Summary
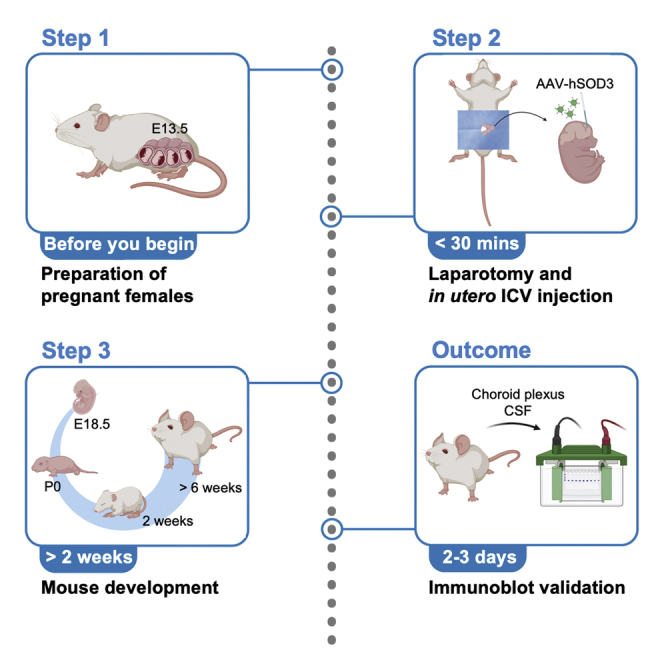
Experimentally targeting mouse choroid plexus (ChP) provides a valuable approach for investigating mechanisms of ChP-cerebrospinal fluid (CSF) biology. Here, we provide a protocol to deliver adeno-associated viral vectors (AAVs) by in utero intracerebroventricular (ICV) injection to ChP epithelial cells. We begin by describing steps for induction anesthesia of the pregnant dam, laparotomy, and in utero ICV injection. We also detail post-surgical care and immunoblot validation.
For complete details on the use and execution of this protocol, please refer to Jang et al. (2022).1
Subject areas: Developmental biology, Model Organisms, Gene Expression, Neuroscience
Graphical abstract
Highlights
-
•
In utero intracerebroventricular delivery of AAVs into the lateral ventricle
-
•
AAV serotype for targeting embryonic mouse choroid plexus
-
•
Persistent protein overexpression by choroid plexus
-
•
Suitable for long-term modulation of CSF composition
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Experimentally targeting mouse choroid plexus (ChP) provides a valuable approach for investigating mechanisms of ChP-cerebrospinal fluid (CSF) biology. Here, we provide a protocol to deliver adeno-associated viral vectors (AAVs) by in utero intracerebroventricular (ICV) injection to ChP epithelial cells. We begin by describing steps for induction anesthesia of the pregnant dam, laparotomy, and in utero ICV injection. We also detail post-surgical care and immunoblot validation.
Before you begin
The following protocol, adapted from Jang et al., describes the injection of adeno-associated viral vectors (AAV) into the lateral ventricle of mouse embryos at E13.5. Variable expression patterns and efficiency can result from different AAV serotypes. We transduced AAVs, particularly the AAV2/5 serotype, due to its tropism for the choroid plexus (ChP) at this age.1,2 The protocol below shows an example using CD1 mice that have large litters. However, we have also used this protocol with C57BL/6 mice or transgenic/knockout mice with C57BL/6J backgrounds.
Institutional permissions
The surgical procedure was approved and performed in accordance with the regulations of the Boston Children’s Hospital Institutional Animal Care and Use Committee. Before beginning, approval must be obtained from each researcher’s own institution.
Acquire pregnant dam from wild-type or specific mouse lines
Timing: >2 weeks
-
1.
Obtain a pregnant dam with the desired strain and embryonic age in accordance with the specific experimental design.
Optional: Consider setting up mating and plug check by the researcher for accurate timing.3
-
2.
Habituate the pregnant dam to the animal facility (e.g., for 3 days, based on your institutional recommendations) before the procedure.
-
3.Weigh each mouse to obtain baseline weight.
-
a.Use this weight to calculate dosages of pre-and/or post-surgical analgesia.Note: Analgesia protocols should follow local animal care committee guidelines.
