Abstract
Nutcracker syndrome (NCS) is a rare condition caused by compression of the left renal vein (LRV) between the aorta and superior mesenteric artery. Surgical treatment is reserved for patients with severe symptoms and failure of conservative treatment. A 31-year-old woman diagnosed in adolescence with NCS has had recurrent pain since age 15. For 30 days, she has had severe left flank pain and microhematuria. The patient underwent extravascular stent placement around the LRV with the Da Vinci Xi. In 30 months of follow-up, the patient has no pain and the LRV remained without compressions or thrombosis.
Keywords: Nutcracker syndrome, Extravascular stent, Robot-assisted surgery
Abbreviations: NCS, Nutcracker Syndrome; LRV, Left Renal Vein; PTFE, Polytetrafluoroethylene; CT, Computed Tomography Scan
1. Introduction
Nutcracker syndrome (NCS) is a rare condition caused by extrinsic compression of the left renal vein (LRV) between the aorta and superior mesenteric artery.1 It is most frequent in young females and can cause renal vein thrombosis, left renal hypertension, pelvic congestion symptoms, hematuria and left flank pain.2 Patients with severe symptoms or signs and anatomic compression of the left renal vein were eligible for intervention.3 Up to now, LRV transposition by open surgical intervention has been considered by many experts the standard of care. However, due to the morbidity and complications of this surgery, other minimally invasive alternatives have been developed. We report a case of NCS treated by robotic polytetrafluoroethylene (PTFE) extravascular stent placement around the left renal vein.
2. Case presentation
A 31-year-old female presents with severe and recurrent pain in the left flank for 30 days and microhematuria. Diagnosed at age 15 with NCS by computed tomography scan (CT), she has bouts of abdominal pain every 2 years, lasting 10–15 days. Preoperative CT showed a compression of the LRV between the aorta and superior mesenteric artery with consequent dilatation of the LRV near to the kidney (Fig. 1). The patient underwent extravascular PTFE stent placement around the LRV with the Da Vinci Xi robotic platform.
Fig. 1.
Preoperative computed tomography scan showing compression of the left renal vein between the aorta and superior mesenteric artery (blue arrow). It is also possible to observe the large dilatation of the left renal vein. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
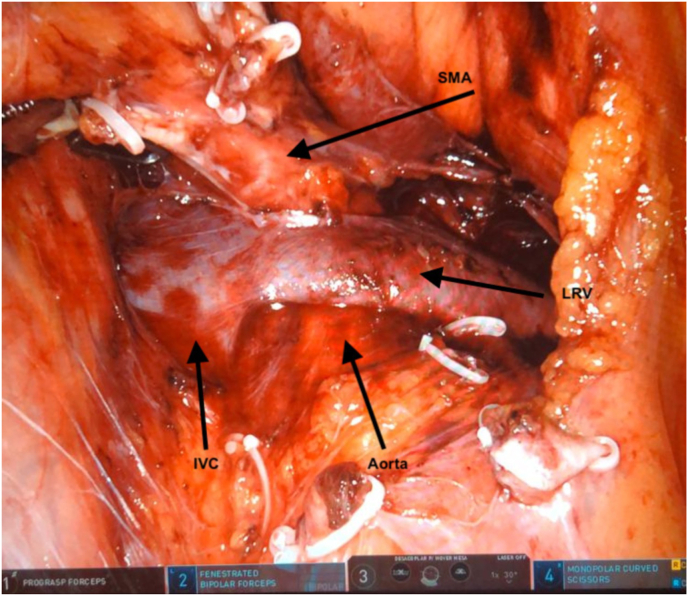
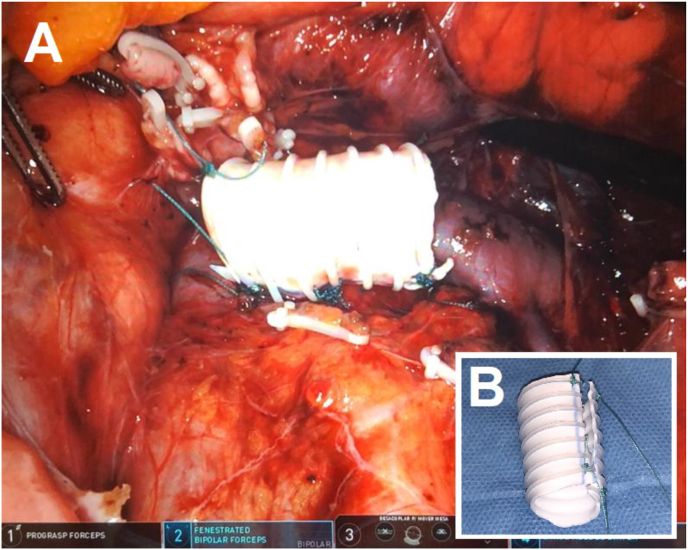
