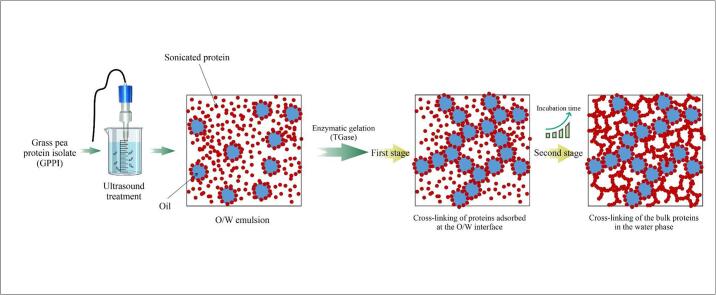
Graphical abstract
Keywords: Emulsion, Gel, Protein, Grass pea, Sonication, Enzyme, Gelation kinetics
Highlights
-
•
HIU promoted the cross-linking efficiency in GPPI emulsion gels during enzymatic gelation.
-
•
The first stage of gelation started by crosslinking of proteins adsorbed at the oil/water interface.
-
•
The second gelation step was caused by crosslinking of the proteins dispersed in the water phase.
-
•
Gel strength and water holding capacity of emulsion gels improved after the sonication of protein.
Abstract
In this study, emulsion gels were prepared by sonicated grass pea protein isolates (GPPI) at different ultrasonic amplitudes (25, 50 and 75 %) and times (5, 10 and 20 min). Formation of emulsion gels was induced by transglutaminase. Enzymatic gelation of emulsions stabilized by sonicated GPPI occurred in two stages. A relatively fast stage led to the formation of a weak gel which was followed by a slow stage that strongly reinforced the gel structure. Emulsion gels fabricated by sonicated GPPIs showed a homogeneous and uniform droplet distribution with higher elastic modulus compared to the native protein. A stiffer emulsion gel with a higher G' was formed after the protein was treated at 75 % amplitude for 10 min. After sonication of GPPI, the water holding capacity (WHC) of emulsion gels increased in accordance with the mechanical properties. Higher intermolecular cross-linking within the gel network increased the thermal stability of emulsion gels fabricated by sonicated GPPI. Although sonicated-GPPI emulsion gels clearly displayed homogenous microstructure in comparison to that made with native GPPI, the microstructures of these gels were nearly identical for all sonication amplitudes and times.
1. Introduction
Emulsion gels or emulgels, are gels embedding oil droplets [1]. Emulsion gels are mainly prepared by a polysaccharide or protein-stabilized oil-in-water emulsion. However, emulsions stabilized by proteins exhibit weak stabilities due to protein aggregation, coalescence, and thermal denaturation [2]. Due to their specific gel-like structure and solid-like mechanical characteristics, emulsion gels, as thermodynamically stable systems, are able to overcome the drawbacks of conventional emulsions. The gel-like structure of emulsion gels improves their physical stability against creaming [3]. Emulsion gels have a wide range of applications in the food industry such as in meat-analogues, reduced-fat food products, and carriers for bioactive ingredients [1], [4].
Gel forming ability is an important functional property of legume proteins [5]. Application of legume proteins in food formulation has attracted a considerable attention due to their digestibility and balance in the composition of polar, nonpolar and charged hydrophobic/hydrophilic amino acids [6]. Grass pea (Lathyrus sativus L.) seed is a new source of legumin protein with up to 30 % protein content. The grass pea protein isolate (GPPI) is easily digestible and has a high nutritional value with high quantity of lysine [6], [7], [8]. However, the compact structure and poor functionality of legume proteins in their native state limits their application in food industries. Therefore, plant proteins need structural modification to improve their surface activity and gelation properties [9]. Application of high intensity ultrasound (HIU) to modify the conformation and functional characteristics of proteins has drawn a lot of attention [4], [9], [10], [11].
High intensity ultrasound (frequency 20–100 kHz) is an efficient, non-thermal and physical technique for modification of the secondary and tertiary structures in protein [12]. Ultrasound waves exert their effects on protein molecules thorough the collapse of cavitation bubbles in liquids [13]. The functionality of proteins such as solubility, emulsifying and gelation properties can be influenced by ultrasonic cavitation, possibly due to the increase in local pressure and temperature, formation of water jets, shock waves and shear forces, which force protein molecules to undergo chemical and physical changes [14]. The structural unfolding of globular proteins, exposes more hydrophobic and sulfhydryl groups to the surface and reinforces the gel forming ability of protein through the formation of intermolecular hydrophobic interactions and disulphide bonds [15].
It has been reported that electrostatic, hydrophobic and disulphide interactions play an important role in the formation of pea protein gel network [16]. According to Hu et al.[17] and Zhang et al. [18], the ultrasonication could improve the gel forming ability of transglutaminase-catalyzed SPI gels. TGase is an enzyme that catalyzes acyl-transfer between γ-hydroxylamine groups of glutamine (Gln) and ε-amino groups of lysine (Lys) and forms a covalent ε-(γ-glutamyl)lysine isopeptide bond [19]. Normally, hydrophobic residues and Gln and Lys groups are located inside the native protein molecules. HIU treatment unfolds the compact structure of protein, exposes embedded residues to the surface of protein, and promotes subsequent enzymatic reactions. Therefore, contribution of more intermolecular interactions, strengthen the emulsion gel network [12]. Moreover, HIU can improve the emulsifying properties of protein through the reduction of its particle size, improvement of protein solubility and reduction of interfacial tension, which accelerate the protein adsorption at the oil/water interface [12]. The hydrophobic interactions between proteins adsorbed at the interface, can produce a thick interfacial layer, and the inter-droplet interactions can create the emulsion gel network [20].
Ultrasonicated proteins could also influence the rheological properties of emulsion gels. The particle size reduction along with the exposure of hydrophobic, free SH groups, and glutamyl and lysine residues could strengthen the intermolecular interactions and subsequently facilitates the formation of a stable gel network with higher elastic modulus [21]. However, as far as we are aware, the effect of different HIU pretreatments of proteins on enzymatic gelation of protein stabilized emulsions has not yet been reported. Recently, we described how HIU affected the properties of GPPI in aqueous solution [9]. It was found that the surface hydrophobicity and the amount of free SH groups increased after HIU. Furthermore, HIU of GPPI led to the formation of gels at lower GPPI concentrations and the gels were stronger for a given protein concentration. Therefore, the object of the present study was to fabricate emulsion gels with uniform microstructure and improved rheological properties using sonicated GPPI prepared at different ultrasonic amplitudes and times. The kinetics of gelation and texture of emulsion gels were also investigated.
2. Materials and methods
2.1. Materials
Grass pea seeds and corn oil were obtained from a local market in Mashhad, Iran. Protein extraction and isolation was performed according to the method described in our previous work [9]. The protein content of the final grass pea protein isolate was approximately 92 %, evaluated by the micro Kjeldahl method (N × 5.8). Commercial TGase was purchased from TAIXIN YIMIN Fine Chemical Industry Co. ltd. (Jiangsu province, China). Fluorescein and Nile red were purchased from sigma Aldrich Co. All other chemicals and materials were of analytical grade.
