Abstract
C-reactive protein (CRP) is a normal constituent of human sera synthesized by hepatocytes and induced by proinflammatory cytokines. The function of this acute-phase reactant includes activation of complement and enhancement of opsonophagocytosis. CRP binds to phosphorylcholine (ChoP), a constituent of eukaryotic membranes that is also found on the cell surface of major bacterial pathogens of the human respiratory tract, including Streptococcus pneumoniae and Haemophilus influenzae. The presence of CRP on mucosal surfaces and role in innate immunity in the human respiratory tract where ChoP-containing organisms reside have not been previously studied. We have shown using a monoclonal antibody to CRP that CRP is present in inflamed (0.17 to 42 μg/ml) and uninflamed (<0.05 to 0.88 μg/ml) secretions from the human respiratory tract in sufficient quantities for an antimicrobial effect. In addition, the CRP gene was expressed in epithelial cells of the human respiratory tract using in situ hybridization on nasal polyps and reverse transcriptase PCR of pharyngeal cells in culture. The complement-dependent bactericidal activity of normal nasal airway surface fluid and sputum against ChoP-expressing H. influenzae was abolished when the secretions were pretreated to remove CRP. In summary, the results indicate that CRP is present in secretions of the human respiratory tract, that human respiratory epithelial cells are capable of CRP expression, and that this protein may contribute to bacterial clearance in the human respiratory tract.
C-reactive protein (CRP) is a constituent of normal human serum (NHS) (33). The concentration of CRP in serum is generally less than 2 μg/ml but increases by as much as 1,000-fold in response to a stimulus such as tissue injury or inflammation (5). Following removal of the inflammatory stimulus, CRP levels decline rapidly. These features have made CRP useful as a clinical marker of an inflammatory process, although the function of this acute-phase reactant and its precise role in host defense remain poorly understood. Until recently there had been no demonstration of a direct antimicrobial effect of CRP in vitro, and the study of CRP using animal models has been limited by the marked differences in the regulation of CRP expression in animals compared to humans (27, 46). There is, however, evidence to suggest that CRP contributes to innate immunity. Mice, which have a constitutively low level of CRP expression, are more resistant to experimental pneumococcal sepsis when carrying the human CRP transgene conferring inducible high-level expression as in humans (34). The protective effect of CRP is thought to be mediated by its ability to act as an opsonin and, when bound, to activate the complement by the classical pathway through interaction with complement component C1q (16, 37). The CRP transgene reduces bacteremia following an intraperitoneal inoculation of pneumococci in both decomplemented and complement-expressing mice, suggesting that there is also a complement-independent pathway for CRP-mediated protection, perhaps through direct opsonization (35).
CRP received its name because it binds to the C polysaccharide or cell wall teichoic acid of Streptococcus pneumoniae. It is now known that CRP binds in a calcium-dependent manner to choline phosphate or phosphorylcholine (ChoP) residues found on C polysaccharide (38). ChoP had been considered to be a highly unusual structural feature in prokaryotes. It is now clear that in addition to S. pneumoniae, many of the bacterial species that normally inhabit the respiratory tract express ChoP, the molecular target of CRP, on their cell surface. These species are now known to include Streptococcus oralis and S. mitis, Haemophilus influenzae and H. somnus, Actinobacillus actinomycetemcomitans, Fusobacterium nucleatum, various Actinomyces species, the commensal Neisseria species, and Mycoplasma species such as M. fermentans (8, 14, 24, 29, 30, 41, 45). The presence of ChoP on a large and diverse collection of species found primarily on the mucosal surface of the airway including gram-positive and gram-negative bacteria, as well as Mollicutes, and its absence from species residing outside the respiratory tract, suggests that this structure contributes to survival in this host environment. Data from both animal models of nasopharyngeal carriage and natural lower respiratory tract infection of humans suggest that ChoP, while not a requirement for survival within the respiratory tract, contributes to the ability of bacteria to persist at these sites (44, 45). The expression of ChoP has been shown to confer resistance to antimicrobial peptides found on the mucosal surface of the upper respiratory tract, such as LL-37, that target structural differences in membranes between host and microbial cells (21). In this regard, the expression of ChoP may allow bacteria to mimic the same structure found on all eukaryotic membrane lipids in the form of phosphatidylcholine. ChoP also allows for bacterial invasion of epithelial cells through interaction with the receptor for platelet-activating factor (rPAF), whose natural ligand, PAF, also contains ChoP (8, 32). CRP, therefore, may contribute to innate immunity by specifically targeting this virulence determinant common to many of the bacterial pathogens of the respiratory tract. A direct antimicrobial effect of CRP, however, has been demonstrated only in the case of H. influenzae, where concentrations of the protein as low as 10 ng/ml bind to ChoP and mediate a complement-dependent bactericidal effect (44). Another striking feature of the ChoP ligand is that there is phase variation in the amount or presence on the bacterial cell surface in many of the species expressing this moiety (17, 40, 42, 45). For H. influenzae, only those phase variants expressing ChoP on their lipopolysaccharide are sensitive to the bactericidal effects of human serum CRP (44). This suggests that these pathogens have developed an efficient means for evasion of clearance mechanisms such as that involving CRP that specifically targets ChoP. The interplay between the expression of ChoP and local amounts of CRP, therefore, may function to maintain the commensal state and limit the pathogenicity of many important respiratory tract bacteria.
Human serum CRP is a cyclic pentameric protein of five identical nonglycosylated subunits of 206 amino acids, each with a molecular mass of 24 kDa, that are noncovalently bound to form the mature CRP molecule (13). Serum CRP is synthesized by hepatocytes in the liver as a single-chain precursor with a cleavable signal sequence at the N terminus (36). CRP was initially thought to be produced and secreted only by hepatocytes under induction primarily by interleukin-6 (IL-6), with a synergistic effect of IL- 1 (39, 48). Tumor necrosis factor alpha, transforming growth factor β, and IL-11 have also been shown to affect hepatic CRP expression (3, 4, 10, 15, 23). There is also evidence of CRP expression by Kupffer cells and peripheral blood mononuclear cells, where it was shown to be a membrane protein that is not secreted (12, 19). Although the source and regulation of serum CRP have been extensively studied, the expression of CRP in the human respiratory tract, particularly the heavily colonized upper respiratory tract where organisms bearing its ChoP target reside, has not been addressed. In this report, we show that CRP is found in secretions from the human airway and that epithelial cells lining the mucosal surface of the upper respiratory tract may be a source of this protein. Furthermore, we demonstrate that CRP isolated from the mucosal surface of the airway has antimicrobial activity.
MATERIALS AND METHODS
Preparation of specimens from the human respiratory tract.
