Abstract
Background
In infertility clinics, preserving high-quality spermatozoa for a long time is a necessity. Pentoxifylline (PT) and L-carnitine (LC) are effective in improving sperm motility as well as protecting the sperm membrane. The present study aimed to investigate the protective impacts of PT and LC on the quality of the normal sperm motility, protamine content, and viability on prolonged storage for 12 days at 4-6°C.
Materials and Methods
The present experimental work included 26 samples, which were first prepared based on the swim-up technique, of normozoospermic men. They were divided into three aliquots as untreated control, LC-treated, and PT-treated groups and incubated for up to 12 days at 4-6°C. Thereafter, chromatin maturity, sperm viability, and motility were assessed on 0, 1, 2, 5, 7, and 12 days. Data were analyzed using a one-way analysis of variance.
Results
The obtained data revealed that PT supplementation increased the percentage of motile spermatozoa in com- parison with control and LC-treated specimens. On the other hand, LC supplementation increased the percentage of viable spermatozoa in comparison with the PT-treated and control samples. During the 12-day storage, the percentage of spermatozoa with a normal protamine content was nearly unchanged in the three groups (P>0.05).
Conclusion
Although LC supplementation can be considered a better alternative than PT for preserving sperm viabil- ity, PT could better preserve sperm motility compared to LC during 12 days at 4-6°C.
Keywords: Carnitine, Pentoxifylline, Preservation, Sperm Motility
Introduction
Subfertile and infertile patients suffer from social and medical problems. According to reports, 10-15% of couples are infertile, and male infertility accounts for approximately 50% (1).
Semen cryopreservation is the most prevalent storing approach in human spermatozoa preservation (2). Nonetheless, cryopreservation reduces some sperm parameters such as viability and motility and damages DNA in the sperm nucleus (3, 4).
The antioxidant content is reduced after cryopreserving the semen, while the reactive oxygen species (ROS) of the spermatozoa are presented an increase (5).
A high ROS content reduces sperm quality, including reducing motility and fertilizing capacity (6). The quality of the sperm can be impacted by the content of the sperm microenvironment (7). For example, antioxidant supplementation can inhibit sperm damage based on neutralizing ROS (5). Adding L-carnitine (LC) to the male reproductive system as an unusual amino acid, to the sperm sample improves sperm motility and viability due to its antioxidant property. The composition of the culture medium is considered an important factor influencing the function of the sperm throughout storage in vitro. LC is a derivative of lysine and methionine and is concentrated in tissues with more energy-consuming, including skeletal and cardiac muscles and reproductive systems, especially the epididymis (8).
According to previous evidence, infertile men have less LC in their semen (9). In addition, LC mostly increases sperm maturation, sperm motility, and spermatogenesis (8) with a noticeable contribution to the oxidation of longchain fatty acids in mitochondria, and thus the generation of energy. The anti-apoptotic, anti-inflammatory, and antioxidant effects of LC are known on treating different pathophysiological disorders (10).
The other most utilized antioxidant in reproductive technologies is pentoxifylline (PT), which is a derivative of methylxanthine. It can enhance sperm motility (11), scavenge ROS, protect the integrity of the plasma membrane (12), and act as a stimulating agent affecting the features of sperm motility by inhibiting cAMP phosphodiesterase (13).
Storage temperature is considered another factor that may affect sperm. Based on a previous study (14), for long-term sample preservation, storing samples at 4-6°C represents much better values in terms of sperm motility and vitality in comparison to 25°C. Considering that, 4-6°C was chosen for sperm storage in the current study.
