Abstract
Subcutaneous panniculitis-like T-cell lymphoma (SPTCL) is a rare subtype of cutaneous T-cell lymphoma that usually presents with tender subcutaneous nodules on the trunk and extremities. Immunosuppressive therapy is considered first-line treatment for SPTCL, while multiagent chemotherapy is used for SPTCL complicated by hemophagocytic lymphohistiocytosis (HLH). Here, we report a 42-year-old Hispanic man that presented with a 5-year history of recurrent painful subcutaneous lesions in the absence of constitutional symptoms, lymphadenopathy, and hepatosplenomegaly. A punch biopsy revealed an atypical lymphoid infiltrate in between subcutaneous adipose lobules. Lymphocytes expressed CD3, CD8, and Beta F-1 and did not express CD4 and CD56. Based on clinical and histologic findings, the patient was diagnosed with SPTCL. In addition, laboratory findings did not demonstrate any evidence of HLH. He was initially started on both prednisone and hydroxychloroquine with no improvement. A trial of cyclosporine and methotrexate yielded no clinical improvement. As the lesions failed to resolve after treatment with multiple immunosuppressive agents, romidepsin, an intravenous histone deacetylase (HDAC) inhibitor, was initiated. After two cycles of romidepsin, the patient achieved complete clinical response. He continues to be in remission 12 months later with monthly maintenance therapy. This case illustrates that romidepsin can be useful as monotherapy for refractory SPTCL without HLH.
Keywords: Subcutaneous panniculitis-like T-cell lymphoma, Romidepsin, Histone deacetylase inhibitor, Case report
Introduction
Subcutaneous panniculitis-like T-cell lymphoma (SPTCL) is a rare form of cutaneous T-cell lymphoma characterized by subcutaneous infiltrates of cytotoxic T cells of the alpha/beta phenotype. It typically presents with tender subcutaneous nodules on the trunk and extremities. It comprises <1% of all cutaneous T-cell lymphomas and typically has an indolent clinical course, consisting of recurrent lesions without extracutaneous involvement [1, 2]. Currently, immunosuppressive agents are considered first-line treatment, and multiagent chemotherapy regimens may be utilized for cases associated with hemophagocytic lymphohistiocytosis (HLH) [3]. There are no current guidelines for the treatment of patients with SPTCL who fail immunosuppressive agents. Romidepsin, an intravenous histone deacetylase (HDAC) inhibitor, has recently been introduced as a promising drug for the treatment of relapsed/refractory peripheral T-cell lymphomas [4]. However, its efficacy in treating refractory SPTCL has not been firmly demonstrated. Here, we report a case of SPTCL without HLH recalcitrant to immunosuppressive agents that responded to romidepsin. This case suggests a potential utility for HDAC inhibitors as a single treatment regimen for immunosuppressive-resistant SPTCL patients.
