Abstract
In recent years, consumer demand for health benefitting, pleasant-tasting rapeseed oil has increased, and so has production. Ireland's climate and agricultural background can support the production of high-quality rapeseed oil. Volatile organic compounds (VOC) can give rise to highly distinctive flavours in rapeseed oils, produced during crop growth and generated during processing. This study performed VOC and sensory evaluation to determine if correlations exist. Samples of Irish rapeseed oils from 6 different producers were analysed. Compounds detected in the oil samples consisted of acids, alcohols, aldehydes, ketones, benzenes, esters, ether, terpenes, and sulphurs. While variations in whole volatile profiles were not considered significant, individual compounds and volatile classes were for hexanal, pentanal, ketones, acids, and sulphurs compounds. Correlations were observed between the VOCs detected and the sensory profile, which indicated the VOC content may influence an oil's sensory profile.
Keywords: Rapeseed oil, VOCs, Sensory, Descriptives producers, Irish
Graphical abstract

Highlights
-
•
Volatile Organic Compound analysis found acids, alcohols, aldehydes, ketones, benzenes, esters, ethers, sulphur compounds, and terpenes were most abundant.
-
•
The main observation from sensory evaluation was that ‘nutty’ & ‘straw-like’flavour mostly influenced acceptability.
-
•
An oil with a nutty flavour, low or moderate odour and lighter colour was determined to be more acceptable.
-
•
Significant correlation was demonstrated between the sensory analysis and the VOC profile.
-
•
Variations were determined between the VOC and sensory evaluation of the Irish rapeseed oils based between producers and within batches.
Abbreviations
- VOC
Volatile Organic Compounds
- SFA
Saturated fatty acids
- USFA
Unsaturated fatty acids
- GCMS
Gas chromatography-mass spectroscopy
- ANOVA
Analysis of variance
- PCA
Principal Component Analysis
1. Introduction
Rapeseed oil is the second leading culinary oil produced globally and continues to expand as consumers become increasingly health-conscious and aware of the benefits of minimally processed foods (Tierney and Zabetakis, 2019). Produced through the pressing of seeds from a cruciferous plant species known as Brassica napus, it is highly desired for its desirable fatty acid composition, bioactive components, and unique flavour due to the specific volatile organic compounds (VOC) it contains (Ivanova-Petropulos et al., 2015; Kraljić et al., 2018; Chew, 2020). Rapeseed oil could play a significant role in the future Irish Agri-economy (Chew, 2020). Currently, Ireland imports most of its culinary oils. However, winter rapeseed is an ideal crop to grow in Ireland as the climate and agricultural conditions are ideal for growth. Research in an Irish context has already proven profitable in cultivating rapeseed and utilizing the seeds for oil extraction. Rapeseed oil looks set to play an important role in Ireland's agriculture as it is proving to be a very acceptable alternative to imported olive oil. A 2021 report published by Teagasc estimated that production of cereal & oilseeds increased by 21% from 2020. With this the winter rapeseed crop increased by 16% in 2021 due to the increased cultivation of approximately 1300 tonnes of rapeseed crop. This increase in rapeseed shows that Ireland has recognised rapeseed as a potential cash crop that can be used to boost Ireland economically (Teagasc, 2021).
VOC are low molecular weight volatile molecules that give rise to oils' distinctive aroma and sensory characteristics. Their impact is dependent upon their concentration and odour threshold. The odour threshold is the concentration limit at which the associated aroma can be detected (Leonardos et al., 1969). The contribution of specific VOC to the aromatic profile is known as the Odour Activation Value (OAV) (Buttery, 1999). Suppose a particular VOC has a significantly higher concentration than another. In that case, the influence on the sensory profile is usually not proportional due to the different odour thresholds of the individual VOC (Buttery, 1999). Table 1 outlines VOC classes, compounds, and theoretical thresholds determined in several non-Irish rapeseed oils (Jia et al., 2020; Matheis and Granvogl, 2019; Petersen et al., 2012; Xu et al., 2018).
Table 1.
Theoretical odour threshold and descriptive words determined for Rapeseed oils from various countries and cultivars based on several scientific publications.
| VOC Class | VOC Compound name | Sensory Descriptive | Odour threshold (ng/L), (mg/kg), (ug/kg) |
|---|---|---|---|
| Acid | Acetic acid | Sour, Vinegara | 1384 ng/L |
| Propanoic acid | Pungent, rancid, fruityb | 289 ng/L | |
| Hexanoic acid | Sour, fatty, sweaty, cheeseb | 3000 ng/L | |
| Alcohol | 1-Pentanol | Balsamica,b | 3590 ng/L |
| 1-Hexanol | Green, floralb | 360 ng/L | |
| 1-Penten-3-ol | Buttery, Pungentb | 2890 ng/L | |
| Aldehyde | Propanal | Pungent, Solventb | 10 ng/L |
| Pentanal | Pungent, almond and Balsamicc | 36 μg/kg | |
| Hexanal | Grass Green, rancid, oily a,b,c | 51 ng/L 1900 μg/kg |
|
| Heptanal | Rancid, fatty, citrusa,b | 46 mg/kg | |
| Octanal | Fatty, soapy. green, oily, fresha,b,c | 9.3 ng/L 360 μg/kg |
|
| Benzaldehyde | Almond, Burnt, sugara,b | 186 ng/L | |
| Benzene | Toluene | Sweet, Alcoholicb | 9481 ng/L |
| Ketone | 6-Methyl-5-hepten-2-one | Fruity, Apple, Musty & Ketonicd | 1 mg/kg |
| 2-Octanone | Earthy, weedy, natural, woody b | 248 ng/L | |
| Sulphur | Dimethyl sulfide | Sulphurous, Burnt, Cabbage-like, Garlic & Onion c,d | 0.001 mg/kg 4.1 μg/kg |
| Terpene | D-Limonene | Lemon, Orange a | 718 ng/L |
Theoretical odour thresholds of VOC in rapeseed oils reported by (Xu et al., 2018).
Theoretical odour thresholds of VOC in rapeseed oils reported by (Petersen et al., 2012).
Theoretical odour thresholds of VOC in rapeseed oils reported by (Matheis and Granvogl, 2019).
Theoretical odour thresholds of VOC in rapeseed oils reported by (Jia et al., 2020).
VOCs are generated during seed growth or produced during oil extraction from the fruit/seed (Zhang et al., 2019). Concentrations of VOC in culinary oils may differ due to species, cultivation, processing and storage conditions of the oil (Gracka et al., 2017; Zhang et al., 2019). VOCs produced during the processing of oils arise from enzymatic reactions during crushing and storage of seeds, fatty acid oxidation, glucosinolate degradation, and the Maillard reaction during roasting and microwaving of seeds (Zhang et al., 2019) (Ivanova-Petropulos et al., 2015). Flavours in the rapeseed oils arise from fatty acid degradation, Maillard or amino acid degradation, while previous studies have reported glucosinolate degradation to correspond with pungent odours in cold pressed rapeseed oil (Zhou et al., 2018).
