ABSTRACT
Background:
Considering the important role of fluoride in preventing caries by reducing the number and activity of cariogenic bacteria and introducing new fluoride-releasing tooth-colored restorative materials, this study was performed to compare and evaluate fluoride ion release and antibacterial activity of resin-modified GI, zirconomer, giomer (Beautifil), and Cention N.
Materials and Methods:
In this experimental in vitro study, eighty samples (20 samples in each group) were prepared, 10 samples for fluoride testing and 10 samples for antibacterial activity. To evaluate the release of fluoride (PPM), fluoride ion-selective electrode was used on days 1st, 3rd, 7th, 14th, and 21st; and antibacterial activities against Streptococcus mutans were examined using direct contact test by absorption of light hourly once every 5 h and days 1st, 2nd, and 7th using an ELISA reader with a microplate reader of 800 TS at a wavelength of 630 nm. The results were analyzed using one-way ANOVA, Tamhane post hoc test, and paired t-test in SPSS software (IBM, Somers, NJ, USA) at a significant level of (P < 0.05).
Results:
The release of fluoride from materials showed a statistically significant difference (P < 0.001). The maximum mean of fluoride released during the 1st, 3rd and 7th days belonged to the zirconomer group and the minimum for the giomer group. During the measurement period of light absorption from 0 to 24 h after planting, light absorption of foursubstances together was found to be statistically significant (P <.001). Zirconomer light absorption increased on the 7th day, unlike other substances, but still, the minimum light absorption was related to zirconomer.
Conclusion:
The highest fluoride release was in zirconomer, Cention N, fuji II LC, and giomer, sequentially. Zirconomer showed maximum and giomer showed minimum antibacterial activities.
Key Words: Anti-bacterial agents, beautifil restorative, dental caries resistance, fluorides
INTRODUCTION
After the introduction of glass ionomer cements (GIC), fluoride-releasing restorative materials were introduced. High brittleness, physicomechanical properties, and poor abrasion resistance and sensitivity to moisture in the early stages of setting limit their use as reliable materials in stress-bearing areas. To overcome these problems, optimization of the composition of glass cements has been considered over the years[1] and some direct restorative materials like resin-modified GIC (RMGIC), zirconomer, prereacted glass ionomers (giomer), and Cention N have been introduced which have different fluoride levels and release rates.[2]
Fluoride-releasing materials such as glass ionomers have antibacterial properties and can prevent the formation of cariogenic biofilms such as Streptococcus mutans by forming fluorapatite, which is more resistant to acid attacks than hydroxyapatite, in addition to re-mineralizing primary caries[3] Therefore, prevent secondary caries, known as one of the most important factors in the failure of dental restorations. It is clear that fluoride is effective in the biological properties of cariogenic streptococci, especially S. mutans.[4] It reduces the production of lactic acid in bacterial plaque by disrupting the process of glucose uptake and glycolysis by bacteria. This ability of fluoride is mediated by a variety of mechanisms including inhibition of enzymes (enolase, sulfatase, and catalase) and proton translocating F-ATPases within the cytoplasm or on the cell membrane surface. In addition, the physiological ability of S. mutans biofilm is affected by fluoride.[5] The pattern of fluoride release in the conventional glass ionomer is the rapid initial release and a rapid decrease in fluoride release occurs after a short time.[2,3] The disadvantage of conventional glass ionomer is its low mechanical properties. To improve it, materials such as resin, alumina, carbon, glass, hydroxyapatite, and fluorapatite nanoparticles have been added, creating resin-modified glass ionomers without any problems in releasing fluoride.[6]
Giomer is the result of a combination of glass ionomer and composite resin, which combines the release and recharging properties of glass ionomer fluoride with the esthetics, polish ability, and high strength of composite resins.[7] Studies have shown that the rate of fluoride release in Giomer is higher than compomer and composite and it has been concluded that glass matrix plays an important role in fluoride release.[8] Zirconia-reinforced glass ionomer (zirconomer), while maintaining the benefits of glass ionomer and eliminating the dangers of mercury, also has the strength and durability of amalgam.[9]
Cention N is an “Alkasite” restorative material, which refers to a new class of restorative materials that are similar to composite materials and are essentially a subset of the resin composite material category. This new class contains an alkaline filler that can release acid-neutralizing ions.[10,11] The release of fluoride and hydroxide ions from a restorative material may help neutralize excess acidity during acid attacks by the cariogenic flora and prevent demineralization. Therefore, both agents may act simultaneously to increase the anti-caries potential of Cention-N.[12] In this study, the effect of antibacterial activity and fluoride release of several tooth-colored restorative materials containing fluoride including resin-modified glass ionomer (Fuji II LC), giomer, zirconomer, and Cention-N were examined due to the important role of fluoride in the prevention of caries and the introduction of new fluoride-releasing tooth-colored restorative materials.