In the present study, the impact of LC and PT supplementation on long-term sperm preservation at a constant temperature is investigated. There are many occasional problems with in vitro fertilization process. For instance, limited access to couples for semen sample collection, the unwillingness of a wife to eggs retrieval process, and painful repetition of sperm-extraction technique. Accordingly, cryopreservation could be considered a useful technique even for short time storage, however, this is an expensive procedure and could not be possible to perform for many laboratories. Besides, the use of cryopreservation has been reported to have negative effects on sperm quality such as motility reduction, survival, and sperm DNA damage (3, 4). Thus, to maintain and improve sperm parameters in andrology laboratories, it is necessary to preserve the sperm with better quality and technique. In this study, the motility, viability, protamine content, and chromatin maturity of sperms were evaluated in the presence of LC and PT reagents and the results were compared with untreated control samples.
Materials and Methods
Study group
Semen samples from healthy men (n=26) aged 28- 34 years were collected after three days of abstinence (normozoospermic men) from the infertility and fertility center in Isfahan, Iran. Informed consent was also obtained from volunteers. The Ethics Committee of Shiraz University (IR.SUMS.REC.1399.769) and the Royan Institute (IR. ACECR.ROYAN.RES.1400.017) approved the study. To perform semen quality analysis, the studied samples were liquefied by placing them at 37°C for 30 minutes.
The samples with normal morphology, sperm count, motility,and viability were included in the study based on the criteria of the World Health Organization (WHO) according to (15). In order to minimize the error of observations, all semen analyzes have been performed by the same technician.
Sperm preparation by the swim-up method
Liquefied semen were moved to a centrifuge tube with a round-shaped bottom. Then, 1 mL of the Ham’s F10 medium (Sigma, USA) with 20% human serum albumin (Kedrion, Italy) was gently added and left in the incubator for 1 hour at an angle of 45° to swim up the spermatozoa. The motile spermatozoa left the pellet and it was submerged into the supernatant. Next, the supernatant containing motile sperms was transferred to another sterile tube with the round-shaped bottom and mixed with the medium, and then it was centrifuged at 500 g for 5 minutes.
Experimental design
Sperm motility and viability:
All samples were divided into three equal aliquots with 10×106 sperms. The control samples were incubated with an equal volume of Ham’s F10 comprising 20% human serum. LC- and PT-supplemented samples were exposed to an equal volume of 3.6 mM LC and 3.6 mM PT (Sigma, USA), respectively. The total molarity was 1.8 mM for both LC and PT after the reconstruction of the samples with treatments (16). All samples were maintained until 12 days at 4-6°C. Sperm viability was evaluated by staining the samples with eosin-nigrosin and calculating the percentage of viable sperms by light microscopy (Nikon, E200, Japan). Moreover, motility was assessed according to the WHO guidelines on 0, 1, 2, 5, 7, and 12 days by a light microscope equipped with the Computer-assisted sperm analysis (CASA) system. Based on the WHO guidelines, sperms can be either progressive motile, move linearly or in a large circle, or be non-progressive, move on a side or slowly without any forward movements, including beating flagellum, moving in small circles, or being immotile (15). The percentage of motile spermatozoa was computed by counting the progressive, non-progressive, and immotile sperms, along with the total number of spermatozoa in 10 randomly selected fields.
Sperm protamine content with chromomycin A3 staining
The fluorescent dye chromomycin A3 (CMA3, Sigma, St Louis, MO, USA) was used for indicating the amount of protamine deficiency in the chromatin structure according to Razavi et al. (17). The sperm with protamine deficiency appears bright yellow.
The samples were fixed by adding Carnoy’s fixative solution (methanol and acetic acid in a ratio of 3:1) with an equal volume to the sample at 4°C for 5 minutes.
First, 20 μL of the fixed sample was placed on the labeled part of a glass slide and allowed to be air-dried, followed by staining the sample with 100 μL of the CMA3 solution [0.25 mg/ml in McIlvaine buffer (7 ml citric acid 0.1 M + 32.9 ml Na2 HPO4 ×7H2 O, 0.2 M, pH=7.0, containing 10 mM MgCl2 )].
In each slide, 200 sperm were assessed utilizing a fluorescence microscope (Olympus, Tokyo, Japan) and 470-460 nm filters at 1000× magnification. Finally, the percentage of mature sperms or sperms with a normal protamine content (CMA3-) was evaluated as well.
