Abstract
Background
Facial emotion identification (FEI) deficits are associated with impaired social functioning in persons living with schizophrenia (PLwS), but the research on emotion-specific FEI deficits remains inconclusive. Furthermore, existing studies on FEI deficits are limited by their small sample sizes. We performed a meta-analysis of studies comparing the FEI abilities between Chinese PLwS and healthy controls in terms of the six basic facial emotions (happiness, sadness, fear, disgust, anger, and surprise), as well as contempt, calmness, and neutral facial expressions.
Methods
Major Chinese- and English-language databases were searched to retrieve case-control studies that compared the FEI task performance between Chinese PLwS and healthy controls (HCs) and reported the emotion-specific correct identification scores for PLwS and HCs. The Joanna Briggs Institute Critical Appraisal Checklist for Case-control Studies (“JBI checklist,” hereafter) was used to assess the risk of bias (RoB) of the included studies. Statistical analysis was performed using the “meta” package of R 4.1.2.
Results
Twenty-three studies with a total of 28 case-control cohorts and 1,894 PLwS and 1,267 HCs were included. The RoB scores of the included studies ranged from two to seven. PLwS had statistically significantly lower FEI scores than HCs and the corresponding emotion-specific pooled standard mean differences (95% confidence intervals) were −0.69 (−0.88, −0.50) for happiness, −0.88 (−1.12, −0.63) for sadness, −1.44 (−1.83, −1.06) for fear, −1.18 (−1.60, −0.76) for disgust, −0.91 (−1.24, −0.57) for anger, −1.09 (−1.39, −0.78) for surprise, −0.26 (−0.51, −0.01) for contempt, −0.31 (−0.52, −0.09) for calmness, and −0.42 (−0.65, −0.18) for neutral. In the analyses of sources of heterogeneity, drug-naïve status, clinical setting, positive and negative psychotic symptoms, and RoB were significant moderators of the magnitudes of FEI deficits.
Conclusions
Chinese PLwS have significant FEI impairments in terms of recognizing the six basic facial emotions, contempt, calmness, and neutral emotions, and the magnitude of impairment varies depending on the type of emotion, clinical characteristics, and the level of RoB of the study. It is necessary to consider the characteristics of FEI deficits and the clinical moderators in the FEI deficits to develop remediation strategies targeting FEI deficits in schizophrenia.
Keywords: facial emotion identification, schizophrenia, case-control studies, Chinese, meta-analysis
Introduction
Facial emotion recognition (FER) impairments are a rather stable trait of schizophrenia, which has been associated with impaired social functioning and predicts subsequent declines in work functioning, social participation, and abilities of independent living in persons living with schizophrenia (PLwS) (1–5). Nevertheless, there has been accumulating evidence that certain psychological and cognitive training interventions are effective for mitigating FER impairments and further result in large improvements in social functioning in PLwS (6–12). Therefore, FER ability has been a promising treatment goal for effective psychosocial rehabilitation in schizophrenia. To optimize the development and selection of remediation strategies targeting FER deficits in schizophrenia, it is necessary to have adequate knowledge of the characteristics of FER difficulties in PLwS.
In the literature, FER deficits in schizophrenia have been extensively examined; however, controversy still exists regarding the specificity of FER deficits (i. e., specific to FER only or in both FER and non-emotional face processing) and the moderating roles of clinical factors on FER deficits (i.e., whether paranoid and non-paranoid schizophrenia differ in FER deficits) (13–17). Two published systematic reviews and meta-analyses pooled effect sizes of the differences in overall FER abilities between PLwS and healthy controls and demonstrated the general deficit in both facial emotion perception and processing in schizophrenia and the significant study-level associations of FER deficits with negative psychotic symptoms, inpatient hospitalization, and late age at onset of schizophrenia (14, 16). Nonetheless, the two systematic reviews focused on the total FER only and directly pooled the effect sizes from different FER tasks together, which ignored the heterogeneity across tasks [i.e., facial emotion identification (FEI) and discrimination], so their meta-analytic findings were still not detailed enough. Since prior studies report conflicting findings on FER deficits in a specific emotion (i.e., happiness) and across a variety of FER tasks (13, 18–20), the specificity of FER deficits with respect to the category of emotion and type of FER task remains inconclusive. For example, two published studies have consistent findings on the significantly lower correct disgust and fear FEI rates in Chinese PLwS than healthy controls but have inconsistent findings on the FEI of happiness: one found comparable rates between Chinese persons with first-onset schizophrenia and healthy controls, and the other found significantly lower rates in Chinese PLwS than healthy controls (18, 21). Importantly, the unstable findings may also be ascribed to the small sample sizes and the inadequate statistical powers of prior studies.
To further clarify the specificity of FER impairments and advance our understanding of the mechanisms of FER impairments in schizophrenia, we performed a meta-analysis of case-control studies using the FEI task to assess the FER deficits in terms of six basic facial emotions (happiness, sadness, fear, disgust, anger, and surprise) and contempt, calmness, and neutral facial expressions in Chinese PLwS. Schizophrenia is typically characterized by language disturbances and semantic deficits and the completion of FEI tasks relies on language and semantic skills (22–25), so experimental FER paradigms of studies to be included were limited to FEI tasks only. To minimize the clinical heterogeneity in FEI deficits caused by race and culture (26–28), the included studies were limited to those with Chinese participants.
Methods
This meta-analysis was reported according to the PRISMA guideline (29). Literature search, the inclusion of eligible studies, data extraction, and risk of bias (RoB) assessment were independently performed by the first and second authors of this study, and disagreements were addressed via discussion and consensus with the corresponding author.
Inclusion and exclusion criteria
Case-control studies that compared the FEI task performance between Chinese PLwS and healthy controls and reported correct identification scores (rates or crude scores, mean ± standard deviations [SDs]) in terms of any of the above-mentioned nine emotions were considered eligible for this study. Studies that did not include healthy controls, used facial emotion discrimination tasks only, examined FER abilities under different conditions, employed eye emotion recognition tasks, adopted prosodic emotion recognition tasks, or did not provide meta-analyzable data were excluded.
Literature search
A literature search was conducted within both Chinese- and English-language databases from their inception to November 13, 2022: CNKI, Wanfang, VIP Information, PubMed, Embase, and PsycINFO. The main search terms were as follows: (“facial emotion” OR “facial affect” OR “emotional face” OR “emotional expression” OR “facial expression”) AND “schizophreni*” AND (“identification” OR “recognition” OR “perception”) AND (“Chin*” OR “Taiwan” OR “Hong Kong”). Reference lists of included studies and related reviews were also manually searched to avoid missing studies.
