol/L (representing microwave time 0 s, microwave power 0 W, l-Phe concentration 0 mmol/L. the same below); T1: 90 s, 250 W, 0 mmol/L; T2: 90 s, 250 W, 1.0 mmol/L; T3: 90 s, 250 W, 2.9 mmol/L; T4: 90 s, 250 W, 5.0 mmol/L; T5: 60 s, 250 W, 2.9 mmol/L; T6: 120 s, 250 W, 2.9 mmol/L; T7: 90 s, 150 W, 2.9 mmol/L; T8: 90 s, 350 W, 2.9 mmol/L; T9: 0 s, 0 W, 2.9 mmol/L
In addition, the contents of total flavonoids and total phenolics in Tartary buckwheat sprouts treated with T2, T3, and T4 were higher than those of T9 and control groups on the 7th day, indicating that the promotion effect of total flavonoids and total phenolics in Tartary buckwheat sprouts was the best when the microwave condition is kept unchanged and the concentration of l-Phe was 2.9 mmol/L. Similarly, it can be seen from T3 and T9 that when l-Phe is at a suitable concentration, certain microwave conditions can effectively promote the production of total flavonoids and total phenolics. Therefore, l-Phe can directly participate in phenylpropane metabolism to produce polyphenols such as flavonoids, and enzymes involved in flavonoids biosynthesis may also be activated by microwave (Kumari et al., 2015) to assist in the production of more flavonoids.
Changes of antioxidant capacity of Tartary buckwheat sprouts
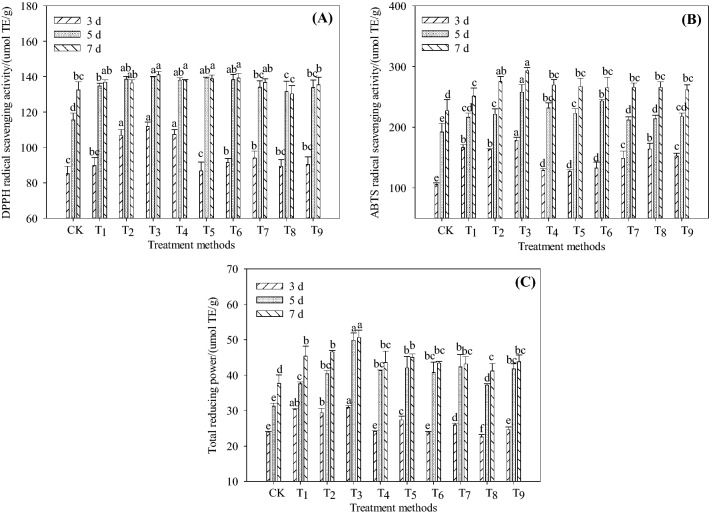
As shown in Fig. 2A, the DPPH radical scavenging ability of Tartary buckwheat sprouts germinated for 5 days under different treatments increased by 24.94–60.39% compared with that for 3 days. On the 7th day of germination, the highest scavenging ability of T3 group was 141.02 ± 1.83 μmol TE/g, which was 6.46% higher than that of CK. The results showed that microwave combined with l-Phe treatment could enhance the DPPH radical scavenging ability of Tartary buckwheat sprouts. This may be related to the increase of ployphenols content in sprouts after microwave and l-Phe treatment.
Fig. 2.
Antioxidant capacity of Tartary buckwheat sprouts germinated at various conditions with microwave and L-Phe. Note Different lowercase letters a, b, c, d, e, f, on the same day indicate significant differences (P < 0.05). CK: 0 s, 0 W, 0 mmol/L (representing microwave time 0 s, microwave power 0 W, l-Phe concentration 0 mmol/L. the same below); T1: 90 s, 250 W, 0 mmol/L; T2: 90 s, 250 W, 1.0 mmol/L; T3: 90 s, 250 W, 2.9 mmol/L; T4: 90 s, 250 W, 5.0 mmol/L; T5: 60 s, 250 W, 2.9 mmol/L; T6: 120 s, 250 W, 2.9 mmol/L; T7: 90 s, 150 W, 2.9 mmol/L; T8: 90 s, 350 W, 2.9 mmol/L; T9: 0 s, 0 W, 2.9 mmol/L
As shown in Fig. 2B, the scavenging ability on ABTS radicals increased significantly with the increase of seed culture time. The scavenging ability on ABTS free radicals in Tartary buckwheat sprouts after 7 days of germination was increased by 51.21–114.91% compared with that for 3 days. Compared with CK, the scavenging ability on ABTS radicals in different treatment groups was significantly increased, and the scavenging ability on ABTS radicals in T3 group (7 days) was the highest, which was 293.20 ± 4.99 μmol TE/g, which was 29.02% higher than CK. The results showed that microwave combined with l-Phe treatment could significantly enhance the scavenging ability of Tartary buckwheat sprouts to ABTS radicals. It is consistent with the result that seed germination can enrich more bioactive compounds such as phenolics and flavonoids (Lobo et al., 2010).
