Abstract
Lyme borreliosis is a multisystemic disorder primarily affecting the skin, nervous system, and joints. It is caused by the spirochete Borrelia burgdorferi sensu lato and is transmitted via ticks of the Ixodidae family. Persistence of borreliae within macrophages has been implicated in the often chronic history of borreliosis. The uptake of B. burgdorferi by professional phagocytes occurs predominantly by coiling phagocytosis, a host cell-driven process in which single pseudopods wrap around and engulf the spirochetes. In the present study, we investigated the molecular machinery and the signal transduction pathways controlling the formation of these unique uptake structures. We found that the phagocytosis of borreliae by primary human macrophages is accompanied by the formation of f-actin-rich structures, which in their morphological organization correspond well to the earlier described coiling pseudopods. Further experiments revealed that Wiskott-Aldrich Syndrome protein and Arp2/3 complex, major regulators of actin polymerization, are also recruited to these sites of actin accumulation. In addition, inhibition of an upstream regulator of Wiskott-Aldrich Syndrome protein, the Rho-family GTPase CDC42Hs, greatly inhibited the occurrence of borrelia-induced phagocytic uptake structures. Inhibition of Rac1, another Rho family GTPase, had a less-pronounced inhibitory effect, while blocking of Rho activity showed no discernible influence. These results suggest that basic mechanisms of actin polymerization that control other types of phagocytosis are also functional in the formation of the morphologically unique uptake structures in coiling phagocytosis. Our findings should enhance the understanding of the infection process of B. burgdorferi and contribute to devising new strategies for countering Lyme disease.
Lyme borreliosis is the most prevalent tick-borne disease in the northern hemisphere. This multisystemic disorder is clinically characterized by acute and chronic stages, primarily affecting the skin, the nervous system, and the joints (33, 40). The causative agent, the spirochete Borrelia burgdorferi sensu lato (8), is transmitted by ticks of the Ixodidae family (15). Although early stages of the spirochetosis can be well treated by antibiotic therapy, a significant number of patients pass over to chronic stages, even years after infection.
Considering the obvious persistence of borreliae within the human body, a major question concerns the role of macrophages and other professional phagocytes in the survival of borreliae (14, 31). As in the case of other pathogens, borreliae may accomplish this by inducing their uptake into an intracellular compartment that is permissive for survival (29).
Previous studies on the interaction of B. burgdorferi with professional phagocytes (37, 38) have observed the occurrence of coiling phagocytosis, a phenomenon initially described for Legionella pneumophila (19). While in conventional phagocytosis cellular protrusions symmetrically enclose the microorganism, in the case of coiling phagocytosis a single phagocyte pseudopod bends around the bacteria in a hooklike fashion and wraps itself around the spirochete. The phagocytes subsequently engulf this entire phagocytic complex. Coiling phagocytosis is host cell driven, since it has been observed with both live and dead bacteria, and seems to be a specific reaction of the phagocyte to the attachment of certain kinds of particles or microorganisms (39). Despite its peculiarities, coiling phagocytosis is a physiologically relevant process, since the uptake of B. burgdorferi by professional phagocytes takes place preferentially via coiling rather than by conventional phagocytosis (38). Interestingly, it has also been speculated that spirochetes internalized by coiling phagocytosis may undergo intracellular processing distinct from that following conventional phagocytosis (38).
In the present study, we investigate the molecular machinery and the signal transduction pathways involved in coiling phagocytosis of B. burgdorferi by primary human macrophages. We present evidence that this process involves the formation of f-actin-rich structures most likely corresponding to coiled pseudopods and is probably driven by actin-regulatory proteins such as Wiskott-Aldrich Syndrome protein (WASp) (5, 19, 43) and the Arp2/3 complex (26), which are also recruited to these structures. A key integrator of signal transduction events involved in coiling phagocytosis seems to be the small GTPase CDC42Hs, which has a distinct influence on the formation of phagocyte whorls. An influence of Rac1, another small GTPase of the Rho family, was also observed. Therefore, although the mechanical and regulatory mechanisms required for coiling phagocytosis are distinct from other types of phagocytosis, the basic mechanisms of actin polymerization seem to share a high degree of similarity.
MATERIALS AND METHODS
Cultivation of borreliae.
The clone of Borrelia afzelii strain PKo used in this study, PKo97 K37, is a clone derived from the European skin isolate PKo (34), well characterized with regard to antigen expression and infectious in mice. (Note that B. afzelii is the most common human pathogenic species of B. burgdorferi sensu lato in Europe [45].) The borreliae were cultivated for 5 to 7 days at 33°C in modified Kelly Medium (MPK-Medium; 34). At this cultivation temperature >80% of the cells express the two major outer surface proteins OspA and OspC (6, 13, 46). Aliquots were stored frozen in liquid nitrogen after the addition of 10% glycerol to the culture. For experiments, aliquots were thawed and cultivated for 5 to 7 days at 33°C in MPK-Medium. Only highly motile bacteria, harvested in the log phase, were used. Prior to incubation with human macrophages, borreliae were pelleted, counted, and resuspended in RPMI supplemented with 20% human serum (not reactive with B. afzelii strain PKo, tested by enzyme-linked immunosorbent assay, immunofluorescence assay, and whole-cell-lysate immunoblot [17, 44]) for serum opsonization.
Monocyte isolation and cell culture.
