Abstract
Background:
Despite significant improvements in the physical and esthetic properties of modern composite resins, there are still concerns about their biocompatibility. The aim of the current study was to evaluate the toxicity of X-tra fil, Grandio, and Admira Fusion composites on dental pulp stem cells (DPSCs) and human gingival fibroblast (HGF) cells.
Materials and Methods:
In this in vitro experimental study, 48 composite disks were made using Grandio, Admira Fusion (2 mm high and 4 mm in diameter), and X-tra fil (4 mm high and 4 mm in diameter) composites and cured for 40 s. The composite blocks were then crushed with a sterile mortar and dissolved in phosphate saline buffer solution. Tetrazolium salt (3-(4,5-dimethyl thiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT_, neutral red (NR) assay, flow cytometry, and quantitative real-time polymerase chain reaction (RT-PCR) tests (n = 5) were used to evaluate the toxicity of the composites on two cell types (HGF, DPSCs). Data were analyzed using one-way ANOVA test followed by Newman–Keuls test. Level of significance was set at P < 0.05.
Results:
According to the results of MTT test, only Grandio showed a significant cytotoxicity in DPSCs, but in HGF cells, Grandio and X-tra fil both showed a significant cytotoxicity. In NR test, Grandio and X-tra fil composites showed a significant cytotoxicity on both HGF and DPSC cells. RT-PCR test results on both DPSC and HGF cells indicated that bax gene expression in the Grandio composite was significant. In this test, the nonexpression of the bcl2 gene in DPSCs was significant in Grandio (100 and 200 μg/ml) and in X-tra fil (200 μg/ml). All of the tests performed in this study showed no significant toxicity of Admira fusion.
Conclusion:
Admira Fusion is suitable for oral cells in terms of biocompatibility and can be used as a suitable restorative material for deep restorations near the pulp or adjacent to the gums.
Keywords: Cells, composite resins, dental pulp, fibroblasts
INTRODUCTION
Today, composite resins are widely used due to their esthetic properties, comparable strength to amalgam, bonding to tooth structure, and conservative preparation.[1] Despite significant improvements in the physical, mechanical, and esthetic properties of modern composite resins, there are still concerns about their biocompatibility.[2]
Because the polymerization of methacrylate-based resin composites is incomplete, about 10% of monomers become dispersed in the oral cavity. Clinical studies have shown that monomer leakage and penetration into intracellular and extracellular fluids cause local and systemic effects. Therefore, it is possible that methacrylates reduce cell proliferation and cause cell mutations that lead to damage to deoxyribonucleic acid (DNA).[3,4] The evidence suggests that bisphenol A-glycidyl methacrylate (Bis-GMA) and other monomers have destructive effects on humans.[5]
Attempts to increase biocompatibility and reduce shrinkage stress have led to the emergence of composite resins without the conventional methacrylates.[3,6] Recently, composites have been introduced whose organic component has been changed; they are called Ormocer (organically modified ceramic) and actually contain the mineral component of silicon dioxide and the polymerizable organic component (aliphatic and aromatic dimethacrylates), which combines the hardness of glass and the properties of resin. In addition to improving esthetics, this material is abrasion resistance, reduces polymerization shrinkage and surface roughness, and protects the teeth against decay. Moreover, the absence of Bis-GMA and other conventional methacrylate monomers reduces toxicity concerns and increases biocompatibility.[7] Admira Fusion is a universal nanohybrid Ormocer. It has also been claimed that Admira Fusion is a true biocompatible due to the removal of conventional methacrylate monomers from its structure, and as a result, its leakage and cytotoxicity have been reduced.[8]
Conventional composites should be placed incrementally in the cavity because of their limited curing depth (2 mm) and to reduce polymerization shrinkage.[9] However, the incremental technique is time-consuming, and there is a possibility of formation of air bubbles and contamination between the restoration layers. Bulk-fill composites are a new category of composites that, according to the manufacturers, can be placed as bulk to a depth of 4 mm with low shrinkage. The main advantages of bulk-fill composites are their increased curing depth resulting from their higher translucency and less polymerization shrinkage due to changes in the filler content (such as the presence of isofillers) or resin matrix (such as the presence of polymerization modulators or plasticizers in the matrix composition).[10,11]
Many laboratory studies have shown that substances released from composite resins during resin degradation, or incomplete polymerization can diffuse into the dentin and reach the dental pulp.[12,13] These substances can affect the viability and regenerative properties of the pulp. The regenerative property of pulp tissue is attributed to the presence of stem cells in the pulp.[13] The toxicity of resin composites has been extensively studied in deep cavities or various pulp cells such as pulp fibroblasts,[14] immortalized odontoblast cell lines,[15] and human transformed pulp-derived cells. However, few studies have examined the cytotoxic effect of these compounds on dental pulp stem cells (DPSCs).[16] Furthermore, in deep composite restorations adjacent to the gingiva, monomer leakage can adversely affect gingival cells. For example, a study has shown that a small amount of triethylene glycol dimethacrylate (TEG-DMA) can reduce the intracellular glutathione in human gingival fibroblasts (HGF) to 30%–50%. Therefore, it can affect cell viability.[17]
Biocompatibility is one of the important properties of composite restorations. In the review of previous literature, few studies were found that have evaluated the cytotoxicity of bulk-fill and Ormocer composites, and those were mostly conducted on gingival mouse fibroblast cells. Only two studies have examined the cytotoxic effect of bulk-fill composites on DPSCs, and the most commonly used cytotoxic test was MTT alone. Therefore, the current study purposed to evaluate the toxicity of X-tra fil (VOCO, Cuxhaven, Germany), Grandio (VOCO, Cuxhaven, Germany), and Admira Fusion (VOCO, Cuxhaven, Germany) composites on DPSCs and HGF cells using MTT, neutral red (NR), flow cytometry, and quantitative real-time polymerase chain reaction (RT-PCR) tests.