MATERIALS AND METHODS
In this experimental in vitro study was approve in research and ethics committee of Isfahan (NO: 397755), 80 samples of the test materials (4 groups of 20) including resin-reinforced glass ionomer (Fuji II LC), Alkasite (Cention N), zirconomer, and giomer (Beautifil) were prepared according to the manufacturer's instructions [Table 1]. In each group, 10 samples were used for fluoride testing and 10 samples for antibacterial activity.
Table 1.
Materials used in the study
| Product | Type | Manufacturer | Composition | Shade | Powder/liquid | Instruction for use |
|---|---|---|---|---|---|---|
| Fuji II LC | RMGI | GC Corporation, Tokyo, Japan | 2-hydroxyethyl methacrylate, Polyacrylic acid and water. 58 weight % fluoro-aluminumsilicate | A2 | 1:1 | Mix the required amount of cement. Working time is 3 min 45 s from the startof mixing at 23°C(73.4°F) Light cure for 20 s using a visible light curing device(470 nm wavelength) |
| Cention N | Alkalisite | Ivoclar Vivadent USA | Liquid: UDMA DCP-Tetramethyl-xylylene-Di UDMA(aromatic aliphatic-UDMA) diisocyanates. PEG-400 DMA Powder: Filler barium aluminum silicate glass. Ytterbium trifluoride. Isofiller(tetric N-Ceram technology). Calcium barium aluminum fluorosilicate glass. Calcium fluoro silicate glass | A2 | 1:1 | Two measuring spoons of powder and 2 drops of resin of Cention N(Ivoclar Vivadent) apply to a mixing pad and mixed manually to a smooth consistency. The mixing time did not exceed 60 s. The material was left for 10min from the start of mixing or light cure for 40 s |
| Zirconomer | Zirconia reinforced GI | Shofo Dental Corporation, Japan | Powder: Alumino-fluoro-silicate glass, zirconium oxide, tartaric acid Liquid: Polyacrylic acid, deionized water | A2 | 2:1 | Divide the dispensed powder into 2 equal portions; first half to the dispensedliquid and mix for 510sec. with the plastic spatula provided. Then, add the remaining half and mix until it reaches a thick putty-like consistency. Mixing must be completed within a total of 30 s |
| Beautifil | Giomer | Shofo Dental Corporation Japan USA | Bis-GMA, UDMA, Bis-MPEPP, TEGDMA. 83.3 weight % fluoro-silicate glass | A2 | - | Each layer should not be>2 mm, and light cure for 20 s |
GI: Glass-ionomer, GIC: GI cements, RMGIC: Resin-modified GIC, UDMA: Urethane dimethacrylate, DCP: Tricyclodecan-dimethanol dimethacrylate, PEG-400 DMA: Polyethylene glycol 400 dimethacrylate, Bis-GMA: Bisphenol A-glycidyl methacrylate, Bis-MEPP: Bisphenole A ethoxylate dimethacrylate, TEGDMA: Triethylene glycol dimethacrylate, GC: Company name, LC: Light cure
Preparation of samples
According to the manufacturer's instructions, the test materials were prepared in plastic cylindrical molds with a diameter of 4 mm and a height of 2 mm while the upper and lower surfaces of the samples were covered with Mylar strip and glass slides. The chemically curing materials were placed at room temperature for 10 min. Light cure materials (Beautifil and Fuji II LC) were cured from the top, bottom, and sides for 20 s for a total of 80 s using a light-curing device (ultradent VALO, 1000 mw/cm2).
