Abstract
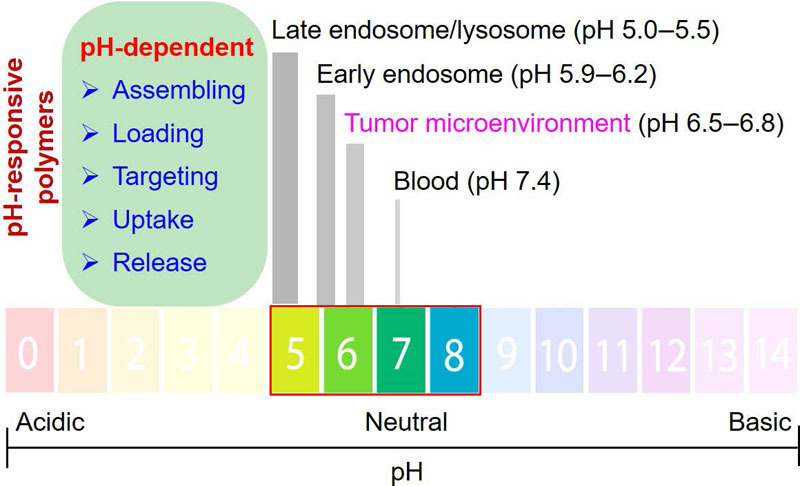
Stimuli-responsive polymers are promising to achieve targeted delivery, improved stability during circulation, and controlled release of therapeutic and diagnostic agents. Among them, pH-responsive polymeric nanocarriers have attracted significant attention as pH varies in different body fluids (e.g., stomach, intestine, and colon) and intracellular organelles (e.g., endosome, lysosome, and mitochondria) to maintain homeostasis, while distinctive pH changes are also found in certain pathological states. For example, the extracellular environment of the tumor is acidic, which can be employed to drive selective delivery. During the internalization process, since most nanocarriers enter cells upon endocytosis where a drop of pH from 6.5 to 5.0 can occur from endosome to lysosome, pH-sensitive groups have been developed for enhanced cargo release. In this review, both non-covalent and covalent interactions responsive to pH changes are introduced, with a focus on the structure-property relationship and their applications in cancer targeting and endosomal escape.
Keywords: cancer targeting, pH-responsive, structure-property relationship, charge shifting, acid-labile linkage
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (No. 81903574) and the Joint Funds of the Zhejiang Provincial Natural Science Foundation of China (No. LTZ22B020001). We are also grateful for the financial support from Zhejiang University.
References
- [1].Park H, Saravanakumar G, Kim J, Lim J, Kim W J. Tumor microenvironment sensitive nanocarriers for bioimaging and therapeutics. Adv. Healthc. Mater. 2021;10:2000834. doi: 10.1002/adhm.202000834. [DOI] [PubMed] [Google Scholar]
- [2].Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013;42:1147–1235. doi: 10.1039/C2CS35265F. [DOI] [PubMed] [Google Scholar]
- [3].Cabral H, Miyata K, Osada K, Kataoka K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018;118:6844–6892. doi: 10.1021/acs.chemrev.8b00199. [DOI] [PubMed] [Google Scholar]
- [4].Sur S, Rathore A, Dave V, Reddy K R, Chouhan R S, Sadhu V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects. 2019;20:100397. doi: 10.1016/j.nanoso.2019.100397. [DOI] [Google Scholar]
- [5].Ruiz A L, Ramirez A, McEnnis K. Single and multiple stimuli-responsive polymer particles for controlled drug delivery. Pharmaceutics. 2022;14:421. doi: 10.3390/pharmaceutics14020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Reineke T M, Raines R T, Rotello V M. Delivery of proteins and nucleic acids: Achievements and challenges. Bioconjug. Chem. 2019;30:261–262. doi: 10.1021/acs.bioconjchem.9b00096. [DOI] [PubMed] [Google Scholar]
- [7].Shi J J, Kantoff P W, Wooster R, Farokhzad O C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang J, Li Y Y, Nie G J. Multifunctional biomolecule nanostructures for cancer therapy. Nat. Rev. Mater. 2021;6:766–783. doi: 10.1038/s41578-021-00315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bobo D, Robinson K J, Islam J, Thurecht K J, Corrie S R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016;33:2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- [10].Danquah M K, Zhang X A, Mahato R I. Extravasation of polymeric nanomedicines across tumor vasculature. Adv. Drug. Deliv. Rev. 2011;63:623–639. doi: 10.1016/j.addr.2010.11.005. [DOI] [PubMed] [Google Scholar]
- [11].Li J J, Kataoka K. Chemo-physical strategies to advance the in vivo functionality of targeted nanomedicine: The next generation. J. Am. Chem. Soc. 2021;143:538–559. doi: 10.1021/jacs.0c09029. [DOI] [PubMed] [Google Scholar]
- [12].AlSawaftah N M, Awad N S, Pitt W G, Husseini G A. pH-responsive nanocarriers in cancer therapy. Polymers. 2022;14:936. doi: 10.3390/polym14050936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dai Y L, Xu C, Sun X L, Chen X Y. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem. Soc. Rev. 2017;46:3830–3852. doi: 10.1039/C6CS00592F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wei M L, Gao Y F, Li X, Serpe M J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017;8:127–143. doi: 10.1039/C6PY01585A. [DOI] [Google Scholar]