MATERIALS AND METHODS
In this in vitro experimental study, HGF and DPSC lines were prepared from the Pasteur Institute of Iran and Iran University of Medical Sciences as frozen vials, and after several weeks of passage and ensuring that the cells were released from stress and returned to normal, they were treated to perform cell survival tests.
In this study, 48 composite disks were fabricated using Grandio, Admira fusion (2 mm high and 4 mm diameter), and X-tra fil (4 mm high and 4 mm diameter) composites. The samples were then cured by a light-emitting diode light curing unit (Kerr, USA) for 40 s at an intensity of1330 mw/cm2. The composite blocks were first crushed with a sterile mortar, and the composite powders were dissolved in a saline phosphate buffer. Then, as explained below, a certain amount of composite was used for direct placement in front of the cells (n = 5) in each test. Cell survival tests included MTT and NR tests.
MTT test
In total, 5000 cells were poured into each well of a 96-well plate (SPL, South Korea) (n = 5); then, 200 microliters of complete Dulbecco Modified Eagles Medium (DMEM) (Biosera, England) was added to each well. After 24 h of incubation, the wells were completely evacuated, and the different cell groups (pulp stem cells and gingival fibroblast cells) were treated with different doses (10, 50, 100, and 200 μg/ml) of composites (Admira Fusion, Grandio, and X-tra fil) and a complete new DMEM medium.
0.05 mg of MTT (Sigma, USA) was added to each well, and after incubation for 4 h, the supernatant culture medium was removed and 100 μl of dimethyl sulfoxide (DMSO) (Sigma, USA) was added to each well (By permeating the cell membrane and dissolving the formazan in itself, DMSO causes its purple color to spread in the wells). The plate was shaken gently several times until the purple color was completely diffused around the well. The light absorption of the purple color of the wells was read by an Elisa Reader (DRG Company, USA) at a wavelength of 490 nm.
The groups tested in the MTT test comprised the DPSCs control group, DPSCs group treated by Admira Fusion (10, 50, 100, and 200 μg/ml), DPSCs group treated by Grandio (10, 50, 100, and 200 μg/ml), DPSCs group treated with X-tra fil (10, 50, 100, and 200 μg/ml), HGF cells control group, HGF cells group treated with Admira Fusion (10, 50, 100, and 200 μg/ml), HGF cells groups treated with Grandio (10, 50, 100, and 200 μg/ml), and HGF cells groups treated with X-tra fil (10, 50, 100, and 200 μg/ml).
In the control group (n = 4), no treatment was performed on the cells. Wells containing control cells were filled with only 200 μl of complete DMEM medium (25 mmol glucose).
In all groups except the control, after treatment, the cells were incubated for 24 h to determine the toxic effect of the composites on them.
All steps were repeated 5 times, with a new sample used each time and mean light absorption considered as mitochondrial activity. The percentage of cell growth and proliferation in each dilution was determined by the following equation:
Percentage of living = 100× Intensity of absorption in the presence of drug-Blank absorption intensity cells/Intensity of control absorption-Blank absorption intensity.
Neutral red assay
First, 5000 cells were poured into each 96-well plate (n = 5); then 200 μl of complete DMEM medium was added. After 24 h of incubation, the well medium was completely drained, and different cell groups were treated with different doses (10, 50, 100, and 200 μg/ml) of composites (Admira Fusion, Grandio, and X-tra fil) and completely new DMEM medium. Next, 200 μl of fixing and rinsing solution (0.5% formaldehyde and 98.5% distilled water) was added to each well.
The wells were emptied of contents, and 200 microliters of desorb solution was added to each well. The NR dye (Sigma, USA) was removed from the cells and accumulated in the wells. Then, the 96-well plates were placed on the mixer for about 10 min. The light absorption of the wells at 540 nm was read by enzyme-linked immunosorbent assay.
The groups tested in the NR test were similar to the MTT test groups. All steps were repeated 5 times, and the mean light absorption was considered as the amount of lysosome absorption. The percentages of cell growth and proliferation in each dilution were determined by the following equation:
Percentage of living = 100 × Intensity of absorption in the presence of drug-Blank absorption intensity cells/Intensity of control absorption-Blank absorption intensity.
Apoptosis tests included flow cytometry and quantitative RT-PCR.
Flow cytometry method
Pulp stem and gingival fibroblast cells were cultured in 12-cell culture plates (SPL, South Korea) at a density of 2105 cells per cell, and composites were incubated for 24 h at the desired concentrations (10, 50, 100, 200 μg/ml). The cell pack was washed twice with phosphate buffered saline (PBS) (comprising: 8 g of sodium chloride, 1.15 g of sodium phosphate, 0.2 g of potassium chloride, and 0.2 g of potassium phosphate in 1L of distilled water). After emptying the supernatant PBS, 100 μl of binding buffer, 5 μl of annexin V-FITC solution, and 5 μl of PI dye solution were added to each cell pack.
