Abstract
Peptones are one of the most expensive components of microbial culture media. The present study was conducted to test the usability of low-cost sheep wool peptone (SWP) as an organic nitrogen source in the production of six industrially important enzymes (lipase, amylase, tannase, pectinase, cellulase and invertase). SWP was prepared by alkaline hydrolysis and acid neutralization. Bacillus licheniformis and Aspergillus niger were selected as test microorganisms for enzyme production. To evaluate the efficacy of SWP in enzyme production, it was compared with commercial tryptone peptone (TP) in the shaking flask cultures of the test microorganisms. The optimum concentration of both SWP and TP was determined to be 8 g/L for the production of B. licheniformis-derived enzymes, but 6 g/L for the production of A. niger-derived enzymes. It was determined that SWP was superior to TP in the production of four enzymes (lipase, amylase, tannase and pectinase) of both B. licheniformis and A. niger. This is the first study about the usage of sheep wool protein hydrolysate (SWP) as an organic nitrogen source or a peptone in fermentative production of microbial enzymes.
Keywords: Bacillus licheniformis, Aspergillus niger, Industrial enzymes, Keratinous proteins, Sheep wool protein hydrolysate
Introduction
Enzymes are large macromolecules found in all living organisms and catalyzing the biological reactions of these organisms. They consist of amino acids linked by amide bonds (Singh et al. 2016; Meghwanshi et al. 2020). Enzymes with industrial importance can be synthesized by microorganisms, plants and animals; however, microorganisms are the most important source of these enzymes. Using microorganisms for enzyme production is more advantageous, since microbial enzymes show higher substrate specificity, lower energy need and higher stability when compared to the plant and animal enzymes (Singh et al. 2016; Bharathi and Rajalakshmi 2019).
Microorganisms which are used for enzyme production include bacteria, filamentous fungi and yeasts. About 60% of industrial enzymes are obtained from fungi, 24% from bacteria, 4% from yeast, and the remaining 10% from plant and animal sources (Raveendran et al. 2018; Fasim et al. 2021). Bacteria most commonly used in the production of industrial enzymes belong to the genera Bacillus and Pseudomonas, while molds belong to the genera Aspergillus and Penicillium (Nigam 2013; Raveendran et al. 2018; Singh et al. 2019; Patil et al. 2021). For example, the fungus A. niger and the bacterium B. licheniformis have been reported to be good producers of industrially important enzymes (Li et al. 2020; Shao et al. 2020; Bellaouchi et al. 2021; Muras et al. 2021; Danmek et al. 2022).
Microbial enzymes are produced by solid-state fermentation or submerged fermentation. In the fermentative production of microbial enzymes, nutritional factors such as carbon, nitrogen and mineral sources are optimized to increase product yield (Singhania et al. 2015; Dinarvand et al. 2017; Niyonzima et al. 2020; Anusree et al. 2020; Patel et al. 2020; Malik et al. 2022). Peptones or protein hydrolysates are one of the most important organic nitrogen sources added into the culture media of microorganisms (Taskin and Kurbanoglu 2011). There are different commercial peptones prepared from proteinaceous materials such as fish, casein, meat and soya bean. The performed studies have demonstrated that when peptones are used as an organic nitrogen source in the microbial media, they significantly increase the synthesis of various microbial metabolites, including enzymes (Teng and Xu 2008; Singh et al. 2011; Orak et al. 2018; Shakir et al. 2019; Vantamuri et al. 2019; Baltaci et al. 2020; Ha et al. 2020; Arslan 2021). However, it is well known that high price of commercial peptones limits their usage in microbial fermentation studies (Taskin 2013a). Therefore, the discovery of new cheap peptones or protein hydrolysates that can be alternatives to commercial peptones will be important in terms of reducing the cost of fermentation medium.
Sheep wool has about 97% protein content (95% pure keratin protein). It contains all of 20 standard amino acids (Arslan and Aydogan 2021). Owing to rich nutritional composition, the protein hydrolyzates from sheep wool are widely utilized as plant fertilizer and animal feed (Abdallah et al. 2019; Petek and Logar 2021). On the other hand, sheep wool protein hydrolysate is used as a peptone for microbial growth or the production of polysaccharides and pigments (Taskin et al. 2016a; Arslan and Aydogan 2021; Arslan 2021; Arslan et al. 2022). However, to the best of our knowledge, there is no attempt about the usage of sheep wool peptone as organic nitrogen source in the synthesis of other microbial metabolites including microbial enzymes.
There are several microbial enzymes with industrial or biotechnological importance, such as protease, lipase, amylase, cellulase, collagenase, pectinase, invertase, xylanase, lactase, laccase, glucose oxidase, inulinase, phytase, peroxidase and tannase (Singh et al. 2016; Bahera et al. 2021; Fasim et al. 2021; Okpara 2022). In the last few decades, there has been a significant increase in applications of enzymes in various industries such as food, agriculture, biofuels, pharmaceuticals, textiles, leather, cosmetics, and waste management (May 2019). For example, six enzymes (tannase, pectinase, invertase, cellulase, lipase and amylase) are widely used in food industry (Raveendran et al. 2018; Kumar et al. 2019). Amylases, lipases and cellulases find also numerous applications in detergent and textile industries (Kumar et al. 2021; Niyonzima 2021). On the other hand, lipases alone are used as biocatalysts in the pharmaceutical and biofuel industries (Vivek et al. 2022).
Therefore, this study was performed to test whether SWP can be used or not as an alternative to commercial peptones for the fermentative production of six industrially important enzymes (tannase, pectinase, invertase, cellulase, lipase and amylase). A. niger and B. licheniformis were selected as test microorganisms since both microorganisms are capable of producing all of six enzymes mentioned.
Materials and methods
Test microorganisms, materials and chemicals
Sheep wool to be used for peptone production was obtained from a local animal farm. The bacterium Bacillus licheniformis A7 (GenBank accession number: KC310458) (Baltaci et al. 2019) and the filamentous fungus Aspergillus niger MT-4 (Taskin et al. 2013a) were selected as test microorganisms for the production of six enzymes (lipase, amylase, invertase, tannase, cellulose and pectinase).
Standard culture media (Potato dextrose agar, potato dextrose broth, nutrient agar and nutrient broth) which were used for the activation of test microorganisms or the preparation of their pre-cultures were purchased from Merck (Germany). The other chemicals which were used for assaying enzyme activities or preparing peptone were purchased from Sigma (USA).
Preparation of wool protein hydrolysate (peptone)
The white-color wool was first washed with distilled water to remove possible impurities. Subsequently, they were dried at 80 °C until the weight became constant and then converted to the peptone, as described in the previous studies (Taskin et al. 2016a; Arslan and Aydogan 2021). For this purpose, washed and dried wool (100 g) was first hydrolyzed inside a heat-resistant bottle containing 150 mL of 2.5 N KOH solution. However, 100 g of wool was hydrolyzed in five steps, not in one step. In the first step, 20 g wool was added to the alkaline solution and the bottle was incubated at 80 °C for 6 h. In the second step, 20 g of wool was added into the alkaline mixture again and the hydrolysis process was started again. The same procedures were repeated in stages 3, 4 and 5. In this way, a total of 100 g of wool was added to the alkaline mixture. In the sixth stage, the bottle was incubated at a higher temperature (120 °C) for 4 h in order to achieve complete degradation of 100 g of wool.
