Highlights
-
•
Paroxysmal chest discomfort is a rare symptom of temporal lobe seizure.
-
•
Home video confirmed slight epileptic features associated with chest discomfort.
-
•
The attacks and amygdala enlargement were improved only by antiseizure medication.
-
•
LTVEM will be diagnostic for patients with chest discomfort of epileptic origin.
Keywords: Chest discomfort, Mesial temporal lobe epilepsy, Amygdala enlargement
Abbreviations: CAD, coronary artery disease; LTVEM, long-term video electroencephalography monitoring; EEG, electroencephalography; CAG, coronary angiography; MRI, magnetic resonance imaging; NMDA, N-methyl-D-aspartate receptor; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; LGI-1, leucine-rich glioma-inactivated ptotein-1; CASPAR2, contactin-associated-protein-receptor-2; GABABR, γ-aminobutyric acid-B receptor; mTLE, mesial temporal lobe epilepsy; TLE, temporal lobe epilepsy
Abstract
Chest discomfort is the representative symptom of dangerous coronary artery disease (CAD), but rarely occurs in patients with seizures. We treated a 74-year-old man with right mesial temporal lobe epilepsy and amygdala enlargement, who was initially suspected of CAD and underwent repeated cardiac angiography because of recurrent episodes of paroxysmal chest discomfort starting from 68 years old. He visited an epileptologist and underwent long-term video electroencephalography monitoring (LTVEM), which confirmed right temporal seizure onset during a habitual episodes of “chest discomfort,” stereotyped movement of chest rubbing with the right hand, followed by impaired conscousness. Brain magnetic resonance imaging revealed right amygdala enlargement. The present case emphasizes the importance of the wide range of symptoms, such as chest discomfort, which may associated with epielpsy and result in a delayed diagnosis. LTVEM is useful for diagnosis of epilepsy with unusual seizure semiology by recording ictal EEG changes during chest discomfort.
1. Introduction
Epilepsy is a common chronic brain disease characterized by recurrent epileptic seizures manifest as various symptoms [1]. Epileptic seizures rarely cause paroxysmal chest discomfort [2], [3], [4], usually a key feature of coronary artery disease (CAD). Several brain areas affecting autonomic function such as the mesial amygdalohippocampal region, ventromedial prefrontal cortex, insula, operculum, and anterior cingulate as well as the somatosensory area are potentially responsible areas for chest discomfort [5]. “Circumjacent” symptoms such as impaired awareness, staring, motion arrest, automatisms, and auras are important guides to diagnosing epilepsy. However, these symptoms are not as apparent in elderly patients with epilepsy and often go unnoticed based only on interview. Therefore, objective identification of “circumjacent” symptoms is essential to distinguish epilepsy from other differential diagnoses, including CAD. We experienced a patient who suffered from spells of paroxysmal chest discomfort for 6 years but cardiologic evaluations were normal, and was finally diagnosed with epilepsy based on video recording at home and long-term video-EEG monitoring (LTVEM).
2. Case presentation
A 74-year-old male started to experience recurrent spells of paroxysmal chest discomfort at the age of 68 years old. His past personal and family histories were unremarkable. The episodes were characterized by sudden left chest discomfort lasting approximately 20 s. No other symptoms occurred, including nausea, diaphoresis, and radiation of the discomfort to other parts of the body. The patient did not respond to any family calls during the episode. Family members assumed his lack of response during his episodes was caused by intense fixation on severe chest discomfort.
Physicians suspected CAD or syncope, leading to multiple cardiologic evaluations, including electrocardiography, echocardiography, and coronary angiography (CAG) four times, all of which showed normal findings. Holter electrocardiography recorded the attack, but showed only sinus tachycardia without any arrhythmia, so providing no evidence of cardiogenic syncope. Consequently, the physicians diagnosed somatoform disorder and administered psychotropic medication without effect. In addition, 1.5-T brain magnetic resonance imaging (MRI) performed during routine medical checkups resulted in no abnormal findings at that time.