After 15 min of incubation, 400 μl of binding buffer was added to the cell suspension and analyzed by flow cytometry (Becton Dickinson, USA).
The groups tested by flow cytometry were similar to the MTT test groups (n = 5). Cells were examined using a flow cytometer with excitation wavelengths of 488 and 540–488 nm and reflection wavelengths of 518 and 617 nm (FL-1) to determine fluorescein and PI, respectively.
For each sample, the specifications of 10,000 cells were recorded and the output information of the device was analyzed using Cell Quest software.
Quantitative real-time polymerase chain reaction
To perform this test, the RNA of cells was first extracted using an RNX-plus™ extraction kit, and after cDNA synthesis, RT-PCR reaction was performed.
Assay of gene expression by real-time polymerase chain reaction
According to the usual PCR program, real-time RT-PCR reaction in 45 cycles and a volume of 15 μl with 1 μl of complementary DNA, 2 μl of primers, 5.7 μl of FastStart SYBR Green Master 6-carboxy-X-rhodamine (ROX) kit solution (Roche, CH), and 4.5 μl of deionized water. Sterilization was performed on a ROTOR GENE 3000 device (Corbett, AU). Data was collected in two channels: fluorescein amidites (FAM)/SYBR green and ROX. Ct samples were taken in logarithmic phase. The raw FAM/SYBR channel data was first normalized against the ROX channel and transferred to Excel and then to LinReg version 2017.1 software (https://LinRegPCR.HFRC.nl). In this program, the linear range, PCR efficiency, and initial value of each sample were obtained. The initial concentration of each sample was normalized against the initial concentration of the corresponding internal control gene to determine the expression value. Then, by selecting the undifferentiated mode as a calibrator, the gene expression changes were obtained in relative terms (percentage). Gene expression was studied and compared before pre-differentiation (zero time) and 4 weeks after differentiation. The groups tested in the by quantitative real-time PCR were similar to the MTT test groups (n = 5).
Statistical analysis of data was analyzed by SPSS software version 20(SPSS Inc., Chicago, Illinois) using one-way ANOVA followed by the Newman–Keuls test. A P < 0.05 was considered as a significant level.
RESULTS
MTT test results
The percentage of cell viability in the MTT test is shown in Table 1. The results showed that in DPSCs cells, only Grandio at the concentrations of 100 and 200 μg/ml had significant cytotoxicity (P = 0.029 and P = 0.021, respectively), but the other two composites showed no significant cytotoxicity at any of the concentrations (P > 0.05).
Table 1.
The percentage of cell viability (mean±standard deviation, minimum and maximum) in MTT test
| Cell type | Composite type | The percentage of cell viability (%) Composite concentration (mg/ml) |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| 10 | 50 | 100 | 200 | Control | ||
| DPSC | Grandio | |||||
| Mean±SD | 92.3±1.7 | 76.4±1.8 | 50.7±5.6 | 30.03±2.4 | 100±0 | |
| Minimum | 90.3 | 75.1 | 46.5 | 28 | 100 | |
| Maximum | 93.6 | 78.4 | 57.1 | 32.7 | 100 | |
| X-tra fil | ||||||
| Mean±SD | 94.9±1.2 | 87.8±1.2 | 81.5±3.2 | 71.6±2.8 | 100±0 | |
| Minimum | 93.4 | 86.7 | 77.8 | 68.3 | 100 | |
| Maximum | 95.9 | 89.1 | 84.0 | 73.7 | 100 | |
| Admira fusion | ||||||
| Mean±SD | 98.1±3.8 | 99±6.5 | 83.5±6.1 | 93.6±6.2 | 100±0 | |
| Minimum | 93.6 | 91.7 | 76.7 | 86.7 | 100 | |
| Maximum | 100.5 | 104.5 | 88.7 | 99.5 | 100 | |
| Gingival fibroblast cells | Grandio | |||||
| Mean±SD | 99.9±4.4 | 84.7±3.1 | 30.4±3.0 | 25.2±2.0 | 100±0 | |
| Minimum | 94.8 | 81.0 | 27.8 | 23.7 | 100 | |
| Maximum | 102.8 | 86.7 | 33.7 | 27.5 | 100 | |
| X-tra fil | ||||||
| Mean±SD | 97.4±1.3 | 86.2±4.7 | 31.3±4.1 | 33.9±2.5 | 100±0 | |
| Minimum | 96 | 82.1 | 27.2 | 31.0 | 100 | |
| Maximum | 98.6 | 91.4 | 35.5 | 36.1 | 100 | |
| Admira fusion | ||||||
| Mean±SD | 101.4±3.7 | 99.6±3.4 | 96.2±3.1 | 82.7±4.9 | 100±0 | |
| Minimum | 97 | 95.5 | 93.1 | 78.6 | 100 | |
| Maximum | 103.8 | 101.7 | 99.5 | 88.1 | 100 | |
SD: Standard deviation; DPSC: Dental pulp stem cells; MTT: Tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
In HGF cells, however, the results showed that Grandio and X-tra fil composites at the concentrations of 100 and 200 μg/ml had significant cytotoxicity (P = 0.018 and P = 0.013, respectively) (P = 0.019 and P = 0.016, respectively), but Admira Fusion showed no significant cytotoxicity in any of the concentrations (P > 0.05).