2.2. GPPI emulsion and emulsion gel preparation
GPPI was dispersed in distilled water (8 % w/v) with 0.02 % (w/v) sodium azide and shaked using a roller shaker at room temperature for 2 h. After preparation, corn oil was added to the dispersions, mixed and homogenized using a laboratory homogenizer (Digital Ultra Turrux T-25, IKA instruments, Germany) at 20000 rpm for 3 min to obtain a uniform emulsion (Φ = 0.2). To obtain emulsion gels, TGase (50 U/g) was added to the emulsions and vortexed for 5 s, then immediately used for confocal and rheological characterization.
2.3. Poly dispersity index (PDI) and droplet size
The average droplet size and polydispersity index (PDI) of emulsion (8 %w/v, Φ = 0.2) fabricated by sonicated and non-sonicated GPPI were measured using a dynamic light scattering particle size analyzer (Cordouan, VASCO 3, France). Samples were diluted with deionized water at a ratio of (1:200 v/v) to prevent multiple scattering particle. Three measurements were done per sample.
2.4. Zeta potential
Zeta potential was measured by a Zeta potential Analyzer (Cordouan Technol, Zetasizer, France) through determining the electrophoretic mobility of emulsion droplets. Emulsions were diluted 200-fold using deionized water. All measurements were done in triplicate.
2.5. Confocal laser scanning microscopy (CLSM)
The microstructure of the emulsion gels was analyzed using CLSM instrument Zeiss LSM800 equipped with three Ar-He-Ne lasers with excitation wavelengths of 488 nm,543 nm and 633 nm, respectively (Carl Zeiss Microscopy GmbH, Germany). To find the relative location of the oil phase and protein phase in the emulsion gel structure, Nile red (1 mg/ml oil or methanol) and Fluorescein (1 mg/ml distilled water) were used before emulsion gel formation to stain the oil and protein phase, respectively. The fluorescence of Fluorescein was exited at 480 nm and detected between 480 and 540 nm. The fluorescence of Nile red was exited at 550 nm and detected between 550 and 630 nm. An aliquot of freshly prepared emulsion (as described in section 2.2) was immediately transferred to a single concave microscope glass slides and covered with glass slips and then kept 20 h at 37 °C for emulsion gel formation. The CLSM images of emulsion gels were obtained by a 63 × magnification water immersed lens (HC × PL APO 63 × (NA = 1.2). The oil phases dyed with the Nile red are normally red, whereas the proteins phase dyed with the fluorescence are green. In fact, most of the protein coated oil droplets appeared predominantly yellow in the images (green + red → yellow).
2.6. Gelation kinetics
The gelation kinetics of sonicated and non-sonicated GPPI stabilized emulsion gels (8 % w/v) induced by TGase were evaluated using a rheometer (AR2000, TA instruments) with plate- plate geometry (diameter = 40.0 mm). Next, 1 ml of the emulsion containing enzyme (as described in section 2.2) immediately loaded between the parallel plates using Pasteur pipette and the gap between the two plates was set to 700 µm. Excess samples around the plates were trimmed of and the geometry was covered by a thin layer of paraffine oil to prevent water evaporation during measurement. The temperature was set to 37 °C through the lower plate. The measurements were performed in the linear viscoelastic region (LVR) at constant strain of 3 %. Dynamic oscillations with a frequency of 1 Hz was applied and elastic modulus (G′) and viscous modulus (G″) were recorded every 30 s as a function of incubation time (Up to 20 h).
2.7. Strain sweep
Strain sweep analysis was carried out to induce a large-scale deformation. The strain was increased from 1 to 1000 % at the constant frequency of 1 Hz. The critical strain was calculated as the G′ was dropped sharply upon strain exceeding the LVR value. The elastic modulus (G′) and loss modulus (G″) were recorded as a function of strain.
2.8. Frequency sweep
The frequency sweep analysis was carried out at the end of the time sweep (after 20 h) using the same rheometer to investigate the effect of frequency on the gel structure. The experiments were oscillated at the frequency ranging from 0.1 to 10 Hz at the identified LVR (i.e., constant stain of 3 %). The elastic modulus (G′), loss modulus (G″) and loss factor (tan δ = G″/G′) were recorded as a function of frequency.
2.9. Water holding capacity (WHC)
The water holding capacity of emulsion gels was evaluated using a previous method described by Liang et al. [15] with slight changes. 3 g of emulsion gel samples carefully transferred into a weighted centrifuged tube and centrifuged (Sigma, 3–30 k refrigerated centrifuge, Germany) at 10000g for 10 min. Any extra water carefully drained off and the residual liquid dried using a paper filter. The centrifuge tubes containing emulsion gel samples were accurately weighted before and after centrifugation. The WHC was calculated using following equation:
| (1) |
where, Wt is the total weight of water in the gel (g) and Wr represents the mass of water released after centrifugation. Each measurement was carried out three times.
2.10. Differential scanning calorimetry (DSC)
The thermal properties of emulsion gels were evaluated using DSC instrument (DSC 214 Polyma, NETZSCH, Germany). 15 mg of freeze-dried emulsion gel transferred into an aluminum pan and sealed with an aluminum cover using a tablet press machine. Samples were heated at the rate of 10 °C/min from 25 to 150 °C. The degradation temperature (Td) and denaturation enthalpy (ΔH) were taken from DSC thermograms. A hermetic aluminum pan was used as a reference.
2.11. FT-IR spectroscopy
Freeze dried emulsion gels were mixed with KBr at a ratio of 1:100 to make a tablet. Infra-red spectra of samples were analyzed using IR spectrometer (Thermo Nicolet Avatar 370 FT- IR, USA) in the region of 4000–400 cm−1 using 64 readings per second at a resolution of 4 cm−1.
2.12. Gel strength
Emulsions containing TGase were transfered into tubes (40 mm × 10 mm, 5 ml) and incubated at 37 °C for 20 h. Gel strength was determined using a texture analyzer (AMETEK lloyd, TA Plus instruments Ltd, USA) with a cylindrical probe (diameter: 4 mm). Emulsion gels were penetrated at a constant prob rate of 1 mm/s and trigger force of 5 g to 50 % of their original height. Gel strength was defined as the maximum force for penetration [22].
2.13. Statistical analysis
Statistical analysis was performed employing SPSS 26.0 statistical software (2020). The measurements were repeated at least three times. The samples were analyzed by a completely randomized factorial design. Results were expressed as means ± standard deviation. The significant differences were analyzed at the P-level of 0.05 using Duncan’s test.
3. Results and discussion
3.1. Emulsion droplet size and zeta potential
The average droplet size of emulsion stabilized by GPPI before gel formation are shown in Table 1. Emulsion stabilized by sonicated GPPIs at higher amplitudes and times exhibited smaller oil droplets. The smaller size and uniform distribution of emulsion droplets could enhance the gel properties when the emulsion is converted to the emulsion gel [23]. The decrease in droplet size could be due to the increase in the surface hydrophobicity of ultrasonically modified GPPI [7], which improves the emulsifying activity of sonicated protein particles and also increases the adsorption rate of proteins at the O/W interface [24]. Zhang et al. [25] also reported that ultrasound treatment, improved the adsorption efficiency of sonicated quinoa protein particles at the interface and reduced the droplet size.
Table 1.