Compounds obtained through lipogenesis (LOX) and thermal processing alter the oil's flavour, colour, and smell by generating undesirable substances. Peroxides and free fatty acids are gradually broken down into smaller chain VOCs such as aldehydes and ketones (Aniołowska et al., 2016; Brühl, 2014; Li et al., 2016; Liu et al., 2018). At higher concentrations, these VOCs can reduce the positive sensory odours of oil, creating a “rancid” aroma and flavour (Ivanova-Petropulos et al., 2015). Long-chain unsaturated fatty acids such as linoleic acid (C18:2) and oleic acid (C18:1) are highly susceptible to oxidation and give rise to VOCs such as aldehydes, ketones and alcohols during thermal oxidation (Xu et al., 2018).
A previous study conducted by the author on a range of Irish rapeseed oil samples found that the fatty acid profile of the oils had significant differences. Irish rapeseed oils were abundant in long-chain unsaturated fatty acids, such as linoleic acid (C18:2) and oleic acid (C18:1) which may be a significant contributor to the variation observed in the volatile profile due to individual fatty acid differences which may have given rise to the differentiation between the oils in terms of sensory properties. Moreover, these individual fatty acids exhibited significant concentration differences (p < 0:05) between rapeseed oil producers and within successive rapeseed oil batches from the same producers. Oleic acid (C18:1) ranged from 34.17% to 66.03%, linoleic acid (C18:2) content ranged from 13.00% to 40.99% and stearic acid (C18:0) content ranged from 1.48% to 4.34%. These fatty acids were considered statistically significant with respect to abundance (p=<0.050) demonstrating variability between producers and batches alike (Coughlan et al., 2022). Hence, fatty acids of different concentrations may be a significant contributor to the variance in VOCs such as aldehydes, ketones, and alcohols.
Hence degradation of these long-chain fatty acids should be minimised during processing through the cold pressing process and optimal storage conditions to prevent the production of negative sensory characteristics while maintaining the favourable properties associated with cold-pressed rapeseed oils.
New pre-pressing methods have been implemented to maximize the retention of important physicochemical and nutritional compounds such as desirable fatty acids and beneficial biochemical compounds (Liu et al., 2018). Microwaving or roasting seeds are the most common pre-treatment methods applied (Koubaa et al., 2016; Liu et al., 2018). Rokosik et al. (2019), suggested that oils should have “nutty, wood-like and astringent” sensory characteristics associated with ketones, furans and aldehydes formed mainly through the Maillard reaction during heat treatments, such as microwaving or roasting (Rokosik et al., 2019). Seed roasting induces the Maillard reaction producing heterocyclic compounds that contribute to the “nutty” and “roasted” flavour associated with many seed oils (Lui and Hou, 2018; Rękas et al., 2017; Ren et al., 2019). Jing et al. (2020) states that the roasted and nutty descriptors of rapeseed oils is developed during the roasting period through the generation of octanal, butanal and nonanal (via fatty acid degradation) which have high OAV resulting in the rapeseed possessing these sensory qualities (Jing et al., 2020).
Lower quality oils are associated with off flavours typically described by sensory panels as “pungent, musty, sulphurous, cabbage and burnt”. Such descriptors are thought to result primarily from the presence of sulphur-containing compounds such as dimethyl sulphide, dimethyl trisulfide and dimethyl sulfoxide generated from glucosinolate degradation, a common reaction during microwaving of whole seeds and flakes (Gracka et al., 2017; Zhou et al., 2018). Seeds storage can also impair the final flavour and aromas of the oils due to the production of VOC associated with “musty” and ""rancid” aromas (Giakoumis, 2018; Zhang et al., 2019). Seeds stored at higher temperatures and humidity may induce lipid oxidation and enzymatic reactions due to microbial contaminations also adversely impacting on sensory character (Bonte et al., 2017a, Bonte et al., 2017b).
While the recognition of rapeseed as a cash crop in Ireland has increased and many studies have been conducted characterizing culinary rapeseed oils, to date, no comprehensive research has been undertaken on rapeseed oil produced exclusively in Ireland. While many studies have reported on the VOC content of culinary oils, including rapeseed oils, this study focuses on rapeseed oil from six Irish producers. By characterizing and understanding the sensory properties of Irish rapeseed oils, this may allow for comprehensive profiling of these culinary oils which may aid in manufacturing a safe and healthy culinary oil sought after by today's consumers. This study presents comprehensive research on the VOC content of Irish rapeseed oils which provide an insight into the inter and intra-batch VOC variability within and between commercial rapeseed oils produced in Ireland, giving an insight into quality that may influence consumer sensory preferences. This information is likely beneficial to producers as it provides indirect information regarding production processes.
2. Methodology
2.1. Sample collection
Commercially available cold pressed Irish rapeseed oils from 6 Irish rapeseed producers were selected in local retail units. Producers were randomly assigned numbers 1 to 6 with the specific codes PRO1 – PRO6. Batches of rapeseed oil from each producer were coded B1-B3. Individual batches were identified by processing dates on the bottles and ranked from earliest to latest.
2.2. Sample preparation: headspace solid phase microextraction (HS- SPME)
Approximately 1 g of rapeseed oil was added to a 20 mL amber screw-capped headspace vial with magnetic screw caps and a silicone/polytetrafluoroethylene septum (Apex Scientific Ltd., Maynooth, Ireland) and equilibrated to 40 °C for 10 min with a 5-s pulsed agitation at 500 rpm. Samples were introduced using a Shimadzu AOC 5000 autosampler (Mason Technology Ltd, Ireland) and a single 50/30 μm Carboxen™/divinylbenzene/polydimethylsiloxane (CAR/DVB/PDMS) SPME fibre (Supelco, Sigma-Aldrich, Arklow, Co. Wicklow, Ireland) was selected as it was assumed to provide the widest range of volatiles based on the properties of the different phases. The SPME fibre was exposed to the headspace above the samples for 20 min at a depth of 1 cm at 40 °C, with 5-sec agitation at 5 rpm. The fibre was then retracted and injected into the GC inlet and desorbed for 2 min at 250 °C.
2.2.1. Standard preparation
2.2.1.1. GCMS mixed standard
A set of external standards were analysed to monitor the performance of the GC-MS and to ensure that the extraction and MS detection was within specification, which comprised of 1-butanol, dimethyl disulphide, butyl acetate and cyclohexanone prepared in methanol at 1000 ppm. An internal stock standard solution of 2-phenyl-d5-ethanol was prepared at 5000 ppm in methanol. These two standard solutions were combined by taking a 1:100 dilution of the external standard and a 1:500 dilution of the internal standard in distilled water and adding 10 μL of this solution to a 20 mL amber headspace vial.
2.2.2. Gas chromatography-mass spectroscopy (HS-GCMS) analysis
Injections of standards and rapeseed oil samples were made on a Shimadzu 2010 Plus GC (Mason Technology Ltd) with an DB-624 UI (60m × 0.32 mm x 1.8 μm) column (Agilent Technologies Ltd, Cork, Ireland) using a split/splitless injector with a 1:10 split ratio using a merlin micro-seal (Agilent Technologies Ltd). The initial temperature of the column oven was held at 40 °C for 5 min, ramped at a rate of 5 °C/min to 230 °C and 15 °C/min to a temperature of 260 °C, and held for 5 min yielding a total GC run time of 65 min. The carrier gas was helium, held at a 1.2 mL/min constant flow rate. The Shimadzu TQ8030 mass spectrometer detector (Mason Technologies Ltd) applied electron ionization at 70 eV and a mass scan range from 35 amu to 250 amu. The ion source temperature was set to 220 °C, and the interface temperature to 260 °C.