After hydrolysis was completed, boiling water (1/1 V) was added to the hydrolysate in the bottle. Subsequently, the alkaline hydrolysate was neutralized using 6 N H3PO4 solution. Then, the neutralized hydrolysate was filtered, evaporated, dried and grinded (Fig. 1A). The final material was termed as sheep wool peptone (SWP) (Fig. 1A–C).
Fig. 1.

Preparation of sheep wool peptone: A the production scheme of the peptone, B washed and dried sheep wool, and C final material (sheep wool peptone)
Preparation of pre-cultures for the test microorganisms
The thermophilic bacterium B. licheniformis A7 was first activated on nutrient agar (NA) at 55 °C for 48 h. For the preparation of pre-culture of the test bacterium, a loop taken from cell biomass on NA was inoculated into 250-mL flask containing 100 mL of the sterilized nutrient broth (NB), and the flasks were then incubated at 55 °C and 200 rpm for 24 h. Before the inoculation of the production, the optical density of the pre-culture was adjusted to 1.0 at 600 nm with sterile saline water.
To prepare the spore suspension of A. niger, MT-4 was prepared, and the fungus was grown on the slant containing potato dextrose agar (PDA) medium at 30 °C for 8 days. Then, the sterile physiological water containing the surfactant (0.2 mL/L Tween 80) was added into the slant. Following this, the slant was vortexed for approximately 5 min and the prepared suspension was then filtrated through three layers of a sterile muslin cloth. The spore concentration was adjusted to about 106 spores per mL using a haemocytometer. During the experiments, 1 mL of the prepared spore suspension was used for inoculation of the production medium (Taskin et al. 2013b; Aidynova et al. 2020).
Production of enzymes by test microorganisms in peptone-based media
The following media for production of enzymes from B. licheniformis were designed: Medium I (lipase production medium) olive oil (20 mL/L) and peptone; Medium II (amylase production medium): starch (20 g/L) and peptone; Medium III (cellulase production medium): cellulose (20 g/L) and peptone; Medium IV (tannase production medium): tannic acid (20 g/L) and peptone; Medium V (invertase production medium): sucrose (20 g/L) and peptone; Medium VI (pectinase production medium): citrus pectin (20 g/L) and peptone.
During the experiments, the concentration of carbon sources (olive oil, starch, cellulose, tannic acid, citrus pectin and sucrose) was kept constant in each production medium but the concentration of the test peptones (SWP, and TP) was changed. That is, while investigating the effects of peptones on enzyme activities, different concentrations (2, 4, 6, 8, 10 and 12 g/L) of each peptone were tested in the medium where the carbon source concentration was kept constant (20 g/L). The initial pH of all the production media was adjusted to 7.0 for B. licheniformis but 6.0 for A. niger using 1 N HCl or 1 N NaOH.
The production experiments were performed in 250-mL flasks containing 100 mL of the production media described above. For this purpose, the flasks were sterilized, cooled at the room temperature and then inoculated with 1 mL of pre-culture or spore suspension. After inoculation, the flasks were incubated at 200 rpm in a shaking incubator. The incubation temperature and incubation time were selected as 55 °C and 48 h for B. licheniformis but 30 °C and 96 h for A. niger, respectively. At the end of specified incubation period, the cell growth and enzyme activities in the cultures were analyzed.
Analysis of enzyme activities
At the end of 48-h incubation period, the cultures of B. licheniformis were centrifuged at 10,000 rpm for 5 min. The precipitated wet cells were discharged and the supernatants were used for analyzing of enzyme activities. At the end of 96-h incubation period, the cultures of A. niger were centrifuged at 5000 rpm for 5 min. Mycelial biomasses were discharged and the supernatants were employed for activity assay of enzymes.
To determine lipase activity, 0.2 mL of the supernatant was mixed with 1 mL of 0.05 M phosphate buffer (pH 7) and 1 mL of substrate (0.013 M p-nitrophenyl palmitate in ethanol), and then, it was incubated at 30 °C for 5 min. To cease the reaction, 2 mL of 0.5 M Na2CO3 was added to the mixtures. Thereafter, the mixtures were centrifuged at 10,000 rpm for 10 min. The absorbance of the final mixture was measured at 410 nm (Hung et al. 2003) using Beckman Coulter DU730 spectrophotometer. One unit (U) of lipase activity was designated as the amount of enzyme required to hydrolyze 1 µmol/min of p-NPP under the assay conditions. A molar extinction coefficient (ε410) of 13,290 M−1 cm−1 for p-nitrophenol was used.
While the amylase activity was determined, soluble starch solution which was prepared in 0.1 M phosphate buffer (pH 7) was used as a substrate. To prepare the reaction mixture, 0.1 mL of supernatant was added to 1 mL of soluble starch solution, and then, the mixture was left to incubate at 30 °C for 10 min. Afterwards, 1 mL of 3,5-dinitrosalicylic acid (DNS) solution was added to the mixture, and the final mixture was incubated in a water bath at 95 °C for 10 min. After cooling to room temperature, the absorbance of the developed color (red-brown) was measured at 540 nm (Miller 1959). The standard curve was prepared using glucose. One unit of amylase activity was defined as the amount of enzyme that released 1 μmol glucose equivalent per minute from soluble starch under the assay conditions.
For determination of cellulase activity, 1% solution of CMC (carboxymethyl cellulose) which was prepared in 1 M phosphate buffer (pH 7) was used as substrate. To prepare the reaction mixture, 0.1 mL of the supernatant which was taken from the culture was mixed with 1 mL of CMC solution. After the mixtures were incubated at 30 °C for 30 min, DNS was incorporated into the mixtures to cease the reaction. The mixtures were incubated again in boiling water for 10 min to develop red-brown color. Then, 0.5 mL of a 40% (w/v) potassium sodium tartrate (Rochelle salt) solution was added to the mixture for the stabilization of the color. After cooling to room temperature, the optical density of the reaction mixture was assayed at 540 nm. One unit of cellulase activity was defined as the amount of enzyme that released 1 μmol glucose equivalent per minute from CMC under the assay conditions (Ghose 1987; Zhang et al. 2009).
Tannase activity was analyzed according to the method described in the previous study (Taskin 2013b). The reaction mixture included 0.3 mL tannic acid solution (1% w/v in 0.2 M phosphate buffer at pH 7) and 0.1 mL supernatant. After the mixtures were incubated at 30 °C for 60 min, 3 mL albumin solution (1 mg/mL) was added into each mixture to terminate the reaction. Following this, the reaction mixtures were centrifuged (5000 rpm, 10 min) and the precipitated fractions were transferred into another tube containing 3 mL of SDS-triethanolamine solution. Then, the tubes were supplemented with 1 mL of FeCl3 reagent (0.01 M FeCl3 in 0.01 N HCl) and the final mixtures were incubated for 15 min. The absorbances were read at 530 nm against the blank. One unit of tannase activity was defined as the amount of enzyme required to hydrolyze 1 μmol of tannic acid in 1 min.
