Abstract
Immune checkpoint inhibitors (ICIs) can cause immune‐related adverse events (irAEs), such as neurological toxicity. A 46‐year‐old man was diagnosed with squamous cell lung cancer. Lung cancer recurred 3 years after he experienced left segmental lung rejection. Therefore, he received atezolizumab as fourth‐line chemotherapy. He experienced fever, headache, and decreased consciousness 10 days after the first dose of atezolizumab. Plain head computed tomography and cerebrospinal fluid examination showed no significant findings. Magnetic resonance imaging (MRI) with a Gadolinium (Gd)‐enhanced Cube fluid‐attenuated inversion recovery (FLAIR) sequence showed nodular abnormalities with contrast enhancement. Thus, aseptic meningitis caused by ICIs was suspected. His consciousness level gradually improved with glucocorticoid therapy. Moreover, most nodular abnormalities observed on cerebral MRI disappeared concurrently. Thus, Gd‐enhanced Cube FLAIR sequence has the unique ability to reveal immune‐related aseptic meningitis
Keywords: aseptic meningitis, Cube FLAIR , immune checkpoint inhibitors, immune‐related adverse event
Cancer treatment with immune checkpoint inhibitors (ICIs) is prevalent and frequent use of ICIs results in increased immune‐related adverse events (irAEs). We present a case of immune‐related aseptic meningitis that occurred following atezolizumab (PD‐L1 inhibitor) treatment. The immune‐related aseptic meningitis was diagnosed using MRI with the Gadolinium (Gd)‐ enhanced Cube fluid‐attenuated inversion recovery (FLAIR) sequence.
INTRODUCTION
Cancer treatment with immune checkpoint inhibitors (ICIs) is prevalent and frequent use of ICIs results in increased immune‐related adverse events (irAEs). To detect neurological immune‐related adverse events (irAEs) and rule out leptomeningeal metastasis, the diagnostic work‐up should include cerebral magnetic resonance imaging (MRI) and cerebrospinal fluid examination. 1 However, brain MRI usually shows no findings specific to aseptic meningitis. We present a case of immune‐related aseptic meningitis that occurred following atezolizumab (PD‐L1 inhibitor) treatment. The immune‐related aseptic meningitis was diagnosed using MRI with the Gadolinium (Gd)‐ enhanced Cube fluid‐attenuated inversion recovery (FLAIR) sequence.
CASE REPORT
A 46‐year‐old man was diagnosed with stage II squamous cell lung cancer. However, lung cancer recurred 3 years after experiencing left segmental lung rejection. Upon recurrence, three lines of chemotherapy were administered (1st: CBDCA+TS‐1, 2nd: VNR, 3rd: DTX + RAM) before administering atezolizumab as fourth‐line chemotherapy.
Ten days after receiving the first dose of atezolizumab, he experienced headache and fever. Upon admission, his consciousness was normal, Glasgow Coma Scale (GCS) E4V5M6. The next day, he experienced nausea and vomiting and his level of consciousness decreased to GCS E4V3M5. As intracranial lesion or leptomeningeal metastasis was suspected, he underwent plain head computed tomography (CT) and cerebrospinal fluid examination. The head CT showed no abnormalities. The cerebrospinal fluid examination showed: total cell count, 26 cells/μl; monocytes, 58%; polynuclear leucocytes, 42%; and protein and glucose levels were 451 mg/dl and 68 mg/dl, respectively. The cerebrospinal fluid contained no bacteria. The differential diagnosis was atezolizumab‐induced aseptic meningitis. Steroid therapy (methylprednisolone, 1000 mg/day for 3 days) was initiated. On the third day, his level of consciousness decreased to E4V2M5; thus, he underwent Gd‐enhanced cerebral MRI. Although the T1‐ and T2‐weighted images showed no significant findings, the Gd‐enhanced Cube FLAIR sequence image showed many nodules exhibiting contrast enhancement scattered on the surface of the cerebrum, cerebellum, and brain stem (Figure 1). There was no finding suggestive of encephalitis. On the fourth day, his level of consciousness improved to E4V5M6. The nodules almost disappeared by the 11th day (Figure 2). An irAE was diagnosed based on the response to steroid therapy and the change of image findings. His symptoms gradually improved, and he was discharged from hospital on the 31st day. No other irAEs except aseptic meningitis occurred. We evaluated aseptic meningitis was critical event for him. Therefore, rechallenge of Atezolizumab wasn't done. The treatment response of Atezolizumab therapy was within stable disease. He received cytotoxic chemotherapy after Atezolizumab.
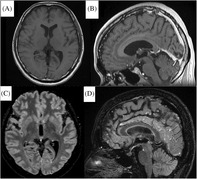
FIGURE 1.