Case Report
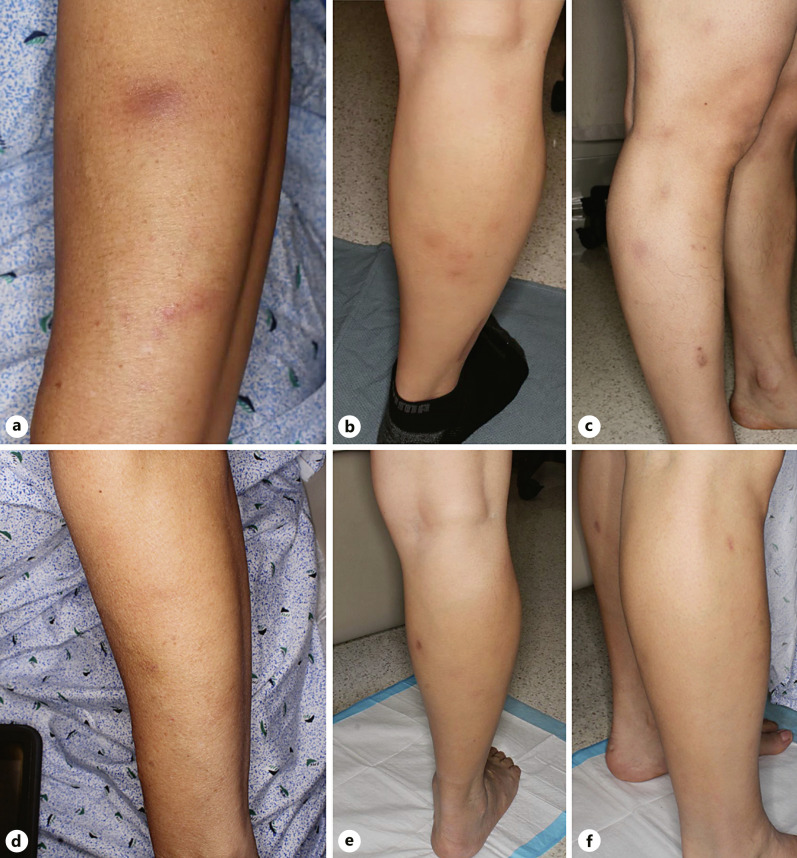
A 42-year-old Hispanic man presented with a 5-year history of recurrent painful subcutaneous lesions that first appeared on the arms and subsequently spread to the legs, buttocks, abdomen, and back. He denied a history of fever, fatigue, or weight loss. Physical exam revealed several violaceous ill-defined subcutaneous painful nodules on the extremities without lymphadenopathy or hepatosplenomegaly (Fig. 1a–c). Previous treatments included topical clindamycin and intermittent oral trimethoprim-sulfamethoxazole for 18 months without improvement.
Fig. 1.
Multiple indurated subcutaneous nodules with minimal surface change on the left forearm (a) and right lower extremity (b, c). Normal skin after resolution of subcutaneous nodules (d–f).
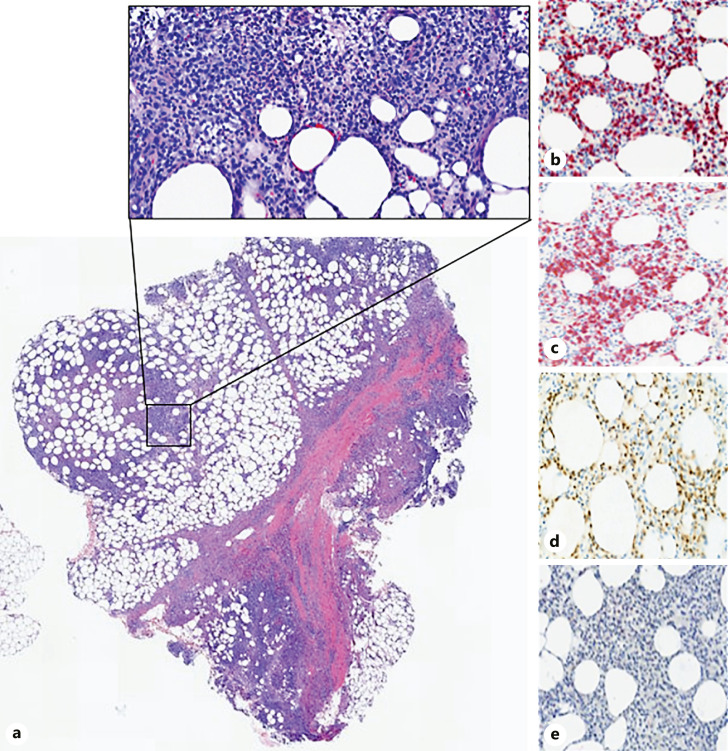
A punch biopsy was performed, revealing a prominent subcutaneous infiltrate of lymphocytes, histiocytes, and neutrophils with a predominance of atypical lymphoid infiltrate in the subcutaneous tissue with characteristic rimming of adipocytes (Fig. 2a). Lymphocytes expressed CD3, CD8, and Beta F-1 and did not express CD4 and CD56 (Fig. 2b–e). High-throughput sequencing of the biopsy identified a dominant T-cell receptor clone, indicating T-cell monoclonality. Laboratory studies including lipid panel, lactate dehydrogenase, antinuclear antibodies, alpha-1-antitrypsin, ferritin, and interleukin-2 receptor levels were unremarkable. Peripheral blood flow cytometry did not reveal an abnormal lymphocyte population. Positive emission tomography (PET) scan demonstrated multiple hypermetabolic subcutaneous lesions within the right flank, gluteus muscles and posterior thighs with no lymph node or visceral involvement. Based on the results of the complete blood count, lipid panel, flow cytometry, ferritin, and interleukin-2 receptor, our patient did not fulfill criteria for HLH [5]. Combining the clinical, laboratory, and histologic findings, the patient was diagnosed with SPTCL without HLH.