- [15].Karimi M, Ghasemi A, Sahandi Zangabad P, Rahighi R, Moosavi Basri S M, Mirshekari H, Amiri M, Shafaei Pishabad Z, Aslani A, Bozorgomid M, et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2011;45:1457–1501. doi: 10.1039/C5CS00798D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cheng R, Meng F H, Deng C, Klok H A, Zhong Z Y. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 2013;34:3647–3657. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
- [17].Xue Y F, Bai H, Peng B, Fang B, Baell J, Li L, Huang W, Voelcker N H. Stimulus-cleavable chemistry in the field of controlled drug delivery. Chem. Soc. Rev. 2021;50:4872–4931. doi: 10.1039/D0CS01061H. [DOI] [PubMed] [Google Scholar]
- [18].Cao Z W, Li W, Liu R, Li X, Li H, Liu L L, Chen Y W, Lv C, Liu Y Y. pH- and enzyme-triggered drug release as an important process in the design of anti-tumor drug delivery systems. Biomed. Pharmacother. 2019;118:109340. doi: 10.1016/j.biopha.2019.109340. [DOI] [PubMed] [Google Scholar]
- [19].Chen H Y, Guo Q, Chu Y C, Li C, Zhang Y W, Liu P X, Zhao Z H, Wang Y, Luo Y F, Zhou Z, et al. Smart hypoxia-responsive transformable and charge-reversible nanoparticles for the deep penetration and tumor microenvironment modulation of pancreatic cancer. Biomaterials. 2022;287:121599. doi: 10.1016/j.biomaterials.2022.121599. [DOI] [PubMed] [Google Scholar]
- [20].Hsieh M H, Wang T H, Hu S H, Hsu T C, Yow J L, Tzang B S, Chiang W H. Tumor site-specific PEG detachment and active tumor homing of therapeutic PEGylated chitosan/folate-decorated polydopamine nanoparticles to augment antitumor efficacy of photothermal/chemo combination therapy. Chem. Eng. J. 2022;446:137243. doi: 10.1016/j.cej.2022.137243. [DOI] [Google Scholar]
- [21].Wang, W. L.; Shen, Q.; Cai, H.; Wang, L. C.; Shao, J. J.; Wang, W. J.; Dong, X. C. Light-responsive organic artificial enzymes: Material designs and bio-applications. Nano Res., in press, 10.1007/s12274-022-4735-2.
- [22].Vander Heiden M G, Cantley L C, Thompson C B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu W, Luo L, Wang Y, Wu Q, Dai H B, Li J S, Durkan C, Wang N, Wang G X. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics. 2018;8:3038–3058. doi: 10.7150/thno.23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sun Q M, Zhu Y Q, Du J Z. Recent progress on charge-reversal polymeric nanocarriers for cancer treatments. Biomed. Mater. 2021;16:042010. doi: 10.1088/1748-605X/abffb5. [DOI] [PubMed] [Google Scholar]
- [25].Deirram N, Zhang C H, Kermaniyan S S, Johnston A P R, Such G K. pH-responsive polymer nanoparticles for drug delivery. Macromol. Rapid Commun. 2019;40:1800917. doi: 10.1002/marc.201800917. [DOI] [PubMed] [Google Scholar]
- [26].Kocak G, Tuncer C, Bütün V. pH-responsive polymers. Polym. Chem. 2017;8:144–176. doi: 10.1039/C6PY01872F. [DOI] [Google Scholar]
- [27].Ratemi E. pH-responsive polymers for drug delivery applications. In: Makhlouf A S H, Abu-Thabit N Y, editors. Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications, Volume 1. Cambridge: Woodhead Publishing; 2018. pp. 121–141. [Google Scholar]
- [28].Wang Y G, Zhou K J, Huang G, Hensley C, Huang X N, Ma X P, Zhao T, Sumer B D, DeBerardinis R J, Gao J M. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat. Mater. 2014;13:204–212. doi: 10.1038/nmat3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou K J, Wang Y G, Huang X N, Luby-Phelps K, Sumer B D, Gao J M. Tunable, ultrasensitive pH-responsive nanoparticles targeting specific endocytic organelles in living cells. Angew. Chem., Int. Ed. 2011;50:6109–6114. doi: 10.1002/anie.201100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Smith S A, Selby L I, Johnston A P R, Such G K. The endosomal escape of nanoparticles: Toward more efficient cellular delivery. Bioconjug. Chem. 2019;30:263–272. doi: 10.1021/acs.bioconjchem.8b00732. [DOI] [PubMed] [Google Scholar]