Sputum specimens were obtained from adults with pneumonia or bronchitis diagnosed according to previously described criteria (25). Nasal airway surface fluid (ASF) was collected from healthy nonsmoking volunteers without dilution or chemical stimulation. Sputum and ASF were solubilized by treatment in acetonitrile (final concentration, 60%) and trifluoroacetic acid (final concentration, 0.1%) for 16 h at 25°C as previously described (2). After insoluble debris was removed by centrifugation at 1,500 × g for 10 min, the solution was lyophilized. The extracted material was resuspended using sonication in deionized water to the original volume; 1.0 M Tris-HCl (pH 7.5) was added until the solution was no longer acidic. Samples were stored at −20°C.
Cell culture.
Detroit 562 cells (CCL 138; American Type Tissue Collection, Manassas, Va.), a human pharyngeal carcinoma cell line, were grown in minimal essential Medium (Gibco BRL, Gaithersburg, Md.) with l-glutamine supplemented with sodium pyruvate (1 mM) and 10% fetal bovine serum (HyClone, VWR Scientific, Philadelphia, Pa.) along with penicillin (10 μg/ml) and streptomycin (10 μg/ml) (Gibco BRL) to confluence and then harvested using trypsin (0.25%, final concentration) and EDTA (0.02%, final concentration) (Gibco BRL). Cells were frozen in fetal bovine serum (HyClone, VWR Scientific) with dimethyl sulfoxide (final concentration, 10%), placed overnight at −70°C in a 1°C freezer container, and then stored in liquid nitrogen.
Treatment to remove CRP.
Solubilized sputum and ASF or tissue culture supernatant were treated with an equal volume of immobilized p-aminophenyl phosphorylcholine-agarose beads (Pierce Chemical Co., Rockford, Ill.) that had been washed in a buffer containing calcium (0.1 M Tris, 0.1 M NaCl, and 1 mM CaCl2 [pH 8.2]) as previously described (44). After incubation with the beads for 1 h at 4°C, the supernatant was removed for analysis.
Western analysis.
Samples for Western analysis were treated at 100°C for 5 min in gel loading buffer (10% glycerol, 2% sodium dodecyl sulfate [SDS], 50 mM Tris-Cl [pH 6.8], 100 mM β-mercaptoethanol, bromophenol blue). Proteins in solubilized sputum and ASF or tissue culture supernatant from confluent monolayers were separated by polyacrylamide gel electrophoresis (PAGE) on an SDS–12.5% polyacrylamide gel, transferred to Immobilon-P membranes (Millipore, Bedford, Mass.), immunoblotted with a monoclonal antibody (MAb) to human CRP, CRP-8 murine immunoglobulin G1 (IgG1; Sigma Chemical Co., St. Louis, Mo.) at a dilution of 1:10,000 or goat CRP antiserum (Sigma) at a dilution of 1:10,000, and detected with alkaline phosphatase conjugated to anti-mouse IgG (Sigma) at a dilution of 1:10,000 or anti goat IgG (Sigma) at a dilution of 1:10,000 as previously described (44). The concentration of CRP in each specimen was determined by comparison to a standard curve consisting of purified human CRP (Sigma) of known concentration on the same blot by digitalization with an AlphaImager gel documentation system (Alpha Innotech Corporation, San Leandro, Calif.). A similar approach was used to quantify amounts of total IgG in solubilized sputum and ASF. In these experiments, a goat anti-human IgG-alkaline phosphatase conjugate (Sigma) was used, and reactivity was compared to a standard curve consisting of purified human IgG (Sigma) of known concentration. Total protein content of the solubilized material was determined by a micro-bicinchoninic acid assay as instructed by the manufacturer (Pierce).
Immunocytochemistry.
Detroit 562 cells grown to confluence were harvested, and 105 cells/ml were allowed to adhere to sterilized glass coverslips in 24-well cell cluster plates (Corning Costar, Cambridge, Mass.) by overnight incubation at 37°C. The coverslips were gently washed in sterile phosphate-buffered saline (PBS), and then the cells were fixed by treatment in 4% paraformaldehyde in PBS for 10 min at room temperature. After the coverslips were washed twice more with PBS, the cells were permeabilized in methanol for 2 min at room temperature. The coverslips were blocked with 1% bovine serum albumin (BSA) in PBS prior to incubation with CRP-8 (Sigma) or an IgG1 isotype control, HASP 4 (against 6A+6B capsular polysaccharide of S. pneumoniae; Statens Seruminstitut, Copenhagen, Denmark) at a dilution of 1:1,000 in 1% BSA in PBS overnight at 37°C. After 10 washes with PBS, fluorescein isothiocyanate-labeled anti-mouse IgG conjugate (Sigma) at a dilution of 1:100 was added in 1% BSA for 30 min at 37°C. The coverslips were then washed 10 times in PBS, mounted on glass slides with Vectashield (Vector Laboratories, Burlingame, Calif.), and viewed with a fluorescence microscope.
RT-PCR.
Poly(A) mRNA was isolated from 5 × 106 to 1 × 107 Detroit 562 pharyngeal carcinoma cells, using an Oligotex kit (Qiagen, Valencia, Calif.). RNA was resuspended in diethyl pyrocarbonate (Sigma)-treated water and stored at −70°C. The reverse transcriptase (RT) reaction mixture consisted of 10 μl of poly(A) mRNA (approximately 1 μg) added to 30 pg of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) reverse primer (sequence listed below) and 30 pg of CRP reverse primer (sequence listed below). The templates were then added to 5 μl of 5× Moloney murine leukemia virus buffer (Promega, Madison, Wis.), 1.25 μl of 10 mM deoxynucleoside triphosphates (Promega), 25 U of RNasin RNase inhibitor (Promega), 200 U of Moloney murine leukemia virus RT (Promega), diethyl pyrocarbonate-treated and H2O to a total volume of 25 μl. The reaction mixture was kept at room temperature for 20 min, at 37°C for 60 min, and at 75°C for 15 min and then placed on ice. For PCR, the RT template was added to PCR buffer (Promega), deoxynucleoside triphosphates (Promega), and Taq polymerase (Promega), plus either 10 pg of CRP forward and reverse primers or 30 pg of GAPDH forward and reverse primers. PCR conditions included an initial denaturation for 3 min at 94°C, followed by 40 cycles of denaturation for 1 min at 94°C, primer annealing for 1 min at 55°C, and elongation for 1 min at 72°C. Poly(A) mRNA as the PCR template was used as the negative control; cDNA obtained from human liver mRNA served as the positive control. The primers were designed based on the human sequence listed in GenBank (accession no. M11725). The sequences of the primers were as follows: CRP forward, 5′-TTTTCTCGTATGCCACCAAG-3′; CRP reverse, 5′-TTTCCAATGTCTCCCACCAG-; GAPDH forward, 5′-AAGGTCGGAGTCAACGGATTTGG; and GAPDH reverse, 5′-GAGATGATGACCCTTTTGGCTCCC-3′.