Human peripheral blood monocytes were isolated from heparinized blood of healthy donors by centrifugation in Ficoll (Seromed, Munich, Germany) as described previously (25). Briefly, monocytic cells were isolated using magnetic anti-CD14 antibody beads and an MS+ Separation Column (Miltenyi Biotec, Bergisch-Gladbach, Germany) and seeded onto Cellocate glass coverslips (Eppendorf, Westbury, N.Y.) at a density of 5 × 104 cells. Cells were cultured in RPMI 1640 medium (Sigma, Deisenhofen, Germany) containing 20% autologous serum at 37°C, 5% CO2, and 90% humidity. Medium was changed every 3 to 4 days. Prior to each experiment, macrophages were washed twice in RPMI 1640 and incubated in RPMI 1640 supplemented with 20% homologous human serum (negative Lyme serology) for 45 min at 37°C in a humified atmosphere of 5% CO2.
Coincubation of borreliae and macrophages.
Human macrophages (7- to 11-days old) derived from monocytes were infected with B. afzelii PKo97 K37 at a borrelia/cell ratio of 10:1 and incubated at 37°C in a humified atmosphere of 5% CO2. Borrelia cells were added to macrophages in medium-filled dishes and allowed to settle. Contact between borreliae and macrophages was therefore not established immediately and was not synchronized. The inducement of structures was observable as early as 10 min and peaked at 45 min after the addition of borreliae. After the times indicated, nonadherent bacteria were removed by dipping the coverslips five times into RPMI (37°C), followed by fixation in 3.7% formaldehyde solution (Sigma, Deisenhofen, Germany) for 10 min at room temperature.
Constructs and protein expression.
GTPase constructs (V12CDC42Hs, N17CDC42Hs, V12Rac, and N17Rac) were kindly provided by Alan Hall. All constructs, including C3 transferase, were expressed in Escherichia coli as glutathione S-transferase (GST) fusions and thrombin cleaved when indicated. Proteins were dialyzed against microinjection buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM MgCl2), concentrated in Centricon (Amicon, Beverly, Mass.), shock-frozen, and stored at −80°C. The purity was tested by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie staining.
Microinjection of proteins.
Monocyte-derived macrophages were cultured for 7 to 11 days. Microinjection was performed by using a Transjector 5246 (Eppendorf) and a Compic Inject Micromanipulator (Cell Biology Trading, Hamburg, Germany). Proteins were injected into the cytoplasm at 2.7 mg/ml, as GST fusions in the case of the CDC42Hs constructs and as thrombin-cleaved proteins in the case of the Rac1 constructs, and at 7 ng/μl in the case of thrombin-cleaved C3 toxin. Injected cells were identified by labeling coinjected rat immunoglobulin G (IgG; 5 mg/ml; Dianova, Hamburg, Germany) with fluorescein isothiocyanate (FITC)-labeled goat anti-rat IgG (Dianova). Control injections were performed with GST.
Immunofluorescence.
Cells were fixed as described above and permeabilized for 15 min in ice-cold acetone. B. afzelii were visualized by labeling OspA with a specific monoclonal antibody, L22 1F11, which recognizes an epitope conserved among B. burgdorferi sensu lato strains (45), and by using DAPI (4′,6′-diamidino-2-phenylindole; Sigma, Deisenhofen, Germany) for staining of bacterial DNA. f-actin was stained with Alexa 568-labeled phalloidin (Molecular Probes, Eugene, Oreg.), WASp was stained with monoclonal antibody 3D8.H5 (41), CDC42Hs was stained with a polyclonal antibody (3), and Arp2/3 complex was stained with a polyclonal antibody against the p41-Arc subunit (24). Secondary antibodies were cyanine dye 3 (Cy3)-, FITC-, and phycoerytherin-conjugated goat anti-mouse antibodies (Caltag, San Francisco, Calif.). Coverslips were mounted in Dako fluorescent mounting medium (DAKO Corporation, Carpinteria, Calif.) and sealed with nail polish.
Specimens of macrophages infected with borreliae were mostly analyzed using confocal laser scanning microscopy (Leica, Wetzlar, Germany). The “z” views of specimens shown in Fig. 2 and 3 were obtained by processing “xy” slices using the NIH image software package.
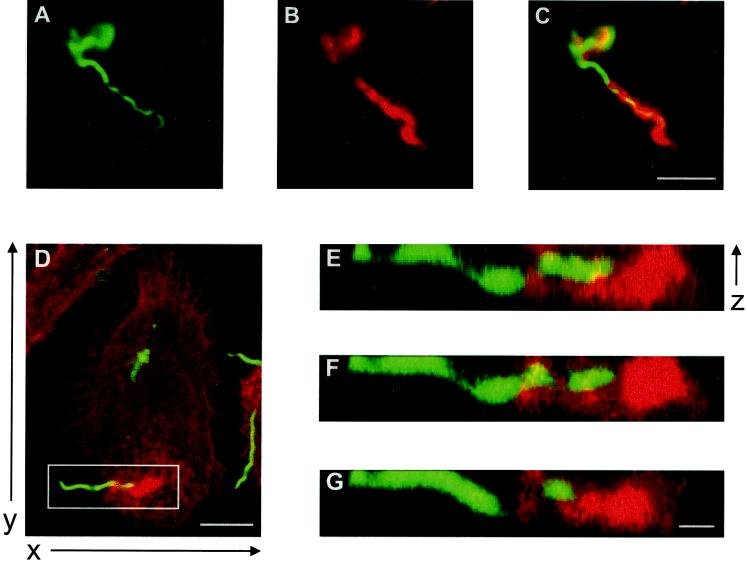
FIG. 2.