- [31].Zhou Q, Shao S Q, Wang J Q, Xu C H, Xiang J J, Piao Y, Zhou Z X, Yu Q S, Tang J B, Liu X R, et al. Enzyme-activatable polymer-drug conjugate augments tumour penetration and treatment efficacy. Nat. Nanotechnol. 2019;14:799–809. doi: 10.1038/s41565-019-0485-z. [DOI] [PubMed] [Google Scholar]
- [32].Chu S L, Shi X L, Tian Y, Gao F X. pH-responsive polymer nanomaterials for tumor therapy. Front. Oncol. 2022;12:855019. doi: 10.3389/fonc.2022.855019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang P, Chen D Y, Li L, Sun K X. Charge reversal nano-systems for tumor therapy. J. Nanobiotechnol. 2022;20:31. doi: 10.1186/s12951-021-01221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li S X, Bennett Z T, Sumer B D, Gao J M. Nano-immune-engineering approaches to advance cancer immunotherapy: Lessons from ultra-pH-sensitive nanoparticles. Acc. Chem. Res. 2020;53:2546–2557. doi: 10.1021/acs.accounts.0c00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dai S, Ravi P, Tam K C. pH-responsive polymers: Synthesis, properties and applications. Soft Matter. 2008;4:435–449. doi: 10.1039/b714741d. [DOI] [PubMed] [Google Scholar]
- [36].Hu J M, Zhang G Y, Ge Z S, Liu S Y. Stimuli-responsive tertiary amine methacrylate-based block copolymers: Synthesis, supramolecular self-assembly and functional applications. Prog. Polym. Sci. 2014;39:1096–1143. doi: 10.1016/j.progpolymsci.2013.10.006. [DOI] [Google Scholar]
- [37].Lackey C A, Press O W, Hoffman A S, Stayton P S. A biomimetic pH-responsive polymer directs endosomal release and intracellular delivery of an endocytosed antibody complex. Bioconjug. Chem. 2002;13:996–1001. doi: 10.1021/bc010053l. [DOI] [PubMed] [Google Scholar]
- [38].Qiu L Y, Bae Y H. Polymer architecture and drug delivery. Pharm. Res. 2006;23:1–30. doi: 10.1007/s11095-005-9046-2. [DOI] [PubMed] [Google Scholar]
- [39].De R, Mahata M K, Kim K T. Structure-based varieties of polymeric nanocarriers and influences of their physicochemical properties on drug delivery profiles. Adv. Sci. 2022;9:2105373. doi: 10.1002/advs.202105373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Peng Y, Zhu X M, Qiu L Y. Electroneutral composite polymersomes self-assembled by amphiphilic polyphosphazenes for effective miR-200c in vivo delivery to inhibit drug resistant lung cancer. Biomaterials. 2016;106:1–12. doi: 10.1016/j.biomaterials.2016.08.001. [DOI] [PubMed] [Google Scholar]
- [41].Bera A, Singh Chandel A K, Uday Kumar C, Jewrajka S K. Degradable/cytocompatible and pH responsive amphiphilic conetwork gels based on agarose-graft copolymers and polycaprolactone. J. Mater. Chem. B. 2015;3:8548–8557. doi: 10.1039/C5TB01251A. [DOI] [PubMed] [Google Scholar]
- [42].Liu C Y, Wan T, Wang H, Zhang S, Ping Y, Cheng Y Y. A boronic acid-rich dendrimer with robust and unprecedented efficiency for cytosolic protein delivery and CRISPR-Cas9 gene editing. Sci. Adv. 2019;5:eaaw8922. doi: 10.1126/sciadv.aaw8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mai Y Y, Eisenberg A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012;41:5969–5985. doi: 10.1039/c2cs35115c. [DOI] [PubMed] [Google Scholar]
- [44].Khan R U, Wang L, Yu H J, Zain-ul-Abdin, Akram M, Wu J L, Haroon M, Ullah R S, Deng Z, Xia X. Poly(organo)phosphazenes: Recent progress in the synthesis and applications in tissue engineering and drug delivery. Russ. Chem. Rev. 2018;87:109–150. doi: 10.1070/RCR4757. [DOI] [Google Scholar]
- [45].Wiradharma N, Zhang Y, Venkataraman S, Hedrick J L, Yang Y Y. Self-assembled polymer nanostructures for delivery of anticancer therapeutics. Nano Today. 2009;4:302–317. doi: 10.1016/j.nantod.2009.06.001. [DOI] [Google Scholar]