Data extraction
A standardized form specifically developed for this study was used to extract data from included studies. Extracted variables included first author, publication year, diagnostic criteria of schizophrenia, numbers of participants in the case and control groups, clinical characteristics of the case group (i.e., mean age, proportion of men, and clinical stage of schizophrenia), characteristics of the FEI task (i.e., facial emotion database and classification of facial emotion), indicators of RoB assessment (i.e., the validity of the FEI task), and emotion-specific correct identification scores of the FEI task (means ± SDs).
RoB assessment
The Joanna Briggs Institute Critical Appraisal Checklist for Case-control Studies (“JBI checklist,” hereafter) was used to assess the RoB of the included studies (30). The JBI has 10 methodology items of a case-control study: comparability, matching, identification of cases and controls, validity of exposure measure, method of exposure measurement, identification of confounders, handling of confounders, validity of outcome measurement, exposure period, and statistical analysis. These items were assessed on four-choice options (yes, no, unclear, and not applicable), and one point was assigned to a “yes” response. Since the item “exposure period” was not applicable and removed from the RoB assessment, the total RoB score in our study ranged from zero to nine, with higher scores suggesting lower RoB.
Statistical analysis
Meta-analysis was used to synthesize standardized mean differences (SMDs) and their 95% confidence intervals (CIs) for the magnitudes of the differences in correct identification scores between schizophrenia patients and healthy controls because the identification abilities were expressed in two distinct ways in included studies: correct rates in some studies and crude correct scores in other studies. Forest plots were generated to show SMDs and the combined estimates. When there was evidence of heterogeneity (I2 > 50% or P < 0.10 for Q statistics), the random-effect model was adopted to combine SMDs; otherwise, the fixed-effect model was used to combine SMDs. In the present study, the SMD was equivalent to the effect size measure, Hedges' g, with absolute values of 0.20–0.49, 0.50–0.79, and 0.80+ denoting small, medium, and large differences, respectively (31–33).
Sources of heterogeneity in the pooled SMDs were examined by using subgroup analyses according to potential categorical moderators (diagnostic criteria, clinical stage of schizophrenia, status of antipsychotic treatment, clinical setting, task, and type of correct identification score) and univariate meta-regression analyses according to potential continuous moderators [publication year, mean age of the schizophrenia sample, mean years of education of the schizophrenia sample, % of males in the schizophrenia sample, mean Positive and Negative Syndrome Scale positive symptom subscale (PANSS-P) score of the schizophrenia sample, mean PANSS negative symptom subscale [PANSS-N] score, and RoB score]. In studies assessing psychotic symptoms by using the Scale for the Assessment of Positive Symptoms (SAPS) and the Scale for the Assessment of Negative Symptoms (SANS), the recommended conversion equations were used to convert SAPS and SANS scores into PANSS-P and PANSS-N scores, respectively (34). Funnel plots and Egger's and Begg's tests were used to test publication bias. Two-sided P < 0.05 was considered statistically significant. All analyses were conducted by using R 4.1.2 (R Development Core Team; Vienna, Austria).
Results
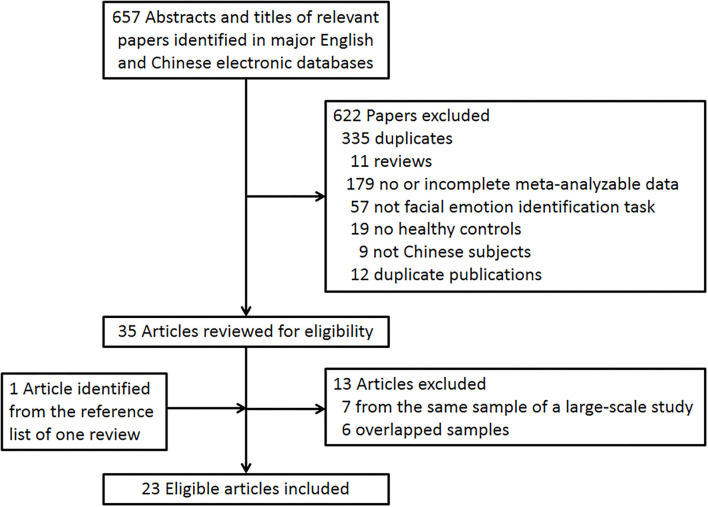
The literature search initially identified 657 records, and finally, 23 studies with a total of 28 case-control cohorts were included (21, 35–56) (Figure 1). There were 1,894 PLwS and 1,267 healthy controls in the included studies. The RoB scores of the included studies ranged between two and seven, with a median score of four. Detailed characteristics and RoB scores of the 23 included studies are shown in Table 1.
Figure 1.
Flowchart of study inclusion.
Table 1.
Characteristics and risk of bias scores of included studies.
| References | Participants (n, mean age, male/female) | Diagnostic criteria | Clinical characteristics | Clinical setting | Facial emotion identification task | Emotion categories | Identification measure | Risk of bias score |
|---|---|---|---|---|---|---|---|---|
| Dong et al. (35) | SCH (65, 28 years, 17/48); HC (67, NR, 20/47) | CCMD-3 | SCH | Outpatient & inpatient | Chinese facial emotion test | Happiness, sadness, fear, disgust, anger, surprise | Correct rate | 4 |
| Chen et al. (36) | SCH (42, 29.7 years, 42/0); HC (37, 32.0 years, 37/0) | CCMD-3 | SCH | Inpatient | Chinese facial expression video system | Happiness, sadness, neutral | Correct rate | 4 |
| Dong et al. (37) | SCH (121, 28.3 years, 35/86); HC (76, 30.2 years, 26/50) | CCMD-3 | Acute SCH | Outpatient & inpatient | Chinese facial emotion test | Happiness, sadness, fear, disgust, anger, surprise | Correct score | 5 |
| Gao et al. (38) | SCH (61, 27.4 years, 17/44); HC (57, 28.9 years, 21/36) | CCMD-3 | SCH | Outpatient & inpatient | Chinese facial emotion test | Happiness, sadness, fear, disgust, anger, surprise | Correct score | 4 |
| Dong et al. (39) | SCH (82, 28.3 years, 22/60); HC (88, 29.3 years, 27/61) | CCMD-3 | Drug-naïve acute SCH | Outpatient & inpatient | Chinese facial emotion test | Happiness, sadness, fear, disgust, anger, surprise | Correct score | 5 |