Droplet size, PDI and ζ-potential values of emulsion stabilized with 1 % w/v native and sonicated GPPI at different sonication amplitudes and times.
| Amplitude (%) | Time (min) | Droplet size (nm) | Zeta potential (mV) | PDI |
|---|---|---|---|---|
| Control | – | 734 ± 50.5a | −2.98 ± 0.73i | 0.53 ± 0.06a |
| 25 | 5 | 645 ± 48.0b | −3.98 ± 0.51 h | 0.33 ± 0.07b |
| 10 | 642 ± 4.00bc | −6.07 ± 0.57 g | 0.35 ± 0.06b | |
| 20 | 569 ± 23.0de | −9.36 ± 1.21d | 0.28 ± 0.02c | |
| 50 | 5 | 572 ± 24.0cde | −6.43 ± 0.34f | 0.35 ± 0.06b |
| 10 | 515 ± 6.50e | −8.50 ± 0.30e | 0.26 ± 0.08d | |
| 20 | 522 ± 5.00e | −11.03 ± 0.48b | 0.16 ± 0.08 g | |
| 75 | 5 | 602 ± 17.0bcd | −9.22 ± 0.07d | 0.23 ± 0.08e |
| 10 | 444 ± 45.0f | −9.63 ± 0.05c | 0.19 ± 0.09f | |
| 20 | 423 ± 21.0f | −15.85 ± 1.53a | 0.19 ± 0.04f |
The PDI of emulsions stabilized with sonicated GPPIs were lower than the non-sonicated GPPI fabricated emulsion. Lower PDI values were also observed for emulsions prepared with protein sonicated at higher ultrasound amplitudes i.e., 50 and 75 %. The enhanced interfacial activity and intermolecular interactions between protein particles and oil droplets could form more uniform emulsion gels with smaller PDI value [26].
The ζ-potential values of emulsion fabricated with native and sonicated GPPIs are shown in Table 1. Both ultrasonic amplitude and time increased the negative surface charge values of emulsion stabilized by sonicated proteins. The denaturation and unfolding of protein during ultrasonication exposed more negatively charged amino acids on the particle’s surfaces [27]. Sha et al. [28] also observed a slight increase in the negative surface charge for emulsion fabricated by sonicated pea protein isolate compared to emulsion fabricated by non-sonicated one.
3.2. Microstructure of emulsion gels
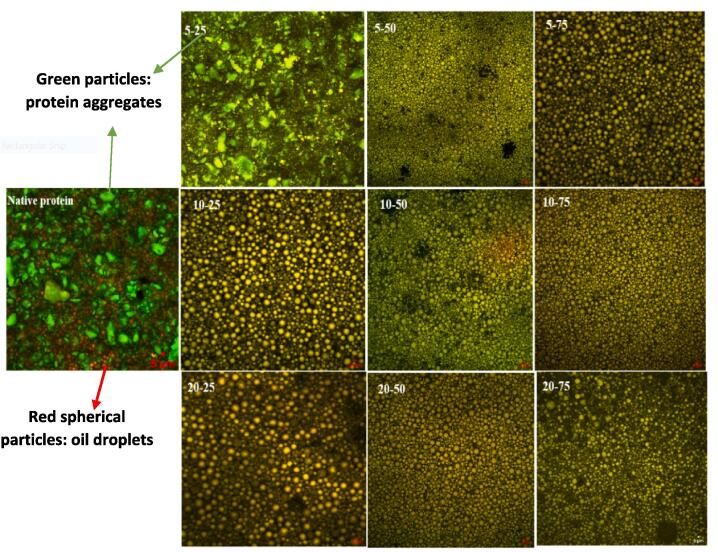
The microstructure of GPPI emulsion gels were analyzed using CLSM technique. Emulsion gel prepared with non-sonicated GPPI showed obvious large protein aggregates while those prepared with sonicated GPPIs had homogeneous microstructures with uniform droplet distributions (Fig. 1). Therefore, sonication of GPPI increased the adsorption efficiency of protein to stabilize the oil/water interface due to their higher surface hydrophobicity [9]. Moreover, HIU-treated proteins produced emulsions with smaller oil droplets (Table 1) and consequently a uniform emulsion gel networks were formed. Geng et al. [12] also reported that emulsion gels stabilized with sonicated soy protein had uniform gel network. No obvious difference was observed among the microstructure of emulsion gels fabricated by sonicated GPPIs at different amplitudes and times. However, similar to the emulsion gel fabricated by native protein, emulsion gels prepared with sonicated sample at 25 % amplitude for 5 min, showed larger protein particles and flocculated oil droplets. It seems that the shear energy caused by ultrasonication was insufficient to disrupt large aggregates during the sonication at this low amplitude and sonication time. As a results, sonicated protein particles at higher amplitudes and times could effectively form a dense gel network with embedded oil droplets within its network structure [29].
Fig. 1.
Microstructure of emulsion gels fabricated from non-sonicated and sonicated GPPIs at different ultrasonic amplitudes and times.
3.3. Gelation kinetics
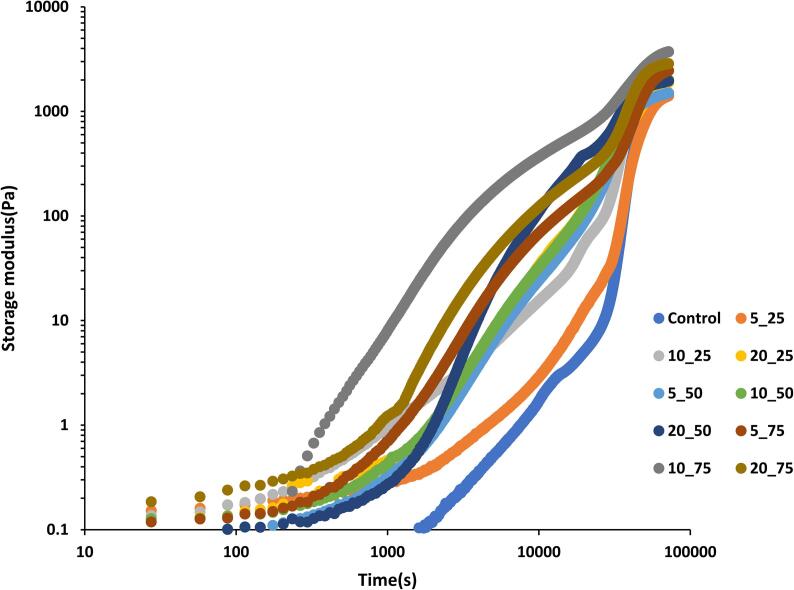
The enzymatic gelation rate of native and sonicated GPPI emulsions at 37 °C was evaluated by measuring the oscillation moduli as a function of time. Fig. 2 shows G′ as a function of time after addition of TGase for the emulsion with proteins treated by ultrasound at 25 %, 50 % and 75 % amplitudes as well as with untreated protein. Gelation appears to occur in two stages. The first stage started immediately after addition of enzyme, whereas the second stage, which caused the upward inflection in the dependency of G′ on time, was clearly visible only after about 8 h. At 75 %, the increase in G′ during the first stage was faster when the proteins were subjected to an ultrasonic treatment for up to 10 min, but it was slower when the treatment lasted for 20 min. This might be due to the oxidation of susceptible SH groups at longer sonication treatment [9]. G′ increased faster with increasing the sonication time to up to 20 min at 25 % and 50 % amplitudes. For a given duration, the first stage of gelation was slower when the sonication amplitude was lower. The subsequent increase of G′ during the second stage occurred at approximately the same rate independent of the ultrasound treatment. The gradual increase in the G′ values of sonicated GPPI emulsion gels at the second stage, reflects the development of exposed ε-(glutamyl) and lysine covalent cross-linking through the TGase reaction, which reinforces the gel networks [30].
Fig. 2.