Fig. 2.
Punch biopsy demonstrating lobular and septal infiltration with prominent medium to small sized lymphocytes accompanied by some histiocytes and neutrophils (a, H&E ×50, ×100). Inset demonstrates characteristic rimming of lymphocytes around individual adipocytes that express CD3 (b), CD8 (c), Beta F-1 (d), and do not stain with CD4 (e).
With an established diagnosis, the patient was initially treated with a 4-week course of prednisone 40 mg daily tapered down by 10 mg weekly and hydroxychloroquine 200 mg twice daily. No clinical improvement was noted after 3 months of treatment. Therefore, the patient was started on cyclosporine 100 mg twice daily and individual lesions were treated with intralesional triamcinolone acetonide injections. Cyclosporine was increased to 150 mg twice daily; however, new lesions continued to appear. After 3 months of treatment, cyclosporine was stopped, and methotrexate was initiated at 7.5 mg weekly dose. Given the lack of response, the dose of methotrexate was increased to 25 mg weekly. Despite 7 months of treatment with methotrexate, a follow-up PET scan demonstrated evidence of active disease defined by the presence of hypermetabolic subcutaneous stranding [6]. Since the patient continued to develop lesions despite receiving multiple different lines of immunosuppressive agents, we initiated treatment with the HDAC inhibitor romidepsin. The patient was started on a cycle of 14 mg romidepsin weekly with three consecutive weeks of treatment followed by 1 week off therapy. Given that the patient initially experienced minor cytopenias, nausea, vomiting, and loss of appetite, the dose of romidepsin was reduced by 20% during the second cycle which led to a reduction of his hematologic and gastrointestinal side effects.
After 2 cycles of treatment, the patient achieved complete clinical response with resolution of all active lesions and no new detectable lesions (Fig. 1d–f). Repeat PET scan after the 4th cycle of romidepsin demonstrated radiographic clinical remission as there was no evidence of hypermetabolic subcutaneous stranding seen on prior imaging [6]. He continues to remain in remission after 12 months, currently undergoing monthly maintenance therapy.
Discussion
SPTCL is an uncommon primary cutaneous T-cell lymphoma that requires a robust workup to ensure an accurate diagnosis and appropriate management. First, it is imperative to distinguish SPTCL from primary cutaneous gamma-delta T-cell lymphoma (PCGD-TCL) as both these malignancies involve subcutaneous adipose tissue. SPTCL is of the alpha/beta phenotype that typically has an indolent course and good prognosis. Unlike SPTCL, PCGD-TCL is an aggressive T-cell lymphoma of the gamma/delta phenotype with a grim prognosis that presents with ulcerated nodules and plaques. CD56 and Beta F-1 staining on histologic examination are used to differentiate between these two entities. CD56 positivity and Beta F-1 negativity are hallmark findings for PCGD-TCL, as it infers a gamma/delta origin [2]. Furthermore, as opposed to more PCGD-TCL patients, only a small subset of SPTCL patients develop HLH, a rare but potentially fatal disorder characterized by nonremitting fevers, lymphadenopathy, hepatosplenomegaly, pancytopenia, liver dysfunction, hypertriglyceridemia, and hyperferritinemia [7].