Six years after the onset, he visited his first neurologist, who recommended video recordings to monitor the episode. The video recorded at home showed that he rubbed his chest with his right hand and had his left upper limb fixed with elbow extended, wrist flexed, and fingers flexed during all his seizures. This stereotypic behavior was recorded during both sleep and wakefulness. The spells typically lasted approximately 20 s. This video evidence suggested possible focal seizure as a differential diagnosis. Routine EEG detected no epileptiform discharges, so he was referred to our department to receive LTVEM.
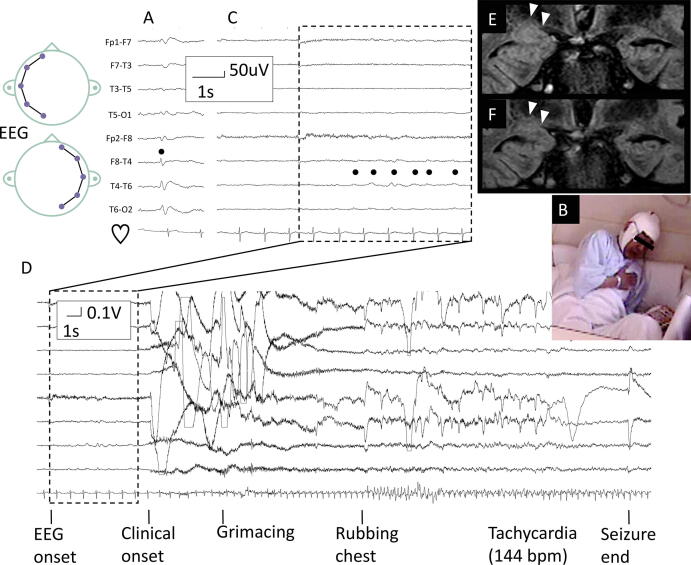
Physical and neurological examinations on admission were unremarkable. LTVEM showed interictal spikes in the right anterior temporal region (Fig. 1A). One habitual spell of chest discomfort was recorded, followed by him rubbing his chest with his right hand (Fig. 1B) accompanied by tachycardia of 144 bpm from the baseline of 65 bpm. He put a bookmark on his book during the seizure but he did not remember this action, indicating impaired awareness. Ictal EEG change started in the right temporal region (Fig. 1C, 1D) 6 s before the clinical onset. 3 T brain MRI showed right amygdala enlargement (Fig. 1E). Examination of the cerebrospinal fluid by indirect immunofluorescence using commercially available kits (Euroimmun, Lübeck, Germany) identified no known autoimmune antibody causing epilepsy, such as antibodies to N-methyl-d-aspartate receptor (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), leucine-rich glioma-inactivated ptotein-1 (LGI-1), contactin-associated-protein-receptor-2 (CASPAR2), and γ-aminobutyric acid-B receptor (GABABR). Therefore, the final diagnosis was right mesial temporal lobe epilepsy (mTLE) with amygdala enlargement. He has been seizure-free until the latest follow-up at 1 year after initiation of 300 mg lacosamide, when MRI showed improvement of the right amygdala enlargement (Fig. 1F).
Fig. 1.
Representative findings of investigations. Longitudinal bipolar montage of electroencephalography (EEG), showing interictal right temporal spikes (A), and right temporal seizure onset (B, C) during paroxysmal chest discomfort associated with chest rubbing with the right hand with sequential consciousness loss (D). Dots represent right anterior temporal interictal spike (A), or ictal seizure onset from the right temporal region (B). Coronal images of fluid-attenuated inversion recovery magnetic resonance imaging taken at admission (E) and one year follow-up after becoming seizure-free (F) showing improvement of the right amygdala enlargement (arrowheads).