Measuring fluoride release
The set samples were taken out of the mold and immersed in 3 ml of distilled water in a polyethylene vial and kept in an incubator with 95% relative humidity at 37°C for 24 h. Fluoride measurements were performed on days 1st, 3rd, 7th, 14th, and 21st using a fluoride ion-selective electrode (Wood Road, Tollesbury, Essex CM9 8SJ U. K.) attached to an ion-selective electrode meter. The instrument was graded according to the manufacturer's instructions using six standard fluoride solutions containing 0.20, 1.00, 2.00, 10.00, 20.00, and 100 ppm fluoride, sequentially. After shaking the containers containing the sample, 1 ml of distilled water per vial was removed and was diluted with 0.1 ml of total ionic strength adjustment buffer. This solution was added to maintain the background ionic strength constant, fluoride decomposition, and PH adjustment. Then, the concentration of fluoride in each solution was measured and recorded in ppm. After each fluoride measurement, the samples were washed and dried in 50 ml of distilled water using ultrasonics for 1 min and immersed in a vial containing 3 ml of freshly distilled water.[13]
Determination of antibacterial activity
The samples were examined using direct contact test on a 96-hole microplate. Direct contact test was performed based on the determination of microbial growth turbidity in microplates using the ELISA method. Optional species of S. Mutants were grown in brain heart infusion (BHI) agar medium. Microorganisms were cultivated secondarily in a suitable cultivation environment and under gaseous conditions to confirm their purity. Optional species were inoculated individually into a tube containing 5 ml of sterile saline phosphate buffer. The suspension with a thickness of 0.5 Mac Farland was prepared by a spectrophotometer with a wavelength of 630 nm. The prepared samples were placed on a plate. 10 μl (approximately 107) of Mutans 0.5 Mac Farland were added to each sample and incubated for 1e h at 37°C. The suspension liquid was evaporated to ensure direct contact between the surface of the test specimens and the Mutates bacterium. Then, 245 μl of sterile BHI medium was added to each well and the plates were gently mixed for 2 min. 15 μl of the bacterial suspension was transferred from the wells to other wells containing fresh BHI (215 μl) and then they were mixed for 2 min. Bacterial growth kinetics were calculated by a spectrophotometer with a wavelength of 630 nm and read by the ELISA method with a microplate reader 800 TS. Densitometer was read every hour for 5 h and three times on days 1st, 2nd, and 7th. Then, the average of three readings of light absorption at different times was calculated and its curve was drawn.[14]
Statistical analysis
Mean and standard deviation values of fluoride release and optical density were calculated. Due to the interaction of time factor and type of material, it was not possible to use two-way ANOVA instead of one-way repeated measure ANOVA. Therefore, data were statistically analyzed by repeated measure one-way ANOVA and for pair comparison between different materials in different times Tamhane post hoc test was done. For intergroup comparison in different times repeated measure one-way ANOVA followed by paired t-test was done. Statistical analysis was performed using SPSS software version 22 (IBM, Somers, NJ, USA) at a significant level of (P < 0.05).
RESULTS
Repeated measure one-way ANOVA test showed a significant difference in fluoride release on different days and substances (P < 0.001). The maximum cumulative fluoride release on days 1-7 was related to zirconomer, followed by Cention N, Fuji II LC, and Beautifil, sequentially, and the minimum was related to Beautifil, which remained stable until day 21 [Figure 1].
Figure 1.
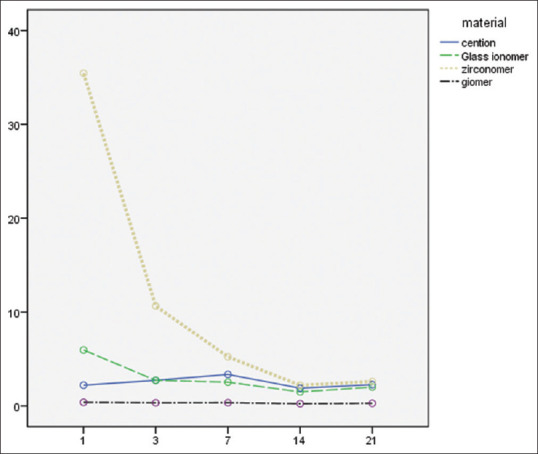
The amount of fluoride released to PPM from the test material over 21 days.
Zirconomer showed an intense decrease in fluoride release during the measurement period. On day 14th, it was approximately equal to the amount of fluoride released from Cention N and Fuji II LC (Zirconomer = 2.20±0.49; Cention N = 1.89 ± 1.07; Fuji II LC = 1.49 ± 0.18).