| Zhang and Chen (40) | SCH (100, 35.7 years, 55/45); HC (100, 34.3 years, 60/40) | CCMD-3 | Remitted SCH | Outpatient & inpatient | Chinese facial emotion test | Happiness, sadness, fear, disgust, anger, surprise | Correct score | 5 |
| Tse et al. (41) | SCH (40, 40 years, 20/20); HC (46, 39 years, NR) | DSM-IV | Remitted SCH | Outpatient | Facial affect perception task | Happiness, sadness, anger, neutral | Correct score | 6 |
| Leung et al. (21) | First-onset SCH (50, 20.7 years, 25/25); HC (26, 21.7 years, 12/14) Chronic SCH (51, 43.5 years, 31/20); HC (28, 44.8 years, 17/11) | DSM-IV | Stable first-onset SCH Stable chronic SCH | Outpatient | Japanese and Caucasian facial expressions of emotion | Happiness, sadness, fear, disgust, anger, surprise | Correct rate | 6 |
| Yu (42) | SCH (88, 23.3 years, 50/38); HC (75, 23.2 years, 33/42) | ICD-10 | Acute paranoid SCH | Inpatient | Japanese and Caucasian facial expressions of emotion | Happiness, sadness, fear, disgust, anger, surprise | Correct rate | 2 |
| Li (43) | SCH (25, 15.2 years, 19/6); HC (25, 15.3 years, 19/6) | DSM-V | Drug-naive type II SCH | Outpatient & inpatient | Basic facial expression cognition test for Chinese | Happiness, sadness, fear, disgust, anger, surprise, neutral | Correct score | 4 |
| Song et al. (44) | SCH (44, 35.5 years, 20/24); HC (41, 32.4 years, 17/24) | DSM-IV | Stable SCH | Inpatient | Computerized facial emotion recognition test | Happiness, sadness, fear, anger, contempt | Correct rate | 7 |
| Wang and Kang (45) | SCH (45, 32 years, 0/45); HC (45, 32 years, 0/45) | ICD-10 | SCH | Inpatient | Ekman-Friesen pictures of facial affect | Happiness, anger, fear | Correct rate | 4 |
| Tang et al. (46) | Deficit SCH (37, 49.2 years, 37/0); non-deficit SCH (57, 46.5 years, 57/0); HC (54, 47.6 years, 54/0) | DSM-IV | Stable deficit SCH Stable non-deficit SCH | Inpatient | Chinese facial emotion test | Happiness, sadness, fear, disgust, anger, surprise | Correct score | 6 |
| Zhu et al. (47) | SCH (30, 33.5 years, 17/13); HC (30, 33.8 years, 15/15) | DSM-IV | Drug-naïve SCH | Inpatient | Chinese facial emotion database | Happiness, sadness, fear, disgust, anger, surprise | Correct rate | 4 |
| Lv (48) | SCH (31, 23 years, 20/11); HC (25, 21.4 years, 17/9) | DSM-IV | Drug-naïve first-onset SCH | Inpatient | Japanese female facial expression dataset | Happiness, fear, anger, neutral | Correct rate | 2 |
| Yang et al. (49) | SCH (30, 22.3 years, 15/15); HC (30, 24.6 years, 15/15) | DSM-IV | First-onset SCH | Inpatient | Ekman-Friesen pictures of facial affect | Happiness, fear, disgust | Correct rate | 6 |
| Zhu (50) | SCH (28, 34.7 years, 15/13); SCH (26, 34.7 years, 12/14); HC (30, 33.8 years, 16/14) | DSM-V | Drug-naive SCH | Inpatient | Chinese emotional face database | Happiness, sadness, fear, disgust, anger, surprise | Correct rate | 4 |
| Liu et al. (51) | Remitted SCH (65, 29.3 years, 35/30); Remitted SCH (45, 31.6 years, 26/19); HC (58, 31.4 years, 37/21) | CCMD-3 | Remitted first-onset SCH Non-remitted first-onset SCH | Outpatient & inpatient | Chinese facial emotion test | Happiness, sadness, fear, disgust, anger, surprise | Correct score | 4 |
| Guo (52) | First-onset SCH (60, 27.6 years, 36/24); Chronic SCH (63, 30.2 years, 40/23); Chronic HC (50, 29.8 years, 27/23) | ICD-10 | Drug-naive first-onset SCH Chronic SCH | Inpatient | Facial emotion recognition test | Happiness, fear, neutral | Correct rate | 2 |
| Zhao et al. (53) | SCH (162, 41.3 years, 74/88); HC (83, 39.7 years, 29/54) | DSM-IV | Stable SCH | Inpatient | Chinese facial emotion test | Happiness, sadness, fear, disgust, anger, surprise, neutral | Correct score | 3 |
| Du et al. (54) | SCH (60, 34.6 years, 18/42); HC (60, 37.3 years, 19/41) | DSM-IV | SCH | Inpatient | Chinese facial emotion images database with intensity classification | Happiness, sadness, fear, disgust, anger, surprise, neutral | Correct score | 4 |
| Gao (55) | SCH (35, 30 years, 14/21); HC (35, 29 years, 16/19) | ICD-10 | Stable SCH | Outpatient & inpatient | Chinese affective picture system | Happiness, sadness, fear, anger | Correct rate | 3 |
| Lee et al. (56) | SCH (351, 45 years, 159/192); HC (101, 23.3 years, 37/64) | DSM-V | SCH | Outpatient & inpatient | Computerized adaptive test of facial emotion recognition | Happiness, sadness, fear, disgust, anger, surprise, neutral | Correct score | 3 |
SCH, schizophrenia; HC, healthy controls; NR, not reported; CCMD-3, Chinese Classification of Mental Disorders, the third version; ICD-10, International Classification of Diseases, Tenth Revision; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, the fourth edition; DSM-V, Diagnostic and Statistical Manual of Mental Disorders, the fifth edition.
Results of the meta-analysis (Table 2) show that PLwS had statistically significantly lower FEI scores than healthy controls in terms of all the nine emotions of interest of this study and their corresponding SMDs (95%CIs) were −0.69 (−0.88, −0.50) for happiness, −0.88 (−1.12, −0.63) for sadness, −1.44 (−1.83, −1.06) for fear, −1.18 (−1.60, −0.76) for disgust, −0.91 (−1.24, −0.57) for anger, −1.09 (−1.39, −0.78) for surprise, −0.26 (−0.51, −0.01) for contempt, −0.31 (−0.52, −0.09) for calmness, and −0.42 (−0.65, −0.18) for neutral (Supplementary Figures 1–9).
Table 2.