2.2.3. Volatile identification
Volatile compounds were identified using mass spectra, and comparisons to the National Institute of Standards and Technology (NIST) 2014 database, a commercial flavour and fragrance library (FFNSC 2, Shimadzu Corporation, Japan), an in-house library created using authentic standards with target and qualifier ions. Spectral deconvolution was also performed to assist in the identification of volatile compounds using AMDIS. In addition, linear retention indices were determined as per van Den Dool and Kratz (1963) for each VOC and checked against peer-reviewed published studies using the same column polarity. Batch processing of samples was carried out using Meta MS (Wehrens et al., 2014), an open-source pipeline for GCMS-based untargeted metabolomics.
An auto-tune of the GCMS was carried out before the analysis to ensure optimal GCMS performance. External standards were run at the start and end of the sample set, and abundances were compared to known amounts to ensure that both the SPME extraction and MS detection were performing within specification.
2.3. Sensory evaluation
2.3.1. Panellists
Three testing sessions with 6 trained panellists evaluated the profiles of 17 Irish rapeseed oils from 6 producers. Panellists were trained to assess oils based on sensory attributes, including taste, texture, flavour, and aftertaste. The methods used included acceptability tests, discrimination tests and descriptive testing.
2.3.2. Training
The panel were trained, and the samples were evaluated using a combination of quantitative descriptive methods similar to those conducted by Hettiarachchi and Illeperuma (Hettiarachchi and Illeperuma, 2015). The first training test consisted of matching tests to determine that each panel member could correctly identify the basic tastes (sugar, salt, etc.). A lexicon of sensory descriptors was developed based on the abundant compounds in the VOC profile of the Irish rapeseed oils determined as outlined in Table 2. The panel was trained to identify these flavours and aromas associated with rapeseed oils in a separate session. Aromas were prepared by adulterating the oils with specific natural materials to impregnate the oil with the corresponding odour. Floral was generated by putting fresh flowers into the oils; oxidised was produced using significantly older oils (12 months passed expiry). Nutty and grassy were prepared by placing peanuts and grass into oils. Samples were presented blind with a glass of water and water crackers on a white tray for palate cleansing.
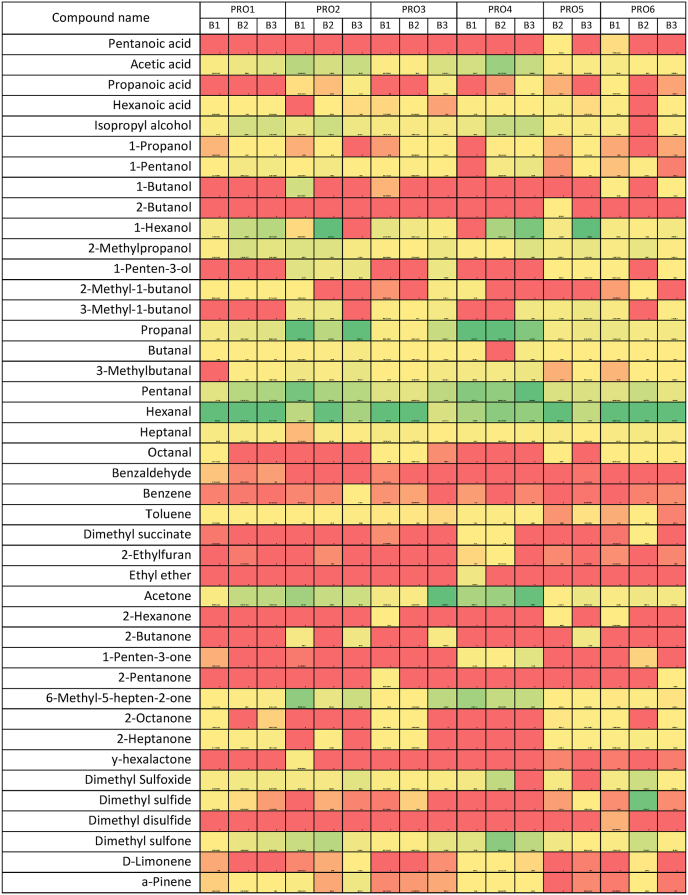
Table 2.
Heat map of the volatile compound composition of Irish rapeseed oil batches.
The lexicon used for the descriptive analysis based on the most abundant VOC as outlined in Table 2 included descriptives such as “green” and “nutty” which were associated with aldehyde compounds such as hexanal. Descriptives such as “fruity” and “earthy” were associated with ketone compounds such as acetone.
2.3.3. Sample evaluation
Datasheets allowed the panel to score each sample on a hedonic scale from 1 to 9 for acceptability and each descriptive sensory test using the rapeseed oil lexicon. A total of 17 samples were similarly presented to the panellists in a similar manner to the training sessions. Each rapeseed oil was given a 3-digit random code to present the samples blind and maintained at room temperature prior to testing. Samples from different producers and different batches were presented at each testing session. After testing, the mean and standard deviation scores were calculated using Microsoft Excel to prepare spider diagrams and bar charts.
2.4. Statistical analysis
Analysis of variance (ANOVA) and Principal Component Analysis (PCA) was carried out using IBM SPSS statistics with a statistical significance of p < 0.05 and a confidence interval of 95%. Post hoc testing, using Duncan's parameters, allowed the identification of samples as significantly different from others by pinpointing the means of differentiation. Additionally, correlation analysis using Pearson's correlation was conducted using IBM SPSS statistics with a statistical significance of p < 0.05 being considered (Dabbou et al., 2012).
3. Results & discussion
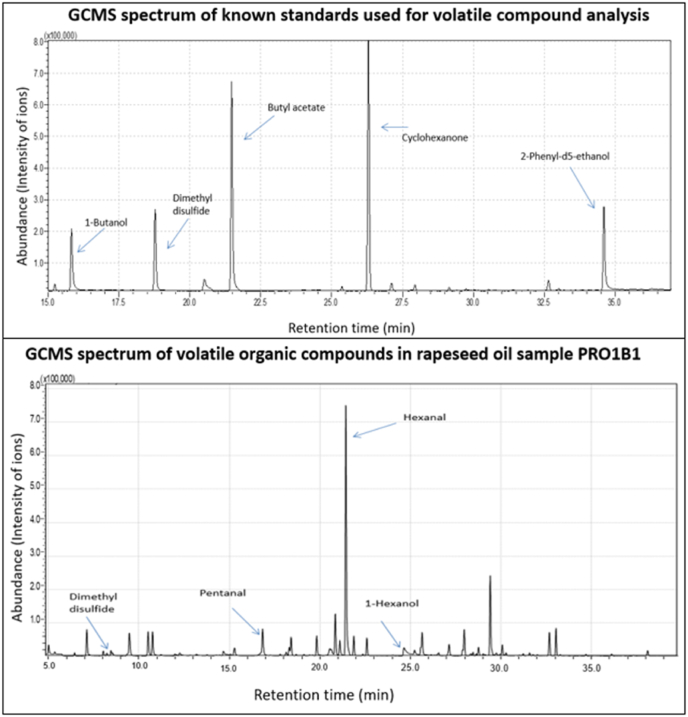
Samples were injected onto the GCMS instrument in triplicate, and the mean ion intensities for each volatile compound were used to generate the compound's percentage abundance. Known standards were used as guidelines for retention time and m/z comparison in addition to Kovats retention indices and library comparison. Fig. 1 (A) contains a GC chromatogram of the known mixed GCMS standards used to determine compound Retention Times (RT) and Retention Indices (RI) and (B) outlines the GC chromatograms of volatile compounds detected in the Irish rapeseed oil sample PRO1B1.