Aniline blue staining
Aniline blue (AB, Sigma, USA) staining for detecting excessive histones in the sperm chromatin was used according to Nasr-Esfahani et al. (18).
To stain the sperms with AB, a smear was prepared from a 10 μL drop of the sample. After air-drying, the smear was fixed in a solution of 3% glutaraldehyde in 0.2 M phosphate buffer (14 mL of 0.2 M NaH2 PO4 +0.2 M Na2 HPO (36 mL), pH=7.2) for 30 minutes.
The slides were stained in the 5% AB solution with 4% acetic acid (pH less than 3.5) for 5 minutes. In each slide, 200 sperm were examined in randomly selected fields under a light microscope (Nikon, E200, Japan) using oil immersion with 90×10 magnification.
The percentage of the sperm with pale blue (normal spermatozoa, AB-) and dark blue (abnormal spermatozoa, AB+) heads was recorded.
Statistical analyses
The data were expressed as mean values ± standard error. The results were statistically analyzed by one-way analysis of variance. SPSS (Version 25, Armonk, New York, USA) and Prism software (version 8, San Diego, CA, USA) were applied to analyze the data and draw figures, respectively, and a P<0.05 was statistically considered significant.
Results
Sperm motility assessment
The motile spermatozoa percentages on 0, 1, 2, 5, 7, and 12 days were assessed in all groups. In the first to seventh days, the percentage of progressive sperm motility was higher significantly in the PT group in comparison with the control group (P<0.001), and this superiority was kept up to the twelfth day. Although the percentage of the progressively motile sperm in the LC group from the first to day 5 was higher compared to the control group, this difference was significant only on the fifth day (P<0.001). The progressive sperms frequency was significantly higher in PT aliquots compared to LC groups (P<0.001) until the twelfth day of analysis. On the 12th day, there were about 0.6% progressively motile sperm in PT-supplemented aliquots, while no progressive motile sperm were observed in the control and LC groups. The data demonstrated that the PT-supplemented group significantly improved sperm progressive motility compared to the LC-supplemented group up to 12 days (P<0.001, Fig.1).
Fig 1.
The trend of changes in the progressive sperm percentage in control, LC, and PT groups on different days of storage. PT; Pentoxifylline, LC; L-carnitine, PT improved the frequency of the progressive sperm compared with LC and control, **; P<0.01, and ***; P<0.001.
On the first and second days, the percentage of nonprogressive motile sperms was higher significantly in the control group compared with the groups receiving LC and PT (P=0.003). However, this observation was reversed on the fifth and seventh days, and the frequency of non-progressive sperm in the LC and PT groups was significantly higher in comparison with the control group (P<0.001, Fig.2), and non-progressive motility in the PT group was higher than in the LC group on the fifth, seventh, and twelfth days (P<0.001). Approximately 2.5% of the sperm were non-progressive in the PT group, while the frequency was zero in the other groups on the twelfth day. The data showed that PT preserved sperm motility up today 12.
Fig 2.
The trend of changes in the percentage of the non-progressive sperm in control, LC, and PT groups on different days of storag. PT; Pentoxifylline, LC; L-carnitine, PT preserved the frequency of the non-progressive sperm compared with LC and control, * ; P<0.05, **; P<0.01, and ***; P<0.001.
The control aliquots contained significantly higher percentages of immotile sperms in comparison with the other groups during the first to twelfth days of incubation (P<0.001). The percentage of immotile sperm was significantly lower in the group receiving LC compared to the control group from the first to the fifth day (P<0.001). On the day fifth, seventh, and twelfth, the immotile sperm percentage was significantly higher in the LCtreated aliquots in comparison with the PT-treated group (P<0.001). On day 12, the percentage of immotile sperm was 96% in the PT-treated group, while it was 100% in the other groups (Fig.3).
Fig 3.