Neutral red test results
The percentage of cell viability in the NR test is shown in Table 2. The results showed that in DPSCs cells, Grandio (100 and 200 μg/ml) had significant cytotoxicity (P = 0.023 and P = 0.019, respectively), but X-tra fil showed significant cytotoxicity only at the concentration of 200 μg/ml (P = 0.038). Admira Fusion had no significant cytotoxicity in any of the concentrations (P > 0.05).
Table 2.
The percentage of cell viability (mean±standard deviation, minimum and maximum) of treated cells in nuteral red test
| Cell type | Composite type | The percentage of cell viability (%) Composite concentration (mg/ml) |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| 10 | 50 | 100 | 200 | Control | ||
| DPSC | Grandio | |||||
| Mean±SD | 99.4±3.1 | 87.7±0.8 | 44.1±3.3 | 31.6±2.1 | 100±0 | |
| Minimum | 96 | 86.6 | 40.6 | 29.6 | 100 | |
| Maximum | 102.2 | 88.3 | 47.2 | 33.8 | 100 | |
| X-tra fil | ||||||
| Mean±SD | 89.9±8.4 | 72.2±7.2 | 69.1±5.2 | 49.9±8.5 | 100±0 | |
| Minimum | 83.7 | 65.1 | 65 | 41.4 | 100 | |
| Maximum | 99.4 | 79.6 | 75.1 | 58.5 | 100 | |
| Admira fusion | ||||||
| Mean±SD | 97.4±2.8 | 101.4±1.3 | 82.6±1.4 | 97.6±1.9 | 100±0 | |
| Minimum | 94.7 | 100.2 | 81.6 | 95.2 | 100 | |
| Maximum | 100.3 | 102.9 | 84.2 | 98.7 | 100 | |
| Gingival fibroblast cells | Grandio | |||||
| Mean±SD | 91.8±3.9 | 85±1.5 | 74.5±0.8 | 53.1±1.3 | 100±0 | |
| Minimum | 89.4 | 83.3 | 73.8 | 52 | 100 | |
| Maximum | 94.4 | 86.1 | 75.4 | 54.6 | 100 | |
| X-tra fil | ||||||
| Mean±SD | 95.4±3.2 | 82.6±1.1 | 71.7±6.5 | 61.7±3.6 | 100±0 | |
| Minimum | 91.6 | 81.2 | 64.5 | 57.7 | 100 | |
| Maximum | 97.7 | 83.5 | 77.4 | 64.7 | 100 | |
| Admira fusion | ||||||
| Mean±SD | 107.9±4.7 | 101±3.6 | 111.5±3.5 | 103.3±3.6 | 100±0 | |
| Minimum | 103 | 95.5 | 107.2 | 99.1 | 100 | |
| Maximum | 112.4 | 107.6 | 113.5 | 105.9 | 100 | |
SD: Standard deviation; DPSC: Dental pulp stem cells
In HGF cells, Grandio and X-tra fil (100 and 200 μg/ml) showed significant cytotoxicity (P = 0.045 and P = 0.039, respectively) (P = 0.043 and P = 0.04, respectively), but Admira Fusion composite had no significant cytotoxicity at any of the concentrations (P > 0.05).
Real-time-polymerase chain reaction test
The relative expression of bax gene in DPSCs and HGF cells with different composites is shown in Tables 3 and 4. Evaluation of bax gene expression showed that with Grandio composite, bax gene expression was significant in both DPSCs and HGF cells (100 and 200 μg/ml) (P < 0.05), but in Admira Fusion and X-tra fil, it was not significant in any of the concentrations (P > 0.05).
Table 3.
The relative expression of bax gene in dental pulp stem cells and human gingival fibroblast cellswith different composites
| Cell type | Composite type | Composite concentration (mg/ml) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 10 | 50 | 100 | 200 | Control | ||
| DPSC | Grandio | 1.4 | 1.77 | 2.15 | 3.42 | 1 |
| X-tra fil | 1.51 | 1.55 | 1.7 | 2.01 | 1 | |
| Admira fusion | 1.32 | 1.4 | 1.28 | 1.8 | 1 | |
| Gingival fibroblast cells | Grandio | 1.1 | 1.9 | 2.8 | 4.1 | 1 |
| X-tra fil | 1.2 | 1.33 | 1.81 | 1.88 | 1 | |
| Admira fusion | 1.5 | 1.6 | 2.0 | 2.1 | 1 | |
DPSC: Dental pulp stem cells
Table 4.