- [46].Stylianopoulos T, Jain R K. Design considerations for nanotherapeutics in oncology. Nanomed. Nanotechnol. Biol. Med. 2015;11:1893–1907. doi: 10.1016/j.nano.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gao M H, Peng Y, Jiang L M, Qiu L Y. Effective intracellular delivery and Th1 immune response induced by ovalbumin loaded in pH-responsive polyphosphazene polymersomes. Nanomed.:Nanotechnol. Biol. Med. 2018;14:1609–1618. doi: 10.1016/j.nano.2018.04.001. [DOI] [PubMed] [Google Scholar]
- [48].Zhang M Z, Chen X X, Li C, Shen X. Charge-reversal nanocarriers: An emerging paradigm for smart cancer nanomedicine. J. Control. Release. 2020;319:46–62. doi: 10.1016/j.jconrel.2019.12.024. [DOI] [PubMed] [Google Scholar]
- [49].Ahmed M. Peptides, polypeptides and peptide-polymer hybrids as nucleic acid carriers. Biomater. Sci. 2017;5:2188–2211. doi: 10.1039/C7BM00584A. [DOI] [PubMed] [Google Scholar]
- [50].Blackman L D, Gunatillake P A, Cass P, Locock K E S. An introduction to zwitterionic polymer behavior and applications in solution and at surfaces. Chem. Soc. Rev. 2019;48:757–770. doi: 10.1039/C8CS00508G. [DOI] [PubMed] [Google Scholar]
- [51].Zheng L C, Sundaram H S, Wei Z Y, Li C C, Yuan Z F. Applications of zwitterionic polymers. React. Funct. Polym. 2017;118:51–61. doi: 10.1016/j.reactfunctpolym.2017.07.006. [DOI] [Google Scholar]
- [52].Erfani A, Seaberg J, Aichele C P, Ramsey J D. Interactions between biomolecules and zwitterionic moieties: A review. Biomacromolecules. 2020;21:2557–2573. doi: 10.1021/acs.biomac.0c00497. [DOI] [PubMed] [Google Scholar]
- [53].Kim J O, Kabanov A V, Bronich T K. Polymer micelles with cross-linked polyanion core for delivery of a cationic drug doxorubicin. J. Control. Release. 2009;138:197–204. doi: 10.1016/j.jconrel.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Evans B C, Fletcher R B, Kilchrist K V, Dailing E A, Mukalel A J, Colazo J M, Oliver M, Cheung-Flynn J, Brophy C M, Tierney J W, et al. An anionic, endosome-escaping polymer to potentiate intracellular delivery of cationic peptides, biomacromolecules, and nanoparticles. Nat. Commun. 2019;10:5012. doi: 10.1038/s41467-019-12906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Xu X D, Wu J, Liu Y L, Saw P E, Tao W, Yu M, Zope H, Si M, Victorious A, Rasmussen J, et al. Multifunctional envelope-type siRNA delivery nanoparticle platform for prostate cancer therapy. ACS Nano. 2017;11:2618–2627. doi: 10.1021/acsnano.6b07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Xu X D, Wu J, Liu Y L, Yu M, Zhao L L, Zhu X, Bhasin S, Li Q, Ha E, Shi J J, et al. Ultra-pH-responsive and tumor-penetrating nanoplatform for targeted siRNA delivery with robust anti-cancer efficacy. Angew. Chem., Int. Ed. 2016;55:7091–7094. doi: 10.1002/anie.201601273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yuan P Y, Yang F, Liew S S, Yan J C, Dong X, Wang J F, Du S B, Mao X, Gao L Q, Yao S Q. Intracellular Co-delivery of native antibody and siRNA for combination therapy by using biodegradable silica nanocapsules. Biomaterials. 2022;281:121376. doi: 10.1016/j.biomaterials.2022.121376. [DOI] [PubMed] [Google Scholar]
- [58].Wang Y G, Wang C S, Li Y, Huang G, Zhao T, Ma X P, Wang Z H, Sumer B D, White M A, Gao J M. Digitization of endocytic pH by hybrid ultra-pH-sensitive nanoprobes at single-organelle resolution. Adv. Mater. 2017;29:1603794. doi: 10.1002/adma.201603794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhou Y, Han S Y, Liang Z Q, Zhao M, Liu G T, Wu J. Progress in arginine-based gene delivery systems. J. Mater. Chem. B. 2020;8:5564–5577. doi: 10.1039/D0TB00498G. [DOI] [PubMed] [Google Scholar]
- [60].Pan Z C, Yang G X, Yuan J F, Pan M W, Li J H, Tan H. Effect of the disulfide bond and polyethylene glycol on the degradation and biophysicochemical properties of polyurethane micelles. Biomater. Sci. 2022;10:794–807. doi: 10.1039/D1BM01422F. [DOI] [PubMed] [Google Scholar]