Gelation kinetics of TGase induced emulsion gels fabricated from non-sonicated and sonicated GPPI at different sonication times and amplitudes.
In all cases, the increase of G′ stagnated after about 16 h. We note that in the absence of enzyme, G′ increased very weakly under these conditions, reaching 2 Pa after 20 h for an emulsion fabricated with proteins treated at 75 % amplitude for 10 min. This means that if any crosslinks other than those created by enzymatic crosslinking contributed to G′ value, they were formed only as a result of enzymatic crosslinking. Qin et al. [19] detected less free SH groups after enzymatic crosslinking of soy and gluten proteins, suggesting that disulfide bridges had been formed during gelation in addition to enzymatic crosslinks.
In order to understand the contribution of the dispersed oil droplets to the gelation kinetics, we repeated the measurements at 25 % and 75 % amplitude without oil. In all cases, only the second stage of the gelation was observed (Fig. 1 supplementary). The onset of gelation was slightly earlier after ultrasonic treatment, but depended little on the duration or amplitude. Most likely, the first stage of the gelation was caused by crosslinking of proteins that were adsorbed at the oil/water interface leading to the formation of a relatively weak network of crosslinked droplets. This network is subsequently reinforced by crosslinking of free proteins in the bulk.
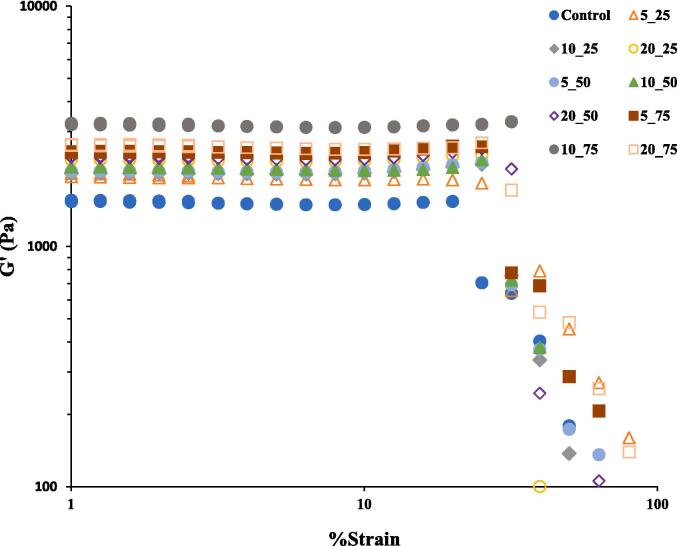
3.4. Strain sweep analysis
Dynamic strain sweep test showed that emulsion gel produced from non-sonicated protein was less resistant to strain compared to the sonicated ones (Fig. 3). With increasing the %strain to a certain amount (fracture point), the elastic modulus remained constant and a sharp decrease in the elastic modulus was observed when the %strain exceeded the linear viscoelastic region (LVR). The G′ values of emulsion gels were independent of the %strain until reaching to a strain of 25 % for sonicated GPPIs and 20 % for non-sonicated one. Therefore, non-sonicated GPPI emulsion gel had weaker and more brittle gel structure, while the sonicated GPPI emulsion gels resisted larger strain and kept their original gel structures [31]. Within LVR, the G′ values increased as the sonication time and amplitude increased. Increasing the G′ values after sonication implies that the gel network structure strengthened as a result of partial unfolding of interior functional groups and contribution of more covalent and hydrophobic bonds in the gel network [32]. However, the proteins treated at 75 % amplitude were the exception, as treatment for 10 min resulted in a stiffer gel than treatment for 20 min. The reduction of G′ value for emulsion gel fabricated from sonicated protein at 75 % amplitude for 20 min is due to the oxidation of some free SH groups at stronger HIU treatment [9].
Fig. 3.
Storage modulus as a function of strain (%) for emulsion gels fabricated from sonicated and non-sonicated GPPIs.
The yield strain of emulsion gels fabricated by sonicated GPPIs were higher than that of non-sonicated one and maintained their deformability at higher levels of strain (Table 2). Increasing the ultrasonic amplitude and time, increased the critical rupture strain. The presence of more covalent bonds [9], which provides more resistant to fracture, could potentially enhance the yield strain of emulsion gels produced from sonicated GPPIs [33]. However, the yield strain reduced as the sonication time increased to 20 min at 75 % amplitude, due to lower content of free SH groups as discussed before. Therefore, the enzymatic gelation of sonicated GPPI could potentially induce the formation of emulsion gel with stiffer gel structure [34].
Table 2.
Yield strain, storage modulus (G′) and loss tangent (tan δ) for emulsion gels fabricated from non-sonicated and sonicated GPPI at different ultrasonic amplitudes and times (f = 1 Hz).
| Amplitude (%) | Time (min) | Yield strain (%) | G′ (Pa) | tan δ |
|---|---|---|---|---|
| Control | – | 20 ± 1.4f | 1475 ± 63i | 0.135 ± 7 × 10-3 a |
| 25 | 5 | 25 ± 1.5e | 1967 ± 32 g | 0.045 ± 2 × 10-2b |
| 10 | 31 ± 2.0 d | 2017 ± 20d | 0.045 ± 3 × 10-3b | |
| 20 | 33 ± 2.1d | 2139 ± 39e | 0.043 ± 7 × 10-3b | |
| 50 | 5 | 31 ± 1.8d | 2039 ± 22d | 0.031 ± 1 × 10-2cd |
| 10 | 31 ± 1.4d | 2089 ± 47d | 0.032 ± 1 × 10-2c | |
| 20 | 37 ± 2.2c | 2280 ± 70c | 0.028 ± 2 × 10-2 d | |
| 75 | 5 | 39 ± 2.0bc | 2346 ± 47c | 0.030 ± 7 × 10-3cd |
| 10 | 49 ± 2.4a | 3822 ± 22a | 0.028 ± 7 × 10-3 d | |
| 20 | 41 ± 0.4b | 2539 ± 39b | 0.032 ± 7 × 10-3c |
3.5. Frequency sweep analysis
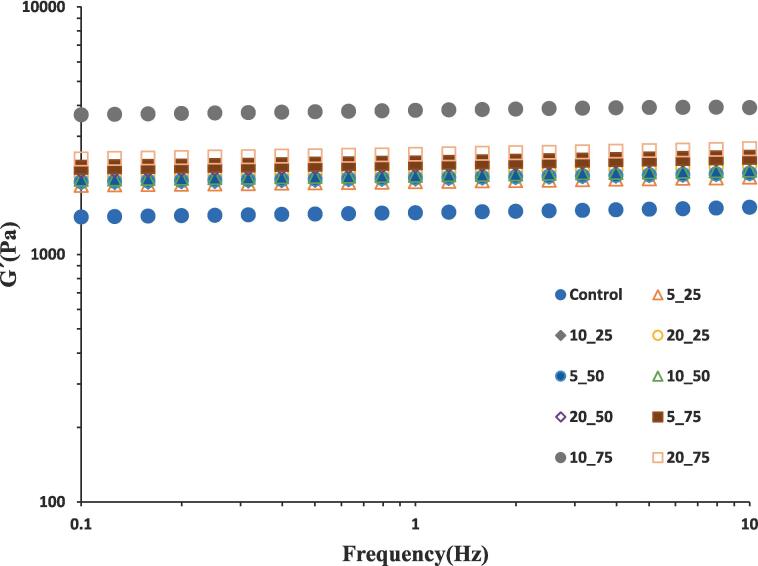
Emulsion gels exhibited very weak frequency dependency (Fig. 4) meaning that all non-sonicated and sonicated GPPIs favored the formation of fine emulsion gel structures [12]. The frequency independence of G′ is an indication of solid like nature of GPPI emulsion gels [35]. The storage moduli were always higher than the loss moduli for all samples, indicating the predominantly elastic behavior of GPPI emulsion gels (the G″ data not provided, due to the consideration of the simplicity within the figure). Hence, the GPPI emulsion gels can be considered as elastic gel network. A maximum G′ was once more discovered with proteins treated at 75 % for 10 min. The observation that the sample treated at 75 % amplitude for 10 min had a stronger gel than the protein treated for 20 min, indicates that there is an optimum ultrasonic treatment for obtaining stiff gels when using a crosslinking enzyme such as TGase.