Patient under general anesthesia and supine position with a gel pad on the torso to prevent movement when placed in the Trendelenburg position. After the transperitoneal laparoscopic access, robotic docking and access to the retroperitoneum, the LRV was dissected until its junction with the inferior vena cava (Fig. 2). An externally reinforced PTFE graft was introduced through the trocar and placed over the LRV (Fig. 3A), being firmly fixed on the extravascular surface with mersilene stitches in order to prevent migration (Fig. 3B). The patient was discharged 34 hours after the surgery and presented a progressive decrease in pain until the fifth month of follow-up, since then she has been without pain and hematuria. In two and a half years of follow-up, there was no stent migration and the left renal vein remained patent, without dilatations, compressions or thrombosis.
Fig. 2.
Left renal vein dissected to inferior vena cava junction after colon reflection. IVC = inferior vena cava; LRV = left renal vein; SMA = superior mesenteric artery.
Fig. 3.
A) Stent around the left renal vein with the edges sutured together and fixed to the extravascular surface to prevent migration. B) Longitudinally open ringed polytetrafluoroethylene vascular stent.
3. Discussion
Treatment of NCS depends on the severity of symptoms and duration of illness. Conservative treatment is recommended for patients with mild symptoms, microscopic hematuria, or mild macroscopic hematuria.4 Patients diagnosed under 18 years of age should be observed for 24 months, as physical development and growth of connective and adipose tissue near the origin of the superior mesenteric artery can relieve LRV compression, resulting in spontaneous remission of symptoms in 75% of cases.4
Surgical treatment is indicated when the patient has significant abdominal or low back pain, anemia, autonomic dysfunction, loss of renal function, varicocele, or failure of conservative treatment. Open surgical approaches, for example LRV or superior mesenteric artery transposition, nephropexy and renal auto-transplantation, have been associated with increased morbidity due to extensive dissection and the need for additional anastomoses.4
In this context, minimally invasive approaches, for example, endovascular and extravascular renal vein stents, transluminal balloon angioplasty or bypass, have gained space in the literature.2 However, the rarity of NCS, the lack of randomized preventive studies and the short-term follow-up prevent the definition of the best surgical treatment strategy.