Fig. 1.
(A) GC chromatogram of the known mixed GCMS standards used to determine compound Retention Times (RT) and Retention Indices (RI) as per the headspace GCMS conditions outlined in the materials and method section. (B)The GC chromatograms of volatile compounds detected in the Irish rapeseed oil sample PRO1B1, conducted per the headspace GCMS conditions outlined in the materials and method section.
Standards detected were 1-Butanol, dimethyl disulphide, butyl acetate, cyclohexane, and 2-phenyl-d5-ethanol. The GCMS separation conditions were as outlined in the materials and method section. Abundant compounds were detected and identified based on the RI of each compound. The most abundant VOCs detected in the rapeseed oil sample PRO1B1 were dimethyl disulphide, pentanal, hexanal and 1-hexanol.
3.1. Volatile compound profile of Irish rapeseed oils
Forty-one compounds were identified in these (17) Irish rapeseed oil samples analysed by HS-SPME GCMS. Table 2 presents a heat map of individual VOC grouped into chemical classes and compounds and compound groups detected, including aliphatic, aromatic hydrocarbons, aliphatic alcohols, aldehydes, ketones, esters ethers, terpenes, and furans.
Table 2 presents the heat map of the volatile compound profile for the Irish rapeseed oils from the 6 different producers. Results are presented as abundance values (intensity of ions of each VOC) as detected by GCMS. The heat map expresses the difference in VOC abundance using a colour gradient. VOC of high abundance are green in colour; low abundance is red, and yellow represents the midpoint value of the total sample abundance, effectively normalising the range of each VOC.
The most abundant compound classes detected were aldehydes (hexanal, pentanal and propanal), ketones (acetone and 6-methyl-5-hepten-2-one), acids (acetic acid) and sulphurs compounds (dimethyl sulphide, dimethyl sulfone, and dimethyl sulfoxide). Kiralan and Ramadan, 2016, also reported hexanal and nonanal as the compounds with the highest concentration in fresh rapeseed oils and derived from the rapeseed oil's high USFA content (oleic acid: 61.78%, linoleic acid: 21.72% and linolenic acid: 8.045). Xu et al. (2018), stated that fatty acid content significantly influences an oil's volatile profile as individual fatty acids are substrates for specific volatile compounds. For example, the oxidation of oils is one of the principal factors in developing an oil flavour profile as specific VOC are generated during fatty acid degradation (Katragadda et al., 2010; Zhang et al., 2019).
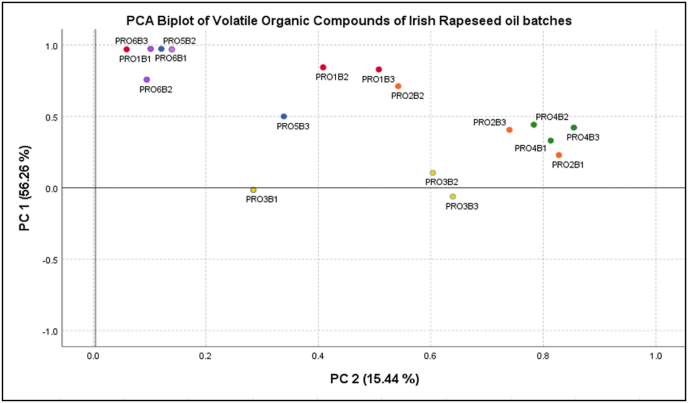
While variations in whole volatile profiles were not considered significant, individual compounds and volatile classes were. Principle component analysis (PCA) was conducted to highlight these differences. Fig. 2 outlines the PCA biplot associated with the VOC profiles generated.
Fig. 2.
Principal component analysis biplot of the volatile profile of Irish rapeseed oils.
Two principal components (PC) were extracted to demonstrate the variance between the rapeseed oil samples. In general, two clusters of data points are evident in the bi-plot. Cluster 1 is located in the top left quadrant of PC1, packed tightly together (PRO6B3, PRO5B2, PRO1B1, PRO6B1 and PRO6B2), and cluster 2 is located in the middle of the bi-plot spreading to the lower right corner of the PCA 1 axis near PCA 2. As previously mentioned, samples did vary in their VOC compound profile mainly in terms of the content of particular VOC. This is clear when comparing the biplot to the heatmap. PC1 explains 56.28% of the total variance between all samples, while PC2 represents 15.44%. Cluster one samples (PRO6B3, PRO5B2, PRO1B1, PRO6B1 & PRO6B2) contain higher abundances of hexanal and pentenal. PRO5B2 and PRO6B1 had more abundances of pentanoic acid and dimethyl sulfide. Cluster 2 (remaining samples) is associated with higher acetone and propanal content and higher 6-Methyl-5-hepten-2-one, 1-penten-3-ol and dimethyl sulfone than the other rapeseed oil samples evaluated.
Differences across batches can be observed, particularly for samples PRO2B2, PRO5B3 and PRO1B1, as these samples are scattered further away from the other batches within the same producer. In comparison, producers 4 and 6 demonstrated consistency within the batch samples; PRO4 and PRO6 samples can be seen close together within respective clusters. The sample from PRO5B3 and PRO3B3 separate more from Cluster 2 as these samples contain a notably lower hexanal content. The rapeseed oil, PRO3B3, spreads closer to the PC 2 section of the bi-plot as it contains notably lower hexanal and contains 1-penten-3-ol, whereas the other batches from producer 3 do not.
Kiralan and Ramadan (2016), reported hexanal and nonanal as the highest VOC identified in fresh rapeseed oils, derived from the high unsaturated fatty acid (USFA) content of the rapeseed oil tested (oleic acid: 61.78%, linoleic acid: 21.72% and linolenic acid: 8.045). Additionally, it was reported that the concentration of these compounds increased over time during storage via oxidation reactions. Aldehydes are the most abundant compound class in these rapeseed oil samples, with hexanal, pentanal, and propanal being the most abundant aldehydes detected across the sample set. Specific aldehydes are formed through lipid oxidation, hexanal is generated through oxidation of linoleic acid while octanal and nonanal, which are present in low amounts in rapeseed oils, are oleic acid derivatives (Ivanova-Petropulos et al., 2015). Furthermore, it has been established that the Irish rapeseed oils tested here are abundant in linoleic acid and oleic acid which can justify the higher aldehyde content observed (Coughlan et al., 2022).