The trend of changes of the immotile sperm percentage in control, LC, and PT groups on different days of storage. PT; Pentoxifylline, LC; L-carnitine, PT decreased the frequency of the immotile sperm in comparison with LC and control, * ; P<0.05, **; P<0.01, and ***; P<0.001.
Sperm viability assessment
During the first 24 hours, the percentage of viable sperm with white head (Fig.4A) was the same among the three groups (P>0.05). After 24 hours until day 12, LC led to a significant improvement in sperm viability compared to control (P<0.001). Additionally, the percentage of viable sperm in the PT group significantly incremented compared to the control group on the twelfth day. Finally, the percentage of viable sperm in the LC group also represented a significant increase compared with the PT group on the twelfth day (P<0.001).
The data revealed that LC-supplemented groups better improve sperm viability than the PT-supplemented group until 12 days (Fig.4B).
Fig 4.
Evaluation of the sperm viability. A. Eosin-nigrosin staining showing that the heads of viable and dead sperms appear white and red, respectively (100x eyepiece magnifications), and B. The trend of changes in sperm viability in control, LC, and PT groups on different days of storage. PT; Pentoxifylline, LC; L-carnitine, LC improved the frequency of the viable sperm compared to PT and control, * ; P<0.05, **; P<0.01, and ***; P<0.001.
Sperm nucleus protamine content
The initial value for the frequency of the sperm with normal protamine content with dull yellow head (CMA3- in Fig.5A) was 98%. The frequency of the sperm with normal protamine content not only was statistically similar in all conditions but also did not change with the progress of time (Fig.5B).
Fig 5.
Evaliuation of protamine content. A. The protamine content of sperm nucleus evaluated by chromomycin A3 (CMA3) staining and B. The trend of changes in the percentage of the sperm with normal protamine contents in control, LC, and PT groups on different days of storage (scale bar: 50 µm). The frequency of the sperms with normal protamine contents did not change in all groups with the progress over time. PT; Pentoxifylline, LC; L-carnitine. Immature sperms were stained bright yellow (CMA3+) 100x eyepiece magnifications.
Sperm chromatin maturity
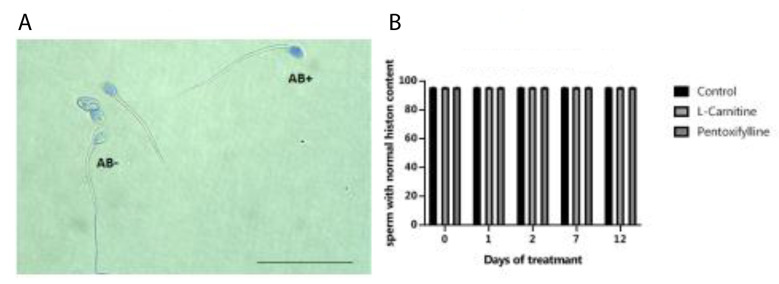
The percentage of the sperm with normal nuclear histone or mature sperm with pale blue head (AB- in Fig.6A) on different days was also similar in different groups (P>0.05). Further, the comparison of the percentages of the sperm with normal histone contents on different days demonstrated no change (Fig.6B)
Fig 6.
Evaliuation of histone content. A. The histone content of sperm nucleus evaluated by AB staining and B. The trend of changes in the percentage of the sperm with normal histone contents in control, LC, and PT groups on different days of storage (scale bar: 50 µm). The frequency of the sperms with normal histone contents represented no change in all groups with the progress over time. AB; Aniline blue, LC; L-carnitine, PT; Pentoxifylline. Mature and immature sperms were stained paler (AB-) and blue (AB+), respectively (100x eyepiece magnifications).
Discussion
The culture medium composition has a key role in prolonging the preservation of spermatozoa with proper quality (19). The excessive generation of ROS may extremely harm the spermatozoa due to a deficiency in antioxidant defenses.