The relative nonexpression of bcl2 in dental pulp stem cells and human gingival fibroblast cells with different composites
| Cell type | Composite type | Composite concentration (mg/ml) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 10 | 50 | 100 | 200 | Control | ||
| DPSC | Grandio | 0.98 | 0.83 | 0.5 | 0.31 | 1 |
| X-tra fil | 0.93 | 0.81 | 0.64 | 0.46 | 1 | |
| Admira fusion | 0.9 | 0.87 | 0.79 | 0.75 | 1 | |
| Gingival fibroblast cells | Grandio | 0.91 | 0.79 | 0.44 | 0.29 | 1 |
| X-tra fil | 0.95 | 0.85 | 0.73 | 0.61 | 1 | |
| Admira fusion | 0.98 | 0.91 | 0.77 | 0.69 | 1 | |
DPSC: Dental pulp stem cells
The relative nonexpression of bcl2 in DPSCs and HGF cells with different composites is shown in table 4. Evaluation of nonexpression of bcl2 (anti-aptotic index) showed that with Grandio (100 and 200 μg/ml) and X-tra fil (200 μg/ml), expression in DPSCs was significant (P = 0.033 and P = 0.021, respectively) (P = 0.045), but in Admira Fusion, the nonexpression of bcl2 gene was not significant (P > 0.05).
In HGF cells, however, the nonexpression of the bcl2 gene was significant only in Grandio composite (100 and 200 μg/ml) (P = 0.028 and P = 0.018, respectively).
Flow cytometry test results
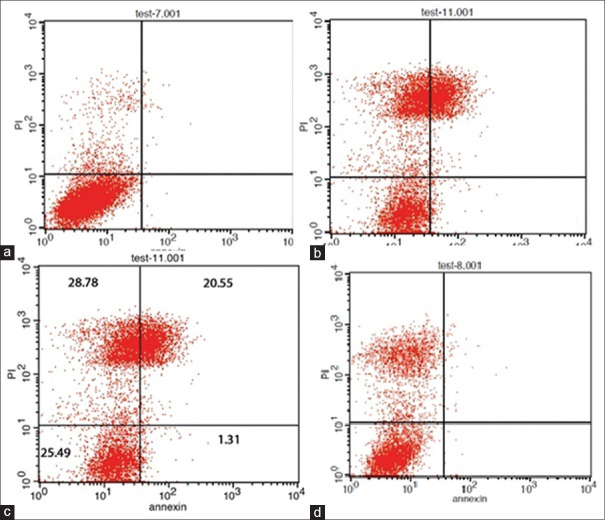
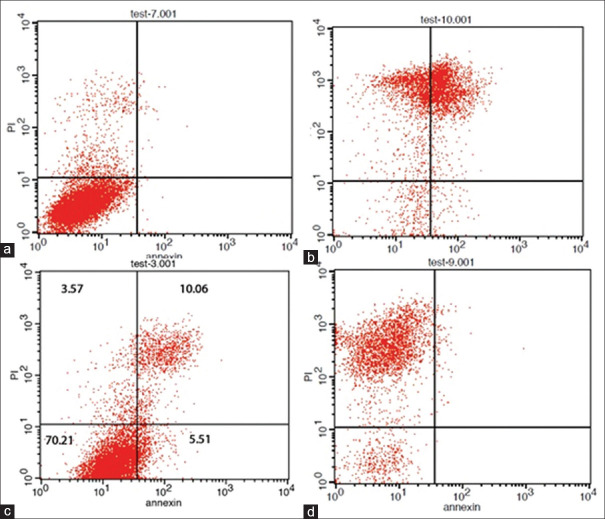
As shown in Figures 1 and 2, in the exposure of HGF and DPSC cells with Grandio and X-tra fil composites, the percentage of annexin PI/V positive cells was significantly increased compared to the nontreated cells (control group), which indicates the acute apoptotic effect of these composites on these cells (P = 0.0042 and P = 0.0064, respectively) (P = 0.023 and P = 0.004, respectively).
Figure 1.
Analysis of DPSC cells treated with different composites using flow cytometry for anxin V and anxin V/PI harvesting (control group (a) X-tra fil (b) Grandio (c) Admira Fusion (d). DPSC: Dental pulp stem cells.
Figure 2.
Analysis of HGF cells treated with different composites using flow cytometry for anxin V and anxin V/PI harvesting (control group (a) X-tra fil (b) Grandio (c) Admira Fusion (d). HGF: Human gingival fibroblast.
DISCUSSION
Among the important characteristics of composites are their biocompatibility and the maintenance of the health of oral cells after composite restoration, especially in cases of deep and subgingival caries.[18] In deep caries where the odontoblast layer is destroyed, the stem cells proliferate and migrate to the affected site and differentiate into odontoblast-like cells, which protect the pulp by secreting reparative dentin as a protective barrier. As a result, stem cell preservation following a restorative treatment plays an important role in maintaining the regenerative properties and pulp vitality.[19]
The toxicity of resin-based restorative materials depends on several factors: The degree of conversion, the amount and type of unbound monomers, ion release, and leachable substances generated by erosion and degradation over time.[12]
In the present study, the samples were examined for cytotoxicity after 24 h of polymerization. Some studies have reported that the greatest release of monomers occurs in the early hours after polymerization or during the 1st week,[20,21] while others have stated that the release of monomers over time can continue up to 30 days[22,23] or even 1 year after polymerization.[24]
The current results showed that Admira Fusion (Ormocer) had the lowest toxicity in all tests performed and was significantly lower than the other composites in most tests.