As shown in Fig. 2C, the total reducing capacity of Tartary buckwheat sprouts increased significantly after germination, and increased by 57.79–82.07% after 7 days of germination (P < 0.05). In addition, the total reducing power of microwave combined with l-Phe treatment for 5–7 days of germination was significantly higher than CK, and the total reducing power of T3 group for 3 and 5 days of germination increased by 29.41% and 59.22% compared with CK, respectively. The results showed that microwave combined with l-Phe treatment could enhance the antioxidant capacity of sprouting Tartary buckwheat. This is because Tartary buckwheat produces more reducing substances under germination conditions, such as polyphenols, amino acids containing sulfhydryl and phenolic hydroxyl groups (Wang et al., 2011), and the combined action of microwave and l-Phe greatly increases the content of these reducing substances, thus significantly increasing the antioxidant capacity of Tartary buckwheat sprouts.
Changes of inhibitory activity on tyrosinase and acetylcholinesterase in Tartary buckwheat sprouts
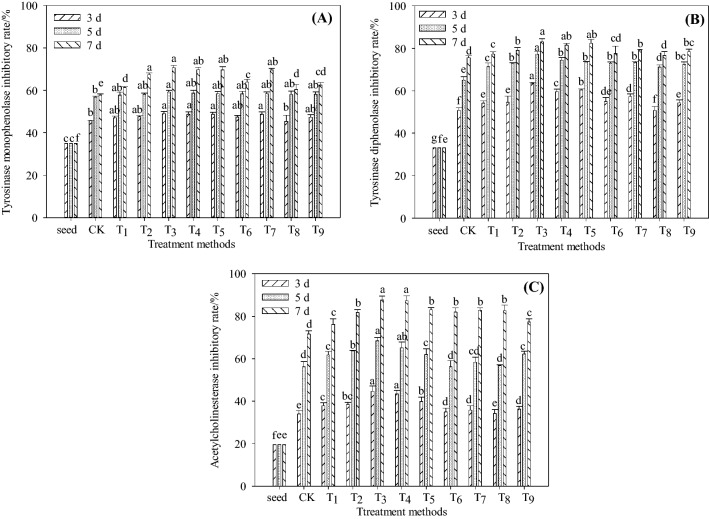
It can be seen from Fig. 3 that the tyrosinase inhibitory activity of Tartary buckwheat sprouts increased during germination. The tyrosinase monophenolase inhibitory activity and diphenolase inhibitory rate of the sprouts on the 7th day after germination were increased by 26.55–44.18% and 30.79–50.88% compared with those of 3rd day of germination, respectively, and the highest values were 101.85% (Fig. 3A) and 150.21% (Fig. 3B) higher than those of seeds, respectively. After germination, the tyrosinase inhibitory activity of sprouts treated with T3 was significantly higher than those of other treatments, indicating that microwave and l-Phe treatment had a certain synergistic effect. On the 3rd and 5th day of germination, the tyrosinase monophenolase inhibitory rate of sprouts treated with T1 and T3 was significantly higher than that of CK and seeds (Fig. 3A), and the inhibitory activity on tyrosinase diphenolase of Tartary buckwheat sprouts treated with T1 and T3 was also significantly higher than that of CK and seeds (Fig. 3B). The results showed that microwave and microwave combined with l-Phe treatment could significantly improve the tyrosinase inhibitory activity of Tartary buckwheat sprouts. This may be due to the enhancement of antioxidant capacity during Tartary buckwheat germination, thus inhibiting the oxidation of tyrosinase. Compared with CK, the tyrosinase monophenolase inhibitory rate of T1 and T3 groups increased by 2.14% and 9.90% respectively, while the diphenolase inhibitory rate increased by 6.07% and 22.21%, respectively. The results showed that microwave and l-Phe treatment had a greater effect on the inhibitory activity of diphenolase.