A widely negative correlation was demonstrated between sperm quality and intracellular ROS levels (20). The lack of antioxidants in the cytoplasmic components of the mature spermatozoa has detrimental effects on resistance to ROS. Thus, antioxidant supplementation prevents the oxidative damage of the sperm due to ROS production throughout storage (21). In the present study, PT and LC, as two antioxidants, were added to preserve stored sperm quality.
A higher LC concentration in the epididymal fluid is suggested for stabilizing the sperm plasma membrane and increasing the survival of sperms (22). In this study, it was attempted to mimic the natural storing environment in the epididymis by adding LC.
Several hypotheses were suggested regarding the role of LC in improving sperm viability. LC has been reported to be able to protect cell membranes from ROS damage by regulating carbohydrate metabolism (23). Access to fats for peroxidation is reduced by LC, and this function may protect the sperm membrane and increase sperm viability (24). Furthermore, LC increases sperm viability by boosting glucose uptake due to its antioxidant properties (25).
Based on the results in the current study, LC supplementation resulted in a noticeably increment in the number of viable spermatozoa throughout storage until 12 days.
The oral administration of LC induced the maturation of spermatozoa and maintenance of sperm viability as confirmed by our in vitro results (26).
On the other hand, according to our results, PT was preferable for improving sperm motility. Prior to IVF/ ICSI, PT supplementation can enhance the flagellar movement of viable sperm (27).
According to the results of Mirshokraei et al. (28) on fresh specimens, PT had a beneficial impact on sperm motility, which is in line with our results.
Milani et al. concluded that PT can be beneficial for improving motility in canine frozen-thawed spermatozoa without any effective sperm longevity (29). Likewise, the PT effect on sperm motility was confirmed in asthenozoospermia samples (30).
Several hypotheses were mentioned concerning the mechanisms of improving sperm motility by PT as follows: i. Studies reported that the endogenous production of nitric oxide (NO) is highly important for sperm motility (31). NO stimulates sperm motility by activating guanylyl cyclase, followed by producing cyclic guanosine monophosphate (cGMP) and activating the cGMP/G kinase protein signaling pathway (32). PT increases the level of NO produced by the sperm and thus improves its motility (33). ii. Some other reports suggested that cAMP in the semen of infertile men significantly reduced compared to its level in fertile men, and there was a significant correlation between the cAMP level and the percentage of motile sperms (P<0.005). This finding implies that cAMP is important for sperm motility (34). On the other hand, PT is an inhibitor of the cAMP phosphodiesterase enzyme, which increases intracellular cAMP concentrations and sperm motility (14). iii. Another hypothesis is that the beneficial effect of PT can partly be explained by its involvement in the regulation of inflammatory mediators in the microenvironment content (35). PT has inhibitory effects on xanthine oxidase, facilitating the formation of oxygen-free radicals in cells (36). Moreover, PT reduces the production of tumor necrosis factor-alpha in the microenvironment. This cytokine increases the production of hydrogen peroxide in the mitochondria (37). Therefore, it is a potent antioxidant that reduces oxidative stress at the cellular level and has beneficial effects such as improved mobility.
Our data confirmed that PT was better than LC in improving sperm motility until 12 days, whereas LC outperformed PT in preserving sperm viability during the same period.
In this study, CMA3 and AB staining were used for measuring the DNA maturity of the sperm and investigating the effects of LC and PT on genetic content in storage time. CMA3 is an effective tool to assess fertility because of its relation to DNA integrity and sperm maturation (38). AB dye discriminates cysteine-and arginine-rich protamine from lysine-rich histones, while CMA3 dye competes with protamines for binding to the DNA minor groove in the sperm (39).
In general, it was investigated that the long-term storage of spermatozoa with or without LC or PT has no impact on the DNA protamine content. It may be attributed to this fact that mature spermatozoa have a small amount of cytoplasm, low levels of cytoplasmic organelles and enzymes, and extremely low cytoplasmic activity (40), thus chromatin maturity and protamine content do not change during the storage. Finally, our results suggested the enhancement of sperm viability and motility, after storing human semen for up to 12 days, by incubation in a common cell culture supplemented antioxidant at a low cost.