In a laboratory study, Schubert et al. examined the toxicity of nanohybrid (Grandio), nanofiller (Filtek Supreme), and Admira Fusion on standard mouse and HGFs. They reported that Admira Fusion toxicity was significantly lower than that of the other composites in the two cell types.[25] Susila and Balasubramanian showed that Ormocer and Silorane release little monomer and are less toxic than the methacrylate-based composites.[26] Kavuncu et al. also showed that the lowest cytotoxicity on HGFs cells was observed in the Admira Fusion group in both 24-h and 1-week intervals.[27]
Fillers do not seem to play a major role in the biocompatibility of composites, and the degree of conversion and the resin content of composite seems to be responsible for most of the reported undesirable cytotoxic effects.[12,28] There are no concerns about the cytotoxicity of conventional monomers such as TEG-DMA and Bis-GMA in Admira Fusion. In fact, this is a great advantage over methacrylate-based resin composites. The lack of cytotoxicity is due to the lack of conventional dimethacrylate monomers and of the unreacted c = c groups at the end of the resin matrix, which leads to improved biocompatibility.[29] Ormocers have been shown to release less monomer, even if they do not show better conversion rates than hybrid composites, because of the lower initial monomer content and, more especially, its three-dimensional network which prevents the release of bonded monomers.[22]
The results of the present study showed that nanohybrid composite (Grandio) had the highest toxicity. In fact, it showed significant toxicity in all tests performed on dental pulp cells and gingival fibroblasts. Alshali et al. evaluated the long-term release of composite monomers by liquid chromatography. Their results showed that Bis-GMA, ethoxylated bisphenol A-dimethacrylate, and TEG-DMA were released from Grandio, and the release of these monomers was the reason for its high cytotoxicity.[30] It has also been shown that more monomers are released from the nanohybrid composite than from Ormocers.[31]
The results of the present study showed that bulk-fill composite was toxic on gingival fibroblast cells, and its toxicity was seen on DPSCs by NT and flow cytometry.
Şişman et al. evaluated the cytotoxicity of bulk-fill composites (SDR, Filtek Bulk Fill, Sonic Fill, X-tra fil, Tetric Evoceram Bulk Fill) and showed that the cell counts were lower in X-tra fil and SDR than in the other groups at different times. They also showed that these two composites had more toxicity than the others, indicating that the amount of monomer remaining after polymerization was higher in these groups. In fact, according to the manufacturer's instructions, the chemical composition of SDR and X-tra fil composites includes TEGDMA and urethane dimethacrylate (UDMA) concurrently, which is a distinguishing feature compared to other composites. TEGDMA and UDMA are more toxic than other chemical compounds, and this may be the reason for the low number of cell counts observed in the SDR and X-tra fil composites.[32] Aydin et al. evaluated the cytotoxic effects of Bulk-fill composites on L929 mouse fibroblast cells and reported that at the end of 72 h, the majority of bulk-fill composites decreased cell viability, but they did not cause unacceptable cytotoxic effects, except Beautiful Bulk Restorative, which was cytotoxic.[28] However, in a similar study on the cytotoxicity of flowable and paste-like bulk-fill composites conducted on L929 mouse fibroblast cells by Demirel et al., it was reported that at the end of 72 h, composite extracts caused a statistically significant decrease in cell viability.[33]
Bulk-fill composites are controversial and belong to the inhomogeneous group. In some cases, less monomers (TEG-DMA and Bis-GMA) were released from bulk-fill composites than from conventional composites,[34] and in another study, this rate was comparable to conventional types.[35] It has also been reported that the release of monomers from bulk-fill composites is present up to 30 days after polymerization and increases over time.[36]
Lee et al. stated that in bulk-fill composites, as the irradiation depth increased, more toxicity occurred, and the highest cytotoxicity was observed in the layer at a depth of 4–6 mm.[37]
Studies have shown that the results of cytotoxicity tests are also affected by the type of test. NR test is more sensitive than MTT. It is directly associated with the integrity of the lysosome membrane, while MTT is related to the mitochondrial integrity. According to other study results, the toxicity of resins at the lysosome level occurs earlier than the effect on mitochondria. This indicates that the NR test can be an important tool in the detection of primary damage at the lysosomal level, which, in fact, distinguishes the cytotoxic effects at the cellular level from damage to cellular organelles and may explain the various results obtained by the two methods in this study.[38,39] For example, the bulk-fill composite did not show toxicity on DPSCs in the MTT assay, but did in the NR test.
The cytotoxicity of the composites depends on the dentin permeability and the residual dentin thickness. Residual dentin absorbs the unreacted monomers and reduces toxicity. When the remaining dentin thickness is <1 mm or acid etching is used, the diffusion of resin monomers through dentinal tubules increases significantly. In low depth cavities, however, fewer effects and reactions are observed in the pulp.[2] In the current study, the specimens were in direct contact with the DPSCs. Therefore, it is suggested that other studies be performed using the dentin barrier test to better simulate clinical situations.[16]
CONCLUSION
According to the results of the present study, Admira Fusion is suitable for oral cells in terms of biocompatibility, and the toxicity of Grandio (nanohybrid composite) is relatively higher. This issue should be considered in deep cavities in order to support pulp and gingival cells.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
Acknowledgment
The present study was financially supported by Kerman University of Medical Sciences, Kerman, Iran.