3. Discussion
This case report shows that chest discomfort can manifest as the main symptom of temporal lobe epilepsy (TLE). Our patient suffered from both chest discomfort and fear of heart attack, so restricting his daily life for a long time. The correct diagnosis and effective treatment can save such patients from the brink of despair. The present report gives some indications about how to differentiate epilepsy from CAD, in particular we should 1) recognize the semiological variability of epileptic seizures, 2) pay attention to “circumjacent” symptoms, such as dystonic posturing and impaired awareness seen in this case, and 3) conduct evaluations for epilepsy diagnosis (Table 1).
Table 1.
Core clinical features of epileptic chest pain/discomfort compared with cardiac origin.
| Condition | Angina pectoris (AP) | Acute myocardial infarction | Ictal chest pain/discomfort |
|---|---|---|---|
| Origin | Cardiac | Cardiac | Epileptic |
| Duration | 5–15 min | >30 min | Seconds to minutes |
| Difference of pain/discomfort features between episodes | Variable | Variable | Stereotypical |
| Accompanying symptoms | Cold sweat, radiating pain/discomfort | Same as AP | Epileptic symptoms (i.e. epileptic auras, consciousness impairment, generalized seizure) |
In this case, we considered that the diagnosis was delayed due to the following two points. First, chest discomfort or chest pain are rare symptoms of epilepsy [2], [3], [4]. Second, “circumjacent” symptoms including dystonic posturing and impaired awareness were misdiagnosed or even overlooked by the attending physicians. Chest discomfort or chest pain followed by consciousness loss can be caused by CAD and arrhythmia [6], [7] and he did not report repetitive chest rubbing movement of the right hand because these symptoms were interpreted as just pain-relieving action.
Amygdala enlargement may be associated with the diagnosis of TLE without hippocampal sclerosis [8], [9], [10] and may be related to the prognosis [11]. Compared with TLE with hippocampal sclerosis, TLE with amygdala enlargement tends to occur more frequently in the elderly and is less likely to progress from focal to bilateral tonic-clonic seizures [8], which are consistent with our case. Amygdala enlargement may be found in healthy people, but is more frequent in patients with TLE [12]. Enlarged amygdala appeared as isointense to slightly hyperintense on fluid-attenuated inversion recovery images without contrast enhancement [9], so was difficult to detect as in our case. In fact, brain MRI at routine medical checkup in our case was considered to show no abnormalities at initial read, but we could retrospectively confirm amygdala enlargement after suspicion of epilepsy. Improvement of amygdala enlargement associated with seizure control, as found in this patient, may indicate that amygdala volume change is not the primary etiology, but just reflects the uncontrolled seizure condition.
Our case had many symptoms that could be explained with epilepsy, but not CAD. Repetitive chest rubbing motion of the right hand with the “fixed” left hand could be interpreted as contralateral dystonic posturing and ipsilateral automatisms, a lateralizing sign implying right hemispheric onset [13], [14]. Tachycardia could be explained as focal impaired awareness seizure, which sometimes causes cardiac manifestations, including rhythm change [2], especially in mTLE, in which 92.4% of seizures were accompanied by tachycardia [15]. Right mTLE may manifest as prominent symptoms of autonomic seizure, such as earlier tachycardia, compared with left mTLE [16]. The mechanism depends on the dominance of the right cerebral hemisphere in the sympathetic network compared to the left. This hypothesis may explain the manifestations of tachycardia in our patient. Impaired awareness seizure is often identified in the mesial temporal lobe [17]. Therefore, dystonic posturing, tachycardia and impaired awareness may be the key to establishing the correct diagnosis in patients with repetitive chest discomfort but normal CAG findings.