Interaction of borreliae with primary human macrophages is associated with the formation of f-actin-rich structures. (A to C) Fluorescence micrographs, obtained 10 min after the addition of borrelia, showing the apical part of the macrophage with the attached spirochete in focus. OspA staining in green (A), actin staining in red (B), and an overlay of images A and B (C). The yellow color indicates colocalization of f-actin accumulation and OspA. (D to G) Overlays of confocal laser scanning micrographs of ventral macrophage parts, obtained 45 min after the addition of borreliae. OspA staining is green; actin staining is red. (D) Superimposition of three horizontal sections (x and y axes are indicated). (E to G) Vertical sections of detail (white box) from panel D (virtual cutting axis parallel to the x axis), showing the association of spirochete with f-actin-rich structure, obtained by vertical sectioning each time of the 16 horizontal sections (The z axis is indicated). The white bars represent 10 μm for all pictures of the same sizes.
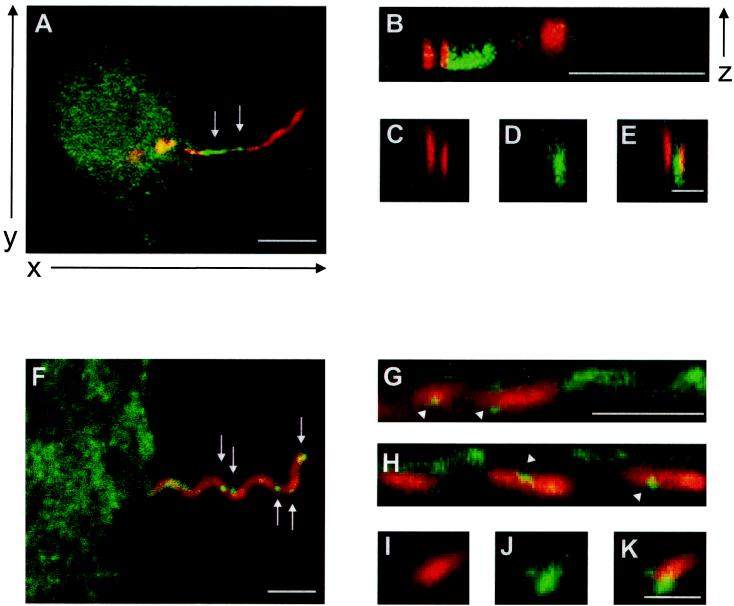
FIG. 3.
Interaction of borreliae with primary human macrophages is associated with the accumulation of WASp and the Arp2/3 complex. (A to E) Confocal laser scanning micrographs, obtained 45 min after the addition of borreliae. OspA staining is red (A, B, C, and E); WASp staining is green (A, B, D, and E). Overlays of OspA and WASp staining (A and B) and an overlay of panels C and D (E) are also shown. (A) Superimposition of three horizontal sections (the x and y axes are indicated); the WASp accumulation is indicated by white arrows. (B to E) Vertical sections of details from panel A, showing association of the spirochete with WASp accumulation, gained by vertical sectioning of each of the 16 horizontal sections (the z axis is indicated), with the virtual cutting axis parallel to the x axis (B) or parallel to the y axis (C to E). The white bars represent 10 μm (A), 5 μm (B), or 0.5 μm (C to E). (F to K) Confocal laser scanning micrographs, with OspA staining in red (F, G, H, I, and K), p41Arc staining in green (F, G, H, J, and K), overlays of OspA and p41Arc staining (F, G, and H), and an overlay of panels I and J (K). (F) Superimposition of three horizontal sections (the x and y axes are as indicated for panel A), with granular Arp2/3 accumulation indicated by the white arrows. (G to K) Vertical sections of details from panel A, showing the association of the spirochete with Arp2/3 accumulation, gained by vertical sectioning of each of the 16 horizontal sections (the z axis is as indicated for panel B), with a virtual cutting axis parallel to the x axis (G and H) or parallel to the y axis (I to K). The white bars represent 5 μm (F to H) or 1 μm (I to K).
For staining of each protein, more than 100 cells of at least three different donors were evaluated. All experiments gave consistent results regarding distribution of f-actin, WASp, and p41-Arc.
Quantification and statistical evaluation.
Values for the formation of f-actin-rich phagocytosis structures were gained by evaluating three times 30 cells microinjected in three separate experiments with the proteins indicated. Mean values and standard deviations (SDs) were determined in relation to GST-injected cells. Mean values for all injected proteins were analyzed by using the Student's t test. A two-tailed P value of <0.05 was considered to be significant.
RESULTS
Interaction of B. burgdorferi with primary human macrophages leads to the formation of f-actin-rich structures.