-
b.This weight will be used as the reference weight to determine post-surgery health.
-
a.
Preparation of AAV-SOD3 solution (for in utero ICV injection; see step-by-step detailed protocol)
Timing: 10 min
-
4.Calculate the amount of virus needed for the experimental batch.Note: We injected 1–5 × 1012 gc/mL of AAV per embryo. The stock AAV-hSOD3 viral vector is stored at −80°C.
-
a.Thaw one aliquot of the stock AAV-hSOD3 viral vector on ice and dilute in sterile saline to adjust the titer.
-
b.Quickly spin down the virus to get rid of any bubbles.
-
a.
Note: Although AAVs are generally considered safe,4 please be sure to follow your institutional guidelines for working with these reagents.
Note: Embryo numbers vary between mouse strains and can range from approximately 6–8 for C57BL/6 to 10–15 for CD1 strains.
Note: To maximize viral transduction efficiency, avoid frequent freeze/thaw cycles of the viral stock solution and discard unused reagents.5
Preparation of glass capillaries for surgery (for in utero ICV injection; see step-by-step detailed protocol)
Timing: 10 min
-
5.Prepare glass capillary needles for virus injection using a micropipette puller.Note: We pulled glass microcapillaries under the following conditions: one-step pulling with heat level 62°C.Note: The exact settings may need to be adjusted based on the micropipette puller machine.
-
a.To inject the virus solution optimally into the ventricle, break the capillary tip using forceps or spring scissors (diameter of the tip is approximately 50 μm).
- b.
-
a.
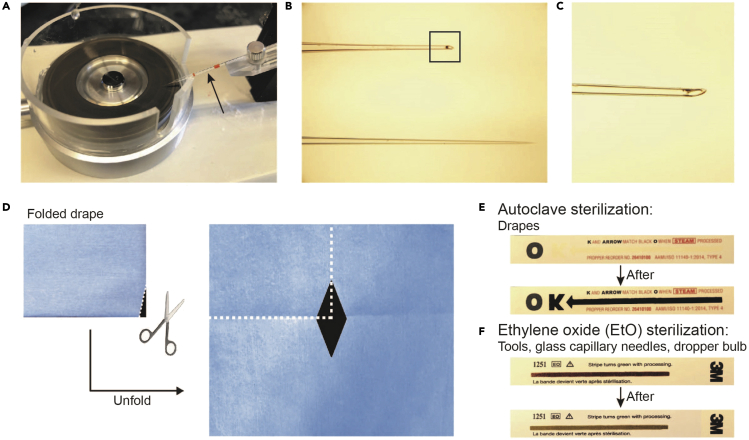
Figure 1.
Preparation of drape, sterilization, and glass capillary needles
(A) Glass capillary needle microgrinder (Cat#EG-45, Narishige). Black arrow in (A) indicates the glass capillary needle that is being prepared.
(B) Example of glass capillary needles before (lower image) and after (upper image) grinding. The black square corresponds to (C).
(C) Higher magnification image of the glass capillary needle tip. Store the needle with the tip pointing up.
(D) The process of cutting and making a diamond-shaped aperture that only exposes the surgical area prior to sterilization.
(E and F) Comparison between autoclave sterilization (E) and ethylene oxide (EtO) sterilization (F) indicators. After sterilization, it will be sterile and ready to use. Figure created with BioRender.com.
Preparation for surgery (for in utero ICV injection; see step-by-step detailed protocol)
Timing: 30 min
-
6.Prepare sterile surgical drapes, tools, glass capillary needles, and bulbs.Note: Surgical drapes should be autoclaved.
-
a.Cut a diamond-shaped incision into a drape to outline the surgical field (Figure 1D).
-
b.Surgical tools, glass capillary needles and bulbs need ethylene oxide (EtO) sterilization to avoid damage from high autoclave temperatures.
-
c.See Figures 1E and 1F to classify the supplies for autoclave and EtO sterilization.
-
a.
-
7.Prepare the surgery station (Figure 2).
-
a.Adjust the heating pad to 37°C.
-
b.Prepare sterile saline and keep warm in a water bath to reach 37°C.
-
c.Turn on the LED illuminator and glass bead sterilizer.
-
a.