Preparation of riboprobe.
The riboprobes were made using primers for amplification based on the full human CRP gene (forward, 5′-CGAGGAAGGCTTTTGTGTTT-3′; reverse, 5′-GGGGTTTGGTGAACACTTCG-3′ ). The PCR product was made using the CRP primers and 0.5 μg of human chromosomal DNA as a template as described above except that the initial denaturation was at 94°C for 4 min, followed by denaturation at 94°C for 10 s, annealing at 50°C for 10 s, and elongation at 74°C for 2 min, for a total of 30 cycles. The PCR product was cloned in both orientations into the pCR2.1 vector (InVitrogen Corp., San Diego, Calif.). The plasmid was linearized by digestion with HindIII and transcribed using T7 RNA polymerase (MBI Fermentas, Amherst, N.Y.) in the presence of [35S]UTP (NEN Life Sciences Products, Boston, Mass.) to create sense and antisense riboprobes for in situ hybridization.
In situ hybridization.
In situ hybridization was performed as previously described (21) on paraformaldehyde-fixed human nasal polyp and explanted liver tissue sections (7 μm) obtained from the pathology department at the Children's Hospital of Philadelphia. The hybridization was performed at 50°C overnight in hybridization buffer containing 50% formamide, 25% dextran sulfate, 0.3 M NaCl, 10 mM NaH2PO4, 5 mM EDTA, 0.2% Ficoll 400, 0.2% polyvinylpyrrolidone, 1 M dithiothreitol, 10 mM Tris-HCl (pH 7.6), 5 mg of polyadenylic acid, 250 μM S-thio-ATP, yeast tRNA (50 μg/ml), and [35S]UTP-labeled sense or antisense riboprobe (0.15 ng/μl). Following hybridization, the slides were washed and dried as previously described prior to development in NTB-2 photoemulsion (Kodak Co., Rochester, N.Y.) (21). The slides were counterstained with a solution of Hoechst 33258 (2 μg/ml; Sigma) for visualization of cell nuclei, mounted, and analyzed by dark-field microscopy and UV fluorescence.
Bactericidal assay.
Bactericidal assays were performed on ChoP-expressing (H418) and nonexpressing (H419) phase variants of the same nontypeable H. influenzae clinical isolate. Assays used 10% pooled NHS obtained from 10 donors as a source of complement as previously described (43). Prior to use in bactericidal assays, this serum was treated with ChoP-coupled agarose beads to remove CRP. The removal of CRP from serum used as a complement source was confirmed by lack of reactivity with a MAb against human CRP in Western analysis. Assays were performed with a suspension of organisms grown to mid-log phase (optical density at 620 nm of 0.3 to 0.4) in brain heart infusion medium supplemented with 2% Fildes enrichment (sBHI; Difco Laboratories, Detroit, Mich.) and NAD (2 μg/ml) diluted to 105 CFU/ml in 20 μl with 60 μl of Hanks balanced salt solution (GIBCO Laboratories, Grand Island, N.Y.), 100 μl of PBS, and 20 μl of pooled NHS depleted of CRP. After incubation for 60 min at 37°C with rotation, the assay was stopped by cooling to 4°C and dilutions were made for quantitative culture. To calculate the percentage survival, colony counts in serial dilutions were determined by plating on sBHI solidified with 1% agar and compared to controls in which complement activity was inactivated by prior heating for 30 min at 56°C. Where indicated, the volume of PBS was reduced by 25 μl, and purified human CRP of known concentration in PBS, the same amount of purified human CRP treated with ChoP-coupled agarose beads in PBS, solubilized sputum, or solubilized ASF was substituted. The concentration of CRP in solubilized sputum and ASF was calculated as described above for Western analysis.
RESULTS
Presence and quantity of CRP in human ASF.
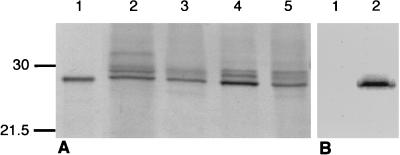
To determine if secretions derived from the human respiratory tract contain CRP, nasal ASF samples from healthy adult volunteers and sputum from patients with acute or chronic bronchitis or pneumonia were examined by Western analysis. Respiratory tract secretions were solubilized, and the proteins were separated by SDS-PAGE. Immunoblotting with a MAb against human CRP, CRP-8, showed a reactive band of the predicted size for denatured CRP (24 kDa) whose migration was indistinguishable from that of CRP purified from human serum (Fig. 1A). Similar results were observed with a polyclonal antibody to human CRP (data not shown). The 24-kDa band recognized by CRP-8 was seen in ASF specimens from four of five volunteers and in sputum samples from 12 of 12 patients (limit of detection, 50 ng/ml). In some samples, less prominent slower-migrating bands were also detected with CRP-8. This triplet pattern of reactive bands was a consistent finding in both ASF and sputum but not in CRP purified from serum. To confirm that these bands represented CRP, ASF and sputum specimens were treated with ChoP-agarose beads, which bind CRP in the presence of calcium (Fig. 1B). Complete loss of reactivity with CRP-8 on Western analysis following treatment with these beads provided functional evidence that the immunoreactive bands were CRP. After treatment with the ChoP-agarose beads in the presence of the same concentration of EDTA rather than calcium, no loss of CRP-8 reactive bands was observed (data not shown).
FIG. 1.
Representative Western analysis demonstrating the presence of CRP in nasal ASF and sputum from four individuals. (A) Nasal ASF (lanes 2 and 4) or sputum (lanes 3 and 5) was solubilized, separated by SDS-PAGE, transferred to a membrane, and immunoblotted with a MAb recognizing CRP. Purified human serum CRP (1.0 ng) is shown in lane 1. Loading of secretions was with 2.5 (lanes 2 and 3) or 12.5 (lanes 4 and 5) μl of specimen per lane. (B) Western analysis of equivalent amounts of ASF untreated (lane 2) or treated (lane 1) with ChoP-agarose beads, which bind CRP. Sizes are indicated in kilodaltons.