Tamhane test showed that there is a significant difference between the fluoride release from the study materials at different times (all P < 0.05). Exceptions, in this case, were between Cention N and Fuji II LC on days 3, 7, 14; and 21 and between Fuji II LC and zirconomer on days 14 and 21. Paired t-test showed a significant difference between the zirconomer group and the Fuji II LC group among all days. Moreover, in Cention N group, except between days 1 and 14 (P = 0.299), 1 and 21 (P = 0.89), 3 and 21 (P = 0.214), and in Beautifil group except between days 1 and 3 (P = 0.13), 1 and 7 (P = 0.17), 3 and 7 (P = 0.57), 3 and 21 (P = 0.10), significant differences were reported among the other days [Table 2].
Table 2.
Mean and standard deviation of fluoride release of experimental restorative materials in (ppm) on days 1, 3, 7, 14 and 21
| Materials/days | Mean±SD | ||||
|---|---|---|---|---|---|
|
| |||||
| 1st | 3rd | 7th | 14th | 21th | |
| Cention | 2.21±0.35 | 2.72±0.74 | 3.35±1.44 | 1.89±1.07 | 2.26±1.41 |
| GI | 5.96±1.05 | 2.72±0.42 | 2.53±0.31 | 1.49±0.18 | 2.00±0.18 |
| Zirconomer | 35.45±5.34 | 10.65±3.06 | 5.23±1.33 | 2.20±0.49 | 2.60±0.53 |
| Giomer | 0.38±0.10 | 0.33±0.11 | 0.34±0.09 | 0.24±0.04 | 0.28±0.02 |
| Total | 11.00±14.67 | 4.10±4.23 | 2.86±2.02 | 1.45±0.95 | 1.78±1.16 |
GI: Glass-ionomer, SD: Standard deviation
The tested materials had a statistically significant difference in light absorption as an indicator of antibacterial activity (P < 0.001). After 48 h, Beautifil had the maximum light absorption and zirconomer had the lowest absorption. Zirconomer light absorption, unlike other materials, increased on the 7th day, but the minimum light absorption was related to zirconomer [Figure 2 and Table 3].
Figure 2.
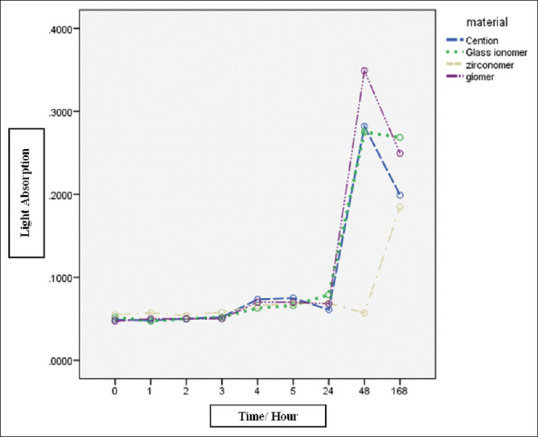
Light absorption of experimental materials in nanometers at different times.
Table 3.
Mean and standard deviation of light absorption of experimental restorative materials cultured in (nanometers) at different times
| Material/times | Hour 0 | Hour 1 | Hour 2 | Hour 3 | Hour 4 | Hour 5 | Hour 24 | Hour 48 | 7thday |
|---|---|---|---|---|---|---|---|---|---|
| Cention | 0.04±0.001 | 0.04±0.001 | 0.05±0.002 | 0.05±0.001 | 0.07±0.001 | 0.07±0.001 | 0.06±0.001 | 0.28±0.08 | 0.19±0.04 |
| GI | 0.05±0.002 | 0.04±0.001 | 0.05±0.002 | 0.05±0.001 | 0.06±0.001 | 0.06±0.001 | 0.07±0.001 | 0.27±0.03 | 0.26±0.04 |
| Zirconomer | 0.05±0.001 | 0.05±0.001 | 0.05±0.001 | 0.05±0.001 | 0.06±0.001 | 0.06±0.001 | 0.06±0.001 | 0.05±0.001 | 0.18±0.05 |
| Giomer | 0.04±0.001 | 0.05±0.001 | 0.05±0.001 | 0.05±0.001 | 0.07±0.001 | 0.07±0.001 | 0.06±0.001 | 0.34±0.03 | 0.24±0.02 |
GI: Glass-ionomer
DISCUSSION
Caries is a biofilm-dependent dental disease that increases with sucrose intake. The bacterial composition of dental biofilm is relatively constant when exposed to small changes in the oral environment. When increased sucrose intake occurs, the formation and evolution of cariogenic biofilms occur due to the growth and dominance of S. mutans. Environmental changes shift toward demineralization. Although other acidogenic and uric acid bacteria are involved in the caries process, S. mutans is the most important bacterium involved. S. mutans is very acidogenic and resistant to acid.[15,16] One solution to reduce the number and severity of caries is to use fluoride-releasing restorative materials.[17] Therefore, in this study, the amount of antibacterial activity and that of fluoride release in new fluoride-releasing restorative materials were investigated.