A few cases of epilepsy have presented with chest manifestation as the primary symptom [2], [3], [4]. One patient is the only example of mTLE confirmed with comprehensive epilepsy evaluation [4]. This 44-year-old woman presented with chest pain as the primary manifestation without other accompanying symptoms, including tachycardia. She underwent extensive cardiological examinations for 3 years and was finally referred to the neurology department with suspected epilepsy based on one episode of confusion. LTVEM clarified right temporal seizure onset during chest pain. Together with this previous case [4], our present case indicates a relationship between chest symptoms and mTLE despite some clinical differences such as chest “discomfort” in our case vs “pain” in the previous case, or presence vs absence of other autonomic symptoms. Appearance of ictal chest discomfort may have complex generation mechanisms which may involve not only various cortices such as the mesial amygdalohippocampal region, ventromedial prefrontal cortex, insula, operculum, and anterior cingulate [5] as well as the somatosensory area, but also the network connecting the cortices.
Evaluation for epilepsy diagnosis can be achieved using the LTVEM system in epilepsy centers and by consumer electronic devices such as a home-video camera or smartphone. In the present case, information from home video was the first indication of the diagnosis. Subsequent LTVEM with heart rate monitoring was crucial for establishing the final diagnosis and may be helpful in diagnosing other conditions manifesting with autonomic symptoms or syncope [18]. For example, a 23-year-old woman suffered episodes of consciousness loss triggered with mental stress from age 11 years [18]. The diagnosis remained unknown until LTVEM captured a habitual episode. LTVEM with heart rate monitoring identified cardiac asystole due to atrioventricular block after mental stress, followed by generalized slowing in the EEG, representing the ischemic state of the cortex during syncope. She was finally diagnosed with emotion-triggered neurocardiogenic syncope. LTVEM will allow recording of the targeted event of autonomic symptoms with various data parameters, including video, EEG, electrocardiography, and electromyography. LTVEM even up to 100 h in our institution, was very useful to diagnose epilepsy and exclude other conditions requiring appropriate intervention.
This study has several limitations. First, we could not clarify the possible epileptogenic zones as the patient underwent neither intracranial recording nor resective surgery. Second, this single case does not allow generalization of the knowledge. However, we hope that this report will make physicians aware of similar cases, through which the specific mechanism of TLE with chest discomfort may be clarified in the future.
4. Conclusion
We experienced a patient presenting with repetitive chest discomfort with normal findings on CAG over 6 years who eventually was diagnosed as mTLE with amygdala enlargement. We suggest LTVEM may be diagnostic when brief episodes of chest pain occur in the appropriate clinical context of patients with a structural brain lesion to prevent onging diagnostic and treatment delays.
Ethical statement
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Declaration of competing Interest
N.K. and K.J. have received speaker’s fees from Daiichi-Sankyo, UCB Japan, and Eisai Co., ltd. H.O., T.S., Y.K., K.U, K.K., T.K., and J.F. have nothing to declare.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors thank to Dr. Kimihiko Kaneko, Department of Neurology, Tohoku University Graduate School of Medicine, for his support.
References
- 1.Thijs R.D., Surges R., O'Brien T.J., Sander J.W. Epilepsy in adults. Lancet. 2019;393(10172):689–701. doi: 10.1016/S0140-6736(18)32596-0. [DOI] [PubMed] [Google Scholar]
- 2.Devinsky O., Price B.H., Cohen S.I. Cardiac manifestations of complex partial seizures. Am J Med. 1986;80(2):195–202. doi: 10.1016/0002-9343(86)90009-4. [DOI] [PubMed] [Google Scholar]