To determine the invertase activity, 0.1 mL of supernatant was mixed with 0.9 mL of 0.1 mol/L M phosphate buffer (pH 7) containing 2% sucrose (w/v). After the mixtures were incubated at 30 °C for 10 min, they were left to incubation at 100 °C for 5 min to stop the reaction. The reducing sugar contents of the mixtures after enzymatic reaction were analyzed using the 3,5-dinitrosalicylic acid (DNS) method. For this purpose, 1 mL of DNS reagent was added to 1 mL of each mixture, the mixtures were incubated at 100 °C for 5 min and then were cooled at room temperature. Following this, the total volume of each mixture was completed to 10 mL with distilled water. Finally, the absorbances of the mixtures were assayed at 550 nm. One unit of invertase activity was defined as the amount of enzyme catalyzing the liberation of 1 µmol of reducing sugars from sucrose per minute. The standard curve was prepared using fructose and glucose at equimolar concentrations (Miller 1959; Taskin et al. 2013b, 2016b).
While pectinase activity was determined, 1.0% (w/v) citrus pectin was used as the substrate. For this, 0.7 mL of substrate prepared in sodium phosphate buffer (50 mM, pH 7.0) was mixed with 0.3 mL of supernatant and the mixture was then incubated at 150 rpm and 30 °C in a shaking incubator for 15 min. After adding 1 mL of DNS reagent, the reaction was stopped by heating the mixture in a water bath for 5 min at 95 °C. After reaction mixture was cooled, 2 mL of distilled water was added into each tube. Finally, the absorbance of each mixture was measured at 550 nm using UV Spectrophotometer. Galacturonic acid was used as standard for the preparation of calibration curve. A unit of total pectinase activity was the amount of enzyme required to efficiently separate 1 µmol of galacturonic acid per min (Ahmed et al. 2021; Jalil and Ibrahim 2021). The activity values for all enzymes were given as U/mL.
During the activity assay for all enzymes, the reaction mixture which was used as the blank included microorganism-free production medium instead of the culture supernatant, as well as the other reaction components such as buffer and substrate solution. For example, while the blank was being prepared during the measurement of amylase activity, 0.1 mL production medium (the production medium that is not inoculated with the microorganism) instead of 0.1 mL culture supernatant was added into the reaction mixture, and the other components were kept constant in the blank. In addition, a different blank was prepared for each concentration of peptones. For example, while the activity of the supernatant of the culture prepared at 4% peptone concentration was determined, the sample (0.1 mL) from the production medium containing 4% peptone was used to prepare the blank. That is, absorbance of each reaction mixture was read against its own blank.
Statistical analysis
Each experiment was repeated at least three times in two replicates. The analysis of variance was conducted using one-way ANOVA test using SPSS 13.0 for Microsoft Windows, and means were compared by Duncan test at the 0.05 level of confidence.
Results
Effects of test peptones on lipase and amylase production
When 100 g of wool was hydrolyzed using 150 mL of 2.5 N KOH solution, 92.8 g of wool could be hydrolyzed. After the prepared alkaline hydrolysate was neutralized using 6 N H3PO4 solution, it was subjected to the filtration, evaporation, drying and powdering processes, respectively. At the end of these processes, 117.6 g of sheep wool peptone (SWP) could be produced.
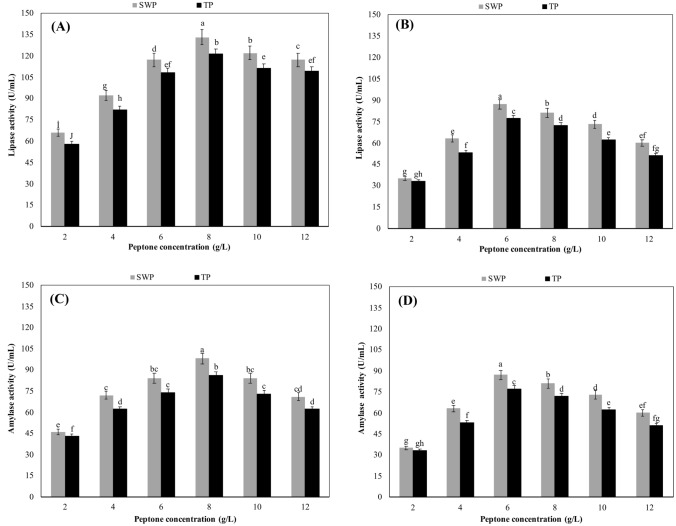
While the effects of SWP and tryptone peptone (TP) on enzyme activities of B. licheniformis and A. niger were investigated, different concentrations of both peptones were tested. As seen in Fig. 2A, both peptones caused the maximum lipase activity in the culture of B. licheniformis when they were added to the medium at the concentration of 8 g/L. Further increases in the concentration of SWP or TP significantly decreased the lipase activity of the bacterium (p ≥ 0.05). At the optimal peptone concentration of 8 g/L, the lipase activities were 133 and 121 U/mL in SWP and TP-based cultures, respectively. As seen from the Fig. 2B, both SWP and TP caused the maximum lipase activity when they were added to the production medium of A. niger at the concentration of 6 g/L. At the peptone concentration of 6 g/L, the lipase activities of the fungus were 93 and 82 U/mL in SWP and TP media, respectively. There were statistically important decreases (p ≥ 0.05) in the lipase activity of the fungus at peptone concentrations above 6 g/L.
Fig. 2.
Effects of test peptones on lipase and amylase production. Culture conditions for B. licheniformis: pH 7.0, temperature 55 °C, shaking speed 200 rpm and incubation time 48 h. Culture conditions for A. niger: pH 6.0, temperature 30 °C, shaking speed 200 rpm and incubation time 96 h. SWP sheep wool peptone, TP tryptone peptone. All values are mean ± standard error of six determinations (n = 6). Same alphabet letters on the columns are not significantly different at p ≤ 0.05
The amylase activity of B. licheniformis increased as peptone concentrations was increased, and the maximum activities could be reached at the peptone concentration of 8 g/L for both SWP and TP (Fig. 2C). Conversely, significant decreases in the amylase activities were detected at the peptone concentrations over 8 g/L (p ≥ 0.05). At the optimal peptone concentration of 8 g/L, the amylase activities of the bacterium in SWP and TP-based cultures were measured as 98 and 86 U/mL, respectively (Fig. 2C). Figure 2D depicts that the optimal concentration of both peptones for the amylase production was determined as 6 g/L. Further concentrations of both peptones were found to cause significant reductions in the amylase activity (p ≥ 0.05). When the experiments were performed at the optimal peptone concentration of 6 g/L, the amylase activities were measured 87 and 77 U/mL in TP and SWP-based cultures, respectively.