To determine if significant differences exist between the VOC chemical classes in the rapeseed oil samples, the combined mass absorbance of each compound class group was used to calculate the overall percentage content of each class group as per Fig. 2.
The aldehyde content ranges from 35.05% to 80.99%, where the samplePRO5B3 had the lowest content (35.05%), and PRO6B3 had the highest aldehyde content (80.99%). This difference is significantly different (p < 0.05), where the compounds propanal, pentenal and hexanal were significantly different throughout. This indicates that the sample from PRO6B3 has a higher oxidation level as aldehyde is formed through fatty acid oxidation.
Similarly, Ivanova-Petropulos et al. (2015) found that rapeseed oils contained an average of 76.8% aldehydes (including: propanal, butanal, 3-methyl-butanal, hexanal, octanal and nonanal). As per Table 1, various publications have associated aldehyde compounds with positive and negative descriptives. Aldehydes such as propanal, heptanal and benzaldehyde are associated with negative sensory descriptions such as “pungent”, “rancid”, and “burnt”. At the same time, aldehydes such as hexanal, octanal and pentanal are associated with positive descriptives such as “green”, “oily”, and “fatty”. The compound concentration and odour threshold influence the intensity in which the aroma is perceived; therefore, the intensity of the aldehyde compounds detected depends on both concentration and OAV.
Ketones are the next most abundant VOC and range from 3.42% to 35.46%, and therefore likely to be quite significant. The rapeseed oil PRO5B2 had the lowest ketone content (3.42%), and PRO3B3 contained the highest amount (35.46%). Acetone also known as 2-propanone, and 6-methyl-5-hepten-2-one were the most significantly different ketones (p = <0.05). Wang et al. (2020) found similar trends within the ketone compounds of rapeseed oils where it was suggested that the 6-methyl-5-hepten-2-one content (7.56 mg/kg) in rapeseed oils were derived from microwaved seeds through β-oxidation of corresponding fatty acids in the seeds. These results are comparable with those reported by Jeleń et al. (2007), where the aldehydes and ketones made up a significant component of the total volatile profile (63%) of rapeseed oils evaluated. As per Table 1, various publications have associated ketone compounds with positive descriptions such as “fruity”, “earthy”, and “weedy”. At the same time, descriptives such as “musty” and “ketonic” are associated with negative sensory characteristics (Jia et al., 2020; Matheis and Granvogl, 2019; Petersen et al., 2012; Xu et al., 2018). Similarly, to aldehydes, for ketones, compound concentration and odour threshold influence the intensity in which the aroma is perceived; ketones, however, generally have lower odour thresholds than aldehydes, therefore, the intensity of the compounds detected depends on both concentration and OAV.
3.2. Sensory evaluation
Based on the sensory evaluation, the acceptability of the rapeseed oils scored from 5 to 8 on the hedonic scale. This indicates the panel liked some rapeseed oils presented more than others. Based on this, the descriptive analysis was conducted to determine why a difference was observed regarding the acceptability of the rapeseed oils. The panel concluded the most predominant sensory qualities were colour, taste, odour, and texture. It was collectively agreed that Light-Dark for colour, Nutty and Straw-like/Floral for taste and light-pungent odour were the appropriate terms for describing the Irish rapeseed oils.
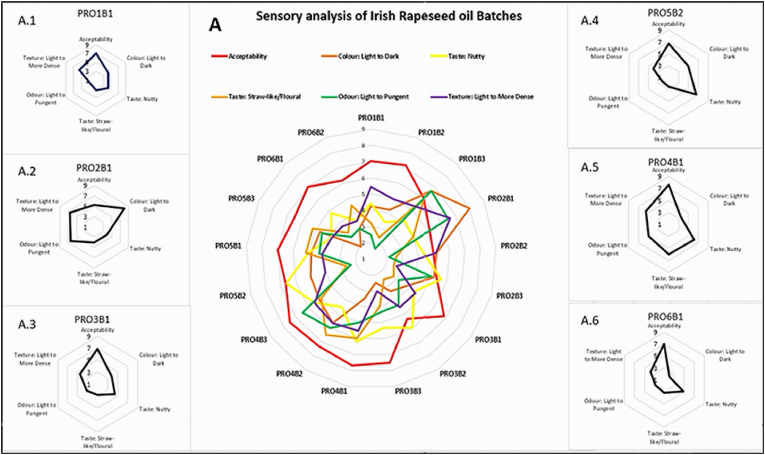
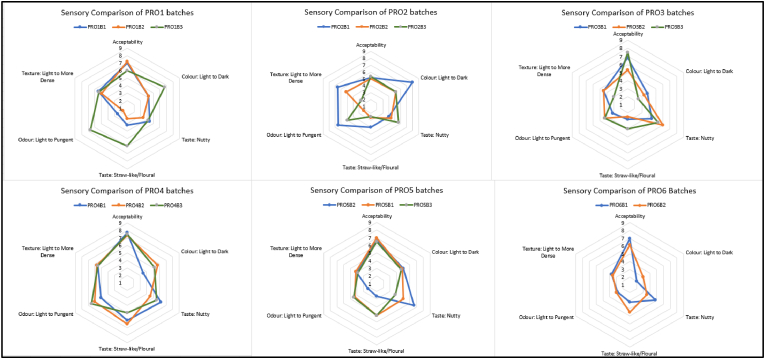
A variation in hedonic scoring was also observed for the descriptive analysis of each rapeseed oil. Fig. 3 demonstrates the variability of the Irish Rapeseed oils' sensory properties. Fig. 3 A demonstrates the sensory differences of the 17 rapeseed oils tested, while Fig. 3 A.1-A.6 represents individual oils from each producer to demonstrate these differences.
Fig. 3.
Sensory evaluation of Irish rapeseed oil batches A presenting the acceptability and descriptive analysis of 17 oils, A.1-A.6 representing one individual rapeseed oil from each producer.
The main observation derived from the sensory evaluation was that flavour was the most influential descriptive of the acceptability scoring of the rapeseed oil. An oil's score for acceptability had a similar scoring for the ‘nutty’ or ‘straw-like’ attributes. Additionally, a relationship can be observed between colour, odour, and texture. Generally, the higher the score for dark, the more pungent odour and thicker the oil texture. This may be representative of a cross-modal association whereby the panellists associate the oils pungency or strength to colour intensity i.e. darker yellow is has a more potent aroma (Stevenson et al., 2012).