Although some authors argue that renal auto-transplantation is the ideal surgical option for NCS, there is a risk of thrombosis and intractable hematuria above 10%, which can lead to nephrectomy. Mesenteric artery transposition is a surgical treatment option with a low risk of renal venous thrombosis, on the other hand, it has a reintervention rate of 27% and can lead to mesenteric ischemia.5 Although minimally invasive, endovascular treatment of NCS has a stent migration rate of approximately 7% and requires antiplatelet aggregation for 2–3 months.1
In a 2010 case series, Zhang et al. described the laparoscopic placement of an extravascular stent in the LRV in three patients with NCS and macrohematuria.3 Within a mean follow-up of 25 months, two patients had complete resolution of the hematuria, without recurrence, while the last patient had a partial resolution, maintaining a microhematuria after the operation.3 In 2015, in a series of 13 laparoscopic cases, Wang et al. achieved complete resolution of symptoms in 10 patients with a median follow-up of 32 months.2 Among the other three patients, two had partial relief of symptoms and one had a recurrence of the condition after 11 months of surgery due to stent migration.2
In 2019, Wang et al. selected 17 patients with NCS and prepared bespoke titanium extravascular stents that were placed in the LRV laparoscopically.1 In this case series, the macroscopic hematuria and low back pain resolved within 5 days, and the microhematuria, proteinuria, and left varicocele disappeared within 2 weeks.1 During the follow-up period, no stent migration occurred.1 One year later, Steinberg et al. published a series of six cases of robotic extravascular stents.5 All patients had a decrease in pain and there was no recurrence of symptoms within a mean follow-up of 24 days.5 The robot offers an ideal minimally invasive approach to extravascular stent placement in the LRV for the treatment of NCS. Magnified three-dimensional imaging and endowrist instruments make a big difference in dissecting the LRV to the inferior vena cava and suturing the graft to the aortic adventitia to prevent migration.
4. Conclusion
Robotic extravascular stenting in LRV appears to be a safe and effective treatment for NCS, avoiding the morbidity associated with vascular manipulation of conventional approaches. However, prospective randomized studies with long-term follow-up are still needed to define the best surgical approach for NCS.
Section headings
Congenital anomalies and anatomical variants.
Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
João Pádua Manzano participated in research design, in the writing of the paper and in the performance of the research, João Henrique Sendrete de Pinho participated in the writing of the paper and in the performance of the research. Thainã de Oliveira Azambuja participated in the writing of the paper. Claudio Ambrogini participated in the writing of the paper. Paulo Collet Bruna participated in the writing of the paper.
All authors contributed to critical revision of the article and final approval.
All authors declare no support received and no conflict of interest.
Declarations of competing interest
None.
Acknowledgements
None.
Contributor Information
João Pádua Manzano, Email: jmanzano@unifesp.br.
João Henrique Sendrete de Pinho, Email: joaohenriquepinho@gmail.com.
Thainã de Oliveira Azambuja, Email: thainaazambuja@gmail.com.
Claudio Ambrogini, Email: ambrogin@uol.com.br.
Paulo Collet Bruna, Email: pauloc_bruna@yahoo.com.br.
References
- 1.Wang H., Guo Y.T., Jiao Y., et al. A minimally invasive alternative for the treatment of nutcracker syndrome using individualized three-dimensional printed extravascular titanium stents. Chin Med J. 2019;132(12):1454–1460. doi: 10.1097/CM9.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S.Z., Zhang W.X., Meng Q.J., Zhang X.P., Wei J.X., Qiao B.P. Laparoscopic extravascular stent placement for nutcracker syndrome: a report of 13 cases. J Endourol. 2015;29(9):1025–1029. doi: 10.1089/end.2014.0411. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q., Zhang Y., Lou S., Liu F., Ye Z., Zhang D. Laparoscopic extravascular renal vein stent placement for nutcracker syndrome. J Endourol. 2010;24(10):1631–1635. doi: 10.1089/end.2010.0001. [DOI] [PubMed] [Google Scholar]
- 4.Daily R., Matteo J., Loper T., Northup M. Nutcracker syndrome: symptoms of syncope and hypotension improved following endovascular stenting. Vascular. 2012;20(6):337–341. doi: 10.1258/vasc.2011.cr0320. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg R.L., Johnson B.A., Garbens A., Cadeddu J.A. Robotic assisted extravascular stent placement for nutcracker phenomenon of the left renal vein: a case series. J Robot Surg. 2020;14(5):781–788. doi: 10.1007/s11701-020-01054-x. [DOI] [PubMed] [Google Scholar]