The amount of fluoride released from these substances was calculated over 21 days. Various factors such as temperature, powder/liquid ratio, mixing time, setting time and porosity affect the rate of fluoride release. In this study, the powder to liquid ratio and mixing method were in accordance with the manufacturer's instructions for each material. In addition, the temperature was determined for all the studied materials and all samples were kept in the incubator at 37°C during the experiment.
In this investigation, distilled water was preferred to artificial saliva due to its high viscosity and the presence of ions in artificial saliva as a storage medium. These ions can affect the release of fluoride ions from restorative materials. Therefore, they lead to a wrong assessment of the released fluoride ions. The storage medium was changed every 24 h due to the possibility of saturation of the released fluoride ions in the medium, which interferes with the additional release of fluoride ions.[6,7]
According to the results of this study, the release of fluoride from four substances showed a statistically significant difference (P > 0.001). The maximum mean of fluoride released during the days 1st, 3rd, and 7th belonged to the zirconomer group and the minimum for the giomer group. The amount of fluoride released by the zirconomer showed an intense decrease during the measurement period and on day 14th, it was approximately equal to the amount of fluoride released from the Cention N and the resin-modified glass ionomer. Throughout the measurement period, the amount of Giomer fluoride release was reported to be almost constant and minimal.
In this study, similar to the study conducted by Tiwari et al., zirconomer had the highest fluoride release and the lowest light absorption (highest antibacterial effect).[8] In this study, zirconomer had a higher fluoride release rate and antibacterial activity than the resin-modified glass ionomer, indicating that differences in antibacterial activity may be related to different fluoride release rates. Some studies have concluded that the amount of fluoride released is associated with antibacterial activity,[8] which confirms this hypothesis. The results of this study also revealed that not only did the rate of fluoride release in Cention N increase on day 7th and was higher than on other days compared to Fuji II LC, but also the amount of light absorption decreased, and this result can confirm the direct relationship between fluoride emission and antibacterial activity. Fluoride release from the material is important due to the formation of fluorapatites as well as the anti-caries property that can prevent the formation of microorganisms.[2,10] The high level of fluoride release from the zirconomer can be explained by its chemical composition, physical properties, and mixing consistency (powder to liquid ratio of 8:1).[8] The reason for the rapid release pattern of fluoride from the zirconomer may be due to the fine glass particles. Various studies have reported that small fine glass particles have a larger surface area for the acid-base reaction. Therefore, it increases the ability to quickly release fluoride from restorative materials.[11,12] According to the results of other studies, the explosive fluoride release was in Fuji II LC.[18] In this study, the zircomer showed a higher explosive release of fluoride than the Fuji II LC. Moreover, the presence of HEMA in Fuji II LC causes it to gradually absorb water to release fluoride ions.[19]
There was a significant difference in the amount of fluoride released on different days and substances, but in all groups, the amount of fluoride released almost decreased over time. The maximum amount of cumulative fluoride release on days 1–7 was related to zirconomer, followed by Cention N, Fuji II LC, and Beautifil, sequentially, and this order remained constant until day 21st. Throughout the measurement period, the giomer fluoride release rate was almost constant and minimal. The fluoride ion content decreased over time. According to other studies by Kiran and Hegde,[20] Neelakantan et al.[21] and Cardoso et al.[22] who compared GIC with various restorative materials, the effect of initial surface wash or the effect of surface wash-off and explosive release of fluoride may lead to a high initial fluoride content, and a rapid fall over the next few days that is probably due only to slow and continuous diffusion through cement pores, fractures, and mass diffusion.[23,24]