Kraljić et al., 2018 reported the conditioning of seeds at different temperatures might influence the flavour of oil by producing volatiles associated with specific flavours. For example, preconditioning seeds at 60 °C for 30 min can enhance the ‘nutty’ flavours associated with rapeseed oils potentially due to increasing octenal content via fatty acid degradation (Kraljić et al., 2018). Wroniak et al., 2016 and Bonte et al., 2017a, Bonte et al., 2017b, reported similar characteristics to cold-pressed rapeseed oils, describing them as ‘rapeseed-like’ and ‘nutty’ due to an increase in aldehyde and ketone content through the generation of more VOC with increased temperature and time (Wroniak et al., 2016; Bonte et al., 2017a). Bonte, Schweiger et al., 2017 associated the flavour “nutty” with the aldehyde and the ketone compounds. Raghavan et al. (1994) stated that the dominant volatile compounds from fresh and aged rapeseed oils were impacted by aldehyde contents, which are generated from the degradation of linolenic acid giving rise to hexanal and are responsible for the fatty & nutty descriptives (Wang et al., 2020). Jing et al. (2020) states that the roasted and nutty descriptors of rapeseed oils is derived from octanal, butanal and nonanal (via fatty acid degradation) which have high OAV resulting in the rapeseed possessing these sensory qualities (Jing et al., 2020). Thus, the difference in “nuttiness” between the rapeseed oils may be attributed to the variations observed in the linolenic acid content of these oils (Coughlan et al., 2022).
Pearson correlation analysis justified why one oil may have been more ‘acceptable’ to the panel than another based on the descriptive analysis. Pearson's correlation allows for measuring the linear relationship between two variables for the positive or negative correlation. Based on the correlation principle, the closer a value is to ± 1, the stronger/weaker the relationship between the two variables. A strong correlation is considered any relationship from ±0.4 to ±1.0. While a moderate relationship is considered from ±0.2 to ±0.4 and poor from ±0.0 to ±0.2. The relationships between the sensory properties tested using Pearson's correlation analysis were as follows. The flavour descriptor ‘nutty’ was the most influential descriptive property influencing an oil's acceptability. If one of the rapeseed oils had a less nutty taste, the oil's acceptability score was impacted. Pearson correlations proved a statistically strong correlation between acceptability and nutty of 52% (r = 0.518; p = <0.05). The descriptor ‘straw-like’ had a moderate correlation to acceptability of 35% (r = 0.349; p = <0.05). Thus, it can be said that the “nutty” flavour was more favourable than the ‘straw-like’. A poor correlation was perceived between colour, odour, and acceptability, indicating these attributes had minimal influence on the acceptability of the rapeseed oils to the consumer. A strong correlation of 42% (r = 0.408; p = <0.05) was observed between colour and odour. The darker the colour of the oil, the more intense the odour. While a strong relationship was observed between these two sensory properties, a poor relationship was perceived between odour and colour with acceptability. Thus, the acceptability of the Irish rapeseed oil's sensory evaluation was strongly influenced by the nutty taste and the odour and colour combination. No significant correlation for ‘texture’ was observed. The comparison of the rapeseed oils produced useful information about their acceptability and sensory properties. Thus, the difference observed between Irish rapeseed oils' sensory properties was explicitly due to the VOC properties.
Substantial differences were observed within successive batches from individual producers. Fig. 4 illustrates the combined radar charts for each producer, comparing the successive batches via sensory evaluation.
Fig. 4.
Comparison of the sensory evaluation of successive batches of rapeseed oil from individual producers.
Fig. 4 presents the sensory charts representing the sensory analysis results of individual producers' rapeseed oils and successive batches from each. The graphs identify differences, and trends emerge within the successive batches.
The rapeseed oils from producer one (PRO1) show a notable difference between batch PRO1B3 and PRO1B1 & PRO1B2. PRO1B3 had a lower acceptability score than PRO1B1 & PRO1B2. Analysis of the descriptive data indicates that PRO1B3 differs from PRO1B1 & PRO1B2. The sensory panel perceived it as darker yellow with a higher straw-like flavour and more pungent odour than PRO1B1 & PRO1B2.
The batches from PRO2 also demonstrated variability based on flavour, the acceptability of the batches was similar, but the descriptive intensities were different. For example, the nuttiness of PRO2B3 was higher but had a lower odour pungency, while PR2OB1 and PRO2B2 had similar nuttiness but demonstrated differences in odour intensity.
The acceptability of PRO3B2 rapeseed oils was lower by the panel. When compared to PRO3B1 and PRO3B3, it was clear that the nutty flavour difference was the cause of the variation in acceptability.
Sensory differences are apparent with PRO4B2 as PRO4B2 had a darker colour, lower nutty and higher straw-like flavour than PRO4B1 and PRO4B3.
PRO5 has similar acceptability within batches, but PRO5B1 had a notably higher nutty flavour and odour intensity.
Only two batches were evaluated from PRO6; a difference was observed between both. PRO6B2 had a lower acceptability score than PRO6B3 due to the lower nutty flavour, dark colour and higher straw-like flavour.
The Irish rapeseed oils presented VOC profiles that varied compound types and concentrations. The variety and concentration of the compounds presented different complexities to the sensory profile of each oil, specifically to the “nutty” and “floral” flavours. Differences in sensory aromas were influenced by the volatile compounds present in the oil. The VOC profile associated with the oils evaluated showed higher aldehyde and ketone compound concentrations.
Pearson's correlation analysis identified the relationship between the sensory analysis tests and the VOC profile; correlation coefficients were converted to percentage values. Aldehyde compounds had an inverse relationship to the sensory properties, specifically to flavour and acceptability. Hence, when the aldehyde content of the rapeseed oils increases, the acceptability of the oils decreases potentially due to off odours and flavours. Thus, they can be acceptable at low levels but become rapidly unacceptable above certain concentrations. The compound concentration and odour threshold of the aldehydes influence the intensity in which the aroma is perceived; therefore, the intensity of the aldehyde compounds detected depends on both concentration and OAV. While these oils contain extensive fatty acid content, they were not associated with sensory oxidation defects. Thus, indicating that all these samples were effective of high quality (Zhu et al., 2016). Volatile compounds exhibit different thresholds and therefore have different odour intensities. This may be why aldehydes did not demonstrate a positive relationship with the sensory profile, as the odour threshold of ketones and acids may exceed aldehydes as the rapeseed oils appear to be of a high standard with lower oxidation levels resulting in less adverse sensory characteristics. The sensory terms “odour” and “straw-like” have the highest correlation, while “odour” and alcohols have the lowest correlation.
The descriptive odour and volatile group acids demonstrated a more substantial relationship 63.2% (r = 0.632; p = <0.05) than odour and ketones 52.1% (r = 0.521; p = <0.05), indicating the acids detected had a more significant influence on odour than the ketones. Ketone compounds are associated with hay or grassy aromas. This may explain the relationship observed between ketones and straw-like. Acid compound presented a stronger relationship to the straw-like descriptor 48% (r = 0.489; p = <0.05) A moderate relationship was apparent between the presence of alcohol compounds and the oil's shade of yellow 28.8% (r = 0.288; p = <0.05). This may indicate that alcohol compounds can influence the intensity of an oil's yellow colour. Remarkably, the shade of the colour in the rapeseed oils varied in a trend like the variation in the alcohol, 1-hexanol. A similar moderate relationship was observed for colour and alcohol 28%, the remaining VOC groups were determined to have a poor relationship with the sensory descriptors and therefor have minimal impact on the sensory profile of the rapeseed oils.