The concentration of CRP was estimated by Western analysis and comparison with a standard curve generated with known quantities of purified serum CRP (Table 1). The concentration of CRP ranged from <0.05 to 0.88 μg per ml of ASF (n = 5) and was generally higher in inflammatory specimens (0.17 to 42.0 μg per ml of sputum). These amounts were also compared to the total protein concentration of the sample. After adjustment for the total protein, there were still >5-fold and 40-fold ranges of CRP concentrations in ASF and sputum, respectively, suggesting that amount of CRP was highly variable and not a simple function of sample viscosity.
TABLE 1.
Concentrations of CRP in human respiratory tract secretions
| Sample | Clinical diagnosisa | CRP (μg/ml)b | Total protein (mg/ml) | CRP (μg)/total protein (mg) |
|---|---|---|---|---|
| Nasal ASF | ||||
| 0513 | 0.84 | 2.80 | 0.30 | |
| 0225 | 0.88 | 6.34 | 0.14 | |
| 0320 | 0.15 | 2.40 | 0.06 | |
| 0845 | 0.88 | NDc | ND | |
| 1207 | <0.05 | 0.70 | ND | |
| Mean ± SEM | 0.56 ± 0.25 | 3.06 ± 1.2 | 0.17 ± 0.07 | |
| Sputum | ||||
| 0514 | Acute bronchitis | 0.20 | 3.50 | 0.06 |
| 0214 | Acute bronchitis | 2.5 | 5.95 | 0.42 |
| 3724 | Chronic bronchitis | 0.17 | 2.50 | 0.07 |
| 4463 | Chronic bronchitis | 3.20 | 4.54 | 0.71 |
| 9051 | Chronic bronchitis | 24.0 | 286 | 0.08 |
| 1039 | NTHi pneumonia | 1.8 | 2.85 | 0.63 |
| 8055 | NTHi pneumonia | 4.80 | 13.63 | 0.35 |
| 8407 | NTHi pneumonia | 12.0 | 6.89 | 1.74 |
| 5703 | NTHi pneumonia | 12.0 | 8.66 | 1.39 |
| 6000 | NTHi pneumonia | 2.8 | 18.52 | 0.15 |
| 6287 | NTHi pneumonia | 20.0 | 8.73 | 2.29 |
| 0713 | NTHi pneumonia | 42.0 | 17.4 | 2.41 |
| Mean ± SEM | 10.45 ± 3.6 | 31.6 ± 12.3 | 0.86 ± 0.25 |
The diagnosis of nontypeable H. influenzae (NTHi) pneumonia was based on previously described criteria (25).
Calculated by Western analysis.
ND, not determined.
Local expression of CRP in the human respiratory tract.
Potential sources for the CRP found on the mucosal surface of the respiratory tract include serum extravasation and local production from resident leukocytes or other components of the airway such as the epithelial cells. The possibility that the CRP detected in respiratory secretions resulted from extravasation from serum was addressed by comparison of the ratio of CRP to IgG, a protein of similar size found on the mucosal surface of the human respiratory tract that originates predominantly from the pool in the serum. The quantity of IgG in ASF and sputum was determined by Western analysis in comparison to purified IgG of known concentration by the same technique and using the same samples as described for measuring quantities of CRP (Table 2). The concentrations of IgG ranged from 0.26 to 0.63 μg per ml of ASF and from 0.36 to 6.36 μg per ml of sputum. Compared to the full range of values of serum CRP from uninflamed normal to severe acute-phase response and IgG, the ratio of CRP to IgG in both ASF and sputum compared with the ratio of CRP to IgG in the serum was 75- to 75,000-fold higher. These results suggested that the CRP detected in the respiratory tract could not be attributable to serum extravasation alone unless there is selective transport of CRP.
TABLE 2.
Quantity of CRP and IgG in nasal ASF and sputum compared with serum
| Specimen | Concn (μg/ml)a
|
CRP/IgG | |
|---|---|---|---|
| CRP | IgG | ||
| Nasal ASF | 0.87 ± 0.02 | 0.38 ± 0.2 | 1.3–3.4 |
| Sputum | 1.5 ± 1.2 | 2.4 ± 3.4 | 0.4–5.0 |
| Serum | 1–38 | (7–15) × 103 | (0.67–54) × 10−4 |
Serum levels are based on the range of uninflamed normal to severe acute-phase response adult serum (5). CRP and IgG concentrations were calculated from the same specimens by Western analysis and are presented as means ± standard errors.
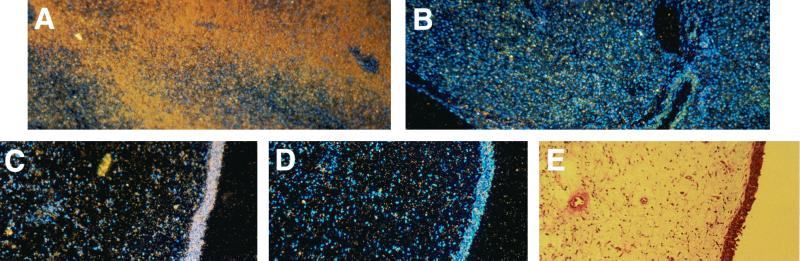
Since it has already been established that human mononuclear leukocytes may produce CRP, we determined whether uninflamed tissue derived from the human respiratory tract expresses this protein. Transcription of the gene for CRP was assessed in excised uninflamed, human nasal polyps by in situ hybridization (Fig. 2). Human liver tissue served as a positive control. Hybridization was seen with the antisense but not the sense riboprobe in epithelial cells lining the nasopharyngeal tissue. This result suggested that epithelial cells lining the upper respiratory tract of humans express the gene for CRP and therefore could be a source of CRP within the airway.
FIG. 2.
In situ hybridization using a human CRP riboprobe labeled with [35S]UTP and hybridized with human liver and nasal polyp tissue. Controls used the antisense (A, positive control) or sense (B, negative control) riboprobe with sections of human liver. Antisense riboprobe with a section of a human nasal polyp shows hybridization of the probe to the epithelial surface (C). No hybridization is seen in the control using the sense riboprobe (D). (E) Hematoxylin-and-eosin staining of a section of the same polyp showing the epithelial cell surface. Magnification, ×100.
Expression of CRP by respiratory epithelial cells.