- [61].Khan R U, Yu H J, Wang L, Zhang Q, Xiong W, Zain-ul-Abdin, Nazir A, Fahad S, Chen X, Elsharaarani T. Synthesis of polyorganophosphazenes and preparation of their polymersomes for reductive/acidic dual-responsive anticancer drugs release. J. Mater. Sci. 2020;55:8264–8284. doi: 10.1007/s10853-020-04595-6. [DOI] [Google Scholar]
- [62].Martinez A P, Qamar B, Fuerst T R, Muro S, Andrianov A K. Biodegradable “smart” polyphosphazenes with intrinsic multifunctionality as intracellular protein delivery vehicles. Biomacromolecules. 2017;18:2000–2011. doi: 10.1021/acs.biomac.7b00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Qian L H, Fu J Q, Yuan P Y, Du S B, Huang W, Li L, Yao S Q. Intracellular delivery of native proteins facilitated by cell-penetrating poly(disulfide)s. Angew. Chem., Int. Ed. 2018;57:1532–1536. doi: 10.1002/anie.201711651. [DOI] [PubMed] [Google Scholar]
- [64].Fu S W, Rempson C M, Puche V, Zhao B W, Zhang F W. Construction of disulfide containing redox-responsive polymeric nanomedicine. Methods. 2022;199:67–79. doi: 10.1016/j.ymeth.2021.12.011. [DOI] [PubMed] [Google Scholar]
- [65].Tan X, Zhou H, Wang C H, Liu X H, Yang X L, Liu W. GSH-responsive camptothecin prodrug-based hybrid micellar nanoparticles enable antitumor chemo-immunotherapy by PD-L1 knockdown. Nano Res. 2023;16:834–848. doi: 10.1007/s12274-022-4739-y. [DOI] [Google Scholar]
- [66].Feng Q, Wilhelm J, Gao J M. Transistor-like ultra-pH-sensitive polymeric nanoparticles. Acc. Chem. Res. 2019;52:1485–1495. doi: 10.1021/acs.accounts.9b00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhang P S, Meng J L, Li Y Y, Wang Z H, Hou Y. pH-sensitive ratiometric fluorescent probe for evaluation of tumor treatments. Materials. 2019;12:1632. doi: 10.3390/ma12101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhao R Y, Fu C H, Wang Z H, Pan M J, Ma B, Yin Q Q, Chen B L, Liu J X, Xia H M, Wan F J, et al. A pH-responsive nanoparticle library with precise pH tunability by Co-polymerization with non-ionizable monomers. Angew. Chem., Int. Ed. 2022;61:e202200152. doi: 10.1002/anie.202200152. [DOI] [PubMed] [Google Scholar]
- [69].Qian Z Q, Dougherty P G, Pei D H. Monitoring the cytosolic entry of cell-penetrating peptides using a pH-sensitive fluorophore. Chem. Commun. 2015;51:2162–2165. doi: 10.1039/C4CC09441G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Luo M, Wang H, Wang Z H, Cai H C, Lu Z G, Li Y, Du M J, Huang G, Wang C S, Chen X, et al. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017;12:648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kitano H, Nagaoka K, Tada S, Gemmei-Ide M. Structure of water in the vicinity of amphoteric polymers as revealed by Raman spectroscopy. J. Colloid Interface Sci. 2007;313:461–468. doi: 10.1016/j.jcis.2007.05.009. [DOI] [PubMed] [Google Scholar]
- [72].Harijan M, Singh M. Zwitterionic polymers in drug delivery: A review. J. Mol. Recognit. 2022;35:e2944. doi: 10.1002/jmr.2944. [DOI] [PubMed] [Google Scholar]
- [73].Song Z, Ma Y H, Chen M, Ambrosi A, Ding C F, Luo X L. Electrochemical biosensor with enhanced antifouling capability for COVID-19 nucleic acid detection in complex biological media. Anal. Chem. 2021;93:5963–5971. doi: 10.1021/acs.analchem.1c00724. [DOI] [PubMed] [Google Scholar]
- [74].Arvizo R R, Miranda O R, Moyano D F, Walden C A, Giri K, Bhattacharya R, Robertson J D, Rotello V M, Reid J M, Mukherjee P. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PLoS One. 2011;6:e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wang Y Q, Huang D, Wang X, Yang F, Shen H, Wu D C. Fabrication of zwitterionic and pH-responsive polyacetal dendrimers for anticancer drug delivery. Biomater. Sci. 2019;7:3238–3248. doi: 10.1039/C9BM00606K. [DOI] [PubMed] [Google Scholar]
