Abstract
McCune-Albright syndrome (MAS) is a rare multisystem disorder characterized by a clinical triad of polyostotic fibrous dysplasia (FD), skin pigmentation, and hyperfunctioning endocrinopathies. A 42-year-old man visited our medical hospital for the treatment of intermittent headaches and was diagnosed with MAS with acromegaly. This patient showed various clinical features of MAS, including pituitary adenoma, polyostotic FD, and hypogonadotropic hypogonadism. The FD lesions showed characteristic radiographic features, such as widespread, sclerotic bony lesions in the cranial bones, mixed radiolucent-radiopaque multilocular lesions in the mandible, and radiolucent lesions in the axial and appendicular skeleton. Over the years, the patient had been hospitalized multiple times due to accidental bony fractures associated with the fragile bony state of FD. This report presents a retrospective description of a case of MAS, with a review of the relevant literature.
Keywords: Fibrous Dysplasia, Polyostotic; Mandible; Acromegaly; Radiography
McCune-Albright syndrome (MAS), first described by McCune (1936)1 and Albright et al. (1937)2 is a heterogeneous disease caused by postzygotic, somatic, and sporadic mutations in the GNAS1 gene. It affects the endocrine system, skin, and bones.3,4 MAS has been classically described as a triad of hyperfunctioning endocrinopathies, café-au-lait spots, and polyostotic FD. Although MAS has various clinical presentations, the most common form of MAS is seen in females with a triad of physical signs.5
MAS presents a wide spectrum of disease manifestations due to mutations of the GNAS1 gene. The rarity of MAS and its variable clinical presentation often lead to misdiagnosis and improper treatments.6 FD results from mutations in the GNAS1 gene, which cause abnormal proliferation and differentiation of osteoblasts together with increased osteoclastic activity.7 Ninety percent of MAS patients have FD lesions in the craniofacial area, resulting in significant orofacial deformities and compromised oral health.8 A pathophysiologic understanding of FD associated with MAS is important for an accurate radiographic diagnosis and for minimizing potential complications.9
Acromegaly is a rare condition characterized by excess growth hormone (GH) that is caused by pituitary tumors.10 Acromegaly affects around 20% of MAS patients, and its prevalence is estimated between 1/100,000 and 1/1,000,000.11
The MAS patient presented in this report showed polyostotic FD, acromegaly, and hypogonadotropic hypogonadism. The purpose of this report was to better characterize the radiographic features of FD associated with MAS accompanied by acromegaly. The characteristic radiographic features of FD and various clinical features in the MAS patient of this report were retrospectively examined, with a review of the relevant literature.
Case Report
To report this case of MAS, the clinical records and radiographic images of 1 patient, which had been obtained for 14 years, were retrospectively examined. This report was approved by the Institutional Review Board of our hospital. Written informed consent could not be obtained from the patient because he died in 2021.
A 42-year-old man visited our hospital for the treatment of intermittent headaches. The headaches started when he was in high school and lasted for 25 years. He had been limping on his left leg since he was 9 years old. The patient had acromegaly since he was a high school student (approximately 23-25 years ago). He showed various features of acromegaly, such as tall height (180 cm), acral enlargement of the hands and feet, prognathism, and forehead protrusion. At the age of 29, he underwent femoral fracture surgery. He had a slight hearing impairment and occasional vision dimness.
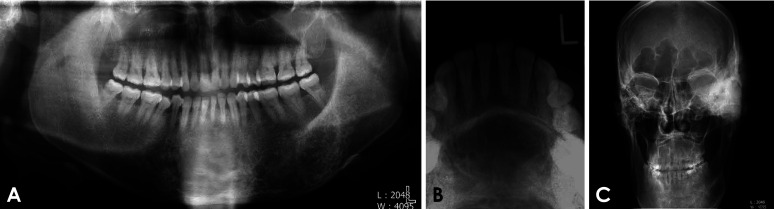
The panoramic and occlusal radiographs (Figs. 1A and B) revealed mixed radiolucent-radiopaque multilocular lesions with poorly defined margins in the lower anterior and left posterior regions of the mandible. The adjacent teeth showed no root resorption or tooth displacement. An increase in the vertical dimensions of the mandible was observed. A posteroanterior skull radiograph (Fig. 1C) revealed an enlarged frontal sinus.