-
8.As the last presurgical step, fill the glass capillary needles with AAV solution.
-
a.Fully immerse the tip into the AAV solution tube and slowly load 1 μL (1–5 × 1012 gc/mL) into the glass capillary needle using a dropper bulb.
-
a.
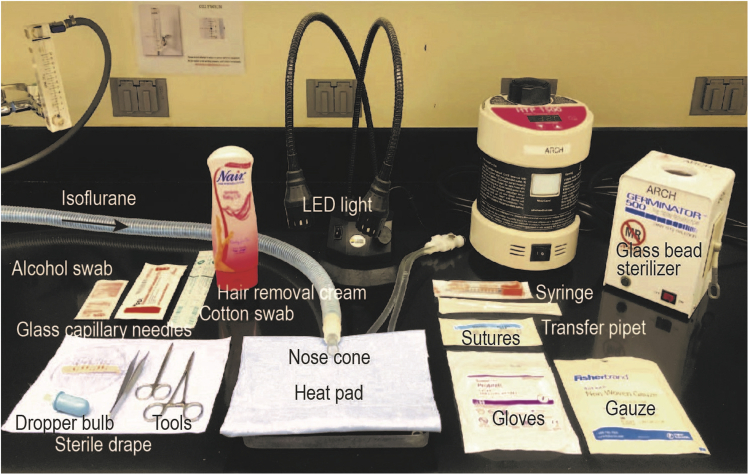
Figure 2.
Image of the experimental setup for in utero ICV injection including equipment and tools
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-Superoxide dismutase 3 (1:1000) | Abcam | Cat#ab171738 |
| Rabbit polyclonal anti-Albumin (1:1000) | Cell Signaling Technology | Cat#4929; RRID: AB_2225785 |
| Rabbit monoclonal anti-Vinculin (1:1000) | Cell Signaling Technology | Cat#13901; RRID: AB_2728768 |
| Anti-rabbit IgG, HRP-linked antibody (1:2000) | Cell Signaling Technology | Cat#7074; RRID: AB_2099233 |
| Bacterial and virus strains | ||
| AAV2/5-CMV-hSOD3-hrGFP-hGH | Boston Children’s Hospital viral core, IDDRC | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Isoflurane | Piramal Critical Care | Cat#6679401710 |
| Pivetal Alloxate (Meloxicam) | Patterson Veterinary | Cat#:07-893-7565 |
| Optixcare eye lubricant | Patterson Veterinary | Cat#07-893-2779 |
| 1× PBS | GIBCO | Cat#10010023 |
| 10× HBSS | GIBCO | Cat#14180-020 |
| Povidone-Iodine swabsticks | Fisher Scientific | Cat#06-669-82 |
| Pierce BCA protein assay | Thermo Fisher Scientific | Cat#23227 |
| RIPA lysis and extraction buffer | Thermo Fisher Scientific | Cat#89900 |
| Halt protease and phosphatase inhibitor cocktail | Thermo Fisher Scientific | Cat#78400 |
| NuPAGE 4%–12% Bis-Tris protein gels | Invitrogen | Cat#NP0336 |
| NuPAGE LDS sample buffer | Invitrogen | Cat#NP0007 |
| NuPAGE sample reducing agent | Invitrogen | Cat#NP0009 |
| GE Healthcare Amersham ECL select | Fisher Scientific | Cat#45-000-999 |
| Amersham Hybond P membranes, PVDF | Millipore Sigma | Cat#GE10600029 |
| Critical commercial assays | ||
| Fast green | Sigma-Aldrich | Cat#F7252 |
| Experimental models: Organisms/strains | ||
| Mouse: CD1 E13.5 pregnant dams | Charles River Laboratories | Cat#022 |
| Recombinant DNA | ||
| pcDNA3.1-myc HisA (-) human SOD3 | Ota et al.6 | N/A |
| Other | ||
| Micropipette puller model PC-10 | Narishige | Cat#PC-10 |
| Microgrinder | Narishige | Cat#EG-45 |
| Isoflurane anesthesia machine with induction chamber and nose cone | VETEQUIP | Cat#901806 |
| Heating pad | Adroit Medical | Cat#HTP-1500 |
| Water bath | VWR | Cat#WB02 |
| LED gooseneck illuminator | LAXCO | Cat#AMPS-ILED-21 |
| Glass bead sterilizer | Cellpoint Scientific | Cat#5-1450 |
| Drummond calibrated micropipets | Fisher Scientific | Cat#21-176-2C |
| Nair hair removal cream | Nair | N/A |