It is important to assess for HLH during SPTCL workup since the presence of HLH will dramatically influence treatment options. Immunosuppressive agents are first line for SPTCL, while systemic chemotherapy is reserved for PCGD-TCL with or without HLH and SPTCL with HLH [8]. In the reported case, the biopsy demonstrated CD56 negativity and Beta F-1 positivity, indicating SPTCL of the alpha/beta origin, not PCGD-TCL. Furthermore, physical examination and laboratory studies did not reveal any laboratory abnormalities concerning for HLH. The patient was diagnosed with SPTCL based on these results and was initially started on immunosuppressive therapy. Subsequently, the patient failed three consecutive immunosuppressive regimens including hydroxychloroquine, cyclosporine, and methotrexate.
Currently, there are no guidelines for treatment of patients with SPTCL who fail immunosuppressive agents. Since HDAC inhibitors have shown efficacy in other types of refractory peripheral T-cell lymphomas, the patient was initiated on an HDAC inhibitor [4, 9, 10]. Romidepsin was chosen over other potential HDAC inhibitors owning to its FDA approval in refractory peripheral T-cell lymphomas [11, 12]. While the precise antitumor mechanisms of romidepsin have not been elucidated, it is likely that romidepsin exerts its effects by induction of cell cycle arrest and apoptosis in tumor cells [12]. In addition, romidepsin selectively modulates critical growth factor pathways such as JAK-STAT involved in cellular proliferation [12]. Important side effects to monitor in patients on romidepsin include cytopenias, gastrointestinal disturbance, and electrolyte abnormalities.
Previous case reports have shown romidepsin to be effective in patients with SPTCL and possible HLH [13, 14]. Additionally, romidepsin has been shown to be effective in refractory SPTCL in a patient who had an initial response to immunosuppressive agents [14]. In patients with relapsed SPTCL, romidepsin was also shown to be effective [10]. Our case differs from these prior studies in that our patient did not have HLH, failed all initial immunosuppressive therapy, and did not have relapsed SPTCL. Another report demonstrated complete remission of systemic SPTCL with chidamide, a different HDAC inhibitor [15]. However, chidamide was used in combination with chemotherapy to achieve remission, so it is unknown whether the positive clinical response was due solely to the HDAC inhibitor. Since the efficacy of romidepsin in treating refractory SPTCL has not been firmly demonstrated, our case reinforces the potential utility of romidepsin as a useful monotherapy for refractory SPTCL without HLH. Additionally, it opens the opportunity for investigation as a first-line therapy for SPTCL.
Statement of Ethics
Ethics approval was not required for this study. This retrospective review of patient data did not require ethical approval in accordance with local/national guidelines. Written, informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Funding Sources
Dr. Neda Nikbakht is the recipient of the Todd Nagel Memorial Award from the Skin Cancer Foundation.
Author Contributions
Laura Gleason and Daniel Joffe wrote, reviewed, and edited the manuscript. Safiyyah Bhatti helped with investigation and methodology. Alexa Cohen, Lauren Banner, and Emily Correia reviewed and edited the manuscript. Onder Alpdogan and Pierluigi Porcu helped with conceptualization. Neda Nikbakht helped with conceptualization, investigation, and supervision.