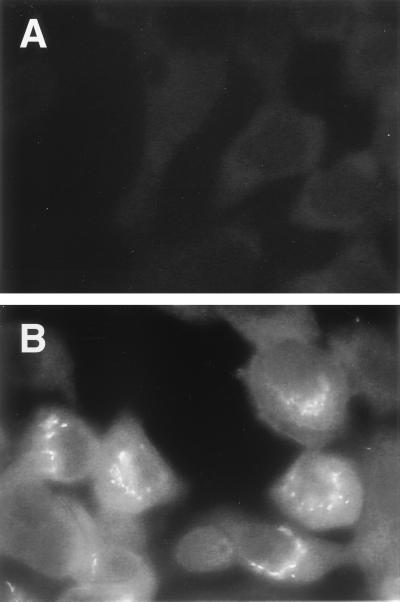
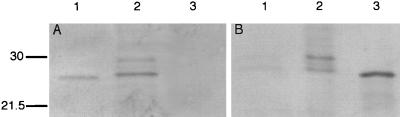
To confirm that transcription of the gene for CRP detected in nasopharyngeal tissue by in situ hybridization correlated with expression of CRP, we examined epithelial cells derived from the human respiratory tract in culture. Immunofluorescence studies were not performed on nasal polyp tissue because of the possibility that reactivity could be due to serum contamination. Immunofluorescence studies on Detroit 562 pharyngeal carcinoma cells using CRP-8 showed diffuse staining which was not observed in controls lacking the anti-CRP antibody (Fig. 3). This reactivity could not be attributed to the presence of fetal calf serum used in cell culture, since Western analysis showed no specific binding to culture medium in the absence of cells (Fig. 4A, lane 3). However, in the presence of Detroit 562 pharyngeal carcinoma cells, Western analysis of tissue culture supernatant revealed a prominent band comigrating with purified serum CRP (Fig. 4A, lane 2). Additional, fainter reactivity was also seen in slower-migrating bands that resembled those observed in ASF and sputum specimens. Treatment of the cell culture medium from the Detroit 562 pharyngeal carcinoma cells with ChoP-agarose beads resulted in a complete loss of the CRP-8 reactive bands (Fig. 4B). Additional evidence that the Detroit 562 pharyngeal carcinoma cells were synthesizing CRP was obtained in RT-PCR experiments (Fig. 5). mRNA was isolated from these cells and from human liver tissue (positive control) to generate cDNA to assess transcription of the genes for CRP and GAPDH as a control for the quality of mRNA. The amplification of a single band of the predicted size in PCRs with the cDNA template and primers based on the sequence of human CRP demonstrated that the reactivity with CRP MAb seen in tissue culture supernatants correlated with transcription of the gene for CRP by these cells. RT-PCR of human liver showed a single band of the same size (data not shown). Negative controls included mRNA in the PCR with either CRP primers or GAPDH primers to demonstrate that there was no DNA contaminating the mRNA samples. It was concluded that epithelial cells derived from the human respiratory tract are capable of transcription of the CRP gene as well as expression of CRP and that these cells could be a source of the CRP detected in ASF and sputum.
FIG. 3.
Immunocytochemistry using a MAb to human CRP and Detroit 562 pharyngeal carcinoma cells. Images show representative views with isotype control antibody (A) or CRP-8 (B) followed by fluorescein isothiocyanate-labeled anti-mouse IgG.
FIG. 4.
Western analysis demonstrating the presence of CRP in tissue culture supernatant from Detroit 562 pharyngeal carcinoma cells. (A) Lane 1, purified human CRP (2 ng); lane 2, tissue culture medium from Detroit 562 pharyngeal carcinoma cells (5 μl); lane 3, tissue culture medium alone (5 μl). (B) Equivalent amounts tissue culture supernatant from Detroit 562 pharyngeal carcinoma cells treated (lane 1) or untreated (lane 2) with ChoP-agarose beads which bind CRP. Lane 3, purified CRP (4 ng). Sizes are indicated in kilodaltons.
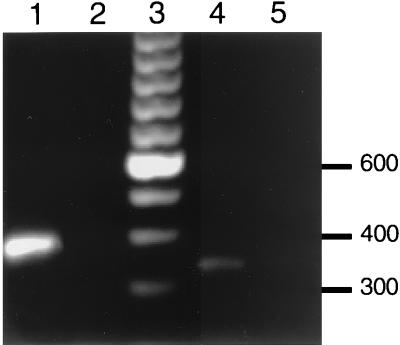
FIG. 5.
Expression of CRP mRNA by Detroit 562 pharyngeal carcinoma cells determined by RT-PCR. Poly(A) mRNA was used to generate cDNA for use as a template in the PCR with primers based on the sequence of human GAPDH (lanes 1 and 2) and human CRP (lanes 4 and 5). PCR products using cDNA templates were visualized on ethidium bromide-stained agarose gels (lanes 1 and 4). The lack of a PCR product when mRNA was used as a template confirmed that these bands were not due to DNA contamination (lanes 2 and 5). The size of PCR products was determined with a 100-bp ladder (lane 3).
Contribution of CRP in respiratory tract secretions to innate immunity.
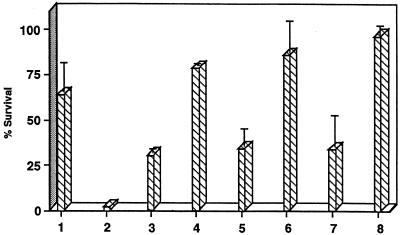
Since serum CRP has been shown to contribute to opsonic and bactericidal activity, we hypothesized that CRP present in ASF and sputum may have a similar function and thereby contribute to innate immunity of the human respiratory tract. In the presence of complement, human serum CRP is bactericidal against H. influenzae phase variants displaying cell surface ChoP but has no effect on phase variants of the same strain lacking ChoP (44). The biological activity of CRP in ASF and sputum was assessed in bactericidal assays with human serum depleted of CRP as a source of complement (Fig. 6). Addition of ASF or sputum to bactericidal assays resulted in an increased killing of ChoP+ H. influenzae. The increased killing associated with addition of ASF or sputum was eliminated by pretreatment of ASF or sputum with ChoP-agarose beads to remove CRP. Analysis of serum proteins bound to ChoP-agarose beads revealed that only a single band of the molecular weight corresponding to CRP was detected by silver staining, indicating that no other proteins were removed in significant amounts (data not shown). The complement activity remained intact with these treatments, as there was no effect on killing of the ChoP− phase variants (data not shown). There was less than 50% survival of bacteria over 60 min at 37°C with ASF or sputum added to give a final CRP concentration of 62.5 or 29.2 ng/ml, respectively. A similar level of killing was observed with 20 ng of purified serum CRP per ml. There was no effect of the solubilized ASF or sputum on bacterial viability in the absence of active complement. These results provided in vitro evidence that CRP in the human respiratory tract may contribute to clearance of organisms such as H. influenzae that reside in this environment and contain ChoP on their surface when complement is present.
FIG. 6.