| Disposable surgical drape | Fisher Scientific | Cat#NC0251373 |
| Weigh scale | Parkland Scientific | Cat#KD-160 |
| Reli silk non-absorbable sutures | VWR | Cat#89219-034 |
| Adson forceps | Fine Science Tools | Cat#11006-12 |
| Halsted-mosquito hemostats | Fine Science Tools | Cat#91308-12 |
| Fine scissors | Fine Science Tools | Cat#14060-10 |
| Nonwoven gauze sponges | Fisher Scientific | Cat#22-028-558 |
| Protexis latex sterile surgical gloves | VWR | Cat#89233-804 |
| Dropper bulb | Fisher Scientific | Cat#03-448-26 |
| Aspirator tube assemblies for microcapillary pipettes | Millipore Sigma | Cat#A5177 |
| Puritan sterile cotton-tipped applicator | VWR | Cat#10805-144 |
| Needle insulin syringe | Fisher Scientific | Cat#22-004-270 |
| Sterile transfer pipette | Sigma-Aldrich | Cat#Z350818 |
| Absorbent underpads | VWR | Cat#56617-016 |
| Pellet pestle cordless motor homogenizer | Fisher Scientific | Cat# 12-141-362 |
Step-by-step method details
Induction anesthesia of the pregnant dam
Timing: 3 min
These steps describe the transition of the pregnant dam from awake to a surgical level of anesthesia (Figure 3A).
-
1.
Check that the anesthesia machine connects to the induction chamber and nose cone properly.
-
2.Place the pregnant dam in an induction chamber where a mixture of 1 L/min oxygen and 3% isoflurane circulates until loss of righting reflex.Note: 2%–4% isoflurane for induction, 1%–3% isoflurane for maintenance in oxygen during surgery via a nose cone; please follow local institutional guidelines.
-
a.Transfer the mouse from the induction chamber to a prone position on the heating pad.
-
b.Apply ophthalmic gel to both eyes using a cotton-tipped applicator to avoid corneal drying.
-
c.Place the mouse on its back and position a nose cone with airflow at 1 L/min and 2% isoflurane as needed to maintain the absence of reflex withdrawal response to toe or tail pinch.
-
a.
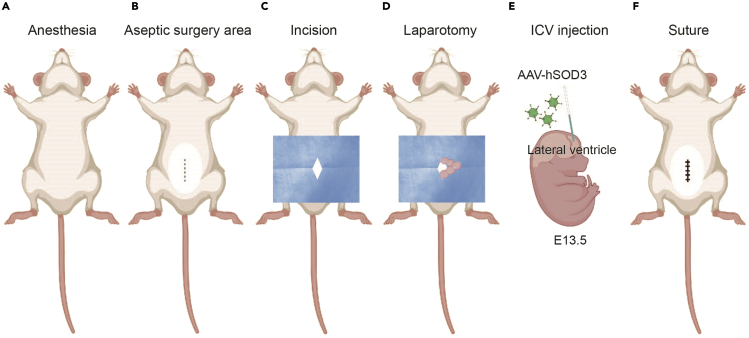
Figure 3.
Outline of in utero ICV injection at E13.5
(A–F) The schematic shows key steps of the surgical procedure: (A) Anesthesia with isoflurane, (B) Abdomen hair removal, (C) Incision of skin and abdominal wall, (D) Expose embryos, and (E) Inject virus into the lateral ventricle.
(F) Injection site: Suture the abdominal cavity and skin followed by recovery and delivery.
Figure created with BioRender.com.
In utero ICV injection procedure
Timing: within 30 min
These steps include the preparation for an aseptic surgical area, laparotomy, ICV injection, and suturing (Figure 3).
-
3.Remove hair from the abdomen by shaving and/or hair-removal cream using a cotton-tipped applicator.
-
a.Shave a larger region of the abdomen than what actual incision size will be.