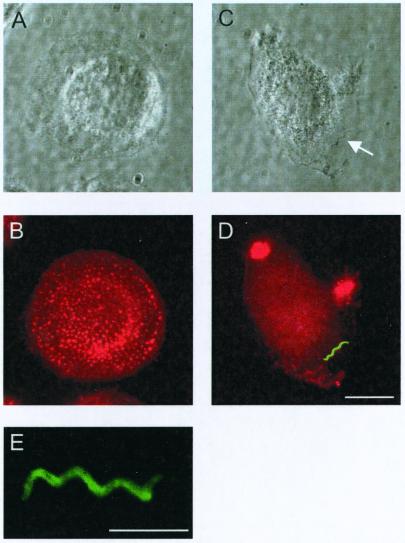
The purpose of our study was to investigate the molecular machinery that organizes actin during coiling phagocytosis of borreliae. For this we infected primary human macrophages with B. burgdorferi spirochetes and visualized the developing uptake structures by fluorescence microscopy. The attachment of spirochetes to macrophages was verified by phase-contrast microscopy (Fig. 1A and C) and was found to be predominantly end-on, as described previously (30). To better discriminate the details of the uptake process, borreliae were for most experiments costained with antibodies against the outer-surface protein OspA (Fig. 1B). The interaction of borreliae with macrophages was found to lead to an extensive alteration of the host cell surface and overall shape, as cells formed numerous ruffles and showed a tendency for contraction. Since remodeling of the cell shape usually involves a rearrangement of the actin cytoskeleton and since phagocytosis is generally thought to require actin polymerization, specimens of borreliae infecting macrophages were stained for f-actin and OspA. In the majority of macrophages (66.1% ± 17.7% of observed; n = 100), massive actin accumulations extending from the host cell surface and enveloping the proximal part of the interacting spirochete cells were observed (one to four structures/macrophage; Fig. 2A to C). Areas with intensive f-actin staining alternated with areas of high OspA fluorescence (Fig. 2C), indicating an uneven envelopment of the borrelia cells.
FIG. 1.
(A to D) Interaction with borreliae induces alterations in cell shape and actin cytoskeleton of primary human macrophages. Seven-day-old macrophages were observed prior to (A and B) and 10 min after (C and D) the addition of borreliae. Phase-contrast (A and C) and fluorescence (B and D) images, f-actin stained with Alexa 568-phalloidin (red) and borrelia stained against OspA (green) are shown. (C) A single spirochete in focus is highlighted by a white arrow. The white bar represents 10 μm. (E) Borrelia cell stained against OspA, as seen on a confocal laser scanning micrograph, with superimposition of four horizontal sections. The white bar represents 5 μm.
Both opsonized and unopsonized borreliae readily attached to macrophages, a finding comparable to that found in previous studies (7). Initial experiments were performed with unopsonized borreliae, i.e., in the absence of serum. However, further experiments revealed that opsonization, either with borrelia-specific antibodies or with serum devoid of B. burgdorferi-specific IgG (see Materials and Methods), enhanced the rate of attachment of borreliae to macrophages when compared to nonopsonized spirochetes (factor 4–5). Additionally, coiling phagocytosis has mainly been described in a context involving complement opsonization (39), and unspecific opsonization by complement mimics more closely the in vivo situation of first-time infection with borreliae. All subsequent experiments were therefore performed using serum-opsonized borreliae. Morphology and protein content of the actin-rich structures as described below was indistinguishable from that gained under IgG-opsonized conditions.
For better resolution, we generated horizontal optical slices by use of a confocal microscope (x and y axes, Fig. 2D). With the aid of an imaging program (see Materials and Methods), stacks of horizontal slices were then assembled and cut perpendicular to the slice plane (z axis; Fig. 2E to G). This allowed us to generate side views of borreliae and to evaluate their spatial relation to the f-actin accumulations of the macrophages. Sequential vertical slices clearly showed that the B. burgdorferi cell body and f-actin-rich folds extending from the macrophage were wrapped around each other, with the most massive actin accumulation occurring at the proximal tip of the spirochete (Fig. 2E to G). Individual coils of the borrelia cell were either deeply buried in the f-actin-rich structure (Fig. 2F) or near the surface of the host cell pseudopod (Fig. 2G), corresponding to the alternate fluorescence intensities of f-actin and OspA as observed in the initial immunofluorescence images (Fig. 2A to C).
In addition to this microscopic evidence, we considered coiling phagocytosis here for several reasons. First, the appearance and disappearance of the f-actin structures corresponds well with a time course of uptake of borreliae by macrophages (not shown). Second, it has been demonstrated that coiling phagocytosis is the predominant phagocytic uptake mechanism of B. burgdorferi by professional phagocytes such as human macrophages (60 to 70% of phagocytosis events) (38). Third, the observed actin accumulations correspond very well to descriptions of coiling pseudopods as shaft-like extensions often more than 15 μm in length that are wrapped around the spirochete cells in a coil-like fashion (39).
WASp and the Arp2/3 complex accumulate at B. burgdorferi cells interacting with macrophages.
In recent years, members of the WASp family of proteins and one of their effectors, the Arp2/3 complex, have emerged as central regulators of f-actin (26; reviewed in reference 18). Participation of the Arp2/3 complex in Fcγ receptor (FcγR)- and complement receptor (CR3)-dependent phagocytosis has been shown, and thus the involvement of WASp-like proteins in these processes can be inferred (28). We therefore asked whether WASp and the Arp2/3 complex may be involved in coiling phagocytosis and in the generation of the observed f-actin-rich structures. For this purpose, we costained specimens of borreliae-infected macrophages for OspA and either WASp (Fig. 3A to E) or p41-Arc, a subunit of the Arp2/3 complex (Fig. 3F to K). As demonstrated in Fig. 3, in side views of the uptake structures, the accumulation of WASp was readily visible, in particular at the proximal tip of the interacting borreliae (Fig. 3A, arrows). Longitudinal (Fig. 3B) or transversal sections (Fig. 3C to E) through the spirochete in Fig. 3A showed an alternation of WASp and OspA staining, a finding similar to the results obtained with f-actin and OspA staining.