Funding Statement
Dr. Neda Nikbakht is the recipient of the Todd Nagel Memorial Award from the Skin Cancer Foundation.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1. Sugeeth MT, Narayanan G, Jayasudha AV, Nair RA. Subcutaneous panniculitis-like T-cell lymphoma. Proc. 2017 Jan;30(1):76–7. 10.1080/08998280.2017.11929537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005 May;105(10):3768–85. 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 3. López-Lerma I, Peñate Y, Gallardo F, Martí RM, Mitxelena J, Bielsa I, et al. Subcutaneous panniculitis-like T-cell lymphoma: clinical features, therapeutic approach, and outcome in a case series of 16 patients. J Am Acad Dermatol. 2018 Nov;79(5):892–8. 10.1016/j.jaad.2018.05.1243. [DOI] [PubMed] [Google Scholar]
- 4. Smolewski P, Robak T. The discovery and development of romidepsin for the treatment of T-cell lymphoma. Expert Opin Drug Discov. 2017 Aug;12(8):859–73. 10.1080/17460441.2017.1341487. [DOI] [PubMed] [Google Scholar]
- 5. Henter J-I, Horne AC, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. Aug 2007;48(2):124–31. 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 6. Kim J-S, Jeong YJ, Sohn M-H, Jeong H-J, Lim ST, Kim DW, et al. Usefulness of F-18 FDG PET/CT in subcutaneous panniculitis-like T cell lymphoma: disease extent and treatment response evaluation. Radiol Oncol. 2012;46(4):279–83. 10.2478/v10019-012-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hung GD, Chen YH, Chen DY, Lan JL. Subcutaneous panniculitis-like T-cell lymphoma presenting with hemophagocytic lymphohistiocytosis and skin lesions with characteristic high-resolution ultrasonographic findings. Clin Rheumatol. 2007 May;26(5):775–8. 10.1007/s10067-005-0193-y. [DOI] [PubMed] [Google Scholar]
- 8. Sullivan C, Loghmani A, Thomas K, Jetly-Shridhar R, Chowdry RP. Hemophagocytic lymphohistiocytosis as the initial presentation of subcutaneous panniculitis-like T-cell lymphoma: a rare case responding to cyclosporine A and steroids. J Investig Med High Impact Case Rep. 2020 Dec;8:2324709620981531. 10.1177/2324709620981531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lü Z, Shi YK, Zhou LQ, Qin Y, Dong M, Yang JL, et al. Primary subcutaneous panniculitis-like T-cell cutaneous lymphoma: clinical presentation, treatment and prognosis. Zhonghua Zhong Liu Za Zhi. 2010 May;32(5):350–3. [PubMed] [Google Scholar]
- 10. Foss F, Horwitz S, Pro B, Miles Prince H, Sokol L, Balser B, et al. Erratum to: romidepsin for the treatment of relapsed/refractory peripheral T cell lymphoma: prolonged stable disease provides clinical benefits for patients in the pivotal trial. J Hematol Oncol. 2017;10(1):154–8. 10.1186/s13045-017-0518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barbarotta L, Hurley K. Romidepsin for the treatment of peripheral T-cell lymphoma. J Adv Pract Oncol. 2015 Jan;6(1):22–36. [PMC free article] [PubMed] [Google Scholar]
- 12. Yoon S, Eom GH. HDAC and HDAC inhibitor: from cancer to cardiovascular diseases. Chonnam Med J. 2016 Jan;52(1):1–11. 10.4068/cmj.2016.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bashey S, Krathen M, Abdulla F, Sundram U, Kim YH. Romidepsin is effective in subcutaneous panniculitis-like T-cell lymphoma. J Clin Oncol. 2012 Aug;30(24):221–5. 10.1200/JCO.2012.41.5976. [DOI] [PubMed] [Google Scholar]
- 14. Jothishankar B, Espinosa ML, Zain J, Parekh V, Di Raimondo C, Abdulla F. Complete response to romidepsin as monotherapy in treatment-resistant subcutaneous panniculitis-like T-cell lymphoma. JAAD Case Rep. 2020 Dec;6(12):1245–7. 10.1016/j.jdcr.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li L, Wu C, Chai Y, Dong C, Zhao L. Chidamide induces long-term remission in rare subcutaneous panniculitis-like T-cell lymphoma: an unusual case report and literature review. Int J Immunopathol Pharmacol. 2021 Apr;35:20587384211009342. 10.1177/20587384211009342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.