REFERENCES
- 1.Ferracane JL. Resin composite-State of the art. Dent Mater. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Bakopoulou A, Papadopoulos T, Garefis P. Molecular toxicology of substances released from resin-based dental restorative materials. Int J Mol Sci. 2009;10:3861–99. doi: 10.3390/ijms10093861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dijken JW, Pallesen U. Durability of a low shrinkage TEGDMA/HEMA-free resin composite system in Class II restorations.A 6-year follow up. Dent Mater. 2017;33:944–53. doi: 10.1016/j.dental.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Song L, Ye Q, Ge X, Misra A, Tamerler C, Spencer P. New silyl-functionalized BisGMA provides autonomous strengthening without leaching for dental adhesives. Acta Biomater. 2019;83:130–9. doi: 10.1016/j.actbio.2018.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rochester JR. Bisphenol A and human health: A review of the literature. Reprod Toxicol. 2013;42:132–55. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Mousavinasab SM, Ghasemi M, Yadollahi M. Evaluation of Enamel and Dentinal Microleakage in Class II Silorane-Based and Methacrylate-Based Resin Composite Restorations Using Specific and Nonspecific Adhesives. J Dent (Tehran) 2018;15:240–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Rajeev V, Arunachalam R, Nayar S, Arunima PR, Ganapathy S, Vedam V. “Ormocer an innovative technology”: A replacement for conventional cements and veneer. A comparative in vitro analysis? Eur J Dent. 2017;11:58–63. doi: 10.4103/ejd.ejd_113_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilie N, Hickel R. Resin composite restorative materials. Aust Dent J. 2011;56(Suppl 1):59–66. doi: 10.1111/j.1834-7819.2010.01296.x. [DOI] [PubMed] [Google Scholar]
- 9.Leprince JG, Palin WM, Hadis MA, Devaux J, Leloup G. Progress in dimethacrylate-based dental composite technology and curing efficiency. Dent Mater. 2013;29:139–56. doi: 10.1016/j.dental.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 10.El-Damanhoury H, Platt J. Polymerization shrinkage stress kinetics and related properties of bulk-fill resin composites. Oper Dent. 2014;39:374–82. doi: 10.2341/13-017-L. [DOI] [PubMed] [Google Scholar]
- 11.Ilie N, Schöner C, Bücher K, Hickel R. An in-vitro assessment of the shear bond strength of bulk-fill resin composites to permanent and deciduous teeth. J Dent. 2014;42:850–5. doi: 10.1016/j.jdent.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg M. In vitro and in vivo studies on the toxicity of dental resin components: A review. Clin Oral Investig. 2008;12:1–8. doi: 10.1007/s00784-007-0162-8. [DOI] [PubMed] [Google Scholar]
- 13.Krifka S, Seidenader C, Hiller KA, Schmalz G, Schweikl H. Oxidative stress and cytotoxicity generated by dental composites in human pulp cells. Clin Oral Investig. 2012;16:215–24. doi: 10.1007/s00784-010-0508-5. [DOI] [PubMed] [Google Scholar]
- 14.Geurtsen W, Lehmann F, Spahl W, Leyhausen G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res. 1998;41:474–80. doi: 10.1002/(sici)1097-4636(19980905)41:3<474::aid-jbm18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 15.de Souza Costa CA, Hebling J, Hanks CT. Effects of light-curing time on the cytotoxicity of a restorative resin composite applied to an immortalized odontoblast-cell line. Oper Dent. 2003;28:365–70. [PubMed] [Google Scholar]
- 16.Shafiei F, Tavangar MS, Razmkhah M, Attar A, Alavi AA. Cytotoxic effect of silorane and methacrylate based composites on the human dental pulp stem cells and fibroblasts. Med Oral Patol Oral Cir Bucal. 2014;19:e350–8. doi: 10.4317/medoral.19340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelmann J, Leyhausen G, Leibfritz D, Geurtsen W. Effect of TEGDMA on the intracellular glutathione concentration of human gingival fibroblasts. J Biomed Mater Res. 2002;63:746–51. doi: 10.1002/jbm.10465. [DOI] [PubMed] [Google Scholar]
- 18.Beltrami R, Colombo M, Rizzo K, Di Cristofaro A, Poggio C, Pietrocola G. Cytotoxicity of different composite resins on human gingival fibroblast cell lines. Biomimetics (Basel) 2021;6:26. doi: 10.3390/biomimetics6020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Téclès O, Laurent P, Zygouritsas S, Burger AS, Camps J, Dejou J, et al. Activation of human dental pulp progenitor/stem cells in response to odontoblast injury. Arch Oral Biol. 2005;50:103–8. doi: 10.1016/j.archoralbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, Taira M, Shintani H, Wakasa K, Yamaki M. Residual monomers (TEGDMA and Bis-GMA) of a set visible-light-cured dental composite resin when immersed in water. J Oral Rehabil. 1991;18:353–62. doi: 10.1111/j.1365-2842.1991.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 21.Pongprueksa P, De Munck J, Duca RC, Poels K, Covaci A, Hoet P, et al. Monomer elution in relation to degree of conversion for different types of composite. J Dent. 2015;43:1448–55. doi: 10.1016/j.jdent.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Polydorou O, Huberty C, Wolkewitz M, Bolek R, Hellwig E, Kümmerer K. The effect of storage medium on the elution of monomers from composite materials. J Biomed Mater Res B Appl Biomater. 2012;100:68–74. doi: 10.1002/jbm.b.31923. [DOI] [PubMed] [Google Scholar]