Fig. 1. A. Panoramic radiograph shows mixed radiolucent-radiopaque, multilocular lesions with poorly defined margins in the lower anterior and left posterior regions. B. Occlusal radiograph shows mixed radiolucent-radiopaque, multilocular lesions in the lower anterior mandible. C. Posteroanterior skull radiograph shows an enlarged frontal sinus. Pronounced macrocephaly made it difficult to position the patient appropriately in the panoramic and skull machine.
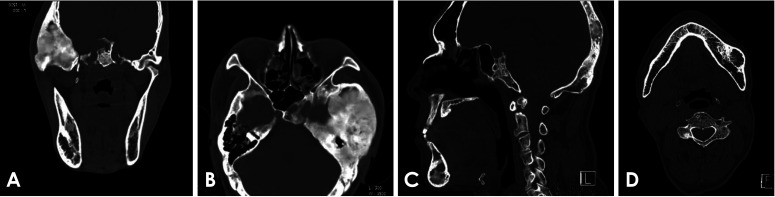
A computed tomography (CT) scan revealed a sclerotic and expansile region with a ground-glass appearance in the left sphenoidal, temporomastoid, and occipital regions, suggestive of FD (Figs. 2A and B). The mandible showed an expansile, multilocular radiolucency with mixed radiopacity in the left posterior and lower anterior regions (Figs. 2C and D). No specific treatment was given for these craniofacial FD lesions.
Fig. 2. A. Computed tomographic image shows a dense, sclerotic, expansile mass and ground-glass appearance. The left temporomastoid region is involved. B. The left sphenoidal and temporomastoid regions are involved. C. The occipital and anterior mandibular regions are involved. D. The mandible shows an expansile and mixed lesion in the left posterior region.
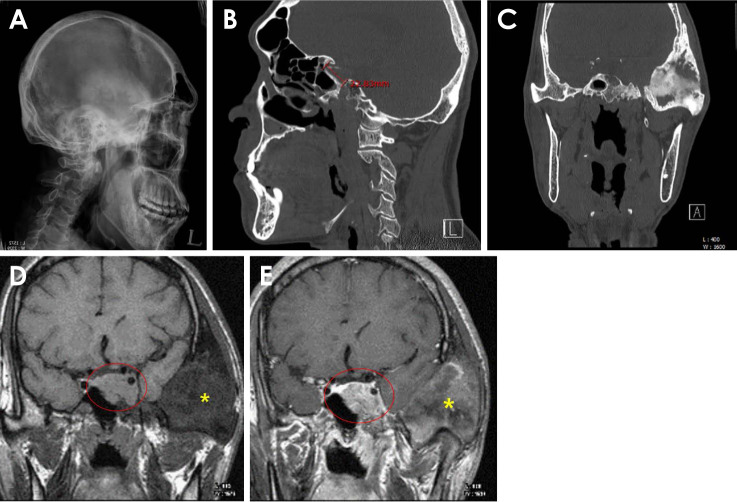
On a lateral skull radiograph and CT scan, slight ballooning and widening of the bony sella turcica were observed (Figs. 3A and B). The sella turcica showed an abnormally enlarged appearance on the radiographs (anteroposterior and depth dimensions: 22 and 8 mm, respectively). An asymmetrical bony erosion of the left sellar floor was also observed on the CT scan (Fig. 3C).
Fig. 3. A. Slight ballooning and widening of the bony sella turcica are seen in lateral skull radiograph. B. The abnormally increased dimensions of the sella turcica (antero-posterior length is approximately 22 mm) are also seen on computed tomography (CT). C. Asymmetrical bony erosion of the left sellar floor is seen on a coronal CT image. D. T1-weighted magnetic resonance (MR) image. MR imaging shows a well-defined homogeneous mass in the left hypophyseal fossa (red circle). FD is also observed in the temporo-mastoid area (asterisk). E. A lobulated mass is clearly detected on a gadolinium-enhanced T1-weighted MR image (red circle: mass in the left hypophyseal fossa). FD (asterisk). FD: fibrous dysplasia.