Fig. 4.
Storage modulus of sonicated and non-sonicated samples at frequency range of 10–0.1 Hz and controlled strain of 3 %.
The loss tangent (tan δ) values were lower than 0.14 (Table 2), indicating that the elastic behavior in GPPI emulsion gels was prominent. The tan δ of emulsion gels fabricated from sonicated GPPIs, decreased as the ultrasonic amplitude increased from 25 to 50 %; while no considerable difference was observed between emulsion gels fabricated by sonicated protein at 50 and 75 % amplitudes. Lin et al. [36], also indicated that sonicated soy bean tofu with different moduli values had similar loss tangents.
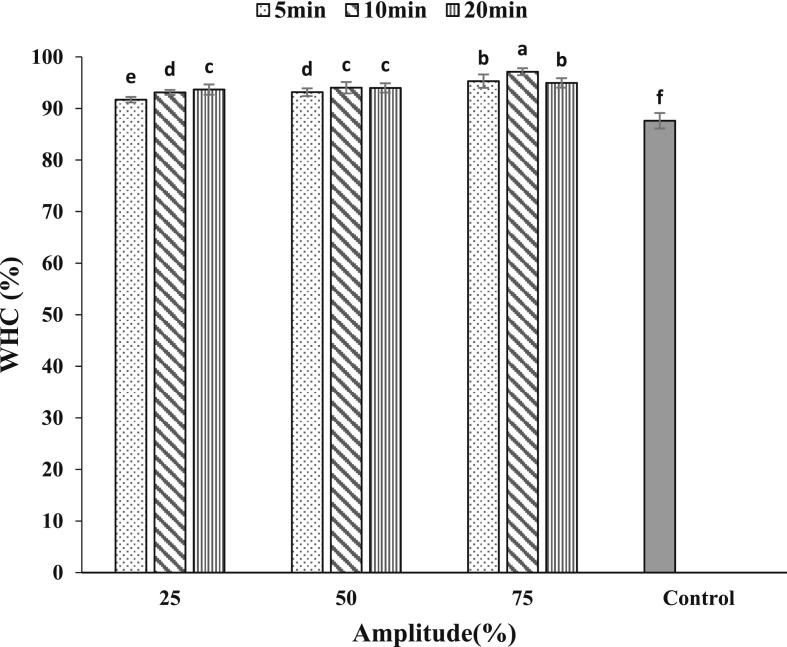
3.6. Water holding capacity
Sonicated GPPIs improved the WHC of emulsion gels due to the formation of more dense and compact gel structure (Fig. 5). This increase in WHC was more pronounced for emulsion gels fabricated by sonicated samples at 75 % amplitude. The smaller particle size and higher solubility of sonicated GPPIs [9] could enhance the formation of a dense and uniform gel network which could bind higher amount of water [30], [37]. Moreover, the formation of intermolecular covalent bonds between glutamine and lysine residues through the action of TGase enzyme could enhance the formation of dense networks with higher WHC [15]. As a result, the denser the gel structure, the more difficult it is for the water molecules to be squeezed out by the external forces. Liang et al. [15] also inferred that TGase can form intermolecular covalent cross-links between glutamyl and lysyl residues, thereby promoting the hydration capacity of whey protein emulsion gel. It can be seen from Fig. 5 that no considerable differences were observed among the WHC of emulsion gels fabricated by GPPI treated at different ultrasonication times.
Fig. 5.
Water holding capacity of emulsion gels fabricated by sonicated GPPIs at different ultrasonic amplitudes and durations.
3.7. Differential scanning calorimetry (DSC)
A characteristic endothermic peak centered around 60 °C was detected in all emulsion gels (Table 3), which indicates the dissociation of intermolecular interactions (hydrogen bonds and weak hydrophobic interactions) in the gel network [8], [26]. The emulsion gel fabricated by untreated sample exhibited two thermal transition temperatures and ΔH values. The second transition temperature is likely related to the disruption of the heat resistant small aggregates within the gel network which are thermo-resistant during exposure to heat treatment [38]. The presence of aggregates in the emulsion gel fabricated with native GPPI was also confirmed by results of CLSM images (Fig. 1). However, this second transition temperature was not detected in emulsion gels fabricated by sonicated GPPIs, demonstrating that ultrasonic treatment disrupted the aggregates and hence removed the second transition temperature. The difference in the degradation temperature (Td) was not considerable among emulsion gels. The highest Td value belonged to the emulsion gel fabricated by sonicated GPPI at 75 % for 10 min due to the higher intermolecular disulphide bonds and ε-(γ-glutamyl) lysine residues within this emulsion gel which increases the thermal resistant of gel network [9], [39].
Table 3.
Degradation temperature (Td) and enthalpy (ΔH) values of emulsion gels fabricated by sonicated GPPI at different ultrasonic amplitudes and times.
|
Ultrasound variables |
Td (°C) | ΔH (J/g) | |
|---|---|---|---|
| Amplitude (%) | Time (min) | ||
| control | – | 61.4 ± 0.1 g | 4.3 ± 0.2f |
| – | 100.6 ± 0.5a | 0.5 ± 0.1 h | |
| 25 | 5 | 63.0 ± 0.2de | 3.6 ± 0.2 g |
| 10 | 63.2 ± 0.2d | 7.3 ± 0.2d | |
| 20 | 63.1 ± 0.4d | 7.4 ± 0.7d | |
| 50 | 5 | 61.9 ± 0.3f | 6.3 ± 0.2e |
| 10 | 62.8 ± 0.3de | 6.1 ± 0.1e | |
| 20 | 63.7 ± 0.3c | 8.7 ± 0.6c | |
| 75 | 5 | 61.5 ± 0.2 fg | 9.6 ± 0.2b |
| 10 | 65.2 ± 0.3b | 10.7 ± 0.1a | |
| 20 | 62.8 ± 0.3de | 9.6 ± 0.1b | |
The development of intermolecular interactions during emulsion gel formation was also confirmed by the increase in ΔH of emulsion gels fabricated by sonicated GPPIs. The higher ΔH value highlights the development of intermolecular interactions (hydrogen bonds, hydrophobic interactions and ɛ-(γ-glutamyl) lysine isopeptide bonds) during enzymatic gelation [40]. Hence, more energy is required to disrupt the highly cross-linked structures [40], [41]. On the other hand, sonicated proteins reinforced the interfacial layer of emulsion; thus, a more stable emulsion gel networks was formed which required higher temperature for the structural disintegration. As a results, the emulsion gels fabricated by sonicated GPPIs were more heat stable and would require more heat absorption [26].