Positive staining for p41-Arc suggests that the Arp2/3 complex is also present in the uptake structures induced by borreliae. In contrast to WASp accumulation, which was more uniform, p41-Arc was found in punctate foci along the borreliae (Fig. 3F, arrows). Sequential longitudinal side views suggest that Arp2/3 is also present in loosely associated folds around the B. burgdorferi cells (Fig. 3G and H). Interestingly, the punctate Arp2/3 accumulations seemed to be more closely associated with borreliae (Fig. 3G and H, arrowheads), which could be particularly well demonstrated in transversal sections (Fig. 3I to K).
Altogether, we conclude that WASp and the Arp2/3 complex are recruited to zones of the macrophage where interaction with borreliae takes place, resulting in the observed phenomenon of coiling phagocytosis.
Influence of the small GTPases CDC42Hs, Rac1, and Rho on the formation of B. burgdorferi-induced f-actin-rich structures.
Regulatory pathways influencing actin polymerization are controlled by members of the Rho family of small GTPases (23, 35, 36). In particular, FcγR-mediated phagocytosis and the associated recruitment of Arp2/3 complex to phagosomes have been shown to depend on CDC42Hs and Rac, while CR3-mediated phagocytosis is controlled by Rho (9, 11, 28). According to the current model, CDC42Hs activates the hematopoietic cell-specific WASp, which then activates the actin-polymerizing activity of the Arp2/3 complex (reviewed in reference 32).
We therefore tested whether CDC42Hs, Rac1, or Rho influence the formation of borrelia-induced f-actin-rich structures in macrophages. For this purpose, we microinjected cells with constitutively active (V12CDC42Hs and V12Rac) or inactive (N17CDC42Hs and N17Rac) GTPase mutants or with C3-transferase, an inhibitor of Rho. (Note that microinjection of constitutively active V14Rho led to contraction of macrophages and was therefore not evaluated.) Subsequently, macrophages were infected with a fixed number of borreliae, and the formation of f-actin-rich structures was evaluated.
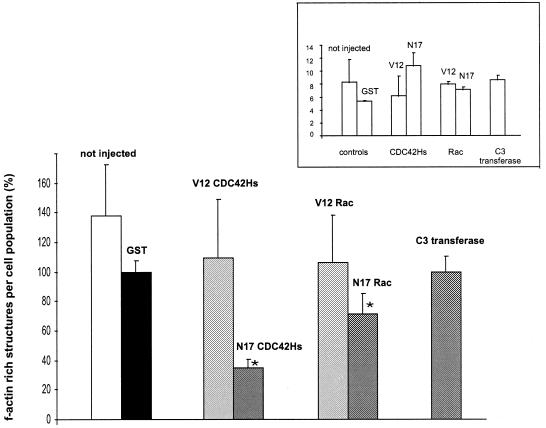
The number (mean ± the SD) of f-actin-rich structures in macrophages injected with GST was set to 100% (±6.2%; Fig. 4), since this control population showed a clear reduction in structure formation compared to uninjected macrophages (138.1% ± 32.9%; Fig. 4), indicating an influence of the microinjection procedure per se. Macrophages injected with constitutively active V12CDC42Hs showed no difference in structure formation compared to GST-injected cells (109.5% ± 38.1%; Fig. 4). Microinjection of constitutively inactive N17CDC42Hs, however, resulted in a marked decrease in structure formation (34.8% ± 5.2%; Fig. 4). A similar tendency emerged for Rac, since macrophages microinjected with constitutively active V12Rac1 yielded values comparable to those of the GST control (106.2% ± 30.5%; Fig. 4), while microinjection of constitutively inactive N17Rac1 resulted in a decrease in structure formation (71.4% ± 13.3%; Fig. 4), although not as pronounced as with the microinjection of N17CDC42Hs. Microinjection of the Rho-inhibitor C3 transferase had no discernible effect on structure formation (100% ± 9.5%; Fig. 4). However, injected macrophages showed a tendency for spreading, which is in line with earlier findings of Rho being a negative regulator of monocyte spreading (2), while at the same time indicating that Rho activity was indeed blocked in these experiments.
FIG. 4.
Formation of borrelia-induced f-actin-rich structures in macrophages is influenced by the small GTPases CDC42Hs and Rac1. Macrophages (7 to 10 days old) were microinjected with the proteins indicated. After 45 min of incubation, cells were infected with B. afzelii, PKo97 K37, at a spirochete/cell ratio of 10:1, washed, and fixed after an additional 45 min of coincubation. The number of borrelia-induced f-actin-rich structures was determined by a triple fluorescence technique (bacterial DNA was stained with DAPI; injected macrophages were stained with anti-rat IgG; f-actin was stained with Alexa 568-phalloidin; see Materials and Methods). Each value represents the mean ± the SD of three independent experiments with 30 cells evaluated per experiment. The mean value of structures formed after the injection of GST was set as 100%. For differences between this standard and values gained for proteins other than GST, a P value of <0.05 was considered to be significant (as indicated by an asterisk). (Inset) Means ± the SD of attached borreliae per macrophage as evaluated for each microinjected protein.