Conclusion
Despite PT's superiority in enhancing sperm motility, LC can be considered a natural factor in the in vivo microenvironment of the spermatozoa, and it was found to be more useful in preserving sperm viability compared to PT. Our founding can be beneficial in fertility clinics for the storage of good quality sperm without cryopreservation.
Acknowledgements
The authors wish to thank the Vice-chancellor of the Research Deputy of Shiraz University of Medical Sciences for the grant (Number: 21160) and the Royan Institute for technical support. There is no conflict of interest in this study.
Authors’ Contributions
E.A., M.H.N.-E., T.T.-Kh., M.T., Z.N.; Contributed to conception and design. Z.N.; Collected and interpreted data. E.A.; Was responsible for overall supervision. Z.N., T.T.-Kh.; Wrote and edited the manuscript, respectively. All authors read and approved the final manuscript.
References
- 1.Poongothai JE, Gopenath TS, Manonayaki SW. Genetics of human male infertility. Singapore Med J. 2009;50(4):336–347. [PubMed] [Google Scholar]
- 2.Oehninger S, Duru NK, Srisombut C, Morshedi M. Assessment of sperm cryodamage and strategies to improve outcome. Mol Cell Endocrinol. 2000;169(1-2):3–10. doi: 10.1016/s0303-7207(00)00343-9. [DOI] [PubMed] [Google Scholar]
- 3.Bagchi A, Woods EJ, Critser JK. Cryopreservation and vitrification: recent advances in fertility preservation technologies. Expert Rev Med Devices. 2008;5(3):359–370. doi: 10.1586/17434440.5.3.359. [DOI] [PubMed] [Google Scholar]
- 4.Rarani FZ, Golshan-Iranpour F, Dashti GR. Correlation between sperm motility and sperm chromatin/DNA damage before and after cryopreservation and the effect of folic acid and nicotinic acid on post-thaw sperm quality in normozoospermic men. Cell Tissue Bank. 2019;20(3):367–378. doi: 10.1007/s10561-019-09775-6. [DOI] [PubMed] [Google Scholar]
- 5.Saraswat S, Jindal SK, Priyadharsini R. The effect of antioxidants supplementation to cryopreservation protocol on seminal attributes and sperm membrane characteristics in Sirohi goat. J Physiol Pharmacol Adv. 2021;2(2):49–58. [Google Scholar]
- 6.Bansal AK, Bilaspuri GS. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int. 2011;2011:686137–686137. doi: 10.4061/2011/686137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talaei-Khozani T, Borzoei Z, Bahmanpour S, Zolghadr J, Dehbashi S, Zareh HR. Effects of sera taken from women with recurrent spontaneous abortion on sperm motility and apoptosis. Iran J Reprod Med. 2011;9(2):125–130. [PMC free article] [PubMed] [Google Scholar]
- 8.Dehghani F, Hassanpour A, Poost-Pasand A, Noorafshan A, Karbalay-Doust S. Protective effects of L-carnitine and homogenized testis tissue on the testis and sperm parameters of busulfan-induced infertile male rats. Iran J Reprod Med. 2013;11(9):693–704. [PMC free article] [PubMed] [Google Scholar]
- 9.Zare Z, Imani H, Mohammadi M, Mofid M, Dashtnavard H. Effect of oral l-carnitine on testicular tissue, sperm parameters and daily production of sperm in adult mouse. Cell J. 2010;11(4):382–389. [Google Scholar]
- 10.Miguel-Carrasco JL, Mate A, Monserrat MT, Arias JL, Aramburu O, Vázquez CM. The role of inflammatory markers in the cardioprotective effect of L-carnitine in L-NAME-induced hypertension. Am J Hypertens. 2008;21(11):1231–1237. doi: 10.1038/ajh.2008.271. [DOI] [PubMed] [Google Scholar]