- [76].Yuan Y Y, Mao C Q, Du X J, Du J Z, Wang F, Wang J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv. Mater. 2012;24:5476–5480. doi: 10.1002/adma.201202296. [DOI] [PubMed] [Google Scholar]
- [77].Han L, Liu C Y, Qi H Z, Zhou J H, Wen J, Wu D, Xu D, Qin M, Ren J, Wang Q, et al. Systemic delivery of monoclonal antibodies to the central nervous system for brain tumor therapy. Adv. Mater. 2019;31:1805697. doi: 10.1002/adma.201805697. [DOI] [PubMed] [Google Scholar]
- [78].Han X J, Alu A, Liu H M, Shi Y, Wei X W, Cai L L, Wei Y Q. Biomaterial-assisted biotherapy: A brief review of biomaterials used in drug delivery, vaccine development, gene therapy, and stem cell therapy. Bioact. Mater. 2022;17:29–48. doi: 10.1016/j.bioactmat.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mizuhara T, Saha K, Moyano D F, Kim C S, Yan B, Kim Y K, Rotello V M. Acylsulfonamide-functionalized zwitterionic gold nanoparticles for enhanced cellular uptake at tumor pH. Angew. Chem., Int. Ed. 2015;54:6567–6570. doi: 10.1002/anie.201411615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Behzadi S, Serpooshan V, Tao W, Hamaly M A, Alkawareek M Y, Dreaden E C, Brown D, Alkilany A M, Farokhzad O C, Mahmoudi M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017;46:4218–4244. doi: 10.1039/C6CS00636A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wang S, Zhang F W, Yu G C, Wang Z T, Jacobson O, Ma Y, Tian R, Deng H Z, Yang W J, Chen Z Y, et al. Zwitterionic-to-cationic charge conversion polyprodrug nanomedicine for enhanced drug delivery. Theranostics. 2020;10:6629–6637. doi: 10.7150/thno.47849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zhou J H, Wu Y D, Wang C R, Cheng Q, Han S C, Wang X X, Zhang J H, Deng L D, Zhao D Y, Du L L, et al. pH-sensitive nanomicelles for high-efficiency sirna delivery in vitro and in vivo: An insight into the design of polycations with robust cytosolic release. Nano Lett. 2011;16:6916–6923. doi: 10.1021/acs.nanolett.6b02915. [DOI] [PubMed] [Google Scholar]
- [83].Cui P F, Zhuang W R, Hu X, Xing L, Yu R Y, Qiao J B, He Y J, Li F Y, Ling D S, Jiang H L. A new strategy for hydrophobic drug delivery using a hydrophilic polymer equipped with stacking units. Chem. Commun. 2018;54:8218–8221. doi: 10.1039/C8CC04363A. [DOI] [PubMed] [Google Scholar]
- [84].MacFarlane L R, Shaikh H, Garcia-Hernandez J D, Vespa M, Fukui T, Manners I. Functional nanoparticles through π-conjugated polymer self-assembly. Nat. Rev. Mater. 2021;6:7–26. doi: 10.1038/s41578-020-00233-4. [DOI] [Google Scholar]
- [85].Kong Y L, Zeng K, Zhang Y, Shao J N, Yan J Q, Liao J Y, Wang W C, Dai X Y, Weng Q J, Yao S Q, et al. In vivo targeted delivery of antibodies into cancer cells with pH-responsive cell-penetrating poly(disulfide)s. Chem. Commun. 2022;58:1314–1317. doi: 10.1039/D1CC06840G. [DOI] [PubMed] [Google Scholar]
- [86].Li Y C, Pang H S, Guo Z F, Lin L, Dong Y X, Li G, Lu M, Wu C B. Interactions between drugs and polymers influencing hot melt extrusion. J. Pharm. Pharmacol. 2014;66:148–166. doi: 10.1111/jphp.12183. [DOI] [PubMed] [Google Scholar]
- [87].Ling D S, Park W, Park S J, Lu Y, Kim K S, Hackett M J, Kim B H, Yim H, Jeon Y S, Na K, et al. Multifunctional tumor pH-sensitive self-assembled nanoparticles for bimodal imaging and treatment of resistant heterogeneous tumors. J. Am. Chem. Soc. 2014;136:5647–5655. doi: 10.1021/ja4108287. [DOI] [PubMed] [Google Scholar]
- [88].Hu X L, Jazani A M, Oh J K. Recent advances in development of imine-based acid-degradable polymeric nanoassemblies for intracellular drug delivery. Polymer. 2021;230:124024. doi: 10.1016/j.polymer.2021.124024. [DOI] [Google Scholar]
- [89].Kim H, Kim S, Kang S, Song Y, Shin S, Lee S, Kang M, Nam S H, Lee Y. Ring opening metathesis polymerization of bicyclic α, β-unsaturated anhydrides for ready-to-be-grafted polymers having tailored pH-responsive degradability. Angew. Chem., Int. Ed. 2018;57:12468–12472. doi: 10.1002/anie.201806763. [DOI] [PubMed] [Google Scholar]