Formation of f-actin-rich structures was subsequently correlated with the number of attached borreliae per macrophage (Fig. 4, inset). Interestingly, the observed reductions in the number of structures upon microinjection of N17CDC42Hs or N17Rac1 were accompanied by higher means of attached borreliae when compared to GST-injected cells (Fig. 4, inset). This clearly indicates that the lower number of f-actin-rich structures in both cases was not due to fewer attached spirochetes but instead was a result of the injected GTPase mutants.
In sum, microinjection of both constitutively inactive N17CDC42Hs and N17Rac1 had a negative effect on the formation of borrelia-induced f-actin accumulations, indicating the involvement of CDC42Hs and Rac1 in coiling phagocytosis of B. burgdorferi by primary human macrophages. Rho activity does not seem to play a part in this process.
DISCUSSION
We investigated here the uptake of B. burgdorferi via coiling phagocytosis by primary human macrophages. We placed particular emphasis on the involvement of the actin polymerization machinery of the host cell in this phenomenon.
Interaction of borreliae with macrophages was associated with the formation of f-actin-rich structures, which resembled rearranged pseudopods extending from the macrophage cell. This is in line with earlier studies wherein localized actin polymerization has been described as the driving force for engulfment in FcγR and CR3-mediated phagocytosis (1, 20). It has been speculated that the generation of unilateral pseudopods, one of the hallmarks of coiling phagocytosis, may result from asymmetrical clustering of receptors at the microbial attachment site and that this possibly applies to CR3-dependent as well as CR-independent conditions (39). Our finding that the observed f-actin-rich structures, which most probably correspond to such unilateral pseudopods, are induced in large numbers (in contrast to nonopsonized conditions) by both, complement- and IgG-opsonized borreliae, fits well with such a model.
We cannot exclude that some of the observed structures are due to uptake processes different from coiling phagocytosis. However, the high incidence of this phenomenon in B. burgdorferi uptake (60 to 70% of phagocytosis events of borreliae by human macrophages) (38) and the microscopic evaluation of a large number of structures (n > 100), most of which showed signs of coiling, should render the statistical error derived from such events negligible.
Recently, a mechanism called tube phagocytosis has been described for the uptake of complement-opsonized borreliae by human neutrophils (42). The eponymous tubes resemble the f-actin-rich structures described here and, ultimately, both phenomena might be different descriptions of the same mechanism. It has to be stressed that so-called tube phagocytosis has only been investigated by use of video-enhanced dark-field microscopy, which has a resolution far below our technique described here.
Entry of B. burgdorferi into macrophages was found to be end-on, as decribed previously (30), with the most massive and probably also initial actin accumulations occurring at the tip of the spirochete contacting the host cell. In this context, it is worth mentioning that spirochete tips have been considered specialized regions, since these are able to bind a variety of host cell factors, for example, plasminogen or fibronectin (12, 21). Binding of plasminogen by B. burgdorferi has also been shown to enhance penetration of endothelial cells (10).
Actin polymerization involves members of the WASp family of proteins and also the actin-nucleating Arp2/3 complex (reviewed in reference 18). Therefore, we went on to look at whether this is also true for coiling phagocytosis of borreliae. Indeed, we could show that both WASp and the Arp2/3 complex are recruited to these borrelia-induced uptake structures, where they presumably induce the observed massive actin accumulations. WASp was mostly present in a uniform distribution, while Arp2/3 complex was also found in the form of distinct dot-like structures very closely associated with the spirochete cell, a finding reminiscent of previously reported punctate foci of Arp2/3 found in CR3-mediated phagocytosis (28).
Particles taken up via FcγR-mediated phagocytosis are engulfed by lamellipodia, which contain more or less uniformely distributed regulatory proteins (4, 28). On the other hand, uptake via CR3-mediated phagocytosis does not involve substantial extensions of the phagocyte surface, while actin-associated proteins such as the Arp2/3 complex are often found in punctate foci at the respective phagosomes (4, 20, 28). Coiling phagocytosis of borreliae by macrophages therefore takes a somewhat intermediate position, since spirochetes are engulfed by huge, although unilateral, pseudopods (39), as in FcγR-mediated phagocytosis, and yet the nucleating Arp2/3 complex is also present in distinct dots in the phagocytosis structure, as described for CR3-mediated phagocytosis (28).
The different properties of all these uptake structures are also reflected by the requirements for different Rho GTPases. FcγR-phagocytosis has been shown to depend on CDC42Hs and Rac1 (9, 27), while CR3-mediated phagocytosis is controlled by Rho (9). At first, this seems to contradict our results of serum-opsonized borreliae taken up via a CDC42Hs- and Rac-controlled pathway. However, the former studies used transfected cell lines as model systems, and requirements for GTPases in phagocytosis by different cell types may be variant. More relevantly, studies in macrophages reported the involvement of CDC42Hs and Rac1 in CR3-dependent phagocytosis (11) and of Rho in FcγR-mediated phagocytosis (16), which corresponds well to our data.