Fig. 3.
Tyrosinase and acetylcholinesterase inhibitory rate of Tartary buckwheat sprouts germinated at various conditions with microwave and l-Phe Note Different lowercase letters a, b, c, d, e, f, g on the same day indicate significant differences (P < 0.05). CK: 0 s, 0 W, 0 mmol/L (representing microwave time 0 s, microwave power 0 W, l-Phe concentration 0 mmol/L. the same below); T1: 90 s, 250 W, 0 mmol/L; T2: 90 s, 250 W, 1.0 mmol/L; T3: 90 s, 250 W, 2.9 mmol/L; T4: 90 s, 250 W, 5.0 mmol/L; T5: 60 s, 250 W, 2.9 mmol/L; T6: 120 s, 250 W, 2.9 mmol/L; T7: 90 s, 150 W, 2.9 mmol/L; T8: 90 s, 350 W, 2.9 mmol/L; T9: 0 s, 0 W, 2.9 mmol/L
As shown in Fig. 3C, the inhibitory activity of Tartary buckwheat sprouts on acetylcholinesterase increased with the increase of germination time. The acetylcholinesterase inhibitory rate of Tartary buckwheat sprouts on the 3, 5, and 7 days increased by 72.75–127.58%, 186.22–248.51%, and 264.60–344.83% compared with that of seeds, respectively. After 7 days of germination, the acetylcholinesterase inhibitory rate of T3 group was 14.95% and 22.19% higher than that of T1 and CK, respectively. The results showed that microwave and l-Phe treatment could significantly enhance the inhibitory activity on acetylcholinesterase in sprouts, and they had a synergistic effect. Zhang et al. (2018) found that the inhibitory effect of Duchesnea Indic polyphenols on acetylcholinesterase increased with the increase of polyphenol concentration. Therefore, it is not difficult to find that the effect of microwave and l-Phe on Tartary buckwheat germination not only increases the content of phenolics in Tartary buckwheat, but also enhances the inhibition of acetylcholinesterase.
Correlation between antioxidant capacity, tyrosinase, and acetylcholinesterase inhibitory activity and total flavonoids and total phenolics
Table 2 showed that total flavonoids content had positive correlation significantly with all indexes except monophenolase inhibitory activity, indicating that these indexes can be used to reflect the antioxidant capacity and two enzymes inhibitory activity of different treatments affected by phenolics on Tartary buckwheat sprouts. It is further explained that the enrichment of phenolic substances will lead to the overall improvement of antioxidant capacity.
Table 2.
Correlation analysis of various indexes of Tartary buckwheat sprouts at various conditions with microwave and l-Phe
| Index | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 |
|---|---|---|---|---|---|---|---|---|
| Total flavonoids | 1.000 | |||||||
| Total phenolics | 0.920** | 1.000 | ||||||
| Total reducing power | 0.637* | 0.642* | 1.000 | |||||
| DPPH radical scavenging ability | 0.622* | 0.724** | 0.452 | 1.000 | ||||
| ABTS radical scavenging ability | 0.825** | 0.896** | 0.574* | 0.454 | 1.000 | |||
| Tyrosinase monophenolase inhibitory rate | 0.532 | 0.497 | 0.499 | 0.582* | 0.147 | 1.000 | ||
| Tyrosinase diphenolase inhibitory rate | 0.641* | 0.553* | 0.510 | 0.610* | 0.190 | 0.923** | 1.000 | |
| Acetylcholinesterase inhibitory rate | 0.584* | 0.642* | 0.563* | 0.786** | 0.278 | 0.760** | 0.876** | 1.000 |
**Indicates extremely significant correlation at 0.01 level (bilateral), *Indicates significant correlation at 0.05 level (bilateral). P1, P2, P3, P4, P5, P6, P7, and P8 represent total flavonoids and total phenolics content, total reducing power, DPPH and ABTS radical scavenging ability, tyrosinase monophenolase, tyrosinase diphenolase, and acetylcholinesterase inhibitory rate. All indicators are statistically analyzed after standardized processing
The aim of PCA was to interpret the matrix of volatile compounds data set in a new system of coordinates (2 or 3 axes or dimensions), where the maximum amount of variation from the original data set can be depicted. Thus, the only few principal components (PC) from the PCA were used to explain the total variation amongst different fermentation processes (Zheng et al., 2013). The eigenvalues represent the contribution of the corresponding eigenvectors to the whole matrix after the orthogonalization of the matrix (Macciotta et al., 2010).