Magnetic resonance (MR) imaging showed a well-defined homogeneous mass in the left hypophyseal fossa (Figs. 3D and E). A T1-weighted MR image (Fig. 3D) showed an isointense lobulate lesion. A gadolinium-enhanced T1-weighted MR image (Fig. 3E) showed an enhanced lesion invading the left cavernous sinus with a downward extension into the left sphenoid sinus. The tumor size was approximately 20 mm×17 mm. These features were consistent with pituitary macroadenoma, and the patient’s acromegaly could have been related to the pituitary tumor.
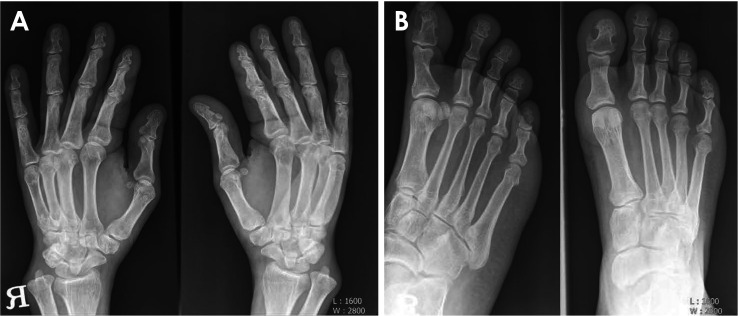
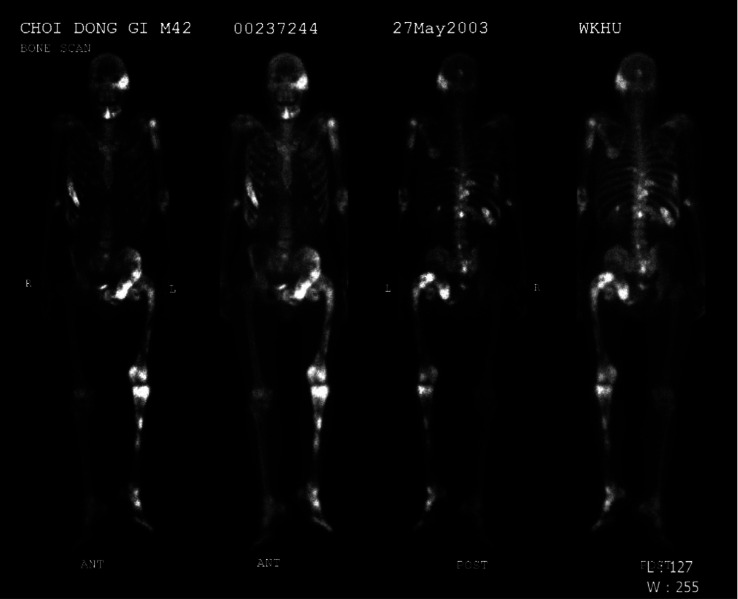
Large tufts and a spade appearance in the distal phalanges of the hand (Fig. 4A) and foot (Fig. 4B) were observed on the radiographs. In a bone scan to assess the bony involvement of polyostotic FD, increased uptake of Tc-99m methyl diphosphonate was observed in the left temporal and sphenoid bones, left humerus, right ribs, left pelvic bones, left femur, and left tibia (Fig. 5). The left femur showed the characteristic “shepherd’s crook” deformity (coxa vera), resulting in an unbalanced limb.
Fig. 4. A. Typical appearance of acromegaly in the phalanges. Large tufts and a spade appearance in the distal phalanges of the hand. B. The distal phalanges of the foot are also involved.
Fig. 5. Multifocal increased uptake of Tc-99m methyldiphosphonate was observed in the left temporal and sphenoid bones, left humerus, right ribs, left pelvic bones, left femur, and left tibia. The “shepherd’s crook” deformity is also seen in the left proximal femur.