4. Conclusion
In total, 41 VOC compounds were detected in these 17 rapeseed oil samples produced in Ireland from different producers. VOC classes consisted of aldehydes, ketones, alcohols, acids, sulphurs, terpenes, ethers, esters, and benzenes. The most abundant compounds detected were hexanal, propanal, 1-hexanol, acetone, 6-Methyl-5-hepten-2-one, and dimethyl sulfone. These compounds were significantly different in terms of abundance. Significant differences (p < 0.05) were observed for individual compounds. PCA endorsed these differences and illustrated that samples from different producers might differ from the successive batch samples from each producer. These differences may be attributed to pre-processing techniques, seed variety, or fatty acid composition (Zhang et al., 2019). Coughlan et al., 2022, previously proved that these Irish rapeseed oils do not contain the same fatty acid profile, which may be a significant contributor to the variation observed in the volatile profile due to individual fatty acid differences which may have given rise to the differentiation between the oils in terms of sensory properties. The Irish rapeseed oils in this study demonstrated variance in fatty acid composition whereby the individual fatty acids were statistically significant with respect to abundance (p=<0.050) demonstrating variability between producers and batches alike (Coughlan et al., 2022).
Additionally, it may be suggested that the VOC composition of oil may be used to identify the sensory profile. The sensory evaluation found that an oil with a nutty flavour, low or moderate odour and a lighter colour is more acceptable to the consumer. Analytical profiling identified these oils as high in aldehyde, ketone, and alcohol compounds. Sensory evaluation coupled with VOC profiling confirmed differences between the Irish rapeseed oils from different producers within successive batches. Cultivation, processing, and storage play a significant role in the VOC content of an oil, which directly influences the sensory profile. Hence VOC profiling could be used to predict the sensory profile of an oil and its acceptability for the consumer.
CRediT authorship contribution statement
Rebecca Coughlan: Methodology, Data, Formal analysis, compiling of initial draft preparation. Kieran Kilcawley: Conceptualization, Methodology, Review. Iwona Skibinska: Formal analysis, Data compilation. Siobhán Moane: Conceptualization, Review, editing. Tracey Larkin: Supervision, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Aiqian Ye
Data availability
The data that has been used is confidential.
References
- Aniołowska M., Zahran H., Kita A. The effect of pan frying on thermooxidative stability of refined rapeseed oil and professional blend. J. Food Sci. Technol. 2016;53(1):712–720. doi: 10.1007/s13197-015-2020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonte A., Brühl L., Vosmann K., Matthäus B. A chemometric approach for the differentiation of sensory good and bad (musty/fusty) virgin rapeseed oils on basis of selected volatile compounds analyzed by dynamic headspace GC-MS. Eur. J. Lipid Sci. Technol. 2017;119(4):1–15. doi: 10.1002/ejlt.201600259. [DOI] [Google Scholar]
- Bonte A., Schweiger R., Pons C., Wagner C., Brühl L., Matthäus B., Müller C. Metabolic changes during storage of Brassica napus seeds under moist conditions and the consequences for the sensory quality of the resulting virgin oil. J. Agric. Food Chem. 2017;65(Issue 50) doi: 10.1021/acs.jafc.7b04149. [DOI] [PubMed] [Google Scholar]
- Brühl L. Fatty acid alterations in oils and fats during heating and frying. Eur. J. Lipid Sci. Technol. 2014;116(6):707–715. doi: 10.1002/ejlt.201300273. [DOI] [Google Scholar]
- Buttery R.G. Flavor chemistry and odor thresholds. Flavor Chem. 1999;1939:353–365. doi: 10.1007/978-1-4615-4693-1_30. [DOI] [Google Scholar]
- Chew S.C. Cold-pressed rapeseed (Brassica napus) oil: chemistry and functionality. Food Res. Int. 2020;131:1–10. doi: 10.1016/j.foodres.2020.108997. 108997. [DOI] [PubMed] [Google Scholar]
- Coughlan R., Moane S., Larkin T. Variability of essential and nonessential fatty acids of Irish rapeseed oils as an indicator of nutritional quality. Int. J. Food Sci. 2022;2022(10):10. doi: 10.1155/2022/7934565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbou S., Chaieb I., Rjiba I., Issaoui M., Echbili A., Nakbi A., Gazzah N., Hammami M. Multivariate data analysis of fatty acid content in the classification of olive oils developed through controlled crossbreeding. JAOCS, J. American Oil Chemists’ Soc. 2012;89(4):667–674. doi: 10.1007/s11746-011-1946-1. [DOI] [Google Scholar]
- Giakoumis E.G. Analysis of 22 vegetable oils' physico-chemical properties and fatty acid composition on a statistical basis, and correlation with the degree of unsaturation. Renew. Energy. 2018;126:403–419. doi: 10.1016/j.renene.2018.03.057. [DOI] [Google Scholar]
- Gracka A., Raczyk M., Hradecký J., Hajslova J., Jeziorski S., Karlovits G., Michalak B., Bąkowska N., Jeleń H. Volatile compounds and other indicators of quality for cold-pressed rapeseed oils obtained from peeled, whole, flaked and roasted seeds. Eur. J. Lipid Sci. Technol. 2017;119(10) doi: 10.1002/ejlt.201600328. [DOI] [Google Scholar]
- Hettiarachchi C.A., Illeperuma D.C.K. Developing a trained sensory panel for comparison of different brands of vanilla ice cream using descriptive sensory analysis. J. Natl. Sci. Found. Sri Lanka. 2015;43(1):45–55. doi: 10.4038/jnsfsr.v43i1.7914. [DOI] [Google Scholar]
- Ivanova-Petropulos V., Mitrev S., Stafilov T., Markova N., Leitner E., Lankmayr E., Siegmund B. Characterisation of traditional Macedonian edible oils by their fatty acid composition and their volatile compounds. Food Res. Int. 2015;77:506–514. doi: 10.1016/j.foodres.2015.08.014. [DOI] [Google Scholar]
- Jeleń H.H., Mildner-Szkudlarz S., Jasińska I., Wąsowicz E. A headspace-SPME-MS method for monitoring rapeseed oil autoxidation. J. Am. Oil Chem. Soc. 2007;84(6):509–517. doi: 10.1007/s11746-007-1072-2. [DOI] [Google Scholar]
- Jia X., Wang L., Zheng C., Yang Y., Wang X., Hui J., Zhou Q. Key odorant differences in fragrant Brassica napus and Brassica juncea oils revealed by gas chromatography-olfactometry, odor activity values, and aroma recombination. J. Agric. Food Chem. 2020;68(50):14950–14960. doi: 10.1021/acs.jafc.0c05944. [DOI] [PubMed] [Google Scholar]