- 23.Tabatabaei MH, Sadrai S, Bassir SH, Veisy N, Dehghan S. Effect of food stimulated liquids and thermocycling on the monomer elution from a nanofilled composite. Open Dent J. 2013;7:62–7. doi: 10.2174/1874210601307010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polydorou O, König A, Hellwig E, Kümmerer K. Long-term release of monomers from modern dental-composite materials. Eur J Oral Sci. 2009;117:68–75. doi: 10.1111/j.1600-0722.2008.00594.x. [DOI] [PubMed] [Google Scholar]
- 25.Schubert A, Ziegler C, Bernhard A, Bürgers R, Miosge N. Cytotoxic effects to mouse and human gingival fibroblasts of a nanohybrid ormocer versus dimethacrylate-based composites. Clin Oral Investig. 2019;23:133–9. doi: 10.1007/s00784-018-2419-9. [DOI] [PubMed] [Google Scholar]
- 26.Susila AV, Balasubramanian V. Correlation of elution and sensitivity of cell lines to dental composites. Dent Mater. 2016;32:e63–72. doi: 10.1016/j.dental.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Kavuncu G, Yilmaz AM, Karademir Yilmaz B, Yilmaz Atali P, Altunok EC, Kuru L, et al. Cytotoxicity of different nano composite resins on human gingival and periodontal ligament fibroblast cell lines: An in vitro study. Biomedicines. 2020;8:48. doi: 10.3390/biomedicines8030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aydin N, Karaoglanoglu S, Oktay E, Sulodlu A. Cytotoxic effects of bulk-fill composites on L929 fibroblast cells. Braz Dent Sci. 2021;24:1–9. [Google Scholar]
- 29.O’Neill C, Kreplak L, Rueggeberg FA, Labrie D, Shimokawa CA, Price RB. Effect of tooth brushing on gloss retention and surface roughness of five bulk-fill resin composites. J Esthet Restor Dent. 2018;30:59–69. doi: 10.1111/jerd.12350. [DOI] [PubMed] [Google Scholar]
- 30.Alshali RZ, Salim NA, Sung R, Satterthwaite JD, Silikas N. Qualitative and quantitative characterization of monomers of uncured bulk-fill and conventional resin-composites using liquid chromatography/mass spectrometry. Dent Mater. 2015;31:711–20. doi: 10.1016/j.dental.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Manojlovic D, Radisic M, Vasiljevic T, Zivkovic S, Lausevic M, Miletic V. Monomer elution from nanohybrid and ormocer-based composites cured with different light sources. Dent Mater. 2011;27:371–8. doi: 10.1016/j.dental.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Şişman R, Aksoy A, Yalçın M, Karaöz E. Cytotoxic effects of bulk fill composite resins on human dental pulp stem cells. J Oral Sci. 2016;58:299–305. doi: 10.2334/josnusd.15-0603. [DOI] [PubMed] [Google Scholar]
- 33.Demirel G, Gür G, Demirsoy FF, Altuntaş EG, Yener-Ilce B, Kiliçarslan MA. Cytotoxic effects of contemporary bulk-fill dental composites: A real-time cell analysis. Dent Mater J. 2020;39:101–10. doi: 10.4012/dmj.2018-336. [DOI] [PubMed] [Google Scholar]
- 34.Lempel E, Czibulya Z, Kovács B, Szalma J, Tóth Á, Kunsági-Máté S, et al. Degree of Conversion and BisGMA, TEGDMA, UDMA Elution from Flowable Bulk Fill Composites. Int J Mol Sci. 2016;17:732. doi: 10.3390/ijms17050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alshali RZ, Salim NA, Sung R, Satterthwaite JD, Silikas N. Analysis of long-term monomer elution from bulk-fill and conventional resin-composites using high performance liquid chromatography. Dent Mater. 2015;31:1587–98. doi: 10.1016/j.dental.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Cebe MA, Cebe F, Cengiz MF, Cetin AR, Arpag OF, Ozturk B. Elution of monomer from different bulk fill dental composite resins. Dent Mater. 2015;31:e141–9. doi: 10.1016/j.dental.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Lee SM, Kim SY, Kim JH, Jun SK, Kim HW, Lee JH, et al. Depth-Dependent Cellular Response from Dental Bulk-Fill Resins in Human Dental Pulp Stem Cells. Stem Cells Int 2019. 2019:1251536. doi: 10.1155/2019/1251536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao M, Antunes F, Eaton JW, Brunk UT. Lysosomal enzymes promote mitochondrial oxidant production, cytochrome c release and apoptosis. Eur J Biochem. 2003;270:3778–86. doi: 10.1046/j.1432-1033.2003.03765.x. [DOI] [PubMed] [Google Scholar]
- 39.Nascimento AS, Lima DB, Fook MV, Albuquerque MS, Lima EA, Sabino MA, et al. Physicomechanical characterization and biological evaluation of bulk-fill composite resin. Braz Oral Res. 2018;32:e107. doi: 10.1590/1807-3107bor-2018.vol32.0107. [DOI] [PubMed] [Google Scholar]