As can be seen from Table 3, the eigenvalue of PC 1 is 5.273, and the contribution rate of variance is 65.91%, which comprehensively reflects the total flavonoids, total phenolics, DPPH radical scavenging ability, total reducing power, acetylcholinesterase, and tyrosinase inhibitory activity of Tartary buckwheat sprouts. That is, it mainly reflects the content of polyphenols and antioxidant capacity of Tartary buckwheat sprouts. The eigenvalue of PC 2 is 1.505, and the contribution rate of variance is 18.82%, which represents the scavenging ability of Tartary buckwheat sprouts to ABTS radicals.
Table 3.
Component matrix and variance explanation of PCA of Tartary Buckwheat sprouts
| Index | Component 1 | Component 2 | Eigenvector of PC F1 | Eigenvector of PC F2 |
|---|---|---|---|---|
| Total phenolics | 0.906 | 0.378 | 0.033 | 0.167 |
| Total flavonoids | 0.889 | 0.320 | 0.032 | 0.141 |
| Acetylcholinesterase inhibitory rate | 0.855 | − 0.389 | 0.031 | -0.172 |
| Tyrosinase diphenolase inhibitory rate | 0.826 | − 0.504 | 0.030 | − 0.223 |
| DPPH radical scavenging ability | 0.812 | − 0.092 | 0.029 | − 0.041 |
| Tyrosinase monophenolase inhibitory rate | 0.767 | − 0.534 | 0.028 | − 0.236 |
| Total reducing power | 0.745 | 0.161 | 0.027 | 0.071 |
| ABTS radical scavenging ability | 0.667 | 0.731 | 0.024 | 0.323 |
| Eigenvalue | 5.273 | 1.505 | ||
| Contribution rate/% | 65.908 | 18.818 | ||
| Cumulative contribution rate/% | 65.908 | 84.726 |
According to the eigenvectors of PC F1 and F2 in Table 3, the following functions are obtained, such as Eqs. 2 and 3:
| 2 |
| 3 |
The comprehensive score F is equal to the sum of the product of each PC and the corresponding contribution rate, such as Eq. 4:
| 4 |
According to the comprehensive score of Table 4, the nine treatments were ranked as follows: . The results showed that the combined treatment of microwave and l-Phe could effectively improve the content and antioxidant capacity of Tartary buckwheat sprouts, and the T3 group had the best effect. Inappropriate microwave power (or treatment time) and l-Phe concentration would affect the phenolic composition and activity of Tartary buckwheat sprouts, and even the results of treatment were lower than those of the two alone treatment groups (T1 and T9).
Table 4.
Comprehensive score and ranking of Tartary buckwheat sprouts at various conditions with microwave and l-Phe
| Treatment methods | PC score | Comprehensive score F | Sorting | |
|---|---|---|---|---|
| F1 | F2 | |||
| CK | − 0.403 | − 0.074 | − 0.280 | 10 |
| T1 | − 0.278 | − 0.058 | − 0.194 | 4 |
| T2 | − 0.246 | − 0.180 | − 0.196 | 6 |
| T3 | − 0.160 | − 0.012 | − 0.108 | 1 |
| T4 | − 0.277 | − 0.046 | − 0.191 | 3 |
| T5 | − 0.300 | 0.059 | − 0.187 | 2 |
| T6 | − 0.332 | − 0.036 | − 0.226 | 9 |
| T7 | − 0.289 | − 0.071 | − 0.204 | 7 |
| T8 | − 0.330 | 0.063 | − 0.206 | 8 |
| T9 | − 0.306 | 0.034 | − 0.195 | 5 |
It was found that phenolic compounds were not only positively correlated with total reducing power, scavenging ability on DPPH radical and ABTS radical (Zheng et al., 2018), but also inhibited tyrosinase to a certain extent (Zolghadri et al., 2019). Phenolic compounds were also the main components of anti-tyrosinase activity (Wang et al., 2020). This is because in plants, flavonoids bind to tyrosinase mainly through hydrogen bonding. In flavonol components, the inhibitory ability of flavonol to tyrosinase is positively correlated with the affinity of flavonol to tyrosinase (Apaza et al., 2021). And the existence of C2 = C3 double bond in flavonoids can enhance their ability of inhibiting tyrosinase and binding affinity to tyrosinase (Fan et al., 2019). Fan (2018) by studying the inhibition of myricetin, dihydromyricetin, and quercetin