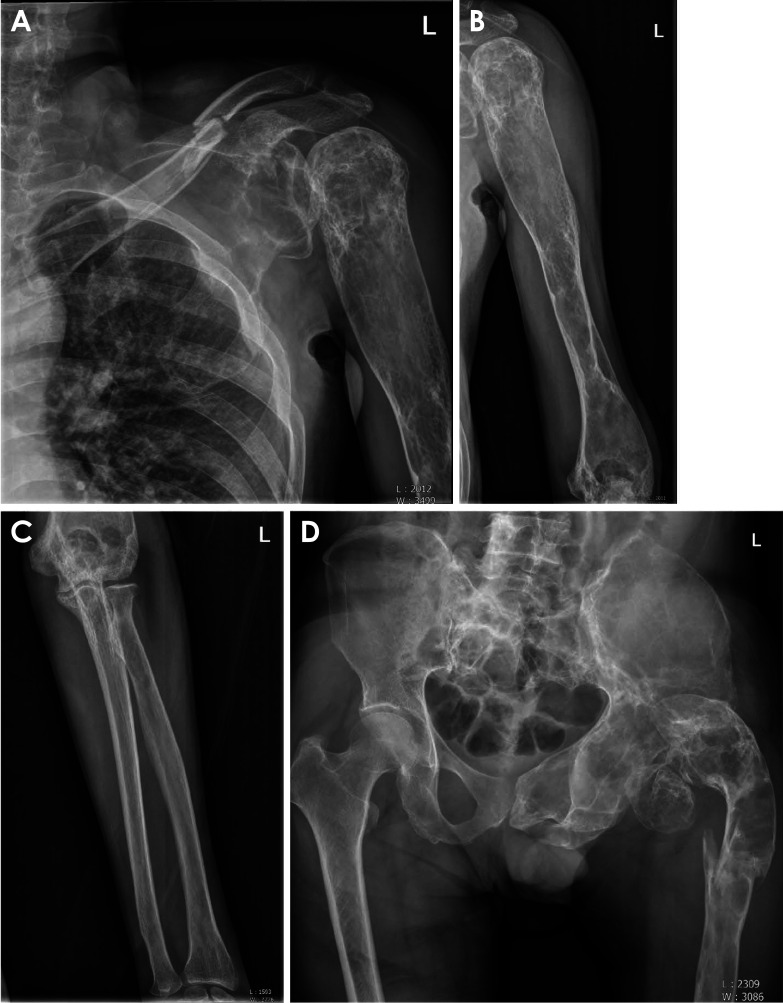
Various axial and appendicular radiographs revealed predominantly radiolucent lesions, with sclerotic lesions in the left scapula and humerus (Figs. 6A and B) and a radiolucent lesion in the left radius and ulna (Fig. 6C). Mixed lesions were also observed in the left femur and left pelvis (Fig. 6D). The patient had experienced multiple accidental bony fractures of the clavicle and femur over the years. These fragile bones were related to the pathologic bony tissues due to FD.
Fig. 6. A. There are predominantly mixed lesions in the left scapula and humerus. A bony fracture line is also seen on the left clavicle. B. There are predominantly radiolucent and mixed lesions in the left humerus. C. The left radius and ulna show radiolucent lesions and mild mixed changes in the proximal portion. D. Mixed lesions in the left femur and left pelvis are observed. A bony fracture line is also seen in the left femur.
Café au lait macules were not observed in association with hyperfunctioning endocrinopathies. However, the patient presented with hypogonadotropic hypogonadism related to a pituitary gland problem. The measured levels of prolactin (4.7 µg/L, normal range: 4.0-15.2 µg/L), testosterone (1.3 µg/L, normal range: 1.8-7.7 µg/L), follicle-stimulating hormone (2.2 IU/L, normal range: 1.5-12.4 IU/L), and luteinizing hormone (0.32 IU/L, normal range: 1.24-7.80 IU/L) helped establish the diagnosis of hypogonadotropic hypogonadism.
The patient underwent transfrontal pituitary adenomectomy and was followed up for 14 years in the endocrine department of the medical hospital. Laboratory tests for preoperative human growth hormone (hGH) and insulin-like growth factor-1 (IGF-1, somatomedin-C) revealed high levels. The hGH and IGF-1 levels were 26-32 µg/L (normal range: 0.4-10 µg/L) and 985-1003 µg/L (normal range: 87-267 µg/L), respectively.
Sandostatin LAR® (somatostatin analogue, octreotide) was administered postoperatively to control the excess growth hormone. The levels of hGH and IGF-1 were reduced and maintained at approximately 4.09-10.73 and 232-349 µg/L, respectively, 14 years after surgery.