Results of meta-analysis on correct identification score differences between schizophrenia patients and healthy controls, as indicated by standardized mean differences (SMDs) and (95% confidence intervals, CIs).
| Emotion | Number of case-control cohorts | Number of participants (schizophrenia, healthy controls) | Heterogeneity (I2, P) | SMD (95% CI) | P | Publication bias | |
|---|---|---|---|---|---|---|---|
| Egger's test (t, P) | Begg's test (z, P) | ||||||
| Happiness* | 28 | 1,894, 1,267 | 81.4%, <0.001 | −0.69 (−0.88, −0.50) | <0.001 | 0.17, 0.866 | −0.28, 0.779 |
| Sadness* | 22 | 1,665, 1,117 | 85.8%, <0.001 | −0.88 (−1.12, −0.63) | <0.001 | −0.56, 0.583 | −0.82, 0.414 |
| Fear* | 26 | 1,812, 1,184 | 92.6%, <0.001 | −1.44 (−1.83, −1.06) | <0.001 | −1.14, 0.264 | −0.37, 0.708 |
| Disgust* | 20 | 1,534, 988 | 87.8%, <0.001 | −1.18 (−1.60, −0.76) | <0.001 | −1.64, 0.119 | 0.00, 1.000 |
| Anger* | 24 | 1,699, 1,150 | 91.5%, <0.001 | −0.91 (−1.24, −0.57) | <0.001 | 0.08, 0.939 | −0.50, 0.620 |
| Surprise* | 19 | 1,504, 958 | 86.3%, <0.001 | −1.09 (−1.39, −0.78) | <0.001 | −1.95, 0.067 | −1.50, 0.133 |
| Contempt** | 2 | 132, 116 | 0.0%, 1.000 | −0.26 (−0.51, −0.01) | 0.040 | Not applicable | Not applicable |
| Calmness** | 2 | 222, 143 | 0.0%, 1.000 | −0.31 (−0.52, −0.09) | 0.005 | Not applicable | Not applicable |
| Neutral* | 6 | 612, 284 | 50.0%, 0.075 | −0.42 (−0.65, −0.18) | 0.001 | Not applicable | Not applicable |
Random-effects model.
Fixed-effects model. Because the number of studies examining contempt, calmness, and neutral emotions are lower than 10, the publication bias of these studies was not tested.
Funnel plots of the six basic facial emotions were visually symmetrical (Supplementary Figures 10–15), and the results of both Egger's and Begg's tests suggested that there was no statistically significant publication bias across the included studies (P = 0.067–0.939, P = 0.133–1.000) (Table 2).
Diagnostic criteria, antipsychotic treatment status, clinical setting, and FEI task were identified as significant categorical moderators, while publication year, mean education years of the schizophrenia sample, % of men in the schizophrenia sample, mean PANSS-P score of the schizophrenia sample, mean PANSS-N score of the schizophrenia sample, and RoB score were identified as significant continuous moderators of the magnitudes of the FEI abilities between PLwS and healthy controls (Table 3). Specifically, the lowest significant pooled SMDs were shown in studies using DSM-V for happiness emotion, in studies using CCMD-3 for fear emotion, and in studies using DSM-IV for both disgust and surprise emotions, compared to studies using other diagnostic criteria from the same emotion-specific subgroups. Significantly lower pooled SMDs were observed in studies recruiting drug-naïve PLwS for the emotion of sadness, in studies enrolling both outpatients and inpatients with schizophrenia for both sadness and fear emotions, in studies recruiting inpatients with schizophrenia for anger emotion, and in studies adopting validated identification tasks in China in comparison to their counterparts from the same subgroups. There were significant positive correlations between happiness-specific pooled SMDs and % of men in the patient sample and fear-specific pooled SMDs and publication year while there were significant negative correlations between mean PANSS-P score in the patient sample and happiness-specific pooled SMDs, between mean PANSS-N score in the patient sample and sadness-specific pooled SMDs, between mean education years in the patient sample and fear-specific pooled SMDs, and between mean PANSS-N score in the patient sample and anger-specific pooled SMDs. RoB scores were significantly and negatively correlated with disgust-specific and surprise-specific pooled SMDs.
Table 3.
Subgroup analyses and univariate meta-regression analyses of sources of heterogeneity in correct identification scores between schizophrenia patients and healthy controls, as indicated by standardized mean differences (95% confidence intervals) and coefficients (95% confidence intervals), respectively.
| Emotion | |||||||
|---|---|---|---|---|---|---|---|
| Happiness | Sadness | Fear | Disgust | Anger | Surprise | ||
| Categorical moderator | |||||||
| Diagnostic criteria | CCMD-3 | −0.73 (−1.11, −0.35) | −1.02 (−1.48, −0.55) | −1.85 (−2.43, −1.28) | −1.06 (−1.31, −0.82) | −0.79 (−1.00, −0.58) | −0.92 (−1.15, −0.69) |
| DSM-IV | −0.45 (−0.72, −0.17) | −0.68 (−0.99, −0.36) | −1.63 (−2.48, −0.78) | −1.65 (−2.77, −0.53) | −1.07 (−1.65, −0.49) | −1.57 (−2.29, −0.86) | |
| ICD-10 | −0.93 (−1.37, −0.49) | −1.24 (−2.79, −0.32) | −1.09 (−1.74, −0.45) | −0.29 (−0.60, 0.02) | −1.19 (−2.36, −0.03) | −0.16 (−0.47, 0.15) | |
| DSM-V | −0.94 (−1.15, −0.73)* | −0.85 (−1.06, −0.65) | −0.82 (−1.29, −0.34)* | −0.95 (−1.24, −0.66)* | −0.53 (−1.81, −0.75) | −0.85 (−1.04, −0.65)* | |
| Stage of schizophrenia | Schizophrenia | −0.79 (−1.11, −0.47) | −0.82 (−1.17, −0.47) | −1.30 (−1.74, −0.85) | −1.09 (−1.24, −0.95) | −0.92 (−1.56, −0.28) | −1.12 (−1.42, −0.83) |
| Acute schizophrenia | −0.82 (−1.34, −0.31) | −1.03 (−1.59, −0.47) | −1.70 (−2.92, −0.48) | −0.94 (−1.60, −0.29) | −0.63 (−1.11, −0.15) | −0.80 (−1.43, −0.17) | |
| Remitted schizophrenia | −0.56 (−1.15, 0.03) | −0.54 (−1.35, 0.28) | −1.36 (−3.40, 0.68) | −0.89 (−1.88, 0.10) | −0.42 (−1.08, 0.24) | −0.84 (−1.53, −0.16) | |
| First-onset schizophrenia | −0.55 (−1.35, −0.39) | −1.01 (−2.28, 0.26) | −2.19 (−3.81, −0.56) | −2.55 (−5.71, 0.61) | −1.52 (−3.04, 0.01) | −1.92 (−5.05, 1.21) | |
| Chronic schizophrenia | −0.87 (−1.35, −0.39) | −0.62 (−1.10, −0.15) | −1.22 (−2.08, −0.36) | −1.56 (−2.08, −1.03) | −0.50 (−0.97, −0.04) | −1.54 (−2.06, −1.02) | |