on tyrosinase activity, it was found that the inhibitory ability of myricetin, dihydromyricetin, and quercetin on tyrosinase was quercetin > dihydromyricetin > myricetin. Khan et al. (2009) pointed out that flavonoids have a strong hydrogen bond interaction with important amino acids of acetylcholinesterase, thus inhibiting the activity of acetylcholinesterase. It was also found that flavonoids and biflavones had better inhibitory activity on acetylcholinesterase than flavonoid glycosides (Nguyen et al., 2021). Li et al. (2020) found that compound 1 has stronger acetylcholinesterase inhibitory activity from 15 flavonoids isolated from plants, and compound 1 is not only a reversible inhibitor but also competes with other compounds to inhibit acetylcholinesterase. It can be seen that the content of flavonoids, polyphenols, and other active substances and the changes of their structural functional groups will affect the inhibition of tyrosinase and acetylcholinesterase activity.
In conclusion, Microwave and exogenous l-Phe treatment could significantly increase the contents of total flavonoids and total phenolics in Tartary buckwheat sprouts, increase the total reducing power, the scavenging rates on DPPH and ABTS free radicals, and the inhibition rates of acetylcholinesterase and tyrosinase enzymes. And there is a certain synergistic effect. According to the results of PCA, the best synergistic effect is T3: microwave 250 W, 90 s; l-Phe 2.9 mmol/L. The results of correlation analysis showed that the antioxidant capacity and the inhibition rates of acetylcholinesterase and tyrosinase were positively correlated with the contents of total phenolics and total flavonoids in Tartary buckwheat sprouts extract. It adds value to the application of sprouted Tartary buckwheat in food, medicine, and cosmetics. However, the antioxidant capacity and enzyme inhibitory activity of Tartary buckwheat sprouts and the types and interaction mechanism of flavonoids in plants are not clear, the screening of tyrosinase and acetylcholinesterase inhibitors from Tartary buckwheat sprouts remains to be done.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 31772025) and the Anhui Natural Science Foundation (Grant No. 1808085MC93).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wenping Peng, Email: 2319389722@qq.com.
Nan Wang, Email: 1494390165@qq.com.
Shunmin Wang, Email: wangshunmin@126.com.
Junzhen Wang, Email: wangjunzhen108@163.com.
Zixiu Bian, Email: 1977671654@qq.com.
References
- Abdel-Aty AM, Elsayed AM, Salah HA, Bassuiny RI, Mohamed SA. Egyptian chia seeds (Salvia hispanica L.) during germination: Upgrading of phenolic profile, antioxidant, antibacterial properties and relevant enzymes activities. Food Science and Biotechnology. 2021;30:723–734. doi: 10.1007/s10068-021-00902-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apaza Ticona L, Thiebaut Estrada C, Rumbero Sánchez Á. Inhibition of melanin production and tyrosinase activity by flavonoids isolated from Loranthus acutifolius. Natural Product Research. 2021;35:4690–4693. doi: 10.1080/14786419.2019.1709185. [DOI] [PubMed] [Google Scholar]
- Balkan IA, Doğan HT, Zengin G, Colak N, Ayaz FA, Gören AC, Kırmızıbekmez H, Yeşilada E. Enzyme inhibitory and antioxidant activities of Nerium oleander L. flower extracts and activity guided isolation of the active components. Industrial Crops and Products. 2018;112:24–31. doi: 10.1016/j.indcrop.2017.10.058. [DOI] [Google Scholar]
- Bao X, Zheng S, Li L, Wang K, Zhang W, Wang W, Wang W, Yu G. Effects of microwave radiation and hormone presoaking seeds on germination and seedling growth of Cyclobalanopsis glaucoides. Seed. 2015;34:12–15. [Google Scholar]