- Jing B., Guo R., Wang M., Zhang L., Yu X. Influence of seed roasting on the quality of glucosinolate content and flavor in virgin rapeseed oil. LWT (Lebensm.-Wiss. & Technol.) 2020;126(September 2019) doi: 10.1016/j.lwt.2020.109301. [DOI] [Google Scholar]
- Katragadda H.R., Fullana A., Sidhu S., Carbonell-Barrachina Á.A. Effect of Fatty acid concentration on volatile compounds. Food Chem. 2010;120(6):59–65. [Google Scholar]
- Kiralan M., Ramadan M.F. Volatile oxidation compounds and stability of safflower, sesame and canola cold-pressed oils as affected by thermal and microwave treatments. J. Oleo Sci. 2016;65(10):825–833. doi: 10.5650/jos.ess16075. [DOI] [PubMed] [Google Scholar]
- Koubaa M., Mhemdi H., Barba F.J., Roohinejad S., Greiner R., Vorobiev E. Oilseed treatment by ultrasounds and microwaves to improve oil yield and quality : An overview. Food Res. 2016;85:59–66. doi: 10.1016/j.foodres.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Kraljić K., Stjepanović T., Obranović M., Pospišil M., Balbino S., Škevin D. Influence of conditioning temperature on the quality, nutritional properties and volatile profile of virgin rapeseed oil. Food Technol. Biotechnol. 2018;56(4):562–572. doi: 10.17113/ftb.56.04.18.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardos G., Kendall D., Barnard N. Odor threshold determinations of 53 odorant chemicals. J. Air Pollut. Control Assoc. 1969;19(2):91–95. doi: 10.1080/00022470.1969.10466465. [DOI] [Google Scholar]
- Li X., Bremer G.C., Connell K.N., Ngai C., Pham Q.A.T., Wang S., Flynn M., Ravetti L., Guillaume C., Wang Y., Wang S.C. Changes in chemical compositions of olive oil under different heating temperatures similar to home cooking. J. Food Chem. Nutri. 2016;4(1):7–15. doi: 10.33687/jfcn.004.01.1532. [DOI] [Google Scholar]
- Liu X., Hoshino N., Wang S., Masui E., Chen J., Zhang H. A novel evaluation index for predicting the degradation rate of frying oils based on their fatty acid composition. Eur. J. Lipid Sci. Technol. 2018;120(7):1–6. doi: 10.1002/ejlt.201700528. [DOI] [Google Scholar]
- Lui C., Hou L.*. Review on volatile flavor components of roasted oilseeds and their products. Grain & Oil Sci. Technol. 2018;1(4):151–156. doi: 10.3724/SP.J.1447.GOST.2018.18052. 2018. [DOI] [Google Scholar]
- Matheis K., Granvogl M. Differentiation between aroma-related bioactives in native cold-pressed rapeseed oils with desired sensory attributes and with a fusty/musty off-flavor using multivariate methods. J. Food Bioact. 2019;8:51–57. doi: 10.31665/jfb.2019.8206. [DOI] [Google Scholar]
- Petersen K.D., Kleeberg K.K., Jahreis G., Busch-Stockfisch M., Fritsche J. Comparison of analytical and sensory lipid oxidation parameters in conventional and high-oleic rapeseed oil. Eur. J. Lipid Sci. Technol. 2012;114(10):1193–1203. doi: 10.1002/ejlt.201200112. [DOI] [Google Scholar]
- Raghavan S.K., Connel D.R., Khayat A. In: Lipids in Food Flavors (ACS Symposium Series. Ho, Hartmann T.G., editors. American Chemical Society; Washington, DC: 1994. Canola oil flavor quality evaluation by dynamic headspace gas chromatography. [Google Scholar]
- Rękas A., Wroniak M., Siger A., Ścibisz I., Derewiaka D., Anders A. Mechanical hulling and thermal pre-treatment effects on rapeseed oil antioxidant capacity and related lipophilic and hydrophilic bioactive compounds. Int. J. Food Sci. Nutr. 2017;68(7):788–799. doi: 10.1080/09637486.2017.1290054. [DOI] [PubMed] [Google Scholar]
- Ren X., Wang L., Xu B., Wei B., Liu Y., Zhou C., Ma H., Wang Z. Influence of microwave pretreatment on the flavor attributes and oxidative stability of cold-pressed rapeseed oil. Dry. Technol. 2019;37(3):397–408. doi: 10.1080/07373937.2018.1459682. [DOI] [Google Scholar]
- Rokosik E., Dwiecki K., Siger A. The quality of cold-pressed rapeseed oil obtained from seeds of Brassica napus L. with increased moisture content. Acta Scientiarum Polonorum, Technologia Alimentaria. 2019;18(2):205–218. doi: 10.17306/J.AFS.2019.0672. [DOI] [PubMed] [Google Scholar]
- Stevenson R.J., Rich A., Russell A. The nature and origin of cross-modal associations to odours. Perception. 2012;41(5):606–619. doi: 10.1068/p7223. [DOI] [PubMed] [Google Scholar]
- Teagasc . 2021. Teagasc Specialist Tillage Crops Report - Harvest Issue 10.https://www.teagasc.ie/media/website/publications/2021/TEAGASC-HARVEST-REPORT-2021.pdf [Google Scholar]
- Tierney A.C., Zabetakis I. Changing the Irish dietary guidelines to incorporate the principles of the Mediterranean diet: proposing the MedÉire diet. Publ. Health Nutr. 2019;22(2):375–381. doi: 10.1017/S136898001800246X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Zhang J., Chen J., Jing B., Zhang L., Yu X. Characterization of Differences in Flavor in Virgin Rapeseed Oils by Using Gas Chromatography–Mass Spectrometry, Electronic Nose, and Sensory Analysis. Eur. J. Lipid Sci. Technol. 2020;122(3):190–205. doi: 10.1002/ejlt.201900205. [DOI] [Google Scholar]
- Wehrens R., Weingart G., Mattivi F. MetaMS: an open-source pipeline for GC-MS-based untargeted metabolomics. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2014;966:109–116. doi: 10.1016/j.jchromb.2014.02.051. [DOI] [PubMed] [Google Scholar]
- Wroniak M., Rekas A., Ratusz K. Influence of impurities in raw material on sensory and physicochemical properties of cold-pressed rapeseed oil produced from conventionally and ecologically grown seeds. Acta Scientiarum Polonorum, Technologia Alimentaria. 2016;15(3):289–297. doi: 10.17306/J.AFS.2016.3.28. [DOI] [PubMed] [Google Scholar]
- Xu L., Yu X., Li M., Chen J., Wang X. Monitoring oxidative stability and changes in key volatile compounds in edible oils during ambient storage through HS-SPME/GC–MS. Int. J. Food Prop. 2018;20(3):S2926–S2938. doi: 10.1080/10942912.2017.1382510. [DOI] [Google Scholar]
- Zhang W., Cao X., Liu S.Q. Aroma modulation of vegetable oils—a review. Crit. Rev. Food Sci. Nutr. 2019:1–14. doi: 10.1080/10408398.2019.1579703. 0(0) [DOI] [PubMed] [Google Scholar]
- Zhou Q., Tang H., Jia X., Zheng C., Huang F., Zhang M. Distribution of glucosinolate and pungent odors in rapeseed oils from raw and microwaved seeds. Int. J. Food Prop. 2018;21(1):2296–2308. doi: 10.1080/10942912.2018.1514632. [DOI] [Google Scholar]
- Zhu H., Wang S.C., Shoemaker C.F. Volatile constituents in sensory defective virgin olive oils. Flavour Fragrance J. 2016;31(1):22–30. doi: 10.1002/ffj.3264. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.