- [90].Du J Z, Li H J, Wang J. Tumor-acidity-cleavable maleic acid amide (TACMAA): A powerful tool for designing smart nanoparticles to overcome delivery barriers in cancer nanomedicine. Acc. Chem. Res. 2018;51:2848–2856. doi: 10.1021/acs.accounts.8b00195. [DOI] [PubMed] [Google Scholar]
- [91].Yu W Q, Liu R, Zhou Y, Gao H L. Size-tunable strategies for a tumor targeted drug delivery system. ACS Cent. Sci. 2020;6:100–116. doi: 10.1021/acscentsci.9b01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sun C Y, Liu Y, Du J Z, Cao Z T, Xu C F, Wang J. Facile generation of tumor-pH-labile linkage-bridged block copolymers for chemotherapeutic delivery. Angew. Chem., Int. Ed. 2016;55:1010–1014. doi: 10.1002/anie.201509507. [DOI] [PubMed] [Google Scholar]
- [93].Kang S, Kim Y, Song Y, Choi J U, Park E, Choi W, Park J, Lee Y. Comparison of pH-sensitive degradability of maleic acid amide derivatives. Bioorg. Med. Chem. Lett. 2014;24:2364–2367. doi: 10.1016/j.bmcl.2014.03.057. [DOI] [PubMed] [Google Scholar]
- [94].Lee Y, Ishii T, Kim H J, Nishiyama N, Hayakawa Y, Itaka K, Kataoka K. Efficient delivery of bioactive antibodies into the cytoplasm of living cells by charge-conversional polyion complex micelles. Angew. Chem., Int. Ed. 2010;49:2552–2555. doi: 10.1002/anie.200905264. [DOI] [PubMed] [Google Scholar]
- [95].Huang Z Y, Huang L F, Huang Y J, He Y F, Sun X Q, Fu X, Xu X Y, Wei G F, Chen D W, Zhao C S. Phthalocyanine-based coordination polymer nanoparticles for enhanced photodynamic therapy. Nanoscale. 2017;9:15883–15894. doi: 10.1039/C7NR05402E. [DOI] [PubMed] [Google Scholar]
- [96].Du J Z, Sun T M, Song W J, Wu J, Wang J. A tumor-acidity-activated charge-conversional nanogel as an intelligent vehicle for promoted tumoral-cell uptake and drug delivery. Angew. Chem., Int. Ed. 2010;49:3621–3626. doi: 10.1002/anie.200907210. [DOI] [PubMed] [Google Scholar]
- [97].Su S, Wang Y Y, Du F S, Lu H, Li Z C. Dynamic covalent bond-assisted programmed and traceless protein release: High loading nanogel for systemic and cytosolic delivery. Adv. Funct. Mater. 2018;28:1805287. doi: 10.1002/adfm.201805287. [DOI] [Google Scholar]
- [98].Su Z W, Xu Y M, Wang Y, Shi W Q, Han S S, Shuai X T. A pH and reduction dual-sensitive polymeric nanomicelle for tumor microenvironment triggered cellular uptake and controlled intracellular drug release. Biomater. Sci. 2019;7:3821–3831. doi: 10.1039/C9BM00825J. [DOI] [PubMed] [Google Scholar]
- [99].Wang W W, Cheng D, Gong F M, Miao X M, Shuai X T. Design of multifunctional micelle for tumor-targeted intracellular drug release and fluorescent imaging. Adv. Mater. 2012;24:115–120. doi: 10.1002/adma.201104066. [DOI] [PubMed] [Google Scholar]
- [100].Zeng X W, Tao W, Mei L, Huang L Q, Tan C Y, Feng S S. Cholic acid-functionalized nanoparticles of star-shaped PLGA-vitamin E TPGS copolymer for docetaxel delivery to cervical cancer. Biomaterials. 2013;34:6058–6067. doi: 10.1016/j.biomaterials.2013.04.052. [DOI] [PubMed] [Google Scholar]
- [101].Neuvonen K, Fülöp F, Neuvonen H, Koch A, Kleinpeter E, Pihlaja K. Comparison of the electronic structures of imine and hydrazone side-chain functionalities with the aid of 13C and 15N NMR chemical shifts and PM3 calculations. The influence of C=N-substitution on the sensitivity to aromatic substitution. J. Org. Chem. 2003;68:2151–2160. doi: 10.1021/jo020608l. [DOI] [PubMed] [Google Scholar]