- 3.Sureshbabu S., Nayak D., Peter S., Sobhana C., Mittal G. Epileptic angina. Epilepsy Behav Case Rep. 2017;7:49–53. doi: 10.1016/j.ebcr.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoo C.S., Lee D., Park K.M., In Lee B., Kim S.E. A rare but treatable cause of recurrent chest pain–Ictal chest pain. BMC Neurol. 2019;19:348. doi: 10.1186/s12883-019-1575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shorvon S., Tomson T. Sudden unexpected death in epilepsy. Lancet. 2011;378(9808):2028–2038. doi: 10.1016/S0140-6736(11)60176-1. [DOI] [PubMed] [Google Scholar]
- 6.Nishizaki M. Life-threatening arrhythmias leading to syncope in patients with vasospastic angina. J Arrhythmia. 2017;33(6):553–561. doi: 10.1016/j.joa.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igarashi Y., Yamazoe M., Suzuki K., Tamura Y., Matsubara T., Tanabe Y., et al. Possible role of coronary artery spasm in unexplained syncope. Am J Cardiol. 1990;65(11):713–717. doi: 10.1016/0002-9149(90)91376-h. [DOI] [PubMed] [Google Scholar]
- 8.Bower S.P.C., Vogrin S.J., Morris K., Cox I., Murphy M., Kilpatrick C.J., et al. Amygdala volumetry in “imaging-negative” temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2003;74:1245–1249. doi: 10.1136/jnnp.74.9.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsueda-Ono T., Ikeda A., Inouchi M., Takaya S., Matsumoto R., Hanakawa T., et al. Amygdalar enlargement in patients with temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2011;82(6):652–657. doi: 10.1136/jnnp.2010.206342. [DOI] [PubMed] [Google Scholar]
- 10.Chakravarty K., Ray S., Kharbanda P.S., Lal V., Baishya J. Temporal lobe epilepsy with amygdala enlargement: A systematic review. Acta Neurol Scand. 2021;144(3):236–250. doi: 10.1111/ane.13455. [DOI] [PubMed] [Google Scholar]
- 11.Na H.K., Lee H.-J., Hong S.-J., Lee D.H., Kim K.M., Lee H.W., et al. Volume change in amygdala enlargement as a prognostic factor in patients with temporal lobe epilepsy: A longitudinal study. Epilepsia. 2020;61(1):70–80. doi: 10.1111/epi.16400. [DOI] [PubMed] [Google Scholar]
- 12.Reyes A., Thesen T., Kuzniecky R., Devinsky O., McDonald C.R., Jackson G.D., et al. Amygdala enlargement: Temporal lobe epilepsy subtype or nonspecific finding? Epilepsy Res. 2017;132:34–40. doi: 10.1016/j.eplepsyres.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotagal P., Luders H., Morris H.H., Dinner D.S., Wyllie E., Godoy J., et al. Dystonic posturing in complex partial seizures of temporal lobe onset: a new lateralizing sign. Neurology. 1989;39(2) doi: 10.1212/wnl.39.2.196. [DOI] [PubMed] [Google Scholar]
- 14.Tufenkjian K., Lüders H.O. Seizure semiology: its value and limitations in localizing the epileptogenic zone. J Clin Neurol. 2012;8:243–250. doi: 10.3988/jcn.2012.8.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leutmezer F., Schernthaner C., Lurger S., Pötzelberger K., Baumgartner C. Electrocardiographic changes at the onset of epileptic seizures. Epilepsia. 2003;44(3):348–354. doi: 10.1046/j.1528-1157.2003.34702.x. [DOI] [PubMed] [Google Scholar]
- 16.Kato K., Jin K., Itabashi H., Iwasaki M., Kakisaka Y., Aoki M., et al. Earlier tachycardia onset in right than left mesial temporal lobe seizures. Neurology. 2014;83(15):1332–1336. doi: 10.1212/WNL.0000000000000864. [DOI] [PubMed] [Google Scholar]
- 17.Hirfanoglu T., Serdaroglu A., Capraz I., Bilir E., Arhan E.P., Aydin K. Comparison of ILAE 2010 and semiological seizure classification in children with epilepsy. Epilepsy Res. 2017;129:41–50. doi: 10.1016/j.eplepsyres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Kato K., Kakisaka Y., Jin K., Fujikawa M., Nakamura M., Suzuki N., et al. Stressful medical explanation may cause syncope in patients with emotion-triggered neurocardiogenic syncope. Pacing Clin Electrophysiol. 2018;41(1):96–98. doi: 10.1111/pace.13199. [DOI] [PubMed] [Google Scholar]