Cention N fillers include barium aluminum-silicate glass, ytterbium trifluoride, an isofiller (Tetric N-Ceram technology), a calcium barium aluminum fluorosilicate glass filler and a calcium fluorosilicate (alkaline) glass. Of this amount, 78.4% of the filler material, only 24.6% of the final restorative material, is responsible for the release of fluoride ions. Moreover, the filler materials in Cention-N are surface modified, so they are resistant to degradation and can lead to the release of small amounts of fluoride ions. GIC has a thick layer of 300 nm silica gel on its surface which increases its thickness after absorbing water. While in Cention-N, due to the formation of calcium fluoride and calcium phosphate, a surface layer with a thickness of 0.5 μm is seen that is resistant to washing with deionized water.[25]
The source of fluoride in giomer is surface prereacted glass ionomer (PRG). Giomer lacks or has a small amount of glass ionomer matrix phase. For this reason, a significant amount of acid-base reaction is not observed in it. Since PRG is prereacted with acid, the acid-based giomer reaction is not critical.[26,27] Another possible explanation for the difference between the release rate of fluoride giomer and glass ionomer is the presence of porosity, which may have a large effect on fluoride release. The porosity in the giomer is less than that in the glass ionomer, resulting in less fluoride release. Giomer also contains resin materials that act as a barrier against the fluoride release and water and it has a filler with variable solubility.[28,29] Many studies have been conducted on the fluoride release from different restorative materials from the past to the present that have had different results.[30,31,32] One of the advantages of GICs that makes them desirable restorative materials is their ability to release fluoride and their antibacterial properties due to their low pH during setting.[33,34] As mentioned in studies, fluoride has a cariogenic property that can inhibit the formation of microorganisms.[10] To prevent caries, the amount of fluoride and its release from the restorative material should remain high without changing the physical properties of the restoration. A high level of fluoride release (also called initial burst) adjacent to the restoration reduces the viability of the bacteria. Therefore, it prevents tooth decay by re-stimulating enamel/dentin remineralization.[35] However, continuous release of fluoride by GICs prevents secondary caries.[29,30] Fuji II LC cement has antibacterial activity against S. mutans, Salivaris and Uralis. Nakajo et al. studied the inhibitory effect of glass ionomer cements on the production of acid by Streptococcus bacteria. They found that the F-released from these cements inhibited the glucose metabolism of S. mutans at concentrations lower than those required for bacteriostatic and bactericidal effects. The obtained variable results can be attributed to the experimental protocols and sample size, storage medium, storage environment change frequency, and the amount of media used to measure fluoride levels.[36] In this study, the fluoride-containing preservative solution was distilled water, similar to the study carried out by Garoushi et al.[18] because it absorbs fluoride more rapidly than artificial saliva. Therefore, in this study, the amount of the released fluoride could not be as much as the amount released from the substance in the oral environment. The oral environment is dynamic and different from in vitro conditions. Despite the importance of laboratory studies to answer short-term questions, the actual performance of restorative materials can only be determined by long-term clinical studies. Therefore, more clinical trial studies with more parameters are recommended to evaluate these characteristics in in vivo environmental conditions.
CONCLUSION
Fluoride is clearly known as an anti-caries agent, and fluoride release is an important part of restorative materials. In this study, zirconomer had the highest fluoride release rate by day 14 and was approximately equal to Fuji II LC and Cention N by day 14. giomer had the lowest fluoride release rate throughout all the period. In 48 h after setting, giomer had minimum and zirconomer had maximum antibacterial effects. The antibacterial effect of zirconomer decreased on the 7th day, unlike other substances, but still, the maximum antibacterial effect was related to zirconomer.