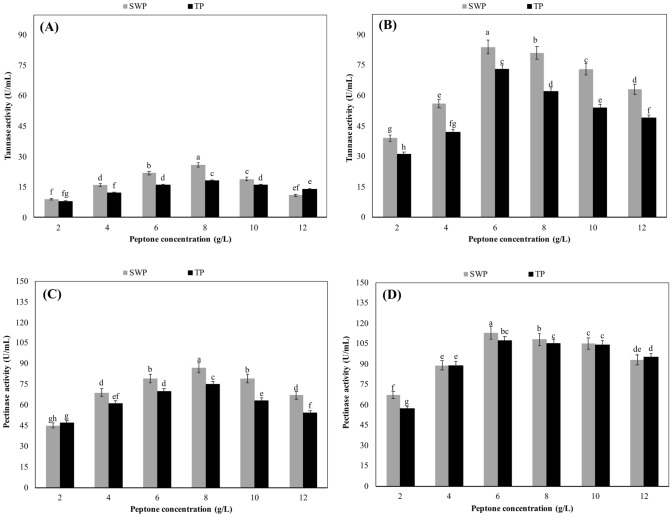
Effects of test peptones on tannase and pectinase production
The results revealed that peptone concentrations up to 8 g/L increased tannase activity of the bacterium, but higher peptone concentrations caused significant decreases in tannase activity (p ≥ 0.05). In short, the optimal concentration of both peptones for tannase production by B. licheniformis was determined as 8 g/L. At this optimal concentration, tannase activities in SWP and TP-based cultures were determined as 26 and 18 U/mL, respectively (Fig. 3A). In contrast to the tannase activity of B. licheniformis, that of A. niger reached to the maximum value when SWP or TP was added into the medium at the concentration of 6 g/L. At this optimal concentration of peptones, the tannase activities which were measured in SWP and TP-based cultures were 84 and 73 U/mL, respectively. The tannase activity of the fungus showed a decreasing trend when the peptone concentration was increased to 8 g/L or over (Fig. 3B).
Fig. 3.
Effects of test peptones on tannase and pectinase production. Culture conditions for B. licheniformis: pH 7.0, temperature 55 °C, shaking speed 200 rpm and incubation time 48 h. Culture conditions for A. niger: pH 6.0, temperature 30 °C, shaking speed 200 rpm and incubation time 96 h. SWP sheep wool peptone, TP tryptone peptone. All values are mean ± standard error of six determinations (n = 6). Same alphabet letters on the columns are not significantly different at p ≤ 0.05
The experiments revealed that the optimal concentration of both test peptones was 8 g/L for pectinase production by B. licheniformis (Fig. 3C) but 6 g/L for pectinase production by A. niger (Fig. 3D). At the optimal peptone concentrations, the pectinase activities of B. licheniformis in SWP and TP-based cultures were determined as 87 and 75 U/mL but those of A. niger were 113 and 107 U/mL, respectively. It was determined that SWP and TP concentrations above 8 g/L for B. licheniformis and 6 g/L for A. niger decreased significantly pectinase production (p ≥ 0.05).
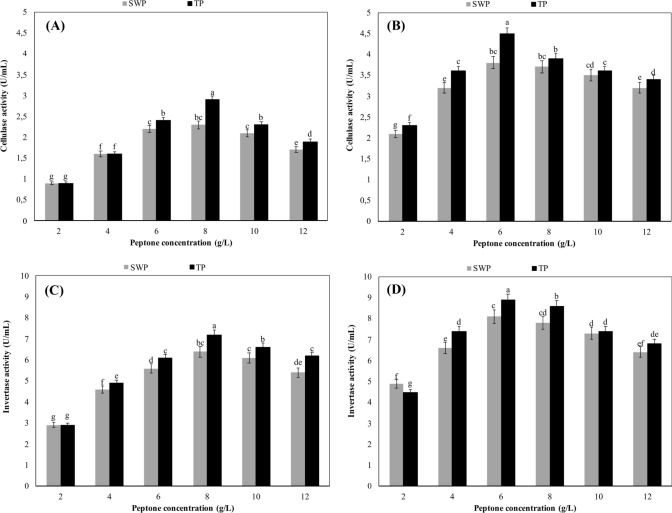
Effects of test peptones on cellulase and invertase production
As seen from Fig. 4A, the cellulase activity of the bacterium reached the maximum value at the peptone concentration of 8 g/L but the activity significantly diminished (p ≥ 0.05) at higher peptone concentrations (10 and 12 g/L). When the experiments were performed at the optimal peptone concentration of 8 g/L, the cellulase activities in SWP and TP-based cultures were found to be 2.3 and 2.9 U/L, respectively. Figure 4B shows that in contrast to cellulase production by B. licheniformis, the most favorable concentration of both SWP and TP was 6 g/L for cellulase production by A. niger. Fungal cellulase activities measured at the optimal concentration of SWP and TP were 3.8 and 4.5 U/L, respectively. On the other hand, peptone concentrations over 6 g/L were determined to decrease the cellulase activity in the culture of the fungus (p ≥ 0.05).
Fig. 4.
Effects of test peptones on cellulase and invertase production. Culture conditions for B. licheniformis: pH 7.0, temperature 55 °C, shaking speed 200 rpm and incubation time 48 h. Culture conditions for A. niger: pH 6.0, temperature 30 °C, shaking speed 200 rpm and incubation time 96 h. SWP sheep wool peptone, TP tryptone peptone. All values are mean ± standard error of six determinations (n = 6). Same alphabet letters on the columns are not significantly different at p ≤ 0.05
The invertase activity of B. licheniformis became maximum when SWP or TP was added into the medium at the concentration of 8 g/L. When the experiments were performed at the optimal peptone concentration of 8 g/L, the invertase activities in SWP and TP-based cultures were measured as 6.4 and 7.2 U/mL, respectively (Fig. 4C). In contrast to B. licheniformis, the invertase activity of A. niger reached to the maximum when any of the test peptones was added into the medium at the concentration of 6 g/L. At the optimal peptone concentration of 6 g/L, the invertase activities in SWP and TP-based cultures were 8.1 and 8.9 U/mL, respectively (Fig. 4D). On the other hand, the experiments revealed that peptone concentrations above optimal decreased significantly invertase activity in both A. niger and B. licheniformis (p ≥ 0.05).