| Stable schizophrenia | −0.60 (−1.18, −0.02) | −1.10 (−1.80, −0.40) | −1.06 (−1.70, −0.41) | −0.80 (−1.24, −0.35) | −1.11 (−1.79, −0.43) | −0.87 (−1.46, −0.27) | |
| Drug-naive | No | −0.67 (−0.91, −0.44) | −0.84 (−1.13, −0.55) | −1.56 (−2.03, −1.09) | −1.27 (−1.85, −0.69) | −0.93 (−1.25, −0.62) | −1.09 (−1.51, −0.68) |
| Yes | −0.84 (−1.03, −0.64) | −1.15 (−1.39, −0.91)* | −1.13 (−1.79, −0.47) | −1.11 (−1.33, −0.88) | −0.83 (−1.91, −0.25) | −1.13 (−1.35, −0.90) | |
| Setting | Outpatient & inpatient | −0.92 (−1.21, −0.63) | −1.22 (−1.58, −0.87) | −1.73 (−2.20, −1.25) | −1.07 (−1.26, −0.88) | −0.84 (−1.39, −0.28) | −0.90 (−1.08, −0.72) |
| Inpatient | −0.56 (−0.80, −0.32) | −0.68 (−1.00, −0.35) | −0.98 (−1.28, −0.67) | −0.86 (−1.16, −0.55) | −1.12 (−1.63, −0.60) | −1.03 (−1.47, −0.60) | |
| Outpatient | −0.39 (−0.82, 0.04) | −0.42 (−0.68, −0.15)* | −3.52 (−7.20, 0.16)* | −3.67 (−7.87, 0.53) | −0.38 (−0.72, −0.03)* | −2.52 (−4.47, −0.56) | |
| Facial emotion identification task | Validated in Chinese | −0.69 (−0.92, −0.45) | −0.98 (−1.26, −0.70) | −1.35 (−1.71, −0.98) | −1.02 (−1.20, −0.84) | −0.91 (−1.27, −0.55) | −0.99 (−1.19, −0.78) |
| Not validated in Chinese | −0.71 (−1.03, −0.38) | −0.43 (−0.64, −0.23)* | −1.73 (−2.79, −0.67) | −2.11 (−4.53, 0.32) | −0.92 (−1.80, −0.05) | −1.72 (−3.63, 0.19) | |
| Outcome measure | Correct rate | −0.74 (−1.03, −0.44) | −0.76 (−1.13, −0.38) | −1.45 (−2.11, −0.80) | −1.50 (−2.63, −0.27) | −0.99 (−1.48, −0.50) | −1.38 (−2.14, −0.63) |
| Correct score | −0.65 (−0.91, −0.40) | −0.77 (−1.30, −0.64) | −1.46 (−1.89, −1.02) | −1.06 (−1.28, −0.84) | −0.84 (−1.31, −0.37) | −0.95 (−1.20, −0.69) | |
| Continuous moderator | |||||||
| Publication year | 0.014 (−0.024, 0.052) | 0.0010 (−0.0477, 0.0497) | 0.092 (0.016, 0.169)* | 0.041 (−0.047, 0.128) | 0.064 (−0.131, 0.002) | 0.020 (−0.043, 0.083) | |
| Mean age of the patient sample | 0.0053 (−0.0225, 0.0331) | 0.0061 (−0.0401, 0.0279) | 0.032 (−0.016, 0.080) | 0.021 (−0.028, 0.070) | −0.025 (−0.065, 0.015) | 0.0042 (−0.0322, 0.0405) | |
| Happiness | Sadness | Fear | Disgust | Anger | Surprise | ||
| Mean education years of the patient sample | 0.052 (−0.098, 0.202) | 0.12 (−0.05, 0.29) | −0.30 (−0.58, −0.03)* | −0.27 (−0.57, 0.03) | −0.029 (−0.228, 0.169) | −0.16 (−0.37, 0.06) | |
| % of males in the patient sample | 0.0089 (0.0013, 0.0166)* | 0.0038 (−0.0077, 0.0153) | 0.014 (−0.004, 0.0031) | 0.0030 (−0.0181, 0.0242) | 0.0052 (−0.0104, 0.0208) | 0.0039 (−0.0110, 0.0188) | |
| Mean PANSS positive subscale score of the patient sample | −0.060 (−0.107, −0.013)* | −0.057 (−0.126, 0.012) | 0.072 (−0.057, 0.202) | 0.11 (−0.03, 0.24) | 0.0021 (−0.1114, 0.1157) | 0.074 (−0.049, 0.197) | |
| Mean PANSS negative subscale score of the patient sample | −0.025 (−0.075, 0.025) | −0.073 (−0.125, −0.021)* | 0.064 (−0.057, 0.186) | 0.12 (−0.03, 0.26) | −0.11 (−0.17, −0.04)* | 0.020 (−0.088, 0.127) | |
| Risk of bias score | −0.0033 (−0.1462, 0.1397) | 0.0135 (−0.1884, 0.2154) | −0.17 (−0.44, 0.11) | −0.40 (−0.73, −0.06)* | 0.19 (−0.06, 0.45) | −0.34 (−0.57, −0.10)* | |
Statistically significant (P < 0.05) differences across subgroups or statistically significant (P < 0.05) coefficients. Because the number of studies examining contempt, calmness, and neutral emotions are lower than 10, sources of heterogeneity of these studies were not tested.
Discussion
This study is a detailed quantitative systematic review of the FEI deficits with respect to nine emotions, which are potentially clinically relevant but have not been systematically examined in previously published studies. The main findings of this meta-analysis are the significantly lower FEI scores in Chinese PLwS than healthy controls in terms of the six basic emotions plus contempt, calm, and neutral, with the magnitudes of impairments being large for fear, disgust, surprise, anger, and sadness, being medium for happiness, and being small for contempt, calmness, and neutral. In the analyses of sources of heterogeneity, clinical factors, such as diagnostic criteria, drug-naïve status, clinical setting, and PANSS-N score, and methodology factors, such as FEI task and RoB score, were significant moderators of schizophrenia-control FEI performance differences.
Findings from empirical studies have shown that PLwS present more severe impairments in recognizing negative and neutral emotions, such as anger, fear, and calmness, while they do not present difficulties in recognizing positive emotions, such as happiness (1, 57, 58). Similarly, the meta-analysis found the greatest levels of impairments in identifying fear, disgust, anger, and sadness emotions in Chinese PLwS. However, the moderate level of impairment in identifying the emotion of happiness and the mild levels of impairment in identifying contempt, calmness, and neutral emotions seem to be not consistent with previous studies. These findings are partly attributed to the attentional biases to emotional scenes in PLwS; that is, compared to controls, PLwS showed increased attention to threatening scenes and paid less attention to happy scenes (59). In addition, the low levels of difficulty of the happiness items in the FEI tasks of prior studies might be the other possible explanation for this inconsistent finding because of the poor discriminant validity of the happiness items (53). As a supporting case in point, studies using validated FEI revealed a severe sadness-specific identification deficit in schizophrenia, but those using un-validated tasks only revealed a moderate sadness-specific identification deficit in our subgroup analyses (Table 3). Due to the limited number of included studies focusing on the FEI of neutral emotions, more empirical studies are warranted to ascertain the severity of impairments in recognizing neutral emotions in schizophrenia.