Effect of CRP in nasal ASF and sputum on killing of H. influenzae. NHS depleted of CRP was used as a source of complement in bactericidal assays examining the survival of H. influenzae phase variants expressing ChoP over 60 min at 37°C. PBS control (bar 1) or 625 (bar 2) or 20 (bars 3 and 4) ng of purified human CRP, sputum (bars 5 and 6), or ASF (bars 7 and 8) was added to bactericidal assays. For bars 4, 6, and 8, after addition of the source of CRP, the sample was treated with ChoP-agarose beads prior to testing. The concentrations of CRP in ASF and sputum were measured as 29.2 and 62.5 ng/ml, respectively. Values represent the means of at least two independent determinations in duplicate ± standard deviation (when n is ≥4).
DISCUSSION
The hypothesis that CRP, well recognized as a serum constituent, is present on the mucosal surface of the human respiratory tract was examined. The presence and significance of CRP on any mucosal surface has not previously been investigated. Specifically, CRP has not been recognized as a component of the innate antimicrobial activity of ASF (6). The expression of CRP in the human upper respiratory tract was of primary interest because of the heavy colonization of this site with organisms that have cell surface ChoP and the potential that CRP could affect the host microbial interaction at this site. It was necessary to test the hypothesis using material of human origin because of marked differences in patterns of CRP expression in animals and humans. CRP was detected using a MAb to the human serum protein (CRP-8), and its identity was confirmed by its ability to bind to ChoP in the presence of calcium. This antibody is highly specific, as it does not recognize related proteins such as human serum amyloid P component, human haptoglobin, human α1-acid glycoprotein, and human IgG (31). CRP was found in the majority of normal, nonpurulent nasal ASF specimens. Because ASF was collected without chemical stimulation, which might alter results through dilution, only small volumes (1 to 5 ml) of this material could be obtained from each individual. Nonetheless, it was possible to calculate the concentration of CRP in Western blots by comparison to a standard curve with known amounts of purified serum protein. In specimens with detectable amounts of CRP, the measured concentration was greater than the levels previously shown to contribute to antimicrobial activity and were only slightly less than those found in human serum in the absence of an inflammatory stimulus (44). CRP on the surface of the airway, therefore, was present in sufficient quantity that it could potentially affect ChoP-expressing flora found in the upper respiratory tract.
CRP in ASF could also serve to maintain the normally sterile airways of the lower respiratory tract whenever pharyngeal contents containing ChoP-expressing bacteria gain access into the lung by aspiration. Normal ASF from the lower airway is difficult to obtain without sample dilution and was not separately determined in this study. However, significant quantities of CRP (0.17 to 42 μg/ml) were detected in all sputum samples tested (n = 12). These were purulent specimens from patients with bronchitis or pneumonia that in contrast to the nasal ASF samples represented infection and inflammation in the host respiratory tract. This finding suggests that CRP could also contribute to the innate defense of the lower airway during infection with ChoP-expressing species as has been demonstrated for experimental invasive infection (34). The major etiologic agents of pneumonia in adults, S. pneumoniae and nontypeable H. influenzae, express cell surface ChoP and bind efficiently to CRP (18, 22).
For sputum, concentrations of CRP were as high or higher than those in nasal ASF, suggesting that there was a response to the inflammatory state in the lower airways affecting CRP levels. Unlike the samples from nasal ASF, amounts of CRP in sputum were not proportional to the total protein content as would be predicted if the source of the CRP was solely a reflection of the viscosity of the specimen or from extravasation from the serum pool. Li et al. detected CRP in bronchoalveolar (BAL) fluid from both healthy human volunteers (4.04 ± 2.2 μg/mg of total protein) and those with acute respiratory distress syndrome (97.8 ± 84.2 μg/mg of total protein) (20). Their study differed from the present study in that they assayed BAL fluid, rather than sputum or upper ASF, using an enzyme-linked immunosorbent assay technique to quantitate CRP levels. The quantities of CRP in normal patients were higher than the values measured in this study. Last, they did not investigate the source of CRP found in the BAL fluid. Another potential source of sputum CRP is leukocytes that have migrated into the site of inflammation. A prior study of pulmonary CRP showed that lavage of the rat lung yielded 33 μg of CRP per ml (11). In rat lung sections, alveolar macrophages were the only cells that showed evidence of CRP expression by immunohistochemistry and in situ hybridization (11). The alveolar epithelial cells (type II pneumocytes) showed no transcription of the CRP gene by RT-PCR in this study (11). The significance of this observation in humans is unknown. Our study focused on the source of CRP in that portion of the airway that is heavily colonized.
Although it was not possible to exclude the possibility that CRP in sputum originated from the serum pool synthesized by the liver, it seemed unlikely that this could explain levels of CRP close to those in the serum in the uninflamed nasal ASF. Likewise, expression by leukocytes is unlikely to be the major source of CRP in these uninflamed specimens. Comparison of the ratio of amounts of CRP to IgG in ASF compared to serum added further circumstantial evidence that the source of CRP in the upper respiratory tract cannot be attributed solely to extravasation from the serum pool. The hypothesis that ASF CRP was a result of local production was then examined. Again these studies used material of human origin exclusively. In situ hybridization experiments on excised nasal polyps demonstrated that there is transcription involving the CRP gene on the epithelial surface. Since polyps are covered with a normal epithelial layer, this result suggests that the normal nasal epithelium expresses CRP. As further confirmation of this finding, the expression of CRP by cells in culture that were derived from the human pharynx were analyzed. Detroit 562 pharyngeal carcinoma cells secreted a protein that was shown to be CRP by both immunological (reactivity with CRP-8 by Western analysis and immunocytochemistry) and functional (binding to ChoP in the presence of calcium) criteria described above. In addition, these cells showed evidence for transcription of the CRP gene by RT-PCR. It was concluded that in addition to hepatocytes and leukocytes, epithelial cells of the human respiratory tract are capable of expressing CRP. Although CRP has been previously detected in human lower airway fluid (20), to the best of our knowledge, this is the first time CRP has been identified in human upper airway secretions and the first report showing that human respiratory tract epithelial cells produce this protein. The epithelial cells lining the mucosal surface of the airway have been shown to be active participants in local airway defense and express other antimicrobial substances such as defensins and other cationic peptides (6, 9). We propose that CRP is another antimicrobial factor secreted by these cells.
In the course of this study, it was noted that there were often higher-molecular-weight forms that were recognized by CRP-8 in protein from nasal ASF and sputum but not serum. This observation suggests the possibility that airway or mucosal CRP may be modified and somehow distinct from the form in serum. Unlike human serum CRP, rat CRP is glycosylated, although the presence of N-linked oligosaccharides do not affect binding to ChoP (26, 28). Since these higher-molecular-weight forms seen in this study were removed following incubation with ChoP linked to agarose beads, there does not appear to be a clear functional significance to the other CRP-like species detected in this study.