Nascent phagosomes have been likened to podosomes, f-actin-rich adhesion structures of monocytic cells such as macrophages and osteoclasts (4, 39), because of their similar protein content (f-actin, talin, protein kinase C, and others). According to our results, this similarity can now be extended: podosomes in primary human macrophages, the phagocytes used in our study, have recently been shown to contain WASp and the Arp2/3 complex and to be controlled by CDC42Hs (24, 25), similar to the f-actin-rich phagocytic structures described here.
In sum, we show here that coiling phagocytosis of B. burgdorferi by primary human macrophages involves massive actin polymerization along the borrelia-triggered pseudopods. This process is probably driven by WASp and the Arp2/3 complex, which are recruited to the engulfment structures. The formation of these structures, in turn, is regulated by the small GTPases CDC42Hs and Rac, but not by Rho. These findings should enhance our understanding of the infection process of B. burgdorferi and therefore contribute to devising new strategies for countering Lyme disease.
ACKNOWLEDGMENTS
C. Heimerl and S. Linder contributed equally to this work.
We are grateful to Peter C. Weber and Jürgen Heesemann for continuous support, Barbara Böhling for expert technical assistance, Alan Hall for providing the GTPase constructs. David L. Nelson for the gift of the 3D8.H5 antibody, Wolf-Dietrich Hardt for help with computer software, and Michael G. Rittig for scientific support and fruitful discussions.
This work was supported by grants of the Deutsche Forschungsgemeinschaft (Graduiertenkolleg “Infektion und Immunität” to C.H. and B.W., SFB413 to S.L. and M.A., and Ae11 to M.A.) and August Lenz Stiftung.
REFERENCES
- 1.Aderem A, Underhill D M. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 2.Aepfelbacher M, Essler M, Huber E, Czech A, Weber P C. Rho is a negative regulator of human monocyte spreading. J Immunol. 1996;157:5070–5075. [PubMed] [Google Scholar]
- 3.Aepfelbacher M, Vauti F, Weber P C, Glomset J A. Spreading of differentiating human monocytes is associated with a major increase in membrane-bound CDC42. Proc Natl Acad Sci USA. 1994;91:4263–4267. doi: 10.1073/pnas.91.10.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen L A, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J Exp Med. 1996;184:627–637. doi: 10.1084/jem.184.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspenström P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr Biol. 1996;6:70–75. doi: 10.1016/s0960-9822(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 6.Barbour A G, Tessier S L, Todd W J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983;41:795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benach J L, Fleit H B, Habicht G S, Coleman J L, Bosler E M, Lane B P. Interactions of phagocytes with the Lyme disease spirochete: role of the Fc receptor. J Infect Dis. 1984;150:497–507. doi: 10.1093/infdis/150.4.497. [DOI] [PubMed] [Google Scholar]
- 8.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 9.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1921. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 10.Coleman J L, Sellati T J, Testa J E, Kew R R, Furie M B, Benach J L. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect Immun. 1995;63:2478–2484. doi: 10.1128/iai.63.7.2478-2484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox D, Chang P, Zahng Q, Reddy P G, Bokoch G M, Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukosytes. J Exp Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson J R, Ellen R P. Clustering of fibronectin adhesins toward Treponema denticola tips upon contact with immobilized fibronectin. Infect Immun. 1994;62:2214–2221. doi: 10.1128/iai.62.6.2214-2221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fingerle V, Laux H, Munderloh U G, Schulte-Spechtel U, Wilske B. Differential expression of outer surface proteins A and C by individual Borrelia burgdorferi in different genospecies. Med Microbiol Immunol. 2000;189:59–66. doi: 10.1007/pl00008257. [DOI] [PubMed] [Google Scholar]
- 14.Georgilis K, Steere A C, Klempner M S. Infectivity of Borrelia burgdorferi correlates with resistance to elimination by phagocytic cells. J Infect Dis. 1991;163:150–155. doi: 10.1093/infdis/163.1.150. [DOI] [PubMed] [Google Scholar]
- 15.Gern L, Burgdorfer W, Aeschlimann A, Krampitz H E. The ecology of Lyme borreliosis in Europe. In: Weber K, Burgdorfer W, editors. Aspects of Lyme borreliosis. Berlin, Germany: Springer; 1993. pp. 59–69. [Google Scholar]
- 16.Hackam D J, Rotstein O D, Schreiber A, Zhang W J, Grinstein S. Rho is required for the initiation of calcium signaling and phagocytosis by Fcγ receptors in macrophages. J Exp Med. 1997;186:955–966. doi: 10.1084/jem.186.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser U, Lehnert G, Lobentanzer R, Wilske B. Interpretation criteria for standardized Western blots for three European species of Borrelia burgdorferi sensu lato. J Clin Microbiol. 1997;35:1433–1444. doi: 10.1128/jcm.35.6.1433-1444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgs H N, Pollard T D. Regulation of actin polymerization by Arp2/3 complex and WASp/Scar proteins. J Biol Chem. 1999;274:32531–32534. doi: 10.1074/jbc.274.46.32531. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz M A. Phagocytosis of the legionnaires' diseases bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984;36:27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan A, Fischer D, Achord D, Sly W. Phosphohexosyl recognition is a general characteristic of pinocytosis of lysosomal glycosidases by human fibroblasts. J Clin Investig. 1977;60:1088–1093. doi: 10.1172/JCI108860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klempner M S, Noring R, Epstein M P, McCloud B, Hu R, Limentani S A, Rogers R A. Binding of human plasminogen and urokinase-type plasminogen activator to the Lyme disease spirochete, Borrelia burgdorferi. J Infect Dis. 1995;171:1258–1265. doi: 10.1093/infdis/171.5.1258. [DOI] [PubMed] [Google Scholar]