- 11.Said TM, Gaglani A, Agarwal A. Implication of apoptosis in sperm cryoinjury. Reprod Biomed Online. 2010;21(4):456–462. doi: 10.1016/j.rbmo.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Zini A, Bielecki R, Phang D, Zenzes MT. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil Steril. 2001;75(4):674–677. doi: 10.1016/s0015-0282(00)01796-9. [DOI] [PubMed] [Google Scholar]
- 13.Sikka SC, Hellstrom WJ. The application of pentoxifylline in the stimulation of sperm motion in men undergoing electroejaculation. J Androl. 1991;12(3):165–170. [PubMed] [Google Scholar]
- 14.Golshan Iranpour F, Nateghian Z, Henkel R, Dashti GR. Effects of temperature and storage time on the motility, viability, DNA integrity and apoptosis of processed human spermatozoa. Andrologia. 2020;52(2):e13485–e13485. doi: 10.1111/and.13485. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 16.Aliabadi E, Karimi F, Talaei-Khozani T. Effects of L-Carnitine and pentoxifylline on carbohydrate distribution of mouse testicular sperm membrane. Iran J Med Sci. 2013;38(2):107–115. [PMC free article] [PubMed] [Google Scholar]
- 17.Razavi S, Nasr-Esfahani MH, Mardani M, Mafi A, Moghdam A. Effect of human sperm chromatin anomalies on fertilization outcome post-ICSI. Andrologia. 2003;35(4):238–243. doi: 10.1046/j.1439-0272.2003.00566.x. [DOI] [PubMed] [Google Scholar]
- 18.Nasr-Esfahani MH, Razavi S, Mardani M. Relation between different human sperm nuclear maturity tests and in vitro fertilization. J Assist Reprod Genet. 2001;18(4):219–225. doi: 10.1023/A:1009412130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed J, Ismail MN, Chou TY, Louis SR, Budin SB. A study of sperm quality characteristics changes in different storage temperatures above freezing point. Int J Collab Res Intern Med Public Health. 2012;4(5):736–743. [Google Scholar]
- 20.Agarwal A, Sharma RK, Sharma R, Assidi M, Abuzenadah AM, Alshahrani S, et al. Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod Biol Endocrinol. 2014;12:33–33. doi: 10.1186/1477-7827-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahrzad E, Zahiri S, Ghasemi F, Jahromi HK. A study of effects of L-carnitine on morphology and apoptosis in cryopreserved Sperm. Adv Environ Biol. 2013;7(9):2126–2134. [Google Scholar]
- 22.Banihani S, Agarwal A, Sharma R, Bayachou M. Cryoprotective effect of lcarnitine on motility, vitality and DNA oxidation of human spermatozoa. Andrologia. 2014;46(6):637–641. doi: 10.1111/and.12130. [DOI] [PubMed] [Google Scholar]
- 23.Gülcin İ. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006;78(8):803–811. doi: 10.1016/j.lfs.2005.05.103. [DOI] [PubMed] [Google Scholar]
- 24.Kalaiselvi T, Panneerselvam C. Effect of L-carnitine on the status of lipid peroxidation and antioxidants in aging rats. J Nutr Biochem. 1998;9(10):575–581. [Google Scholar]
- 25.Abd-Elrazek AM, Ahmed-Farid OA. Protective effect of L-carnitine and Larginine against busulfan-nduced oligospermia in adult rat. Andrologia. 2018;50(1):e12806–e12806. doi: 10.1111/and.12806. [DOI] [PubMed] [Google Scholar]
- 26.Dokmeci D. Oxidative stress, male infertility and the role of carnitines. Folia Med (Plovdiv). 2005;47(1):26–30. [PubMed] [Google Scholar]