In line with earlier studies, the significant moderating roles of several clinical factors on the FEI abilities in schizophrenia were further confirmed (14, 16). Nonetheless, the findings are detailed enough, specific to the emotion in the FEI task. Although the effectiveness of antipsychotic treatment is limited for improving the facial affect processing deficits in schizophrenia, antipsychotic treatment still has a significant positive effect on FER deficits, and some second-generation antipsychotics can effectively relieve FER deficits in schizophrenia, particularly in terms of some negative emotions (60, 61). In keeping with this, more sadness-specific FEI impairments were found in drug-naïve than medicated PLwS in our subgroup analyses. Psychotic symptoms, both positive and negative symptoms, can negatively influence FER and processing (1, 15); therefore, our meta-regression analyses show significant negative correlations of the mean PANSS-P score with happiness-specific pooled SMDs, and the mean PANSS-N score with sadness-specific and anger-specific pooled SMDs. In general, inpatients have more psychotic symptoms than outpatients. In accordance with the negative associations between psychotic symptoms and pooled SMDs in the meta-regression analyses, the subgroup analyses found greater levels of sadness-, fear-, and anger-specific FEI difficulties in studies enrolling inpatients and both outpatients and inpatients than those enrolling outpatients only. Finally, one interesting finding from the subgroup analyses is the non-significant differences in pooled SMDs across clinical stages of schizophrenia, again confirming the trait characteristic of FEI impairment in PLwS.
The negative correlations between the RoB score and disgust- and surprise-specific FEI SMDs deserve to be emphasized because it suggests that the RoB of the included studies influences the magnitude estimates of FEI deficits in schizophrenia and that the magnitude of FEI impairments would be larger if more low-RoB studies were included in this meta-analysis.
This meta-analysis has several limitations. First, to minimize the own-race bias for FEI, the included studies were limited to those with participants of ethnic Chinese origin only. It is necessary to repeat our analyses in studies with participants from Western countries. The second significant limitation is the high RoB of the included studies since no included studies scored nine in the JBI checklist assessment. Third, the number of studies focusing on facial emotions other than the six basic emotions was small (n = 2–6), and our estimates of the magnitudes of FEI impairments in terms of these facial emotions might be unstable.
In summary, Chinese PLwS have FEI deficits in terms of all nine emotions of interest in this study, and the deficits are severe in terms of fear, disgust, surprise, anger, and sadness emotions, moderate in terms of happiness emotions, and mild in terms of contempt, calmness, and neutral emotions. Drug-naïve status, clinical setting, positive psychotic symptoms, and negative psychotic symptoms are potential moderators of the magnitudes of FEI deficits. It is necessary to consider these characteristics of FEI deficits and the clinical moderators of the FEI deficits when developing remediation strategies targeting FER deficits in schizophrenia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
Y-MX: acquisition and analysis of data for the study, drafting the paper, and interpretation of data for the study. Y-MX and FD: design and acquisition of data for the study. B-LZ: drafting the paper, revising the paper for important intellectual content, and interpretation of data for the study. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank all the research staff for their team collaboration work.
Funding
This work was supported by the National Natural Science Foundation of China (grant number: 71774060), the Health Commission of Hubei Province Scientific Research Project (grant number: WJ2019F012), and the Wuhan Health and Family Planning Commission (grant numbers: WX17Q30, WG16A02, and WG14C24). The funding source listed had no role in the study design; in the collection, analysis, and interpretation of data, in the writing of the report; and in the decision to submit the paper for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.1097350/full#supplementary-material
References
- 1.Gao Z, Zhao W, Liu S, Liu Z, Yang C, Xu Y. Facial emotion recognition in schizophrenia. Front Psychiatry. (2021) 12:633717. 10.3389/fpsyt.2021.633717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behere RV. Facial emotion recognition deficits: the new face of schizophrenia. Indian J Psychiatry. (2015) 57:229–35. 10.4103/0019-5545.166641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gica S, Poyraz BC, Gulec H. Are emotion recognition deficits in patients with schizophrenia states or traits? A 6-month follow-up study. Indian J Psychiatry. (2019) 61:45–52. 10.4103/psychiatry.IndianJPsychiatry_307_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan YJ, Chen SH, Chen WJ, Liu SK. Affect recognition as an independent social function determinant in schizophrenia. Compr Psychiatry. (2009) 50:443–52. 10.1016/j.comppsych.2008.11.003 [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Wang Z, Lin G, Qian H, Gao Z, Wang X, et al. Neurological soft signs and neurocognitive deficits in remitted patients with schizophrenia, their first-degree unaffected relatives, and healthy controls. Eur Arch Psychiatry Clin Neurosci. (2020) 270:383–91. 10.1007/s00406-019-01024-x [DOI] [PubMed] [Google Scholar]
- 6.Bordon N, O'Rourke S, Hutton P. The feasibility and clinical benefits of improving facial affect recognition impairments in schizophrenia: systematic review and meta-analysis. Schizophr Res. (2017) 188:3–12. 10.1016/j.schres.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 7.Marsh PJ, Polito V, Singh S, Coltheart M, Langdon R, Harris AW, et al. quasi-randomized feasibility pilot study of specific treatments to improve emotion recognition and mental-state reasoning impairments in schizophrenia. BMC Psychiatry. (2016) 16:360. 10.1186/s12888-016-1064-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Statucka M, Walder DJ. Efficacy of social cognition remediation programs targeting facial affect recognition deficits in schizophrenia: a review and consideration of high-risk samples and sex differences. Psychiatry Res. (2013) 206:125–39. 10.1016/j.psychres.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 9.Tsotsi S, Kosmidis MH, Bozikas VP. Improved facial affect recognition in schizophrenia following an emotion intervention, but not training attention-to-facial-features or treatment-as-usual. Psychiatry Res. (2017) 254:135–42. 10.1016/j.psychres.2017.04.038 [DOI] [PubMed] [Google Scholar]