-
b.Clean the skin with alternating solutions of 70% ethanol and Povidone-iodine 3 times (Figure 3B).
-
a.
Note: Excess hair can induce infection following surgery.
Note: To avoid skin irritation, be sure to rinse hair-removal cream thoroughly.
Note: Apply the 70% ethanol and Povidone-iodine in a spiral manner moving from the center towards the periphery of the surgical field.
Note: Standard laboratory gloves are recommended at all times up to this point.
-
4.
Replace standard laboratory gloves with sterile surgical gloves.
Tip: To minimize the need for glove changes, wrap frequently touched surfaces such as the LED illuminator with autoclaved aluminum foil.
-
5.
Place a sterile surgical drape with a diamond-shaped aperture exposing the surgical field.
-
6.
Place sterile gauze on top of the drape to absorb extra fluids.
-
7.
Make a 1.5 cm incision in the skin and carefully separate the underlying abdominal muscle fibers overlying the uterus (Figure 3C).
-
8.
Use forceps to grasp one uterine horn and place it on the sterile gauze outside the mouse (Figure 3D).
Note: Avoid compression of the placenta or the blood vessels. Troubleshooting 1.
-
9.
Using fingers or forceps, position an embryo to access the dorsal surface of the head. Troubleshooting 2.
-
10.
Insert the microcapillary into the lateral ventricle and inject 1 μL AAV by slowly releasing it from the glass capillary needle using a dropper bulb (Figure 3E). Troubleshooting 3.
CRITICAL: Practice this procedure ahead of time by injecting a filtered solution of 0.1% Fast green dye to visually confirm that solution fills both ventricles and not in other brain regions. Troubleshooting 4.
CRITICAL: Between each injection, apply warm sterile saline to keep the embryos and uterine horns moist.
-
11.After the desired number of embryos are injected, gently reintroduce the uterine horn to its original position into the abdomen.Note: This avoids tangling the related vasculature system, which can cause post-surgery bleeding.
-
a.Gently pull the other uterine horn.
-
b.Repeat the same procedure 9–10.
-
a.
-
12.
Suture the abdominal muscle and then the skin (Figure 3F).
Note: When performing surgery on multiple pregnant dams on the same day, use a glass bead sterilizer between the dams to resterilize the surgery tools.
Post-surgical care
Timing: 15 min and follow-up daily for 3 days
Post-surgical care describes both immediate care and 3 days of follow-up care.
-
13.
Administer post-operative analgesia by subcutaneous injection in the interscapular area.
Note: We inject Meloxicam (5 mg/kg) every 24 h for 3 days.
Note: Analgesia protocols should follow local animal care committee guidelines.
-
14.
Record analgesia administration on a surgery card for post-surgical care. An example analgesia documentation card is shown in Figure 4A.
-
15.Following surgery, place mice in clean cages to lessen the chance of an infection.
-
a.Place cages on a heating pad until mice begin to move spontaneously.
-
a.
Note: Mice should move upon stimulation within 5 min and spontaneously within 15 min.
-
16.
Follow-up care: monitor and record the mouse’s weight, gross appearance, and behavior daily following surgery.
Note: Check the suture region (mice may remove the stitches). If properly performed, laparotomy for in utero injection is unlikely to cause post-surgery discomfort or pain.
Note: Lack of appetite, hunched posture, ruffled fur, and other posturing are examples of pain or distress.
Note: Weight loss can occur as a result of dehydration.
Note: Additional post-surgery complications include bleeding and infection near the surgical site. Any of these signs would warrant immediate attention and appropriate veterinary care.
Pause point: In general, we highly recommend finishing the entire injection procedure without delays, and as efficiently as possible.
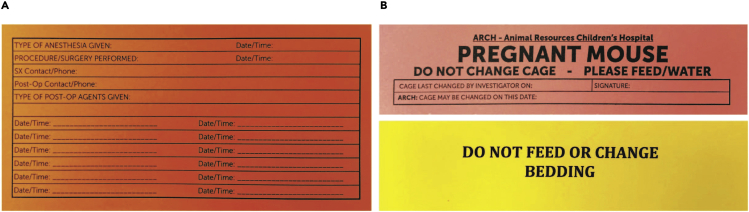
Figure 4.