Discussion
The definition of MAS has recently been broadened to include not only precocious puberty, but other hyperfunctioning endocrinopathies as well.11 Precocious puberty is the most common presenting symptom in females.5 Other endocrinopathies such as hyperthyroidism, Cushing’s syndrome, hyperprolactinemia, hypophosphatemia, rickets, and acromegaly have been associated with the syndrome.12 In this patient, as café-au-lait spots were not observed, MAS was diagnosed based on the coexistence of polyostotic FD and hyperfunctioning endocrinopathies, such as excess growth hormone and hypogonadotropic hypogonadism.
FD is the most common component of MAS13, and it usually involves the craniofacial bones.14 Acromegaly is almost always associated with the skull base FD.15 In the patient presented herein, the skull base showed FD involvement. A CT scan (Fig. 2) showed that the left skull base, including the sphenoid, temporo-mastoid, and occipital areas had a sclerotic and ground-glass appearance.
Excess GH is particularly detrimental in patients with MAS because it may accelerate FD, especially in the craniofacial bones, potentially causing vision and hearing loss.16 The present patient complained of intermittent headaches, hearing impairment, and frequent dimness of vision. These symptoms were thought to be related to FD of the skull base.
In general, FD shows a site-specific radiographic appearance. Lesions in the long bones have a lytic appearance;11 craniofacial FD typically demonstrates dense and sclerotic lesions,9,11 and jaw lesions usually show a ground-glass appearance. The radiological features of FD vary with the amount and degree of the mineralized tissue within the lesion.17 For example, in the femur, the classic radiographical appearance of radiolucent ground-glass is changed to a more dense and sclerotic lesion according to the patient’s age.9,18,19 These reported radiographic features show similar patterns to the histologic features of FD concerning specific sites.20 The patient presented in this report also showed similar site-specific radiographic features, with FD manifesting as sclerotic lesions in the cranial bone, radiolucent-radiopaque mixed lesions with multilocular appearance in the mandible, and more radiolucent lesions in the axial and appendicular skeleton. The mandible showed a multilocular appearance. In the authors’ opinion, this was an interesting radiographic feature of this case, compared with the common “ground-glass” appearance of the jaw.
To evaluate the skeletal extent of FD, whole-body bone scintigraphy should be considered.6 Multiple foci of increased Tc-99m uptake are typical signs of polyostotic FD.21 In this study, multiple bones showed hot-spot lesions and characteristic radiographic features of the “shepherd’s crook” deformity of left femur. It is presumed that the FD on the left femur had been present since childhood (9 years old). Symptomatic bone dysplasia has been reported to be mostly present during the first decade, with limb deformities.11
The remarkable features of acromegaly related to facial appearance include enlarged supraorbital ridges, a wide nose, and prognathism. Additionally, patients often show interdental separation, macroglossia, and enlarged hands and feet.22 Radiographs of the sella turcica in this study showed an abnormally enlarged appearance on lateral skull and CT scans. Weisberg et al.23 evaluated 100 patients with enlargement of the sella turcica, and the most common cause of sellar expansion was a primary intrasellar pituitary tumor.
The radiographs of this patient also showed large tufts and a spade appearance in the distal phalanges of the hand and foot (Fig. 4). These features were consistent with the findings of a previous study24 associated with acral enlargement. An interesting radiographic feature of this case associated with acromegaly was the radiographic appearance of the enlarged frontal sinus (Fig. 1C). Steinbach et al.24 reported that frontal sinus enlargement in males is related to acromegaly.
Currently, the treatment of acromegaly includes surgical removal of the tumor, radiation therapy, and medication for the pituitary tumor.25 Somatostatin analogues reduced the GH and IGF-1 levels in most cases, although remission of acromegaly was only achieved in 30% of patients.26 It is not easy to completely control excess GH. The reported acromegaly cases responded poorly to surgery and medical treatments.22
Hypogonadotropic hypogonadism probably reflects a reduction in hypothalamic gonadotropin-releasing hormone secretion related to the presence of a pituitary tumor.27 This patient showed hypogonadotropic hypogonadism related to pituitary gland problems, constituting another distinctive endocrine problem related to MAS.