Financial support and sponsorship
This study was conducted as thesis for the degree of specialization in Operative Dentistry (#397755). Dentistry school, Isfahan University of Medical Sciences, support the study.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Sun L, Yan Z, Duan Y, Zhang J, Liu B. Improvement of the mechanical, tribological and antibacterial properties of glass ionomer cements by fluorinated graphene. Dent Mater. 2018;34:e115–27. doi: 10.1016/j.dental.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Berg JH. Glass ionomer cements. Pediatr Dent. 2002;24:430–8. [PubMed] [Google Scholar]
- 3.Maldonado A, Swartz ML, Phillips RW. An in vitro study of certain properties of a glass ionomer cement. J Am Dent Assoc. 1978;96:785–91. doi: 10.14219/jada.archive.1978.0195. [DOI] [PubMed] [Google Scholar]
- 4.Marquis RE, Clock SA, Mota-Meira M. Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol Rev. 2003;26:493–510. doi: 10.1111/j.1574-6976.2003.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 5.Koo H. Strategies to enhance the biological effects of fluoride on dental biofilms. Adv Dent Res. 2008;20:17–21. doi: 10.1177/154407370802000105. [DOI] [PubMed] [Google Scholar]
- 6.Gupta N, Jaiswal S, Nikhil V, Gupta S, Jha P, Bansal P. Comparison of fluoride ion release and alkalizing potential of a new bulk-fill alkasite. J Conserv Dent. 2019;22:296–9. doi: 10.4103/JCD.JCD_74_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mungara J, Philip J, Joseph E, Rajendran S, Elangovan A, Selvaraju G. Comparative evaluation of fluoride release and recharge of pre-reacted glass ionomer composite and nano-ionomeric glass ionomer with daily fluoride exposure: An in vitro study. J Indian Soc Pedod Prev Dent. 2013;31:234–9. doi: 10.4103/0970-4388.121820. [DOI] [PubMed] [Google Scholar]
- 8.Tiwari S, Kenchappa M, Bhayya D, Gupta S, Saxena S, Satyarth S, et al. Antibacterial activity and fluoride release of glass-ionomer cement, compomer and zirconia reinforced glass-ionomer cement. J Clin Diagn Res. 2016;10:ZC90–3. doi: 10.7860/JCDR/2016/16282.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva Neto RD. University of Sao Paulo; 2019. Evaluation of the antimicrobial activity of temporary restorative materials: agar diffusion test, biofilm and root canal microorganism quantification submitted to in situ. [Google Scholar]
- 10.Hicks MJ, Flaitz CM. Resin-modified glass-ionomer restorations and in vitro secondary caries formation in coronal enamel. Quintessence Int. 2000;31:570–8. [PubMed] [Google Scholar]
- 11.Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials-fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007;23:343–62. doi: 10.1016/j.dental.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Karantakis P, Helvatjoglou-Antoniades M, Theodoridou-Pahini S, Papadogiannis Y. Fluoride release from three glass ionomers, a compomer, and a composite resin in water, artificial saliva, and lactic acid. Oper Dent. 2000;25:20–5. [PubMed] [Google Scholar]
- 13.Malik S, Ahmed MA, Choudhry Z, Mughal N, Amin M, Lone MA. Fluoride release from glass ionomer cement containing fluoroapatite and hydroxyapatite. J Ayub Med Coll Abbottabad. 2018;30:198–202. [PubMed] [Google Scholar]
- 14.Huang Q, Huang S, Liang X, Qin W, Liu F, Lin Z, et al. The antibacterial, cytotoxic, and flexural properties of a composite resin containing a quaternary ammonium monomer. J Prosthet Dent. 2018;120:609–16. doi: 10.1016/j.prosdent.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Marsh PD. Controlling the oral biofilm with antimicrobials. J Dent. 2010;38(Suppl 1):S11–5. doi: 10.1016/S0300-5712(10)70005-1. [DOI] [PubMed] [Google Scholar]
- 16.Marsh PD, Bradshaw DJ. Physiological approaches to the control of oral biofilms. Adv Dent Res. 1997;11:176–85. doi: 10.1177/08959374970110010901. [DOI] [PubMed] [Google Scholar]
- 17.Pretty IA, Smith PW, Edgar WM, Higham SM. Detection of in vitro demineralization adjacent to restorations using quantitative light induced fluorescence (QLF) Dent Mater. 2003;19:368–74. doi: 10.1016/s0109-5641(02)00079-9. [DOI] [PubMed] [Google Scholar]