Discussion
Peptones are one of the most expensive components which are added to the microbial media for the cultivation of microorganisms or the synthesis of microbial metabolites. Earlier studies revealed that in comparison with other organic nitrogen sources or inorganic nitrogen sources, peptones caused more production of microbial enzymes (Aiyer 2004; Adham and Ahmed 2009; Singh et al. 2011; Akhter et al. 2011; Aboubakr et al. 2013; Lugani et al. 2015; Oyedeji et al. 2017; Baltaci et al. 2020; El-Ghomary et al. 2021; Mirjatt et al. 2021). However, as seen from Table 1, commercial peptones were mainly preferred for enzyme production in previous studies. Considering the fact that the prices of commercial peptones are high, the development of inexpensive peptones as an alternative to commercial peptones is important for microbial biotechnology studies. For example, sheep wool peptone, whose effectiveness has been proven in previous microbial studies (Taskin et al. 2016a; Arslan and Aydogan 2021; Arslan 2021; Arslan et al. 2022), can be also used as an alternative to commercial peptones in the production of microbial enzymes.
Table 1.
Peptones and other organic nitrogen sources used for production of microbial enzymes
| Enzymes | Enzyme source | Organic nitrogen sources | References |
|---|---|---|---|
| Amylase | B. licheniformis | Peptone*, proteose peptone, casein, tryptone | Aiyer (2004) |
| Amylase | A. niger | Peptone*, yeast extract, casein, meat extract | Mirjatt et al. (2021) |
| Lipase | B. licheniformis | Feather peptone*, fish peptone, proteose peptone | Baltaci et al. (2020) |
| Lipase | A. niger | Peptone*, yeast extract | Adham and Ahmed (2009) |
| Tannase | A. niger | Peptone*, beef extract, soy bean meal, yeast extract | |
| Cellulase | Bacillus sp. Y3 | Peptone*, yeast extract | Lugani et al. (2015) |
| Cellulase | A. niger | Peptone*, beef extract, yeast extract | Kumar et al. (2018) |
| Pectinase | A. niger | Peptone*, yeast extract | Akhter et al. (2011) |
| Pectinase | Aspergillus sp. | Peptone*, yeast extract | El-Ghomary et al. (2021) |
| Invertase | A. niger | Peptone*, glutamic acid, yeast extract | Oyedeji et al. (2017) |
(*) indicates which is the best organic nitrogen source for enzyme production
Therefore, the present study was performed to investigate whether sheep wool protein hydrolyzate can be used or not as an alternative peptone to commercial peptones in production of six microbial enzymes (lipase, amylase, invertase, tannase, cellulase and pectinase) with industrial importance.
To appraise the effectiveness of sheep wool peptone (SWP) on the production of the enzymes mentioned, it was compared with commercial tryptone peptone (TP). The previous studies have demonstrated that the bacterium Bacillus licheniformis and the filamentous fungus Aspergillus niger have the ability to produce the target enzymes mentioned above (Aiyer 2004; Kumar et al. 2011; Aboubakr et al. 2013; Ghani et al. 2013; Aslam et al. 2020; Baltaci et al. 2020; Mohapatra et al. 2020). Therefore, B. licheniformis and A. niger were chosen as the test microorganisms while testing the effects of SWP and TP on the synthesis of the target enzymes. SWP was re-produced according to the method given in our previous studies (Taskin et al. 2016a; Arslan and Aydogan 2021). The amount of produced SWP was determined as 117.6 g. SWP yield was similar to the those reported in the previous studies (Taskin et al. 2016a; Arslan and Aydogan 2021).
In the literature, it has been stated that the concentration of nitrogen sources affects the synthesis of microbial metabolites including microbial enzymes, therefore, determining the optimal concentration of the nitrogen source is a very important optimization step (Teng and Xu 2008; Taskin et al. 2011, 2012; Taskin 2013a; Baltaci et al. 2020; Arslan 2021). Considering this information, different concentrations of both SWP and TP were tested to achieve the maximum production of the target enzymes. As seen from Figs. 1, 2, and 3, the optimal concentration of both SWP and TP was determined as 8 g/L for the synthesis of B. licheniformis-derived enzymes but 6 g/L for the synthesis of A. niger-derived enzymes. In brief, it was found that the production of the target enzymes was strictly depended on the concentration of the test peptones. This result may be due to carbon:nitrogen (C/N) ratio of the production medium, as reported in the previous studies (Aiyer 2004; Coradi et al. 2013; Oliveira et al. 2017; El Enshasy et al. 2018; Balakrishnan et al. 2021).
The results also demonstrated that the target enzymes except two enzymes (invertase and cellulase for both test microorganisms) were produced more in SWP-based culture compared to TP-based culture. In other words, it was determined that SWP was a better peptone source for the production of four enzymes (lipase, amylase, tannase and pectinase). In the previous studies (Taskin et al. 2016a; Arslan and Aydogan 2021), the protein content of SWP was reported to be lower than that of TP. However, the same studies demonstrated that SWP was better peptone source than TP for the cell growth and/or the metabolite synthesis in some microorganisms. Similarly, the present study demonstrated that even if the protein content of SWP was lower than that of TP, SWP was superior to TP for the production of four enzymes. In the literature (Arslan and Aydogan 2021), it was reported that SWP had higher contents of some amino acids (arginine, cystine glycine, tyrosine, threonine, serine and proline) and minerals (K, Mg, Ca, P, S and Fe) when compared to TP. Therefore, we deduced that aforementioned amino acids and/or minerals of SWP might have been responsible for enhancement of synthesis of four enzymes. This hypothesis can be supported by the fact that especially K, P Mg, Ca, Na and Fe ions are capable of enhancing the synthesis of lipases, tannases, pectinases and amylase (Srivastava and Baruah 1986; Pokorny et al. 1994; Belmares et al. 2004; Singh and Mandal 2012; Jahan et al. 2017).
After the wool fibers are processed by the textile industry, a high amount of wool-based wastes such as fibers and fabric leftovers are generated. Therefore, raw wool or wool wastes required for the production of SWP can be obtained from sheep farms or textile industries (Arslan 2021). In short, the raw material needed for the production of SWP is easy to supply. In addition, it can be considered that production cost of SWP is lower than those of commercial peptones, since SWP is prepared using cheap chemicals in contrast to commercial peptones which are prepared using expensive hydrolytic enzymes. Furthermore, converting wool-based wastes such as fibers and fabric leftovers into the peptone may help the reduction of environmental pollution problem.
In conclusion, the present study demonstrated that SWP from sheep wool could be effectively used as an organic nitrogen source in the production of industrially valuable microbial enzymes. Even, it was seen that SWP was superior to commercial tryptone peptone in the production of four enzymes (lipase, amylase, tannase and pectinase). Considering the low cost of SWP as well as its high efficiency in enzyme production, we propose to use SWP as an alternative peptone in the production of microbial enzymes.
Author contributions
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MAO, SA, NPA, HO, AA and MT. The first draft of the manuscript was written by MT, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Funding
This study was supported by the project (FBG-2020–8779) (Ataturk University, Erzurum, Turkey).
Data availability
All data are included in this manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study does not contain any experiments with human participants or animals.