The craniofacial form of MAS is the most common type of FD, and also the most difficult form to manage.9 Bone changes in MAS are often severe and have a more complicated course of the disease than in polyostotic FD without extra-skeletal manifestations.28 Dentists and radiologists play a crucial role in all the steps of the management of FD associated with MAS, since bone imaging provides essential information for the diagnosis and prognosis.18
In summary, this male patient was diagnosed with MAS in middle age based on polyostotic FD, acromegaly, and hypogonadotropic hypogonadism. Polyostotic FD presents with a wide range of radiographic features. These features of MAS with acromegaly have seldom been reported and might contribute to the various radiographic features of polyostotic FD and MAS.
Acknowledgments
The authors thank Prof. Choi Si-Sung (MD) and Prof. Lee Young-Jin (MD) for their insightful comments.
Footnotes
This work was supported by Wonkwang University in 2022. The funder had no role in the design of the study, data collection, analysis, interpretation of data, and writing of the manuscript.
Conflicts of Interest: None
References
- 1.McCune DJ. Osteitis fibrosa cystica: The case of a 9-year-old girl who also exhibits precocious puberty, multiple pigmentation of the skin and hyperthyroidism. Am J Dis Child. 1936;52:743–744. [Google Scholar]
- 2.Albright F, Butler AM, Hampton AO, Smith P. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females - report of five cases. N Engl J Med. 1937;216:727–746. [Google Scholar]
- 3.Spencer T, Pan KS, Collins MT, Boyce AM. The clinical spectrum of McCune-Albright syndrome and its management. Horm Res Paediatr. 2019;92:347–356. doi: 10.1159/000504802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takayasu S, Makita K, Kageyama K, Okawa Y, Oki Y, Yamagata S, et al. Presence of aberrant adrenocorticotropic hormone precursors in two cases of McCune-Albright syndrome. Endocr J. 2020;67:353–359. doi: 10.1507/endocrj.EJ19-0449. [DOI] [PubMed] [Google Scholar]
- 5.Pina Rivera Y, Rwegerera GM, Sesay S. Short stature and growth hormone deficiency: unexpected manifestations of McCune-Albright syndrome. BMJ Case Rep. 2018;2018:bcr2018225709. doi: 10.1136/bcr-2018-225709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javaid MK, Boyce A, Appelman-Dijkstra N, Ong J, Defabianis P, Offiah A, et al. Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: a consensus statement from the FD/MAS international consortium. Orphanet J Rare Dis. 2019;14:139. doi: 10.1186/s13023-019-1102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lima-Martínez MM, Gil V, Mederico M, Gómez-Pérez R. Hypogonadotropic hypogonadism in a male with McCune-Albright syndrome. Endocrinol Nutr. 2013;60:145–147. doi: 10.1016/j.endonu.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Akintoye SO, Lee JS, Feimster T, Booher S, Brahim J, Kingman A, et al. Dental characteristics of fibrous dysplasia and McCune-Albright syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:275–282. doi: 10.1016/s1079-2104(03)00225-7. [DOI] [PubMed] [Google Scholar]
- 9.Kushchayeva YS, Kushchayev SV, Glushko TY, Tella SH, Teytelboym OM, Collins MT, et al. Fibrous dysplasia for radiologists: beyond ground glass bone matrix. Insights Imaging. 2018;9:1035–1056. doi: 10.1007/s13244-018-0666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coopmans EC, Postma MR, Wolters TL, van Meyel SW, Netea-Maier R, van Beek AP, et al. Predictors for remission after transsphenoidal surgery in acromegaly: a dutch multicenter study. J Clin Endocrinol Metab. 2021;106:1783–1792. doi: 10.1210/clinem/dgab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumitrescu CE, Collins MT. McCune-Albright syndrome. Orphanet J Rare Dis. 2008;3:12. doi: 10.1186/1750-1172-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Premawardhana LD, Vora JP, Mills R, Scanlon MF. Acromegaly and its treatment in the McCune-Albright syndrome. Clin Endocrinol (Oxf) 1992;36:605–608. doi: 10.1111/j.1365-2265.1992.tb02272.x. [DOI] [PubMed] [Google Scholar]