Example of surgery and post-surgery cards for documentation
(A) The surgery card contains anesthesia and analgesia information.
(B) Post-surgery cards are used to indicate request to reduce mouse handling following the procedure.
Expected outcomes
Here, we provide a detailed step-by-step protocol to transduce AAV into the lateral ventricles of mouse embryos, a simple and fast surgical procedure aimed to target the ChP. Based on our experience, when injections are performed at E13.5, >80% of injected embryos are typically born healthy (at E18.5-E19.5, depending on mouse strain). Troubleshooting 5. Pups develop normally and show regular behavior compared to the non-injected colonies.
The in utero ICV injection procedure has been used to manipulate gene expression to study the physiological role of ChP during brain development.7,8,9 AAV vectors have tropism based on their serotype, and different serotypes result in various transduction outcomes in the brain region. We used AAV5 for targeting ChP from embryonic stage E13.5 injection. AAV9 has also been shown to target ChP after E15 injection and to persist for months, while other serotypes have not shown ChP specificity (Table 2 in Jang and Lehtinen2).
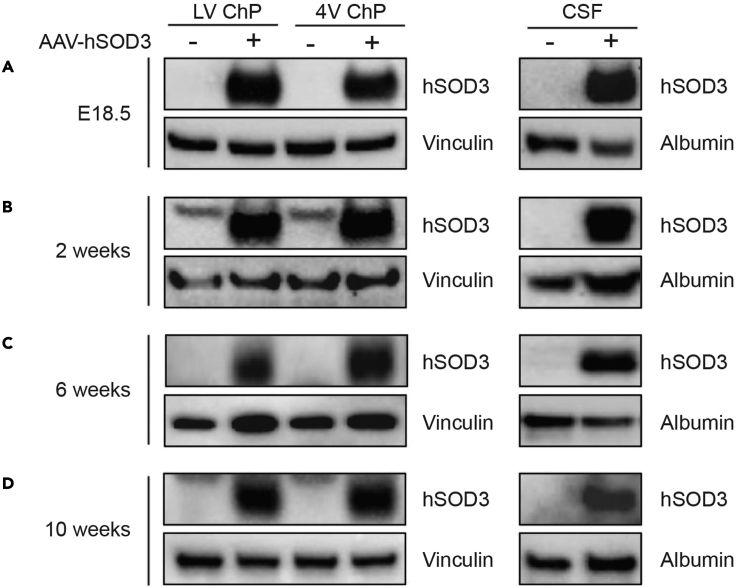
ChP secretes nutrients and signaling molecules into the CSF.10,11 We tracked the transduction of AAV-SOD3 in ChP and subsequent SOD3 secretion into CSF using immunoblotting (Figure 5). We confirmed ChP-SOD3 expression and CSF-SOD3 levels in CSF at E18.5, 5 days post injection (dpi) (Figure 5A). ChP epithelial cells are long-lived.12 Thus, when expression was monitored at 2, 6, and 10 weeks of age, SOD3 was persistently overexpressed in the ChP and SOD3 levels remained elevated in the CSF (Figures 5B–5D).
Figure 5.
In utero ICV injection achieves long-term targeting of postnatal ChP and CSF
Lysates of ChP (left panels) or CSF (right panels) transduced with either AAV-hSOD3 or control (AAV-GFP) were probed with hSOD3 or Vinculin antibodies at (A) E18.5, (B) 2 weeks, (C) 6 weeks, and (D) 10 weeks.
Limitations
This protocol provides a simple and fast approach to manipulating gene expression. However, the successful delivery of AAVs is highly dependent upon the researcher’s surgical skills. Therefore, the choice of strain and number of embryos to inject should be considered before performing the surgery to reduce procedure time. Prolonged procedure time decreases the survival rate of pups and increases post-surgery complications.
We typically used E13.5 embryonic aged mice and performed injections at ages ranging from E12 to E16. At younger ages below E12, such as E10-E11, in utero ICV injection is more challenging because of small embryo sizes and difficulty visualizing the ventricles. In these circumstances, we recommend using ultrasound to visualize the ventricle and the ICV injection. We recently reported successful targeting of ChP at E10.5 to evaluate ChP development.13
If the purpose of the study relies on targeting other cell types including cortical neurons or hippocampal pyramidal neurons, we recommend other virus types or in utero electroporation. The in utero electroporation technique involves the delivery of plasmid DNA into the embryonic lateral ventricles followed by electroporation for incorporation into apical progenitors.14,15,16
Troubleshooting
Problem 1
Amniotic pouch (sac) breakage (refer to step 8 of in utero ICV injection procedure).