- 10.Bozikas VP, Dardagani A, Parlapani E, Ntouros E, Lagoudis A, Tsotsi S. Improved facial affect recognition in patients with first-episode psychosis. Early Interv Psychiatry. (2019) 13:977–83. 10.1111/eip.12738 [DOI] [PubMed] [Google Scholar]
- 11.Byrne LK, Pan L, Mc CM, Mellor D, Xu Y. Assessment of a six-week computer-based remediation program for social cognition in chronic schizophrenia. Shanghai Arch Psychiatry. (2015) 27:296–306. 10.11919/j.issn.1002-0829.215095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Sun K, Liu D, Chen MX Li G, Ma J, et al. The effects of combined social cognition and interaction training and paliperidone on early-onset schizophrenia. Front Psychiatry. (2020) 11:525492. 10.3389/fpsyt.2020.525492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan CC, Wong R, Wang K, Lee TM. Emotion recognition in Chinese people with schizophrenia. Psychiatry Res. (2008) 157:67–76. 10.1016/j.psychres.2006.03.028 [DOI] [PubMed] [Google Scholar]
- 14.Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. (2010) 36:1009–19. 10.1093/schbul/sbn192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkl SJ, Lah S, Harris AW, Williams LM. Facial emotion identification in early-onset and first-episode psychosis: a systematic review with meta-analysis. Schizophr Res. (2014) 159:62–9. 10.1016/j.schres.2014.07.049 [DOI] [PubMed] [Google Scholar]
- 16.Chan RC Li H, Cheung EF, Gong QY. Impaired facial emotion perception in schizophrenia: a meta-analysis. Psychiatry Res. (2010) 178:381–90. 10.1016/j.psychres.2009.03.035 [DOI] [PubMed] [Google Scholar]
- 17.Bora E, Pantelis C. Social cognition in schizophrenia in comparison to bipolar disorder: a meta-analysis. Schizophr Res. (2016) 175:72–8. 10.1016/j.schres.2016.04.018 [DOI] [PubMed] [Google Scholar]
- 18.Yang C, Zhang T, Li Z, Heeramun-Aubeeluck A, Liu N, Huang N, et al. The relationship between facial emotion recognition and executive functions in first-episode patients with schizophrenia and their siblings. BMC Psychiatry. (2015) 15:241. 10.1186/s12888-015-0618-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang S, Chen S, Zhao L, Miao D. Categorization of emotional faces in schizophrenia patients: an ERP study. Neurosci Lett. (2019) 713:134493. 10.1016/j.neulet.2019.134493 [DOI] [PubMed] [Google Scholar]
- 20.Li H, Chan RC, Zhao Q, Hong X, Gong QY. Facial emotion perception in Chinese patients with schizophrenia and non-psychotic first-degree relatives. Prog Neuropsychopharmacol Biol Psychiatry. (2010) 34:393–400. 10.1016/j.pnpbp.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 21.Leung JS, Lee TM, Lee CC. Facial emotion recognition in Chinese with schizophrenia at early and chronic stages of illness. Psychiatry Res. (2011) 190:172–6. 10.1016/j.psychres.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 22.Wang K, Cheung EF, Gong QY, Chan RC. Semantic processing disturbance in patients with schizophrenia: a meta-analysis of the N400 component. PLoS ONE. (2011) 6:e25435. 10.1371/journal.pone.0025435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Boer JN, van Hoogdalem M, Mandl RCW, Brummelman J, Voppel AE, Begemann MJH, et al. Language in schizophrenia: relation with diagnosis, symptomatology and white matter tracts. NPJ Schizophr. (2020) 6:10. 10.1038/s41537-020-0099-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu YM, Li F, Liu XB, Zhong BL. Depressive symptoms in Chinese male inpatients with schizophrenia: prevalence and clinical correlates. Psychiatry Res. (2018) 264:380–4. 10.1016/j.psychres.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Yu XL, Xiao X, Li M, Li Y. Joint-tissue integrative analysis identified hundreds of schizophrenia risk genes. Mol Neurobiol. (2022) 59:107–16. 10.1007/s12035-021-02572-x [DOI] [PubMed] [Google Scholar]
- 26.Reyes BN, Segal SC, Moulson MC. An investigation of the effect of race-based social categorization on adults' recognition of emotion. PLoS ONE. (2018) 13:e0192418. 10.1371/journal.pone.0192418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohan SN, Mukhtar F, Jobson L. An exploratory study on cross-cultural differences in facial emotion recognition between adults from Malaysia and Australia. Front Psychiatry. (2021) 12:622077. 10.3389/fpsyt.2021.622077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack RE, Garrod OG Yu H, Caldara R, Schyns PG. Facial expressions of emotion are not culturally universal. Proc Natl Acad Sci U S A. (2012) 109:7241–4. 10.1073/pnas.1200155109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan MD, Wang ZQ, Fei L, Zhong BL. Prevalence of prolonged grief disorder and its symptoms in Chinese parents who lost their only child: a systematic review and meta-analysis. Front Public Health. (2022) 10:1016160. 10.3389/fpubh.2022.1016160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z. editors. Joanna Briggs Institute Reviewer's Manual. Adelaide, SA: The Joanna Briggs Institute; (2017). Available online at: https://reviewersmanual.joannabriggs.org/ (accessed November 11, 2022). [Google Scholar]
- 31.Zhong BL, Xu YM, Xie WX, Li Y. Can P300 aid in the differential diagnosis of unipolar disorder versus bipolar disorder depression? A meta-analysis of comparative studies. J Affect Disord. (2019) 245:219–27. 10.1016/j.jad.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 32.Chen WC, Chen SJ, Zhong BL. Sense of alienation and its associations with depressive symptoms and poor sleep quality in older adults who experienced the lockdown in Wuhan, China, during the COVID-19 pandemic. J Geriatr Psychiatry Neurol. (2022) 35:215–22. 10.1177/08919887221078564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo W, Zhong BL, Chiu HF. Prevalence of depressive symptoms among Chinese university students amid the COVID-19 pandemic: a systematic review and meta-analysis. Epidemiol Psychiatr Sci. (2021) 30:e31. 10.1017/S2045796021000202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Erp TG, Preda A, Nguyen D, Faziola L, Turner J, Bustillo J, et al. Converting positive and negative symptom scores between PANSS and SAPS/SANS. Schizophr Res. (2014) 152:289–94. 10.1016/j.schres.2013.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong Y, Li X, Wang K, Wang C, Xiong Z, Xie S. A pilot study on the emotional cognition disorders in schizophrenia patients. Chin J Psychiatry. (2005) 38:108. 10.3760/j:issn:1006-7884.2005.02.018 [DOI] [Google Scholar]