- 13.Collins MT, Singer FR, Eugster E. McCune-Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet J Rare Dis. 2012;7 Suppl 1:S4. doi: 10.1186/1750-1172-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leet AI, Collins MT. Current approach to fibrous dysplasia of bone and McCune-Albright syndrome. J Child Orthop. 2007;1:3–17. doi: 10.1007/s11832-007-0006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salenave S, Boyce AM, Collins MT, Chanson P. Acromegaly and McCune-Albright syndrome. J Clin Endocrinol Metab. 2014;99:1955–1969. doi: 10.1210/jc.2013-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akintoye SO, Chebli C, Booher S, Feuillan P, Kushner H, Leroith D, et al. Characterization of gsp-mediated growth hormone excess in the context of McCune-Albright syndrome. J Clin Endocrinol Metab. 2002;87:5104–5112. doi: 10.1210/jc.2001-012022. [DOI] [PubMed] [Google Scholar]
- 17.Fitzpatrick KA, Taljanovic MS, Speer DP, Graham AR, Jacobson JA, Barnes GR, et al. Imaging findings of fibrous dysplasia with histopathologic and intraoperative correlation. AJR Am J Roentgenol. 2004;182:1389–1398. doi: 10.2214/ajr.182.6.1821389. [DOI] [PubMed] [Google Scholar]
- 18.Bousson V, Rey-Jouvin C, Laredo JD, Le Merrer M, Martin-Duverneuil N, Feydy A, et al. Fibrous dysplasia and McCune-Albright syndrome: imaging for positive and differential diagnoses, prognosis, and follow-up guidelines. Eur J Radiol. 2014;83:1828–1842. doi: 10.1016/j.ejrad.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Kuznetsov SA, Cherman N, Riminucci M, Collins MT, Robey PG, Bianco P. Age-dependent demise of GNAS-mutated skeletal stem cells and “normalization” of fibrous dysplasia of bone. J Bone Miner Res. 2008;23:1731–1740. doi: 10.1359/jbmr.080609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riminucci M, Liu B, Corsi A, Shenker A, Spiegel AM, Robey PG, et al. The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gs alpha gene: site-specific patterns and recurrent histological hallmarks. J Pathol. 1999;187:249–258. doi: 10.1002/(SICI)1096-9896(199901)187:2<249::AID-PATH222>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 21.Brockmann H, Joe A, Palmedo H, Biersack HJ. A patient with acromegaly presenting with polyostotic fibrous dysplasia on bone scan: McCune-Albright syndrome. Clin Nucl Med. 2005;30:813–815. doi: 10.1097/01.rlu.0000187612.09377.e0. [DOI] [PubMed] [Google Scholar]
- 22.Reddy R, Hope S, Wass J. Acromegaly. BMJ. 2010;341:c4189. doi: 10.1136/bmj.c4189. [DOI] [PubMed] [Google Scholar]
- 23.Weisberg LA, Zimmerman EA, Frantz AG. Diagnosis and evaluation of patients with an enlarged sella turcica. Am J Med. 1976;61:590–596. doi: 10.1016/0002-9343(76)90136-4. [DOI] [PubMed] [Google Scholar]
- 24.Steinbach HL, Feldman R, Goldberg MB. Acromegaly. Radiology. 1959;72:535–549. doi: 10.1148/72.4.535. [DOI] [PubMed] [Google Scholar]
- 25.Kwon O, Song YD, Kim SY, Lee EJ Rare Disease Study Group, Science and Research Committee, Korean Endocrine Society. Nationwide survey of acromegaly in South Korea. Clin Endocrinol (Oxf) 2013;78:577–585. doi: 10.1111/cen.12020. [DOI] [PubMed] [Google Scholar]
- 26.Bodakçi E, Tuna MM, Kılınç F, Pekkolay Z, Soylu H, TuzcuŞA, et al. A rare cause of acromegaly: McCune-Albright syndrome. Dicle Med J. 2015;42:242–244. [Google Scholar]
- 27.Swislocki AL, Camargo CA, Hoffman AR. McCune-Albright syndrome. A case of primary hypogonadism obscured by hyperprolactinemic hypogonadotropic hypogonadism. West J Med. 1990;153:653–656. [PMC free article] [PubMed] [Google Scholar]
- 28.Belsuzarri TA, Araujo JF, Melro CA, Neves MW, Navarro JN, Brito LG, et al. McCune-Albright syndrome with craniofacial dysplasia: clinical review and surgical management. Surg Neurol Int. 2016;7(Suppl 6):S165–S169. doi: 10.4103/2152-7806.178567. [DOI] [PMC free article] [PubMed] [Google Scholar]