Finally, we show that the CRP in secretions from the human airway has antibacterial activity. It was not possible to obtain enough nasal ASF to extract sufficient quantities of CRP of mucosal origin. Nasal ASF, however, contained bactericidal activity against a respiratory pathogen, nontypeable H. influenzae, which was eliminated by pretreatment with ChoP-agarose to remove CRP. The level of bactericidal activity was similar to that of the equivalent amount of human serum CRP. Further evidence that this antimicrobial activity was due to CRP in these specimens was the finding that it was specific for phase variants expressing ChoP, the molecular target for CRP. The bactericidal activity of CRP isolated from the mucosal surface has yet to be investigated. The in vitro assay in this report required complement. Functionally active complement components such as C1 (the precursor of C1q) as well as C3, C4, and factor B have been found in human saliva and may indicate that the necessary factors for airway CRP to have bactericidal activity are present on the mucosal surface of the human respiratory tract (1). There is also evidence for complement-independent clearance mechanisms involving CRP (33). Moreover, CRP may have multiple other effects, besides it role in opsonization, on resident bacteria in the upper respiratory tract. The rPAF-mediated attachment and invasion of epithelial cells by S. pneumoniae and nontypeable H. influenzae requires interaction with ChoP (7, 32). CRP on the mucosal surface could block this interaction between ChoP-expressing bacteria and the rPAF. CRP may also act to modulate the immune response. Mice carrying the transgene for human CRP, for example, are more resistant to endotoxemia (47). If these mice express pulmonary CRP, they could prove to be a useful model to examine the role of CRP in innate immunity of the airway.
ACKNOWLEDGMENTS
We thank Eduardo Ruchelli for providing sections of nasal polyps, Daniel Musher for providing sputum specimens, Rebecca Oakey for guidance with in situ hybridization experiments, and Sandra Watsworth for assistance with immunocytochemistry experiments.
This work was supported by Public Heath Service grants AI38436 and AI44231.
REFERENCES
- 1.Andoh A, Fujiyama Y, Kimura T, Uchihara H, Sakumoto H, Okabe H, Bamba T. Molecular characterization of complement components (C3, C4, and factor B) in human saliva. J Clin Immunol. 1997;17:404–407. doi: 10.1023/a:1027320425291. [DOI] [PubMed] [Google Scholar]
- 2.Bals R, Wang X, Zasloff M, Wilson J M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann H, Gauldie J. Regulation of hepatic acute phase plasma protein genes by hepatocyte stimulating factors and other mediators of inflammation. Mol Biol Med. 1990;7:147–159. [PubMed] [Google Scholar]
- 4.Baumann H, Schendel P. Interleukin-11 regulates the hepatic expression of the same plasma protein genes as interleukin-6. J Biol Chem. 1991;266:20424–20427. [PubMed] [Google Scholar]
- 5.Claus D, Osmand A, Gewurz H. Radioimmunoassay of human C-reactive protein and levels in normal sera. J Lab Clin Med. 1976;87:120–128. [PubMed] [Google Scholar]
- 6.Cole A, Dewan P, Ganz T. Innate antimicrobial activity of nasal secretions. Infect Immun. 1999;67:3267–3275. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cundell D R, Gerard N P, Gerard C, Idanpaan-Heikkila I, Tuomanen E I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 8.Deutsch J, Salman M, Rottem S. An unusual polar lipid from the cell membrane of Mycoplasma fermentans. Eur J Biochem. 1995;227:897–902. doi: 10.1111/j.1432-1033.1995.tb20216.x. [DOI] [PubMed] [Google Scholar]
- 9.Diamond G, Jones D E, Bevins C L. Airway epithelial cells are the site of expression of a mammalian antimicrobial peptide gene. Proc Natl Acad Sci USA. 1993;90:4596–4600. doi: 10.1073/pnas.90.10.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinarello C. Interleukin-1 and the pathogenesis of the acute phase response. N Engl J Med. 1984;311:1413–1418. doi: 10.1056/NEJM198411293112205. [DOI] [PubMed] [Google Scholar]
- 11.Dong Q, Wright J R. Expression of C-reactive protein by alveolar macrophages. J Immunol. 1996;156:4815–4820. [PubMed] [Google Scholar]
- 12.Egenhofer C, Alsdorf K, Fehsel K, Kolb-Bachofen V. Membrane associated C-reactive protein on rat liver macrophages is synthesized within the macrophages, expressed as neo-C-reactive protein and bound through a C-reactive protein-specific membrane receptor. Hepatology. 1995;18:1216–1223. [PubMed] [Google Scholar]
- 13.Gewurz H, Zhang X, Lint T. Structure and function of the pentraxins. Curr Opin Immunol. 1995;7:54–64. doi: 10.1016/0952-7915(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 14.Gmur R, Thurnheer T, Guggenheim B. Dominant cross-reactive antibodies generated during the response to a variety of oral bacterial species identification. J Dent Res. 1999;78:77–85. doi: 10.1177/00220345990780011201. [DOI] [PubMed] [Google Scholar]
- 15.Gresser I, Delers F, Tran Quangs N, Marion S, Engler R, Maury C. Tumor necrosis factor induces acute phase protein in rats. J Biol Regul Homeost Agents. 1987;1:173–176. [PubMed] [Google Scholar]
- 16.Kaplan M, Volankis J. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reactions of CRP with pneumococcal CPS and with the choline phosphatides lecithin and sphingnomyelin. J Immunol. 1974;12:2135. [PubMed] [Google Scholar]
- 17.Kim J, Weiser J. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis. 1998;177:368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 18.Kim J O, Romero-Steiner S, Sørensen U, Blom J, Carvalho M, Barnardi S, Carlone G, Weiser J N. Relationship between cell-surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect Immun. 1999;67:2327–2333. doi: 10.1128/iai.67.5.2327-2333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuta A, Baum L. C-reactive protein is produced by a small number of normal human peripheral blood lymphocytes. J Exp Med. 1986;164:321–326. doi: 10.1084/jem.164.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Sanders R, McAdam K, Hales C, Thompson B, Gelfand J, Burke J. Impact of C-reactive protein (CRP) on surfactant function. J Trauma. 1989;29:1690–1697. doi: 10.1097/00005373-198912000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Lysenko E, Gould J, Bals R, Wilson J, Weiser J. Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP 18 expressed in the upper respiratory tract. Infect Immun. 2000;68:1664–1671. doi: 10.1128/iai.68.3.1664-1671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lysenko E, Richards J, Cox A, Kapoor M, Weiser J. The position of phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae affects binding and sensitivity to C-reactive protein mediated killing. Mol Microbiol. 2000;35:234–245. doi: 10.1046/j.1365-2958.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- 23.Mackiewicz A, Ganapathi M, Schultz D, Brabenec A, Weinstein J, Kelley M. Transforming growth factor B1 regulates production of acute phase proteins. Proc Natl Acad Sci USA. 1990;87:1491–1498. doi: 10.1073/pnas.87.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosser J L, Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an enzyme. J Biol Chem. 1970;245:287–298. [PubMed] [Google Scholar]