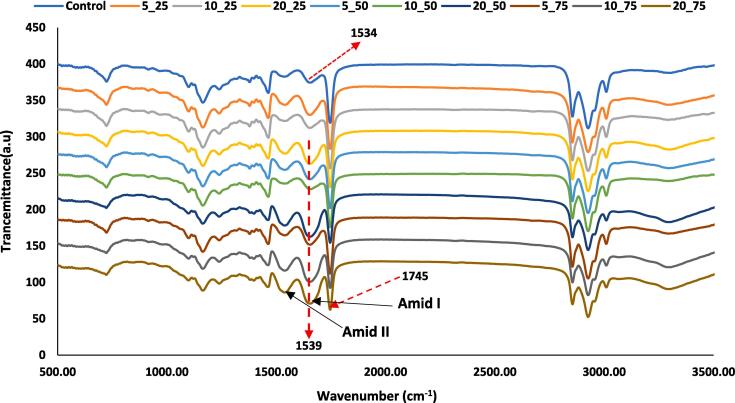
3.8. FT-IR spectroscopy
The FT-IR spectra of emulsion gels are shown in Fig. 6. The main spectral bands consisted of three intense bands including amid I (1700–1575 cm−1), amid II (1550–1510 cm−1) and amid III (1238 cm−1). Amid I region (1600–1700 cm−1) is mainly associated with the stretching vibration of carbonyl group (C O), C—N, and N—H bending contributions. Amid I region directly represents the backbone conformation and hydrogen bonding of protein secondary structure [14]. Ultrasonication of GPPI increased the intensity of amid I region. This increase could be associated to the changes occurred in the secondary structure of protein under sonication. Ultrasonication stretches the molecular structure of the protein and exposes more functional groups to the surface which results in the change in the intensity of amid I region [42]. Similarly, the changes in transmittance intensity and peak positions for amide I region were previously reported by Malik and Sani [35] for sonicated sunflower meal.
Fig. 6.
FTIR spectroscopy of emulsion gels fabricated from sonicated GPPIs at different ultrasonic amplitudes and times.
Compared to the spectrum of emulsion gel fabricated by non-sonicated GPPI, the peak position of amid II bands for those prepared by sonicated samples at amplitudes of 50 and 75 %, shifted from 1534 cm−1 to 1539 cm−1 and their intensities increased. These changes in the amid II bands, indicate the alteration in the secondary structure of GPPI due to the breakup of internal interactions thorough the ultrasonic cavitation effect [35]. The absorption peak at 1745 cm−1 corresponding to the C O stretching vibration, was exhibited in the FT-IR spectra of emulsion gels. This peak can be attributed to residue of corn oil in the emulsion [2]. Sonication had no considerable effect on the peak intensity at 1745 cm−1 and amid III region.
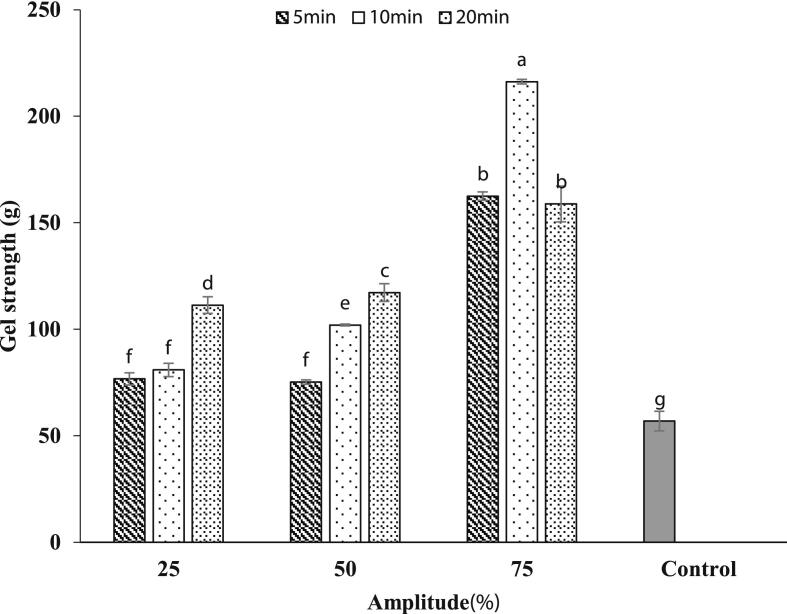
3.9. Gel strength
Ultrasonic treatment improved the strength of GPPI-emulsion gels (Fig. 7). The lower gel strength of native GPPI emulsion gel is due to its weaker network structure. The aggregate form of native GPPI, weakens the intermolecular interactions within the emulsion gel network [43]. While, higher gel strength for the emulsion gels fabricated by sonicated GPPIs is related to the larger number of exposed hydrophobic groups which favors the intermolecular interaction between protein particles and oil droplets in the emulsion gels. Besides, when glutamyl and lysyl residues are exposed through the ultrasonic cavitation effect, the accessibility of TGase to the glutamyl and lysyl residues increases, and consequently, the strength of the gel structure is improved. The sonicated GPPIs could enhance the protein–protein interactions during gel formation and consequently increase the gel forming ability of protein within the emulsion gel [44]. On the other hand, the particle size of emulsion droplets reduced after the ultrasonication of GPPI. Therefore, a compact emulsion gel network with improved gel strength can be formed [26]. Similarly, Zhang et al. [26] also found that the emulsion gel stabilized by SPI and pectin exhibited a compact and dense network with higher gel strength when SPI was subjected to HIU treatment.
Fig. 7.
Gel strength of emulsion gels fabricated by sonicated samples at different ultrasonic amplitudes and duration.
The emulsion gel strength significantly increased with increasing the ultrasonication amplitude and time. Stronger ultrasonic treatment unfolds the protein molecules and exposes more susceptible reactive groups to the surface of protein which increases the gel strength by reinforcement of intermolecular interactions within the emulsion gel network [45]. Similarly, Qin et al. [19], reported that the SPI gel strength and its mixture with wheat gluten, increased when sonication time increased to 30 min. They stated that the particle size reduction along with the exposure of hydrophobic and free SH groups facilitated the TGase cross-linking and strengthened the gel elasticity [19]. The strength of emulsion gel decreased when GPPI was subjected to ultrasonication amplitude of 75 % for 20 min. This decrease might be due to the oxidation of reactive SH groups at longer sonication time [9]. In this case the emulsion gel breaks more easily when exposed to the external forces. Therefore, it can be concluded that the gel strength of sonicated-GPPI emulsion gel is mainly influenced by the content of SH groups, glutamyl and lysyl residues, and the emulsions droplets size.
4. Conclusion
Emulsion gels fabricated by sonicated GPPI exhibited a homogeneous and uniform gel network. Enzymatic gelation of emulsions stabilized by GPPI occurs in two steps. A relatively fast stage leading to relatively weak gels is followed by a much slower stage during which the gels are reinforced by more than an order of magnitude. The first gelation step was not observed in the absence of oil from which we deduce that it was caused by crosslinking of proteins adsorbed at the oil/water interface. The second step occurred at the same rate with and without oil and was caused by crosslinking of the proteins in the water phase. The rate of gel formation was faster for sonicated proteins during incubation. The emulsion gels fabricated by sonicated GPPI at higher ultrasonic amplitudes and times showed elastic behavior and more resistant to strain due to the presence of permanent intermolecular cross-links (disulphide bonds and ε-(γ-glutamyl) lysine residues). The WHC and gel strength of emulsion gels prepared from sonicated GPPI were higher due to the formation of a dense and uniform gel network. Higher intermolecular cross-links promoted the thermal stability of emulsion gels. As a results, HIU treatment played a critical role in formation of stable protein-emulsion gels with improved microstructure and elasticity which can be used in food applications as a structuring agent and delivery of hydrophobic bioactive compounds.