References
- Abdallah AM, Ugolini F, Baronti S, Maienza A, Ungaro F, Camilli F. Assessment of two sheep wool residues from textile industry as organic fertilizer in sunflower and maize cultivation. J Soil Sci Plant Nutr. 2019;19:793–807. doi: 10.1007/s42729-019-00079-y. [DOI] [Google Scholar]
- Aboubakr HA, El-Sahn MA, El-Banna AA. Some factors affecting tannase production by Aspergillus niger Van Tieghem. Braz J Microbiol. 2013;44:559–567. doi: 10.1590/S1517-83822013000200036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adham NZ, Ahmed EM. Extracellular lipase of Aspergillus niger NRRL3; production, partial purification and properties. Indian J Microbiol. 2009;49:77–83. doi: 10.1007/s12088-009-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed T, Rana MR, Zzaman W, Ara R, Aziz MG. Optimization of substrate composition for pectinase production from Satkara (Citrus macroptera) peel using Aspergillus niger-ATCC 1640 in solid-state fermentation. Heliyon. 2021;7:e08133. doi: 10.1016/j.heliyon.2021.e08133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aidynova R, Arslan N, Aydoğan MN. Use of mulberry pomace as substrate for citric acid production by Aspergillus niger MT-4. Trak Univ J Nat Sci. 2020;21:159–165. [Google Scholar]
- Aiyer PD. Effect of C: N ratio on alpha amylase production by Bacillus licheniformis SPT 27. Afr J Biotechnol. 2004;3:519–522. doi: 10.5897/AJB2004.000-2103. [DOI] [Google Scholar]
- Akhter N, Morshed MA, Uddin A, Begum F, Sultan T, Azad AK. Production of pectinase by Aspergillus niger cultured in solid state media. Int J Biosci. 2011;1:33–42. [Google Scholar]
- Anusree M, Swapna K, Aguilar CN, Sabu A. Optimization of process parameters for the enhanced production of fibrinolytic enzyme by a newly isolated marine bacterium. Bioresour Technol Rep. 2020;11:100436. doi: 10.1016/j.biteb.2020.100436. [DOI] [Google Scholar]
- Arslan NP. Use of wool protein hydrolysate as nitrogen source in production of microbial pigments. J Food Process Preserv. 2021;45(7):e15660. doi: 10.1111/jfpp.15660. [DOI] [Google Scholar]
- Arslan NP, Aydogan MN. Evaluation of sheep wool protein hydrolysate and molasses as low-cost fermentation substrates for hyaluronic acid production by Streptococcus zooepidemicus ATCC 35246. Waste Biomass Valor. 2021;12:925–935. doi: 10.1007/s12649-020-01062-w. [DOI] [Google Scholar]
- Arslan NP, Cinar-Yilmaz H, Vural-Keles D, Doymus M, Yilmaz F, Taskin M. Exopolysaccharide production with high antibacterial efficiency from Lentinus edodes using sheep wool protein hydrolysate. Biomass Convers Biorefin. 2022;12:537–546. doi: 10.1007/s13399-021-01864-5. [DOI] [Google Scholar]
- Aslam F, Ansari A, Aman A, Baloch G, Nisar G, Baloch AH, Rehman HU. Production of commercially important enzymes from Bacillus licheniformis KIBGE-IB3 using date fruit wastes as substrate. J Genet Eng Biotechnol. 2020;18:1–7. doi: 10.1186/s43141-020-00060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahera BK, Prasad R, Behera S. Life sciences industry. Singapore: Springer; 2021. Industrial Enzymes; pp. 29–88. [Google Scholar]
- Balakrishnan M, Jeevarathinam G, Kumar S, Muniraj I, Uthandi S. Optimization and scale-up of α-amylase production by Aspergillus oryzae using solid-state fermentation of edible oil cakes. BMC Biotechnol. 2021;21(1):1–11. doi: 10.1186/s12896-021-00686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltacı MÖ, Tuysuz E, Ozkan H, Taskin M, Adiguzel A. Lipase production from thermophilic bacteria using waste frying oil as substrate. Tek Bil Der. 2019;9:23–27. [Google Scholar]
- Baltaci MO, Orak T, Taskin M, Adiguzel A, Ozkan H. Enhancement of amylase and lipase production from Bacillus licheniformis 016 using waste chicken feathers as peptone source. Waste Biomass Valori. 2020;11:1809–1819. doi: 10.1007/s12649-018-0468-6. [DOI] [Google Scholar]
- Bellaouchi R, Abouloifa H, Rokni Y, Hasnaoui A, Ghabbour N, Hakkou A, Asehraou A. Characterization and optimization of extracellular enzymes production by Aspergillus niger strains isolated from date by-products. J Genet Eng Biotechnol. 2021;19(1):1–8. doi: 10.1186/s43141-021-00145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmares R, Contreras-Esquivel JC, Rodriguez-Herrera R, Coronel AR, Aguilar CN. Microbial production of tannase: an enzyme with potential use in food industry. LWT-Food Sci Technol. 2004;37:857–864. doi: 10.1016/j.lwt.2004.04.002. [DOI] [Google Scholar]
- Bharathi D, Rajalakshmi G. Microbial lipases: an overview of screening, production and purification. Biocatal Agric Biotechnol. 2019;22:101368. doi: 10.1016/j.bcab.2019.101368. [DOI] [Google Scholar]
- Coradi GV, da Visitação VL, de Lima A, et al. Comparing submerged and solid-state fermentation of agro-industrial residues for the production and characterization of lipase by Trichoderma harzianum. Ann Microbiol. 2013;63:533–540. doi: 10.1007/s13213-012-0500-1. [DOI] [Google Scholar]
- Danmek K, Ruenwai R, Sorachakula C, Jung C, Chuttong B. Occurrence of an invertase producing strain of Aspergillus niger LP5 isolated from longan pollen and its application in longan syrup production to feed honey bees (Apis mellifera L.) J Ecol Environ. 2022;46:13. doi: 10.5141/jee.22.002. [DOI] [Google Scholar]
- Dinarvand M, Rezaee M, Foroughi M. Optimizing culture conditions for production of intra and extracellular inulinase and invertase from Aspergillus niger ATCC 20611 by response surface methodology (RSM) Braz J Microbiol. 2017;48:427–441. doi: 10.1016/j.bjm.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Enshasy HA, Elsayed EA, Suhaimi N, Malek RA, Esawy M. Bioprocess optimization for pectinase production using Aspergillus niger in a submerged cultivation system. BMC Biotechnol. 2018;18(1):1–13. doi: 10.1186/s12896-018-0481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghomary AE, Shoukry AA, EL-Kotkat MB. Productivity of pectinase enzymes by Aspergillus sp. isolated from Egyptian soil. Azhar J Agric Res. 2021;46(2):79–87. doi: 10.21608/ajar.2021.245617. [DOI] [Google Scholar]