- 27.Nassar A, Mahony M, Morshedi M, Lin MH, Srisombut C, Oehninger S. Modulation of sperm tail protein tyrosine phosphorylation by pentoxifylline and its correlation with hyperactivated motility. Fertil Steril. 1999;71(5):919–923. doi: 10.1016/s0015-0282(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 28.Mirshokraei P, Hassanpour H, Mehdizadeh A, Taheri MA. Pentoxifylline induces capacitation and acrosome reaction and improves quality of motility in canine ejaculated spermatozoa. Res Vet Sci. 2011;91(2):281–284. doi: 10.1016/j.rvsc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Milani C, Fontbonne A, Sellem E, Stelletta C, Gérard O, Romagnoli S. Effect of post-thaw dilution with caffeine, pentoxifylline, 2’-deoxyadenosine and prostatic fluid on motility of frozen-thawed dog semen. Theriogenology. 2010;74(1):153–164. doi: 10.1016/j.theriogenology.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 30.Henkel RR, Schill WB. Sperm preparation for ART. Reprod Biol Endocrinol. 2003;1:108–108. doi: 10.1186/1477-7827-1-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramya T, Misro MM, Sinha D, Nandan D, Mithal S. Altered levels of seminal nitric oxide, nitric oxide synthase, and enzymatic antioxidants and their association with sperm function in infertile subjects. Fertil Steril. 2011;95(1):135–140. doi: 10.1016/j.fertnstert.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 32.Miraglia E, De Angelis F, Gazzano E, Hassanpour H, Bertagna A, Aldieri E, et al. Nitric oxide stimulates human sperm motility via activation of the cyclic GMP/protein kinase G signaling pathway. Reproduction. 2011;141(1):47–54. doi: 10.1530/REP-10-0151. [DOI] [PubMed] [Google Scholar]
- 33.Banihani SA, Abu-Alhayjaa RF, Amarin ZO, Alzoubi KH. Pentoxifylline increases the level of nitric oxide produced by human spermatozoa. Andrologia. 2018;50(2):e12859–e12859. doi: 10.1111/and.12859. [DOI] [PubMed] [Google Scholar]
- 34.Malachi T, Bichachu S, Halbrecht I. Prostaglandins and cyclic-AMP in human semen. Prostaglandins Leukot Med. 1982;8(1):55–61. doi: 10.1016/0262-1746(82)90127-5. [DOI] [PubMed] [Google Scholar]
- 35.Saleh RA, HCLD AA. Oxidative stress and male infertility: from research bench to clinical practice. J Androl. 2002;23(6):737–752. [PubMed] [Google Scholar]
- 36.Hammerman C, Goldschmidt D, Caplan MS, Kaplan M, Schimmel MS, Eidelman AI, et al. Amelioration of ischemia-reperfusion injury in rat intestine by pentoxifylline-mediated inhibition of xanthine oxidase. J Pediatr Gastroenterol Nutr. 1999;29(1):69–74. doi: 10.1097/00005176-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Bilsborough W, Stanton K, Weerasooriya R, Dembo L, Taylor R, Green D. Effect of lowering tumour necrosis factor-α on vascular endothelial function in type II diabetes. Clin Sci (Lond). 2002;103(2):163–169. doi: 10.1042/cs1030163. [DOI] [PubMed] [Google Scholar]
- 38.Nasr-Esfahani MH, Aboutorabi R, Razavi S. Credibility of chromomycin A3 staining in prediction of fertility. Int J Fertil Steril. 2009;3(1):5–10. [Google Scholar]
- 39.Bassiri F, Nasr-Esfahani MH, Forozanfar M, Tavalaee M. Relationship between sperm parameters with sperm function tests in infertile men with at least one failed intracytoplasmic sperm injection cycle. Int J Fertil Steril. 2020;13(4):324–329. doi: 10.22074/ijfs.2020.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rengan AK, Agarwal A, van der Linde M, du Plessis SS. An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Reprod Biol Endocrinol. 2012;10:92–92. doi: 10.1186/1477-7827-10-92. Effects of PT and LC on Quality of Sperms. [DOI] [PMC free article] [PubMed] [Google Scholar]