- 25.Musher D M, Kubitschek K R, Crennan J, Baughn R E. Pneumonia and acute febrile tracheobronchitis due to Haemophilus influenzae. Ann Intern Med. 1983;99:444–450. doi: 10.7326/0003-4819-99-4-444. [DOI] [PubMed] [Google Scholar]
- 26.Nagpurkar A, Saxena U, Mookerjea S. Interaction of rat serum phosphorylcholine-binding protein with phospholipid-containing liposomes. J Biol Chem. 1983;258:10518–10523. [PubMed] [Google Scholar]
- 27.Pepys M, Baltz M, Gomer K, Davies A, Doenhoff M. Serum amyloid P component is an acute-phase reactant in the mouse. Nature. 1979;278:259–261. doi: 10.1038/278259a0. [DOI] [PubMed] [Google Scholar]
- 28.Sambasivam H, Rassouli M, Murray R, Nagpurkar A, Mookerjea S, Azadi P, Dell A, Morris H. Studies on the carbohydrate moiety and on the biosynthesis of rat C-reactive protein. J Biol Chem. 1993;268:10007–10016. [PubMed] [Google Scholar]
- 29.Schenkein H, Barbour S, Berry C, Kipps B, Tew J. Invasion of human vascular endothelial cells by Actinobacillus actinomycetemcomitans via the receptor for platelet-activating factor. Infect Immun. 2000;68:5416–5419. doi: 10.1128/iai.68.9.5416-5419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schenkein H, Gunsolley J, Best A, Harrison M T, Hahn C, Wu J, Tew J. Antiphosphorylcholine antibody levels are elevated in humans with periodontal diseases. Infect Immun. 1999;67:4814–4818. doi: 10.1128/iai.67.9.4814-4818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigma Chemical Co. Monoclonal anti-human C-reactive protein, clone CRP-8, product no. C1688. St. Louis, Mo: Sigma Chemical Co.; 1993. [Google Scholar]
- 32.Swords W, Buscher B, Van der Steeg K, Preston A, Nichols W, Weiser J, Gibson B, Apicella M. Nontypeable Haemophilus influenzae adhere to and invade airway epithelial cells via interaction of lipooligosaccharide with the PAF receptor. Mol Microbiol. 2000;37:13–27. doi: 10.1046/j.1365-2958.2000.01952.x. [DOI] [PubMed] [Google Scholar]
- 33.Szalai A J, Agrawal A, Greenhough T J, Volanakis J E. C-reactive protein. Immunol Res. 1997;16:127–136. doi: 10.1007/BF02786357. [DOI] [PubMed] [Google Scholar]
- 34.Szalai A J, Briles D E, Volanakis J E. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in transgenic mice. J Immunol. 1995;155:2557–2563. [PubMed] [Google Scholar]
- 35.Szalai A J, Briles D E, Volanakis J E. Role of complement in C-reactive-protein-mediated protection of mice from Streptococcus pneumoniae. Infect Immun. 1996;64:4850–4853. doi: 10.1128/iai.64.11.4850-4853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tucci A, Goldberger G, Whitehead A S, Kay R M, Woods D E, Colten H R. Biosynthesis and postsynthetic processing of human C-reactive protein. J Immunol. 1983;131:2416–2419. [PubMed] [Google Scholar]
- 37.Volanakis J E, Kaplan M H. Interaction of C-reactive protein complexes with the complement system. II. Consumption of guinea pig complement by CRP complexes. Requirement for human C1q. J Immunol. 1974;113:9–17. [PubMed] [Google Scholar]
- 38.Volanakis J E, Kaplan M H. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc Soc Exp Biol Med. 1971;136:612. doi: 10.3181/00379727-136-35323. [DOI] [PubMed] [Google Scholar]
- 39.Weinhold B, Ruther U. Interleukin-6-dependent and independent regulation of the human C-reactive protein gene. Biochem J. 1997;327:425–429. doi: 10.1042/bj3270425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiser J N, Austrian R, Sreenivasan P K, Masure H R. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun. 1994;62:2582–2589. doi: 10.1128/iai.62.6.2582-2589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiser J N, Goldberg J B, Pan N, Wilson L, Virji M. The phosphorylcholine epitope undergoes phase variation on a 43-kilodalton protein in Pseudomonas aeruginosa and on pili of Neisseria meningitidis and Neisseria gonorrhoeae. Infect Immun. 1998;66:4263–4267. doi: 10.1128/iai.66.9.4263-4267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiser J N, Love J M, Moxon E R. The molecular mechanism of phase variation of H. influenzae lipopolysaccharide. Cell. 1989;59:657–665. doi: 10.1016/0092-8674(89)90011-1. [DOI] [PubMed] [Google Scholar]
- 43.Weiser J N, Pan N. Adaptation of Haemophilus influenzae to acquired and innate humoral immunity based on phase variation of lipopolysaccharide. Mol Microbiol. 1998;30:767–775. doi: 10.1046/j.1365-2958.1998.01108.x. [DOI] [PubMed] [Google Scholar]
- 44.Weiser J N, Pan N, McGowan K L, Musher D, Martin A, Richards J C. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med. 1998;187:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiser J N, Shchepetov M, Chong S T H. Decoration of lipopolysaccharide with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. Infect Immun. 1997;65:943–950. doi: 10.1128/iai.65.3.943-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitehead A, Zahedi K, Rits M, Mortensen R, Lelias J. Mouse C-reactive protein: generation of complementary DNA clones, structural analysis, and induction of messenger RNA during inflammation. Biochem J. 1990;266:283–290. doi: 10.1042/bj2660283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia D, Samols D. Transgenic mice expressing rabbit C-reactive protein are resistant to endotoxemia. Proc Natl Acad Sci USA. 1997;18:2575–2580. doi: 10.1073/pnas.94.6.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang D, Jiang S, Rzewnicki D, Samols D, Kushner I. The effect of interleukin-1 on C-reactive protein expression in Hep3B cells is exerted at the transcriptional level. Biochem J. 1995;310:143–148. doi: 10.1042/bj3100143. [DOI] [PMC free article] [PubMed] [Google Scholar]