- Ghani M, Ansari A, Aman A, Zohra RR, Siddiqui NN, Qader SAU. Isolation and characterization of different strains of Bacillus licheniformis for the production of commercially significant enzymes. Pak J Pharm Sci. 2013;26(4):691–697. [PubMed] [Google Scholar]
- Ghose TK. Measurement of cellulase activities. Pure Appl Chem. 1987;59(2):257–268. doi: 10.1351/pac198759020257. [DOI] [Google Scholar]
- Fasim A, More VS, More SS. Large-scale production of enzymes for biotechnology uses. Curr Opin Biotechnol. 2021;69:68–76. doi: 10.1016/j.copbio.2020.12.002. [DOI] [PubMed] [Google Scholar]
- Ha G, Kim J, Im S, Shin SJ, Yang HJ, Jeong DY. Application of response surface methodology in medium optimization to improve lactic acid production by Lactobacillus paracasei SRCM201474. J Life Sci. 2020;30(6):522–531. [Google Scholar]
- Hung TC, Giridhar R, Chiou SH, Wu WT. Binary immobilization of Candida rugosa lipase on chitosan. J Mol Catal B Enzym. 2003;26(1–2):69–78. doi: 10.1016/S1381-1177(03)00167-X. [DOI] [Google Scholar]
- Jahan N, Shahid F, Aman A, Mujahid TY, Qader SAU. Utilization of agro waste pectin for the production of industrially important polygalacturonase. Heliyon. 2017;3(6):e00330. doi: 10.1016/j.heliyon.2017.e00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalil MTM, Ibrahim D. Partial purification and characterisation of pectinase produced by Aspergillus niger LFP-1 grown on pomelo peels as a substrate. Trop Life Sci Res. 2021;32(1):1. doi: 10.21315/tlsr2021.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Sharma HK, Sarkar BC. Effect of substrate and fermentation conditions on pectinase and cellulase production by Aspergillus niger NCIM 548 in submerged (SmF) and solid state fermentation (SSF) Food Sci Biotechnol. 2011;20(5):1289–1298. doi: 10.1007/s10068-011-0178-3. [DOI] [Google Scholar]
- Kumar SS, Sreekumar R, Sabu A. Green Bio-processes. Singapore: Springer; 2019. Tannase and its applications in food processing; pp. 357–381. [Google Scholar]
- Kumar BA, Amit K, Alok K, Dharm D. Wheat bran fermentation for the production of cellulase and xylanase by Aspergillus niger NFCCI 4113. Res J Biotechnol. 2018;13:5. [Google Scholar]
- Kumar D, Bhardwaj R, Jassal S, Goyal T, Khullar A, Gupta N. Application of enzymes for an eco-friendly approach to textile processing. Environ Sci Pollut Res. 2021 doi: 10.1007/s11356-021-16764-4. [DOI] [PubMed] [Google Scholar]
- Li C, Zhou J, Du G, Chen J, Takahashi S, Liu S. Developing Aspergillus niger as a cell factory for food enzyme production. Biotechnol Adv. 2020;44:107630. doi: 10.1016/j.biotechadv.2020.107630. [DOI] [PubMed] [Google Scholar]
- Lugani Y, Singla R, Sooch BS. Optimization of cellulase production from newly isolated Bacillus sp. Y3. J Bioprocess Biotech. 2015;5(11):1. doi: 10.4172/2155-9821.1000264. [DOI] [Google Scholar]
- Malik WA, Khan HM, Javed S. Bioprocess optimization for enhanced production of bacterial cellulase and hydrolysis of sugarcane bagasse. Bioenergy Res. 2022;15(2):1116–1129. doi: 10.1007/s12155-021-10259-3. [DOI] [Google Scholar]
- May O. Industrial enzyme applications. John Wiley & Sons; 2019. Industrial enzyme applications–overview and historic perspective; pp. 1–24. [Google Scholar]
- Meghwanshi GK, Kaur N, Verma S, Dabi NK, Vashishtha A, Charan PD, Kumar R. Enzymes for pharmaceutical and therapeutic applications. Biotechnol Appl Biochem. 2020;67(4):586–601. doi: 10.1002/bab.1919. [DOI] [PubMed] [Google Scholar]
- Mohapatra PKD, Biswas I, Mondal KC, Pati BR. Concomitant yield optimization of tannase and gallic acid by Bacillus licheniformis KBR6 through submerged fermentation: An industrial approach. Acta Biol Szeged. 2020;64(2):151–158. doi: 10.14232/abs.2020.2.151-158. [DOI] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Mirjatt AN, Khushk I, Zahid H, Qureshi AS, Bhutto MA, Watoor B. Environmental friendly production of amylase from Aspergillus niger EFRL-FC-024 using corn waste as carbon source. Pak J Anal Environ Chem. 2021;22(1):165–171. doi: 10.21743/pjaec/2021.06.17. [DOI] [Google Scholar]
- Muras A, Romero M, Mayer C, Otero A. Biotechnological applications of Bacillus licheniformis. Crit Rev Biotechnol. 2021;41(4):609–627. doi: 10.1080/07388551.2021.1873239. [DOI] [PubMed] [Google Scholar]
- Nigam PS. Microbial enzymes with special characteristics for biotechnological applications. Biomolecules. 2013;3(3):597–611. doi: 10.3390/biom3030597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyonzima FN, Veena SM, More SS. Microbial enzymes: roles and applications in industries. Singapore: Springer; 2020. Industrial production and optimization of microbial enzymes; pp. 115–135. [Google Scholar]
- Niyonzima FN. Detergent-compatible fungal cellulases. Folia Microbiol. 2021;66(1):25–40. doi: 10.1007/s12223-020-00838-w. [DOI] [PubMed] [Google Scholar]
- Okpara MO. Microbial enzymes and their applications in food industry: a mini-review. Adv Enzyme Res. 2022;10:23–47. doi: 10.4236/aer.2022.101002. [DOI] [Google Scholar]
- Oyedeji O, Bakare MK, Adewale IO, Olutiola PO, Omoboye OO. Optimized production and characterization of thermostable invertase from Aspergillus niger IBK1, using pineapple peel as alternate substrate. Biocatal Agric Biotechnol. 2017;9:218–223. doi: 10.1016/j.bcab.2017.01.001. [DOI] [Google Scholar]
- Oliveira F, Salgado JM, Abrunhosa L, Pérez-Rodríguez N, Domínguez JM, Venâncio A, Belo I. Optimization of lipase production by solid-state fermentation of olive pomace: from flask to laboratory-scale packed-bed bioreactor. Bioprocess Biosyst Eng. 2017;40(7):1123–1132. doi: 10.1007/s00449-017-1774-2. [DOI] [PubMed] [Google Scholar]
- Orak T, Caglar O, Ortucu S, Ozkan H, Taskin M. Chicken feather peptone: a new alternative nitrogen source for pigment production by Monascus purpureus. J Biotechnol. 2018;271:56–62. doi: 10.1016/j.jbiotec.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Patel H, Ray S, Patel A, Patel K, Trivedi U. Enhanced lipase production from organic solvent tolerant Pseudomonas aeruginosa UKHL1 and its application in oily waste-water treatment. Biocatal Agric Biotechnol. 2020;28:101731. doi: 10.1016/j.bcab.2020.101731. [DOI] [Google Scholar]