- 22.Kolluri R, Tolias K F, Carpenter C L, Rosen F S, Kirchhausen T. Direct interaction of the Wiskott-Aldrich syndrome protein with the GTPase Cdc42. Proc Natl Acad Sci USA. 1996;93:5615–5618. doi: 10.1073/pnas.93.11.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linder S, Higgs H, Hüfner K, Schwarz K, Pannicke U, Aepfelbacher M. The polarization defect of Wiskott-Aldrich syndrome macrophages is linked to dislocalization of the Arp2/3 complex. J Immunol. 2000;165:221–225. doi: 10.4049/jimmunol.165.1.221. [DOI] [PubMed] [Google Scholar]
- 25.Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci USA. 1999;96:9648–9653. doi: 10.1073/pnas.96.17.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machesky L M, Insall R H. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol. 1998;8:1347–1956. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 27.Massol P, Montcourrier P, Guillemot J C, Chavrier P. Fc receptor-mediated phagocytosis requires CDC42 and Rac1. EMBO J. 1998;17:6219–6229. doi: 10.1093/emboj/17.21.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May R C, Caron E, Hall A, Machesky L M. Involment of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat Cell Biol. 2000;2:246–248. doi: 10.1038/35008673. [DOI] [PubMed] [Google Scholar]
- 29.Méresse S, Stelle-Mortimer O, Moreno E, Desjardins M, Finlay B, Gorvel J-P. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat Cell Biol. 1999;1:E183–E188. doi: 10.1038/15620. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery R R, Malawista S E. Entry of Borrelia burgdorferi into macrophages is end-on and leads to degradation in lysosomes. Infect Immun. 1996;64:2867–2872. doi: 10.1128/iai.64.7.2867-2872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery R R, Nathanson M H, Malawista S E. The fate of Borrelia burgdorferi, the agent for Lyme disease, in mouse macrophages. J Immunol. 1993;150:909–915. [PubMed] [Google Scholar]
- 32.Mullins R D. How WASP-family proteins and the Arp2/3 complex convert intracellular signals into sytoskeletal structures. Curr Opin Cell Biol. 2000;12:91–96. doi: 10.1016/s0955-0674(99)00061-7. [DOI] [PubMed] [Google Scholar]
- 33.Pfister H-W, Wilske B, Weber K. Lyme borreliosis: basic science and clinical aspects. Lancet. 1994;343:1013–1016. doi: 10.1016/s0140-6736(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 34.Preac-Mursic V, Wilske B, Schierz G. European Borrelia burgdorferi isolated from humans and ticks: culture conditions and antibiotic susceptibility. Zentbl Bakteriol Hyg A. 1986;263:112–118. doi: 10.1016/s0176-6724(86)80110-9. [DOI] [PubMed] [Google Scholar]
- 35.Ridley A J, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 36.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 37.Rittig M G, Jagoda J C, Wilske B, Murgia R, Cinco M, Repp R, Burmester G R, Krause A. Coiling phagocytosis discriminates between different spirochetes and is enhanced by phorbol myristate acetate and granulocyte-macrophage colony-stimulating factor. Infect Immun. 1998;66:627–635. doi: 10.1128/iai.66.2.627-635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rittig M G, Krause A, Häupl T, Schaible U E, Modolell M, Kramer M D, Lütjen-Drecoll E, Simon M M, Burmester G R. Coiling phagocytosis is the preferential phagocytic mechanism for Borrelia burgdorferi. Infect Immun. 1992;60:4205–4212. doi: 10.1128/iai.60.10.4205-4212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rittig M, Wilske B, Krause A. Phagocytosis of microorganisms by means of overshooting pseudopods: where do we stand? Microbes Infect. 1999;1:727–735. doi: 10.1016/s1286-4579(99)80074-4. [DOI] [PubMed] [Google Scholar]
- 40.Steere A C. Medical progress—Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 41.Stewart D M, Treiber-Held S, Kurman C C, Facchetti F, Notarangelo L D, Nelson D L. Studies of the expression of the Wiskott-Aldrich syndrom protein. J Clin Investig. 1996;97:2627–2634. doi: 10.1172/JCI118712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suhonen J, Hartiala K, Viljanen M K. Tube phagocytosis, a novel way for neutrophils to phagocytize Borrelia burgdorferi. Infect Immun. 1998;66:3433–3435. doi: 10.1128/iai.66.7.3433-3435.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Symons M, Derry J M, Karlak B, Jiang S, Lemahieu V, McCormick F, Francke U, Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is inplicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- 44.Wilske B, Fingerle V, Herzer P, Hofmann A, Lehnert G, Peters H, Pfister H-W, Preac-Mursic V, Soutschek E, Weber K. Recombinant immunoblot in the serodiagnosis of Lyme borreliosis. Med Microbiol Immunol. 1993;182:255–270. doi: 10.1007/BF00579624. [DOI] [PubMed] [Google Scholar]
- 45.Wilske B, Preac-Mursic V, Göbel U B, Graf B, Jauris-Heipke S, Soutschek E, Schwab E, Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31:340–350. doi: 10.1128/jcm.31.2.340-350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]