- 36.Chen Y, Yao Z, Xie S, Du J, Cao Y, Chen Q. A pilot study of discernment dysfunction in facial expression in male schizophrenia. J Clin Psychol Med. (2007) 17:298–300. 10.3969/j.issn.1005-3220.2007.05.004 [DOI] [Google Scholar]
- 37.Dong Y, Li X, Wang K, Xiong Z, Xie S, Gao J, et al. A study on characteristics and correlative factors of facial emotion recognition in schizophrenics. Chin J Nerv Ment Dis. (2007) 33:412–5. 10.3969/j.issn.1002-0152.2007.07.007 [DOI] [Google Scholar]
- 38.Gao J, Li X, Wang K, Li Z, Dong Y, Xiong Z, et al. Relationship between emotion recognition deficit and symptomatology in schizophrenia. China J Health Psychol. (2007) 15:971–4. 10.3969/j.issn.1005-1252.2007.11.007 [DOI] [Google Scholar]
- 39.Dong Y, Wang K, Li X, Gao J, Wu L, Xie S, et al. Relationship between emotion recognition deficit, alexithymia and flat affect in schizoprenic patients. Chin J Behav Med Brain Sci. (2009) 18:34–6. 10.3760/cma.j.issn.1674-6554.2009.01.013 [DOI] [Google Scholar]
- 40.Zhang C, Chen S. A correlation study of emotion recognition, alexithymia and flat affect in recovery schizophrenic patients. Med J Chin Peoples Health. (2010) 22:1353–5. 10.3969/j.issn.1672-0369.2010.11.001 [DOI] [Google Scholar]
- 41.Tse WS, Yan L, Bond AJ, Chan RC, Tam DW. Facial emotion linked cooperation in patients with paranoid schizophrenia: a test on the Interpersonal Communication Model. Int J Soc Psychiatry. (2011) 57:509–17. 10.1177/0020764010371276 [DOI] [PubMed] [Google Scholar]
- 42.Yu S. The Executive Function and Personality Basis of Facial Emotion Recognition and alexithymia In Paranoid Schizophrenia. Hangzhou: Zhejiang University; (2012). [Google Scholar]
- 43.Li X. The Study of the Resting–state fMRI and Facial Expression Cognitive Function on Adolescents Patients with Autism and Type II Schizophrenia. Xinxiang: The Second Clinical College of Xinxiang Medical University; (2015). [Google Scholar]
- 44.Song Y, Xiang YT, Huang Y, Wang X, Wang X, Zhang F, et al. Impairments in negative facial emotion recognition in chinese schizophrenia patients detected with a newly designed task. J Nerv Ment Dis. (2015) 203:718–24. 10.1097/NMD.0000000000000358 [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Kang W. A pilot study on the face recognition disorder among female patients with schizophrenia. J Mod Med Health. (2015) 31:556–8. 10.3969/j.issn.1009-5519.2015.04.02729216439 [DOI] [Google Scholar]
- 46.Tang XW, Yu M, Duan WW, Zhang XR, Sha WW, Wang X, et al. Facial emotion recognition and alexithymia in Chinese male patients with deficit schizophrenia. Psychiatry Res. (2016) 246:353–9. 10.1016/j.psychres.2016.09.055 [DOI] [PubMed] [Google Scholar]
- 47.Zhu Y, Zhou Z, Yuan G. A related study of facial emotion recognition function in patients with schizophrenia. J Psychiatry. (2016) 29:182–5. 10.3969/j.issn.2095-9346.2016.03.007 [DOI] [Google Scholar]
- 48.Lv Y. Multimodal Magnetic Resonance Imaging in Patients with First-episode Schizophrenia. Nanjing: Nanjing Medical University; (2017). [Google Scholar]
- 49.Yang C, Zhang T, Li Z, Heeramun-Aubeeluck A, Liu N, Huang N, et al. Changes in event-related potentials in patients with first-episode schizophrenia and their siblings. BMC Psychiatry. (2017) 17:20. 10.1186/s12888-016-1189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Y. The Control Study of Olanzapine and Aripiprazole on Cognitive Function in Schizophrenia. Nanjing: Nanjing Medical University; (2017). [Google Scholar]
- 51.Liu P, Chen K, Tang X, Xiao W. Neurocognitive and social cognitive functions in first-episode schizophrenic patients in remission. J Clin Psychiatry. (2018) 28:45–7. 10.3969/j.issn.1005-3220.2018.01.016 [DOI] [Google Scholar]
- 52.Guo X. Study on Social Cognition and Social Function of Schizophrenia. Jining: Jining Medical University; (2019). [Google Scholar]
- 53.Zhao Y, Fan H, Zhang Y, Qu W, Li D, Li Y, et al. Chinese facial emotion recognition test: reliability and validity. Chin J Psychiatry. (2020) 53:512–9. 10.3760/cma.j.cn113661-20200228-00065 [DOI] [Google Scholar]
- 54.Du X, Fan H, Wang Y, Zhang J, Zhu X, Zhao Y, et al. Difference of facial emotion perception in patients with bipolar disorder and schizophrenia. Chin J Nerv Ment Dis. (2021) 47:404–8. 10.3969/j.issn.1002-0152.2021.07.005 [DOI] [Google Scholar]
- 55.Gao Z. Investigation of the Processing Features of Facial-emotion Recognition deFect in Schizophrenia. Taiyuan: Shanxi Medical University; (2021). [Google Scholar]
- 56.Lee SC, Lin GH, Shih CL, Chen KW, Liu CC, Kuo CJ, et al. Error patterns of facial emotion recognition in patients with schizophrenia. J Affect Disord. (2022) 300:441–8. 10.1016/j.jad.2021.12.130 [DOI] [PubMed] [Google Scholar]
- 57.Muros NI, Garcia AS, Forner C, Lopez-Arcas P, Lahera G, Rodriguez-Jimenez R, et al. Facial affect recognition by patients with schizophrenia using human avatars. J Clin Med. (2021) 10:1904. 10.3390/jcm10091904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tremeau F. A review of emotion deficits in schizophrenia. Dialogues Clin Neurosci. (2006) 8:59–70. 10.31887/DCNS.2006.8.1/ftremeau [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Navalon P, Serrano E, Almansa B, Perea M, Benavent P, Dominguez A, et al. Attentional biases to emotional scenes in schizophrenia: an eye-tracking study. Biol Psychol. (2021) 160:108045. 10.1016/j.biopsycho.2021.108045 [DOI] [PubMed] [Google Scholar]
- 60.Gabay AS, Kempton MJ, Mehta MA. Facial affect processing deficits in schizophrenia: a meta-analysis of antipsychotic treatment effects. J Psychopharmacol. (2015) 29:224–9. 10.1177/0269881114560184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Behere RV, Venkatasubramanian G, Arasappa R, Reddy N, Gangadhar BN. Effect of risperidone on emotion recognition deficits in antipsychotic-naive schizophrenia: a short-term follow-up study. Schizophr Res. (2009) 113:72–6. 10.1016/j.schres.2009.05.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.