- Patil AG, Khan K, Aishwarya S, Padyana S, Huchegowda R, Reddy KR, Zameer F. Industrially important fungi for sustainable development. Cham: Springer; 2021. Fungal amylases and their industrial applications; pp. 407–434. [Google Scholar]
- Petek B, Logar RM. Management of waste sheep wool as valuable organic substrate in European Union countries. J Mater Cycles Waste Manag. 2021;23(1):44–54. doi: 10.1007/s10163-020-01121-3. [DOI] [Google Scholar]
- Pokorny D, Friedrich J, Cimerman A. Effect of nutritional factors on lipase biosynthesis by Aspergillus niger. Biotechnol Lett. 1994;16(4):363–366. doi: 10.1007/BF00245052. [DOI] [Google Scholar]
- Raveendran S, Parameswaran B, Ummalyma SB, Abraham A, Mathew AK, Madhavan A, Pandey A. Applications of microbial enzymes in food industry. Food Technol Biotechnol. 2018;56(1):16. doi: 10.17113/ftb.56.01.18.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakir HA, Mahmood R, Irfan M, Deeba F, Javed I, Qazi JI. Protease production from Bacillus safensis in submerged fermentation using response surface methodology. Rev Mex Ing Quim. 2019;18(1):375–385. doi: 10.24275/uam/izt/dcbi/revmexingquim/2019v18n1/Shakir. [DOI] [Google Scholar]
- Shao Y, Zhang YH, Zhang F, Yang QM, Weng HF, Xiao Q, Xiao AF. Thermostable tannase from Aspergillus niger and its application in the enzymatic extraction of green tea. Molecules. 2020;25(4):952. doi: 10.3390/molecules25040952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Mandal SK. Optimization of processing parameters for production of pectinolytic enzymes from fermented pineapple residue of mixed Aspergillus species. Jordan J Biol Sci. 2012;147(625):1–7. [Google Scholar]
- Singh R, Kapoor V, Kumar V. Influence of carbon and nitrogen sources on the α-amylase production by a newly isolated thermophilic Streptomyces sp. MSC702 (MTCC 10772) Asian J Biothechnol. 2011;3(6):540–553. doi: 10.3923/ajbkr.2011.540.553. [DOI] [Google Scholar]
- Singh R, Kumar M, Mittal A, Mehta PK. Microbial enzymes: industrial progress in 21st century. 3 Biotech. 2016;6:1–15. doi: 10.1007/s13205-016-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RS, Singh T, Pandey A. Microbial enzymes—an overview. In: Singh RS, Singh RR, Larroche C, editors. Advances in enzyme technology. Elsevier; 2019. pp. 1–40. [Google Scholar]
- Singhania RR, Patel AK, Thomas L, Goswami M, Giri BS, Pandey A. Industrial biorefineries & white biotechnology. Elsevier; 2015. Industrial enzymes; pp. 473–497. [Google Scholar]
- Srivastava RAK, Baruah JN. Culture conditions for production of thermostable amylase by Bacillus stearothermophilus. Appl Environ Microbiol. 1986;52(1):179–184. doi: 10.1128/aem.52.1.179-184.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskin M, Kurbanoglu EB. Evaluation of waste chicken feathers as peptone source for bacterial growth. J Appl Microbiol. 2011;111:826–834. doi: 10.1111/j.1365-2672.2011.05103.x. [DOI] [PubMed] [Google Scholar]
- Taskin M, Sisman T, Erdal S, Kurbanoglu EB. Use of waste chicken feathers as peptone for production of carotenoids in submerged culture of Rhodotorula glutinis MT-5. Eur Food Res Technol. 2011;233(4):657–665. doi: 10.1007/s00217-011-1561-2. [DOI] [Google Scholar]
- Taskin M. A new strategy for improved glutathione production from Saccharomyces cerevisiae: use of cysteine-and glycine-rich chicken feather protein hydrolysate as a new cheap substrate. J Sci Food Agric. 2013;93(3):535–541. doi: 10.1002/jsfa.5818. [DOI] [PubMed] [Google Scholar]
- Taskin M. Co-production of tannase and pectinase by free and immobilized cells of the yeast Rhodotorula glutinis MP-10 isolated from tannin-rich persimmon (Diospyros kaki L.) fruits. Bioprocess Biosyst Eng. 2013;36:165–172. doi: 10.1007/s00449-012-0771-8. [DOI] [PubMed] [Google Scholar]
- Taskin M, Tasar GE, Incekara U. Citric acid production from Aspergillus niger MT-4 using hydrolysate extract of the insect Locusta migratoria. Toxicol Ind Health. 2013;29(5):426–434. doi: 10.1177/0748233712436646. [DOI] [PubMed] [Google Scholar]
- Taskin M, Ozkan B, Atici O, Aydogan MN. Utilization of chicken feather hydrolysate as a novel fermentation substrate for production of exopolysaccharide and mycelial biomass from edible mushroom Morchella esculenta. Int J Food Sci Nutr. 2012;63(5):597–602. doi: 10.3109/09637486.2011.640309. [DOI] [PubMed] [Google Scholar]
- Taskin M, Esim N, Genisel M, Ortucu S, Hasenekoglu I, Canli O, Erdal S. Enhancement of invertase production by Aspergillus niger OZ-3 using low-intensity static magnetic fields. Prep Biochem Biotechnol. 2013;43(2):177–188. doi: 10.1080/10826068.2012.713431. [DOI] [PubMed] [Google Scholar]
- Taskin M, Unver Y, Firat A, Ortucu S, Yildiz M. Sheep wool protein hydrolysate: a new peptone source for microorganisms. J Chem Technol Biotechnol. 2016;91:1675–1680. doi: 10.1002/jctb.4971. [DOI] [Google Scholar]
- Taskin M, Ortucu S, Unver Y, Tasar OC, Ozdemir M, Kaymak HC. Invertase production and molasses decolourization by cold-adapted filamentous fungus Cladosporium herbarum ER-25 in non-sterile molasses medium. Process Saf Environ Prot. 2016;103:136–143. doi: 10.1016/j.psep.2016.07.006. [DOI] [Google Scholar]
- Teng Y, Xu Y. Culture condition improvement for whole-cell lipase production in submerged fermentation by Rhizopus chinensis using statistical method. Bioresour Technol. 2008;99(9):3900–3907. doi: 10.1016/j.biortech.2007.07.057. [DOI] [PubMed] [Google Scholar]
- Vantamuri AB, Manawadi SI, Guruvin SK, Holeyannavar VM, Shettar DS. Production of laccase by Ganoderma sp. in submerged fermentation. J Adv Sci Res. 2019;10(04):67–71. [Google Scholar]
- Vivek K, Sandhia GS, Subramaniyan S. Extremophilic lipases for industrial applications: a general review. Biotechnol Adv. 2022;60:108002. doi: 10.1016/j.biotechadv.2022.108002. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Hong J, Ye X. Biofuels. Humana Press; 2009. Cellulase assays; pp. 213–231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in this manuscript.