- Fan M. Study on the inhibition mechanism of flavonoids towards tyrosinase and structure-activity relationship. Nanchang University. (2018)
- Fan M, Ding H, Zhang G, Hu X, Gong D. Relationships of dietary flavonoid structure with its tyrosinase inhibitory activity and affinity. LWT-Food Science and Technology. 2019;107:25–34. doi: 10.1016/j.lwt.2019.02.076. [DOI] [Google Scholar]
- Gao B. Studies on components identification and biological activities of flavonoids and essential oils in PCR'Chachi' peels. Huazhong Agricultural University. (2011)
- Ingkaninan K, Temkitthawon P, Chuenchom K, Yuyaem T, Thongnoi W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. Journal of Ethnopharmacology. 2003;89:261–264. doi: 10.1016/j.jep.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Ji H, Tang W, Zhou X, Wu Y. Combined effects of blue and ultraviolet lights on the accumulation of flavonoids in Tartary buckwheat sprouts. Polish Journal of Food and Nutrition Sciences. 2016;66:93. doi: 10.1515/pjfns-2015-0042. [DOI] [Google Scholar]
- Jo Y-H, Seo G-U, Yuk H-G, Lee S-C. Antioxidant and tyrosinase inhibitory activities of methanol extracts from Magnolia denudata and Magnolia denudata var purpurascens flowers. Food Research International. 2012;47:197–200. doi: 10.1016/j.foodres.2011.05.032. [DOI] [Google Scholar]
- Khan MTH, Orhan I, Şenol F, Kartal M, Şener B, Dvorská M, Šmejkal K, Šlapetová T. Cholinesterase inhibitory activities of some flavonoid derivatives and chosen xanthone and their molecular docking studies. Chemico-Biological Interactions. 2009;181:383–389. doi: 10.1016/j.cbi.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Kim S-L, Kim S-K, Park C-H. Introduction and nutritional evaluation of buckwheat sprouts as a new vegetable. Food Research International. 2004;37:319–327. doi: 10.1016/j.foodres.2003.12.008. [DOI] [Google Scholar]
- Kumari S, Krishnan V, Sachdev A. Impact of soaking and germination durations on antioxidants and anti-nutrients of black and yellow soybean (Glycine max L.) varieties. Journal of Plant Biochemistry and Biotechnology. 2015;24:355–358. doi: 10.1007/s13562-014-0282-6. [DOI] [Google Scholar]
- Lee JH, Kim HJ, Jee Y, Jeon Y-J, Kim HJ. Antioxidant potential of Sargassum horneri extract against urban particulate matter-induced oxidation. Food Science and Biotechnology. 2020;29:855–865. doi: 10.1007/s10068-019-00729-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim HJ. Antioxidant activities of premature and mature mandarin (Citrus unshiu) peel and juice extracts. Food Science and Biotechnology. 2022;18:1–7. doi: 10.1007/s10068-022-01064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Baek N, Nam TG. Natural, semisynthetic and synthetic tyrosinase inhibitors. Journal of Enzyme Inhibition and Medicinal Chemistry. 2016;31:1–13. doi: 10.3109/14756366.2015.1004058. [DOI] [PubMed] [Google Scholar]
- Li M, Gao X, Lan M, Liao X, Su F, Fan L, Zhao Y, Hao X, Wu G, Ding X. Inhibitory activities of flavonoids from Eupatorium adenophorum against acetylcholinesterase. Pesticide Biochemistry and Physiology. 2020;170:104701. doi: 10.1016/j.pestbp.2020.104701. [DOI] [PubMed] [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Reviews. 2010;4:118. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Bian Z, Chen X, Chu Y, Wang S. Effects of Microwave and L-phe treatment on main nutritional components in germination Tartary Buckwheat. Journal of Anhui Polytechnic University. 2019;34:1–7. [Google Scholar]
- Macciotta NPP, Gaspa G, Steri R, Nicolazzi EL, Dimauro C, Pieramati C, Cappio-Borlino A. Using eigenvalues as variance priors in the prediction of genomic breeding values by principal component analysis. Journal of Dairy Science. 2010;93:2765–2774. doi: 10.3168/jds.2009-3029. [DOI] [PubMed] [Google Scholar]