CRediT authorship contribution statement
Rassoul Mozafarpour: Formal analysis, Methodology, Writing – original draft. Arash Koocheki: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors greatly thank Ferdowsi University of Mashhad for financial and laboratory support (grant number: 3/52573).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.106278.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Li P., Li X., Nisar T., Yang X., Sun J., Yang X., Guo Y. Structural characteristics of binary biopolymers-based emulsion-filled gels: A case of mixed sodium caseinate/methyl cellulose emulsion gels. Food Struct. 2021;30 doi: 10.1016/j.foostr.2021.100233. [DOI] [Google Scholar]
- 2.Hu S., Wu J., Zhu B., Du M., Wu C., Yu C., Song L., Xu X. Low oil emulsion gel stabilized by defatted Antarctic krill (Euphausia superba) protein using high-intensity ultrasound. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Y., Mao L., Zheng H., Chen H., Gao Y. Characterization of β-carotene loaded emulsion gels containing denatured and native whey protein. Food Hydrocoll. 2020;102 doi: 10.1016/j.foodhyd.2019.105600. [DOI] [Google Scholar]
- 4.Mao L., Lu Y., Cui M., Miao S., Gao Y. Design of gel structures in water and oil phases for improved delivery of bioactive food ingredients. Crit. Rev. Food Sci. Nutr. 2020;60:1651–1666. doi: 10.1080/10408398.2019.1587737. [DOI] [PubMed] [Google Scholar]
- 5.Gharibzahedi S.M.T., Smith B. The functional modification of legume proteins by ultrasonication: A review. Trends Food Sci. Technol. 2020;98:107–116. [Google Scholar]
- 6.Mahdavian Mehr H., Koocheki A. Effect of atmospheric cold plasma on structure, interfacial and emulsifying properties of Grass pea (Lathyrus sativus L.) protein isolate. Food Hydrocoll. 2020;106 doi: 10.1016/j.foodhyd.2020.105899. [DOI] [Google Scholar]
- 7.Mozafarpour R., Sani M.A., Koocheki A., McClements D.J., Mehr H.M. Ultrasound modified protein colloidal particles: Interfacial activity, gel property and encapsulation efficiency. Adv. Colloid Interface Sci. 2022;102768 doi: 10.1016/j.cis.2022.102768. [DOI] [PubMed] [Google Scholar]
- 8.Feyzi S., Milani E., Golimovahhed Q.A. Grass Pea (Lathyrus sativus L.) Protein Isolate: The Effect of Extraction Optimization and Drying Methods on the Structure and Functional Properties. Food Hydrocoll. 2018;74:187–196. doi: 10.1016/j.foodhyd.2017.07.031. [DOI] [Google Scholar]
- 9.Mozafarpour R., Koocheki A., Nicolai T. Modification of grass pea protein isolate (Lathyrus sativus L.) using high intensity ultrasound treatment: Structure and functional properties. Food Res. Int. 2022;158 doi: 10.1016/j.foodres.2022.111520. [DOI] [PubMed] [Google Scholar]
- 10.Kahraman O., Petersen G.E., Fields C. Physicochemical and Functional Modifications of Hemp Protein Concentrate by the Application of Ultrasonication and pH Shifting Treatments. Foods. 2022;11:587. doi: 10.3390/foods11040587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y., Cheng Y., Zhang Z., Wang Y., Mintah B.K., Dabbour M., Jiang H., He R., Ma H. Modification of rapeseed protein by ultrasound-assisted pH shift treatment: Ultrasonic mode and frequency screening, changes in protein solubility and structural characteristics. Ultrason. Sonochem. 2020;69 doi: 10.1016/j.ultsonch.2020.105240. [DOI] [PubMed] [Google Scholar]
- 12.Geng M., Wang Z., Qin L., Taha A., Du L., Xu X., Pan S., Hu H. Effect of ultrasound and coagulant types on properties of β-carotene bulk emulsion gels stabilized by soy protein. Food Hydrocoll. 2022;123 doi: 10.1016/j.foodhyd.2021.107146. [DOI] [Google Scholar]
- 13.Shen X., Fang T., Gao F., Guo M. Effects of ultrasound treatment on physicochemical and emulsifying properties of whey proteins pre- and post-thermal aggregation. Food Hydrocoll. 2017;63:668–676. doi: 10.1016/j.foodhyd.2016.10.003. [DOI] [Google Scholar]
- 14.Martínez-Velasco A., Lobato-Calleros C., Hernández-Rodríguez B.E., Román-Guerrero A., Alvarez-Ramirez J., Vernon-Carter E.J. High intensity ultrasound treatment of faba bean (Vicia faba L.) protein: Effect on surface properties, foaming ability and structural changes. Ultrason. Sonochem. 2018;44:97–105. doi: 10.1016/j.ultsonch.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Liang X., Ma C., Yan X., Zeng H., McClements D.J., Liu X., Liu F. Structure, rheology and functionality of whey protein emulsion gels: Effects of double cross-linking with transglutaminase and calcium ions. Food Hydrocoll. 2020;102 [Google Scholar]
- 16.Sun X.D., Arntfield S.D. Molecular forces involved in heat-induced pea protein gelation: Effects of various reagents on the rheological properties of salt-extracted pea protein gels. Food Hydrocoll. 2012;28:325–332. doi: 10.1016/j.foodhyd.2011.12.014. [DOI] [Google Scholar]
- 17.Hu H., Zhu X., Hu T., Cheung I.W.Y., Pan S., Li-Chan E.C.Y. Effect of ultrasound pre-treatment on formation of transglutaminase-catalysed soy protein hydrogel as a riboflavin vehicle for functional foods. J. Funct. Foods. 2015;19:182–193. doi: 10.1016/j.jff.2015.09.023. [DOI] [Google Scholar]
- 18.Zhang P., Hu T., Feng S., Xu Q., Zheng T., Zhou M., Chu X., Huang X., Lu X., Pan S. Effect of high intensity ultrasound on transglutaminase-catalyzed soy protein isolate cold set gel. Ultrason. Sonochem. 2016;29:380–387. doi: 10.1016/j.ultsonch.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Qin X.-S., Luo S.-Z., Cai J., Zhong X.-Y., Jiang S.-T., Zhao Y.-Y., Zheng Z. Transglutaminase-induced gelation properties of soy protein isolate and wheat gluten mixtures with high intensity ultrasonic pretreatment. Ultrason. Sonochem. 2016;31:590–597. doi: 10.1016/j.ultsonch.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Torres O., Murray B., Sarkar A. Emulsion microgel particles: Novel encapsulation strategy for lipophilic molecules. Trends Food Sci. Technol. 2016;55:98–108. doi: 10.1016/j.tifs.2016.07.006. [DOI] [Google Scholar]
- 21.Kumar M., Tomar M., Potkule J., Reetu S., Punia J., Dhakane-Lad S., Singh S., Dhumal P.C., Pradhan B., Bhushan T., Anitha O., Alajil A., Alhariri R., Amarowicz J.F.K. Functional characterization of plant-based protein to determine its quality for food applications. Food Hydrocoll. 2022;123 doi: 10.1016/j.foodhyd.2021.106986. [DOI] [Google Scholar]