- [102].Kalia J, Raines R T. Hydrolytic stability of hydrazones and oximes. Angew. Chem., Int. Ed. 2008;47:7523–7526. doi: 10.1002/anie.200802651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Liu B, Thayumanavan S. Substituent effects on the pH sensitivity of acetals and ketals and their correlation with encapsulation stability in polymeric nanogels. J. Am. Chem. Soc. 2017;139:2306–2317. doi: 10.1021/jacs.6b11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Chauhan V P, Chen I X, Tong R, Ng M R, Martin J D, Naxerova K, Wu M W, Huang P G, Boucher Y, Kohane D S, et al. Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc. Natl. Acad. Sci. USA. 2019;116:10674–10680. doi: 10.1073/pnas.1819889116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Bachelder E M, Beaudette T T, Broaders K E, Dashe J, Fréchet J M J. Acetal-derivatized dextran: An acid-responsive biodegradable material for therapeutic applications. J. Am. Chem. Soc. 2008;130:10494–10495. doi: 10.1021/ja803947s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Bachelder E M, Pino E N, Ainslie K M. Acetalated dextran: A tunable and acid-labile biopolymer with facile synthesis and a range of applications. Chem. Rev. 2017;117:1915–1926. doi: 10.1021/acs.chemrev.6b00532. [DOI] [PubMed] [Google Scholar]
- [107].Braga C B, Perli G, Becher T B, Ornelas C. Biodegradable and pH-responsive acetalated dextran (Ac-Dex) nanoparticles for NIR imaging and controlled delivery of a platinum-based prodrug into cancer cells. Mol. Pharm. 2019;16:2083–2094. doi: 10.1021/acs.molpharmaceut.9b00058. [DOI] [PubMed] [Google Scholar]
- [108].Parrott M C, Finniss M, Luft J C, Pandya A, Gullapalli A, Napier M E, DeSimone J M. Incorporation and controlled release of silyl ether prodrugs from PRINT nanoparticles. J. Am. Chem. Soc. 2012;134:7978–7982. doi: 10.1021/ja301710z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Raj W, Jerczynski K, Rahimi M, Przekora A, Matyjaszewski K, Pietrasik J. Molecular bottlebrush with pH-responsive cleavable bonds as a unimolecular vehicle for anticancer drug delivery. Mater. Sci. Eng.:C. 2021;130:112439. doi: 10.1016/j.msec.2021.112439. [DOI] [PubMed] [Google Scholar]
- [110].Yan Y, Fu J, Wang T F, Lu X Y. Controlled release of silyl ether camptothecin from thiol-ene click chemistry-functionalized mesoporous silica nanoparticles. Acta Biomater. 2017;51:471–478. doi: 10.1016/j.actbio.2017.01.062. [DOI] [PubMed] [Google Scholar]
- [111].Wang Y M, Fan S Y, Xiao D, Xie F, Li W, Zhong W, Zhou X B. Novel silyl ether-based acid-cleavable antibody-MMAE conjugates with appropriate stability and efficacy. Cancers. 2019;11:957. doi: 10.3390/cancers11070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Xu P S, Van Kirk E A, Zhan Y H, Murdoch W J, Radosz M, Shen Y Q. Targeted charge-reversal nanoparticles for nuclear drug delivery. Angew. Chem., Int. Ed. 2007;46:4999–5002. doi: 10.1002/anie.200605254. [DOI] [PubMed] [Google Scholar]
- [113].Kang S, Noh C, Kang H, Shin J Y, Kim S Y, Kim S, Son M G, Park E, Song H K, Shin S, et al. Dynamics and entropy of cyclohexane rings control pH-responsive reactivity. JACS Au. 2021;1:2070–2079. doi: 10.1021/jacsau.1c00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Choy C J, Geruntho J J, Davis A L, Berkman C E. Tunable pH-sensitive linker for controlled release. Bioconjug. Chem. 2016;27:824–830. doi: 10.1021/acs.bioconjchem.6b00027. [DOI] [PubMed] [Google Scholar]
- [115].Roberts D A, Pilgrim B S, Dell T N, Stevens M M. Dynamic pH responsivity of triazole-based self-immolative linkers. Chem. Sci. 2020;11:3713–3718. doi: 10.1039/D0SC00532K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ong S Y, Zhang C Y, Dong X, Yao S Q. Recent advances in polymeric nanoparticles for enhanced fluorescence and photoacoustic imaging. Angew. Chem., Int. Ed. 2021;60:17797–17809. doi: 10.1002/anie.202101964. [DOI] [PubMed] [Google Scholar]
- [117].Lang W J, Liew S S, Wang S Y, Hong D W, Zhu L Q, Du S B, Jiang L Y, Yao S Q, Ge J Y. Cell-penetrating poly(disulfide)-based nanoquenchers (qCPDs) for self-monitoring of intracellular gene delivery. Chem. Commun. 2022;58:1792–1795. doi: 10.1039/D1CC07020G. [DOI] [PubMed] [Google Scholar]
- [118].Du S B, Liew S S, Li L, Yao S Q. Bypassing endocytosis: Direct cytosolic delivery of proteins. J. Am. Chem. Soc. 2018;140:15986–15996. doi: 10.1021/jacs.8b06584. [DOI] [PubMed] [Google Scholar]