Potential solution
This can be observed during the stabilization step and/or during the ICV injection step. A damaged amniotic sac will affect embryo survival rate. Hold the embryos very gently with one’s fingers when manipulating them.
Problem 2
The placement of the embryo for injection is not optimal (refer to step 9 of in utero ICV injection procedure).
Potential solution
The embryos are quite mobile in the amniotic sac. Apply warm sterile saline to the selected embryo and gently turn the embryo within the uterus until the head is positioned upward and is in a good position to inject. During this process, take care to not injure or touch the placenta. If locating one embryo proves challenging, it is preferable to go on to the next embryo so that the surgery can be completed within 30 min of the incision.
Problem 3
Difficulty with injection and release of the AAV (refer to step 10 of in utero ICV injection procedure).
Potential solution
The quality of the glass capillary needle is important. If the glass capillary needle is not beveled properly, it can lead to difficulties penetrating the uterine wall. The needle with a broken tip should touch the surface of the beveling machine very gently to sharpen and optimize the glass capillary needle tip. Since this procedure uses a bulb to load and release the AAV solution, this technique should be practiced before performing the actual procedure.
Problem 4
Uncertain if the virus is delivered into the lateral ventricle (refer to step 10 of in utero ICV injection procedure).
Potential solution
0.1% fast green dye can be used to verify injection location. Both ventricles will be filled green in case of successful injection. If the injection is too shallow or too deep, the AAV may be delivered into another part of the brain. Avoid using these embryos for further analysis.
Problem 5
Poor viability rate (refer to expected outcomes).
Potential solution
There are two possible explanations for low survival rate such as fewer pups are born than initially injected:
-
•
Complications with embryo health and survival:
Based on our experience, limiting surgery duration is critical for increasing the health of the pups. Try to reduce the injection time between the embryos and keep hydrating the uterus throughout the procedure. Immediately following surgery, place the dam on a thermostatically controlled heating pad to enhance recovery and monitor carefully for any complications.
-
•
Maternal care:
Some female mice reject litters following delivery. This neglect can occur, owing to potential stress before/during the delivery. To reduce stress, we recommend limiting access to the mouse cage and avoiding mouse handling during days immediately before and after delivery. We recommend placing a special care card (See for the example in Figure 4B) on the cage indicating this request to others. In addition, environmental enrichment in the cage such as nest building materials may help reduce maternal stress, thereby improving the overall survival and health of the pups. A foster dam may also be helpful.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Maria K. Lehtinen (maria.lehtinen@childrens.harvard.edu).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
We thank the Lehtinen lab for helpful discussions, Nancy Chamberlin for advice on the manuscript, Miriam Zawadzki for help with photography, Naoyuki Taniguchi and Yasuhiko Kizuka for sharing the human SOD3 expression vector, and the BCH Viral Core. Graphical abstract and figures were created with BioRender.com. We are grateful for the following support: NIH NINDS R01 NS088566 (M.K.L.), the New York Stem Cell Foundation (M.K.L.); BCH IDDRC 1U54HD090255; BCH Viral Core P30EY012196. M.K.L. is a New York Stem Cell Foundation – Robertson Investigator.
Author contributions
A.J. designed the study and performed all experiments; A.J. and M.K.L. wrote the manuscript.
Declaration of interests
M.K.L. and A.J. are co-inventors on a provisional patent application related to this manuscript.
Contributor Information
Ahram Jang, Email: ahram.jang@childrens.harvard.edu.
Maria K. Lehtinen, Email: maria.lehtinen@childrens.harvard.edu.
Data and code availability
The published article includes all datasets/code analyzed during this study.