- 18.Garoushi S, Vallittu PK, Lassila L. Characterization of fluoride releasing restorative dental materials. Dent Mater J. 2018;37:293–300. doi: 10.4012/dmj.2017-161. [DOI] [PubMed] [Google Scholar]
- 19.Bansal R, Bansal T. A comparative evaluation of the amount of fluoride release and re-release after recharging from aesthetic restorative materials: An in vitro study. J Clin Diagn Res. 2015;9:ZC11–4. doi: 10.7860/JCDR/2015/11926.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiran A, Hegde V. A short term comparitive analysis of fluoride release from a newly introduced Glass Ionomer Cement in deionised water and lactic acid. J Int Oral Health. 2010;2:71–7. [Google Scholar]
- 21.Neelakantan P, John S, Anand S, Sureshbabu N, Subbarao C. Fluoride release from a new glass-ionomer cement. Oper Dent. 2011;36:80–5. doi: 10.2341/10-219-LR. [DOI] [PubMed] [Google Scholar]
- 22.Cardoso AM, de Sousa Leitão A, Neto JC, de Almeida TL, Lima DM, Brandt LM, et al. Evaluation of fluoride release, pH and microhardness of glass ionomer cements. Pesqui Bras Odontopediatria Clin Integr. 2015;15:23–9. [Google Scholar]
- 23.Gandolfi MG, Chersoni S, Acquaviva GL, Piana G, Prati C, Mongiorgi R. Fluoride release and absorption at different pH from glass-ionomer cements. Dent Mater. 2006;22:441–9. doi: 10.1016/j.dental.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 24.Jingarwar MM, Pathak A, Bajwa NK, Sidhu HS. Quantitative assessment of fluoride release and recharge ability of different restorative materials in different media: An in vitro study. J Clin Diagn Res. 2014;8:ZC31–4. doi: 10.7860/JCDR/2014/9985.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Todd J. N. Schaan, Liechtenstein: Ivoclar-Vivadent Press; 2016. Scientific Documentation: Cention; pp. 1–58. [Google Scholar]
- 26.Forsten L. Resin-modified glass ionomer cements: Fluoride release and uptake. Acta Odontol Scand. 1995;53:222–5. doi: 10.3109/00016359509005976. [DOI] [PubMed] [Google Scholar]
- 27.Tay FR, Pashley EL, Huang C, Hashimoto M, Sano H, Smales RJ, et al. The glass-ionomer phase in resin-based restorative materials. J Dent Res. 2001;80:1808–12. doi: 10.1177/00220345010800090701. [DOI] [PubMed] [Google Scholar]
- 28.Itota T, Carrick TE, Rusby S, Al-Naimi OT, Yoshiyama M, McCabe JF. Determination of fluoride ions released from resin-based dental materials using ion-selective electrode and ion chromatograph. J Dent. 2004;32:117–22. doi: 10.1016/j.jdent.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn AT, Wilson AD. The dissolution mechanisms of silicate and glass-ionomer dental cements. Biomaterials. 1985;6:378–82. doi: 10.1016/0142-9612(85)90096-1. [DOI] [PubMed] [Google Scholar]
- 30.Francci C, Deaton TG, Arnold RR, Swift EJ, Jr, Perdigão J, Bawden JW. Fluoride release from restorative materials and its effects on dentin demineralization. J Dent Res. 1999;78:1647–54. doi: 10.1177/00220345990780101001. [DOI] [PubMed] [Google Scholar]
- 31.Hicks J, Garcia-Godoy F, Donly K, Flaitz C. Fluoride-releasing restorative materials and secondary caries. J Calif Dent Assoc. 2003;31:229–45. [PubMed] [Google Scholar]
- 32.Momoi Y, McCabe JF. Fluoride release from light-activated glass ionomer restorative cements. Dent Mater. 1993;9:151–4. doi: 10.1016/0109-5641(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 33.Gao W, Smales RJ, Yip HK. Demineralisation and remineralisation of dentine caries, and the role of glass-ionomer cements. Int Dent J. 2000;50:51–6. doi: 10.1111/j.1875-595x.2000.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 34.Vermeersch G, Leloup G, Delmée M, Vreven J. Antibacterial activity of glass-ionomer cements, compomers and resin composites: Relationship between acidity and material setting phase. J Oral Rehabil. 2005;32:368–74. doi: 10.1111/j.1365-2842.2004.01300.x. [DOI] [PubMed] [Google Scholar]
- 35.Freedman R, Diefenderfer KE. Effects of daily fluoride exposures on fluoride release by glass ionomer-based restoratives. Oper Dent. 2003;28:178–85. [PubMed] [Google Scholar]
- 36.Nakajo K, Imazato S, Takahashi Y, Kiba W, Ebisu S, Takahashi N. Fluoride released from glass-ionomer cement is responsible to inhibit the acid production of caries-related oral streptococci. Dent Mater. 2009;25:703–8. doi: 10.1016/j.dental.2008.10.014. [DOI] [PubMed] [Google Scholar]