- 22.Hu H., Fan X., Zhou Z., Xu X., Fan G., Wang L., Huang X., Pan S., Zhu L. Acid-induced gelation behavior of soybean protein isolate with high intensity ultrasonic pre-treatments. Ultrason. Sonochem. 2013;20:187–195. doi: 10.1016/j.ultsonch.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y., Wang Y., Li K., Bai Y., Li B., Xu W. Effect of high intensity ultrasound on physicochemical, interfacial and gel properties of chickpea protein isolate. LWT. 2020;129 doi: 10.1016/j.lwt.2020.109563. [DOI] [Google Scholar]
- 24.Cheng Y., Ofori Donkor P., Yeboah G.B., Ayim I., Wu J., Ma H. Modulating the in vitro digestion of heat-set whey protein emulsion gels via gelling properties modification with sequential ultrasound pretreatment. LWT. 2021;149 doi: 10.1016/j.lwt.2021.111856. [DOI] [Google Scholar]
- 25.Zhang X., Zuo Z., Ma W., Yu P., Li T., Wang L. Assemble behavior of ultrasound-induced quinoa protein nanoparticles and their roles on rheological properties and stability of high internal phase emulsions. Food Hydrocoll. 2021;117 doi: 10.1016/j.foodhyd.2021.106748. [DOI] [Google Scholar]
- 26.Zhang X., Chen X., Gong Y., Li Z., Guo Y., Yu D., Pan M. Emulsion gels stabilized by soybean protein isolate and pectin: Effects of high intensity ultrasound on the gel properties, stability and β-carotene digestive characteristics. Ultrason. Sonochem. 2021;79 doi: 10.1016/j.ultsonch.2021.105756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang L., Wang J., Li Y., Wang Z., Liang J., Wang R., Chen Y., Ma W., Qi B., Zhang M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014;62:595–601. [Google Scholar]
- 28.Sha L., Koosis A.O., Wang Q., True A.D., Xiong Y.L. Interfacial dilatational and emulsifying properties of ultrasound-treated pea protein. Food Chem. 2021;350 doi: 10.1016/j.foodchem.2021.129271. [DOI] [PubMed] [Google Scholar]
- 29.Mao L., Roos Y.H., Miao S. Study on the Rheological Properties and Volatile Release of Cold-Set Emulsion-Filled Protein Gels. J. Agric. Food Chem. 2014;62:11420–11428. doi: 10.1021/jf503931y. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z., Regenstein J.M., Zhou P., Yang Y. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrason. Sonochem. 2017;34:960–967. doi: 10.1016/j.ultsonch.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Zhao X., Li D., Wang L., Wang Y. Rheological properties and microstructure of a novel starch-based emulsion gel produced by one-step emulsion gelation: Effect of oil content. Carbohydr. Polym. 2022;281 doi: 10.1016/j.carbpol.2021.119061. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Harb S., Panouillé M., Huc-Mathis D., Moulin G., Saint-Eve A., Irlinger F., Bonnarme P., Michon C., Souchon I. The rheological and microstructural properties of pea, milk, mixed pea/milk gels and gelled emulsions designed by thermal, acid, and enzyme treatments. Food Hydrocoll. 2018;77:75–84. doi: 10.1016/j.foodhyd.2017.09.022. [DOI] [Google Scholar]
- 33.Ben-Harb S., Panouille M., Huc-Mathis D., Moulin G., Saint-Eve A., Irlinger F., Bonnarme P., Michon C., Souchon I. The rheological and microstructural properties of pea, milk, mixed pea/milk gels and gelled emulsions designed by thermal, acid, and enzyme treatments. Food Hydrocoll. 2018;77:75–84. [Google Scholar]
- 34.Wang X., Luo K., Liu S., Zeng M., Adhikari B., He Z., Chen J. Textural and rheological properties of soy protein isolate tofu-type emulsion gels: influence of soybean variety and coagulant type. Food Biophys. 2018;13:324–332. [Google Scholar]
- 35.Malik M.A., Saini C.S. Rheological and structural properties of protein isolates extracted from dephenolized sunflower meal: Effect of high intensity ultrasound. Food Hydrocoll. 2018;81:229–241. doi: 10.1016/j.foodhyd.2018.02.052. [DOI] [Google Scholar]
- 36.Lin H.-F., Lu C.-P., Hsieh J.-F., Kuo M.-I. Effect of ultrasonic treatment on the rheological property and microstructure of tofu made from different soybean cultivars. Innov. Food Sci. Emerg. Technol. 2016;37:98–105. doi: 10.1016/j.ifset.2016.08.013. [DOI] [Google Scholar]
- 37.Hu H., Li-Chan E.C.Y., Wan L., Tian M., Pan S. The effect of high intensity ultrasonic pre-treatment on the properties of soybean protein isolate gel induced by calcium sulfate. Food Hydrocoll. 2013;32:303–311. doi: 10.1016/j.foodhyd.2013.01.016. [DOI] [Google Scholar]
- 38.Khatkar A.B., Kaur A., Khatkar S.K., Mehta N. Characterization of heat-stable whey protein: Impact of ultrasound on rheological, thermal, structural and morphological properties. Ultrason. Sonochem. 2018;49:333–342. doi: 10.1016/j.ultsonch.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 39.Chandrapala J., Zisu B., Palmer M., Kentish S., Ashokkumar M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011;18:951–957. doi: 10.1016/j.ultsonch.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Shahbazi M., Jäger H., Chen J., Ettelaie R. Construction of 3D printed reduced-fat meat analogue by emulsion gels. Part II: Printing performance, thermal, tribological, and dynamic sensory characterization of printed objects. Food Hydrocoll. 2021;121 doi: 10.1016/j.foodhyd.2021.107054. [DOI] [Google Scholar]
- 41.Lo B., Kasapis S., Farahnaky A. Effect of low frequency ultrasound on the functional characteristics of isolated lupin protein. Food Hydrocoll. 2022;124 doi: 10.1016/j.foodhyd.2021.107345. [DOI] [Google Scholar]
- 42.Wang Y., Zhang Z., He R., Liu D., Kumah Mintah B., Dabbour M., Ma H. Improvement in enzymolysis efficiency and changes in conformational attributes of corn gluten meal by dual-frequency slit ultrasonication action. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.105038. [DOI] [PubMed] [Google Scholar]
- 43.Jambrak A.R., Mason T.J., Lelas V., Herceg Z., Herceg I.L. Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J. Food Eng. 2008;86:281–287. doi: 10.1016/j.jfoodeng.2007.10.004. [DOI] [Google Scholar]
- 44.Wen C., Zhang J., Yao H., Zhou J., Duan Y., Zhang H., Ma H. Advances in renewable plant-derived protein source: The structure, physicochemical properties affected by ultrasonication. Ultrason. Sonochem. 2019;53:83–98. doi: 10.1016/j.ultsonch.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 45.Li M., Yang R., Feng X., Fan X., Liu Y., Xu X., Zhou G., Zhu B., Ullah N., Chen L. Effects of low-frequency and high-intensity ultrasonic treatment combined with curdlan gels on the thermal gelling properties and structural properties of soy protein isolate. Food Hydrocoll. 2022;127 doi: 10.1016/j.foodhyd.2022.107506. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.