- Nguyen TTH, Nguyen VT, Van Cuong P, Nguyen Thanh T, Le Thi TA, Mai Huong DT, Truong BN, Litaudon M, Ninh The S. A new flavonoid from the leaves of Garcinia mckeaniana Craib and α-glucosidase and acetylcholinesterase inhibitory activities. Natural Product Research. 2021;24:1–11. doi: 10.1080/14786419.2021.1916019. [DOI] [PubMed] [Google Scholar]
- Seo J-M, Arasu MV, Kim Y-B, Park SU, Kim S-J. Phenylalanine and LED lights enhance phenolic compound production in Tartary buckwheat sprouts. Food Chemistry. 2015;177:204–213. doi: 10.1016/j.foodchem.2014.12.094. [DOI] [PubMed] [Google Scholar]
- Su Y, Wang Q, Wang C, Chan K, Sun Y, Kuang H. The treatment of Alzheimer's disease using Chinese medicinal plants: from disease models to potential clinical applications. Journal of Ethnopharmacology. 2014;152:403–423. doi: 10.1016/j.jep.2013.12.053. [DOI] [PubMed] [Google Scholar]
- Szwajgier D. Anticholinesterase activities of selected polyphenols–a short report. Polish Journal of Food and Nutrition Sciences. 64: 59-64 (2014)
- Tian J-L, Liu T-L, Xue J-J, Hong W, Zhang Y, Zhang D-X, Cui C-C, Liu M-C, Niu S-L. Flavanoids derivatives from the root bark of Broussonetia papyrifera as a tyrosinase inhibitor. Industrial Crops and Products. 2019;138:111445. doi: 10.1016/j.indcrop.2019.06.008. [DOI] [Google Scholar]
- Vian A, Davies E, Gendraud M, Bonnet P. Plant responses to high frequency electromagnetic fields. BioMed Research International. 2016: (2016) [DOI] [PMC free article] [PubMed]
- Wang M, Yan H, Tian H, Mao Q, Zhang Q, Li M. Antioxidant and enzyme inhibitory activities of Lavandula angustifolia Mill. extracts. Science and Technology of Food Industry. 2019;40:14–20. [Google Scholar]
- Wang R, Wang G, Sui W, Zhou C, Li S, Ji Y, Si C. Tyrosinase inhibitory performance of hydrolysate from post-washing liquor of steam exploded corn stalk and its fractionation enhancement. Industrial Crops and Products. 2020;154:112652. doi: 10.1016/j.indcrop.2020.112652. [DOI] [Google Scholar]
- Wang S, Meckling KA, Marcone MF, Kakuda Y, Tsao R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. Journal of Agricultural and Food Chemistry. 2011;59:960–968. doi: 10.1021/jf1040977. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang J, Guo Y. Microwave irradiation enhances the germination rate of tartary buckwheat and content of some compounds in its sprouts. Polish Journal of Food and Nutrition Sciences. 2018;68:25. doi: 10.1515/pjfns-2017-0025. [DOI] [Google Scholar]
- Zhang C, Liu J, Wu X, Shen W, Li J, Shi H, Wang Z. Microwave-assisted extraction, in vitro antioxidant activity, α-glucosidase inhibition and acetylcholinesterase inhibition of Duchesnea indica polyphenols. Southwest China Journal of Agricultural Sciences. 2018;31:1171–1179. [Google Scholar]
- Zheng C, Hao J, Song S, Jiang Z, Liu H. Effect of slightly acidic electrolyzed water on the bioactive compounds and antioxidant activity of Tartary buckwheat sprouts. Food Science. 2018;39:20–25. [Google Scholar]
- Zheng J, Wu C-D, Huang J, Zhou R-Q, Liao X-P. Analysis of volatile compounds in Chinese soy sauces moromi cultured by different fermentation processes. Food Science and Biotechnology. 2013;22:605–612. doi: 10.1007/s10068-013-0121-x. [DOI] [Google Scholar]
- Zheng Z-P, Tan H-Y, Wang M. Tyrosinase inhibition constituents from the roots of Morus australis. Fitoterapia. 2012;83:1008–1013. doi: 10.1016/j.fitote.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Zolghadri S, Bahrami A, Hassan Khan MT, Munoz-Munoz J, Garcia-Molina F, Garcia-Canovas F, Saboury AA. A comprehensive review on tyrosinase inhibitors. Journal of Enzyme Inhibition and Medicinal Chemistry. 2019;34:279–309. doi: 10.1080/14756366.2018.1545767. [DOI] [PMC free article] [PubMed] [Google Scholar]