References
- 1.Jang A., Petrova B., Cheong T.C., Zawadzki M.E., Jones J.K., Culhane A.J., Shipley F.B., Chiarle R., Wong E.T., Kanarek N., Lehtinen M.K. Choroid plexus-CSF-targeted antioxidant therapy protects the brain from toxicity of cancer chemotherapy. Neuron. 2022;110:3288–3301.e8. doi: 10.1016/j.neuron.2022.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jang A., Lehtinen M.K. Experimental approaches for manipulating choroid plexus epithelial cells. Fluids Barriers CNS. 2022;19:36. doi: 10.1186/s12987-022-00330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://www.jax.org/news-and-insights/jax-blog/2014/september/six-steps-for-setting-up-timed-pregnant-mice
- 4.Dismuke D.J., Tenenbaum L., Samulski R.J. Biosafety of recombinant adeno-associated virus vectors. Curr. Gene Ther. 2013;13:434–452. doi: 10.2174/15665232113136660007. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y., Jiang B., Samai P., Tank S.M., Shameem M., Liu D. Genome DNA leakage of adeno-associated virus under freeze-thaw stress. Int. J. Pharm. 2022;615:121464. doi: 10.1016/j.ijpharm.2022.121464. [DOI] [PubMed] [Google Scholar]
- 6.Ota F., Kizuka Y., Kitazume S., Adachi T., Taniguchi N. N-glycosylation is essential for the secretion of extracellular superoxide dismutase. FEBS Lett. 2016;590:3357–3367. doi: 10.1002/1873-3468.12378. [DOI] [PubMed] [Google Scholar]
- 7.Shipley F.B., Dani N., Xu H., Deister C., Cui J., Head J.P., Sadegh C., Fame R.M., Shannon M.L., Flores V.I., et al. Tracking calcium dynamics and immune surveillance at the choroid plexus blood-cerebrospinal fluid interface. Neuron. 2020;108:623–639.e10. doi: 10.1016/j.neuron.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui J., Shipley F.B., Shannon M.L., Alturkistani O., Dani N., Webb M.D., Sugden A.U., Andermann M.L., Lehtinen M.K. Inflammation of the embryonic choroid plexus barrier following maternal immune activation. Dev. Cell. 2020;55:617–628.e6. doi: 10.1016/j.devcel.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H., Fame R.M., Sadegh C., Sutin J., Naranjo C., Della S., Cui J., Shipley F.B., Vernon A., Gao F., et al. Choroid plexus NKCC1 mediates cerebrospinal fluid clearance during mouse early postnatal development. Nat. Commun. 2021;12:447. doi: 10.1038/s41467-020-20666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehtinen M.K., Zappaterra M.W., Chen X., Yang Y.J., Hill A.D., Lun M., Maynard T., Gonzalez D., Kim S., Ye P., et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lun M.P., Monuki E.S., Lehtinen M.K. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 2015;16:445–457. doi: 10.1038/nrn3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkho B.Z., Monuki E.S. Proliferation of cultured mouse choroid plexus epithelial cells. PLoS One. 2015;10:e0121738. doi: 10.1371/journal.pone.0121738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser K., Jang A., Kompanikova P., Lun M.P., Prochazka J., Machon O., Dani N., Prochazkova M., Laurent B., Gyllborg D., et al. MEIS-WNT5A axis regulates development of fourth ventricle choroid plexus. Development. 2021;148:dev192054. doi: 10.1242/dev.192054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellender T.J., Avery S.V., Mahfooz K., Scaber J., von Klemperer A., Nixon S.L., Buchan M.J., van Rheede J.J., Gatti A., Waites C., et al. Embryonic progenitor pools generate diversity in fine-scale excitatory cortical subnetworks. Nat. Commun. 2019;10:5224. doi: 10.1038/s41467-019-13206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitali I., Fièvre S., Telley L., Oberst P., Bariselli S., Frangeul L., Baumann N., McMahon J.J., Klingler E., Bocchi R., et al. Progenitor hyperpolarization regulates the sequential generation of neuronal subtypes in the developing neocortex. Cell. 2018;174:1264–1276.e15. doi: 10.1016/j.cell.2018.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mairet-Coello G., Courchet J., Pieraut S., Courchet V., Maximov A., Polleux F. The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Abeta oligomers through Tau phosphorylation. Neuron. 2013;78:94–108. doi: 10.1016/j.neuron.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The published article includes all datasets/code analyzed during this study.