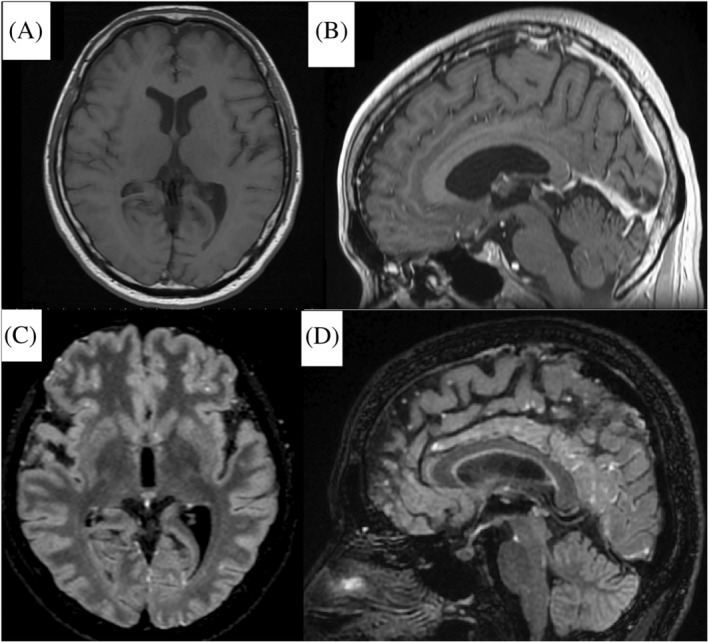
Cerebral magnetic resonance images obtained on the third day: (A) and (B) Axial and sagittal images of the Gd‐enhanced T1‐weighted sequence show no abnormal findings. (C) and (D) Axial and sagittal images with the Gd‐enhanced Cube FLAIR sequence show nodular lesions with high intensity scattered on the meninges of the frontal and occipital lobes
FIGURE 2.
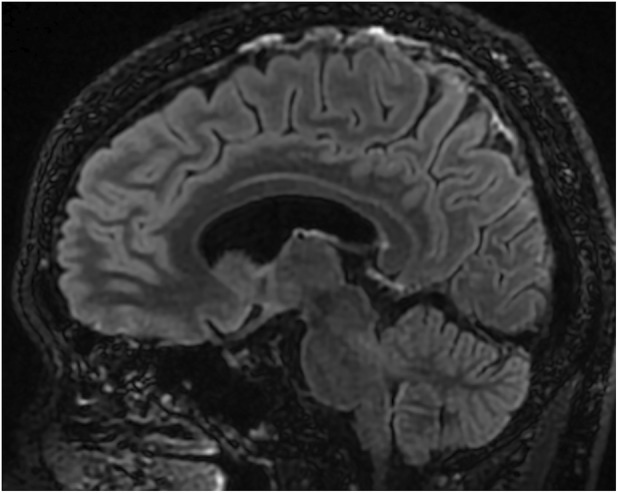
A cerebral magnetic resonance image obtained on the 11th day after glucocorticoid therapy: A sagittal image with the Gd‐enhanced Cube FLAIR sequence shows no abnormal findings
DISCUSSION
ICIs cause irAEs, which are adverse drug reactions due to excessive immunoreactions. Neurological irAEs occur less frequently than pneumoniae, hepatitis, kidney injury, colonitis, or endocrinopathy. Most patients with neurological irAEs respond to high‐dose steroids. However, in severe cases, neurological irAEs can lead to morbidity or death. 1 Some patients with neurological irAEs experience persistent neurological symptoms. 2 Therefore, neurological irAEs should be detected early and appropriate treatment initiated at the earliest. A lumbar puncture can help rule out progression of the underlying cancer, seizure, infection, and metabolic derangement to diagnose immune‐related aseptic meningitis. However, the paraneoplastic syndrome cannot be completely denied because the specific antibody was not measured. As diagnosis by exclusion often needs considerable time, examination findings specific to neurological irAEs would facilitate an earlier diagnosis.
Cerebral MRI may not show findings specific to aseptic meningitis as an irAE. Even in the cases of encephalitis as irAEs, only 43% of the cases showed findings on MRI. 3 Few reports show that imaging modalities can assist in diagnosing aseptic meningitis; in most meningoencephalitis cases, images with the FLAIR sequence of cerebral MRI showed that the area of leptomeninges exhibited contrast enhancement. 4
In the Cube sequence, signal attenuation can be minimized with contrast‐to‐noise ratio, which improves spatial resolution. 5 Autocalibration reconstruction for Cartesian (ARC) imaging improves scanning efficiency and generates a large image volume with a smaller isotropic voxel. ARC enables image reconstruction with sufficient image quality in coronal, sagittal, and axial planes. The Cube FLAIR sequence produces almost no cerebrospinal fluid flow artefacts when applied to brain imaging. 5 In addition, the superficial blood flow signal is suppressed with a T2‐shortening effect by Gd enhancement in the Cube FLAIR sequence. Therefore, in our case, the nodular meningeal abnormalities were clearly highlighted due to the favourable suppressive effect of the blood flow signal, thereby improving the spatial resolution to detect smaller nodular lesions. Multiple nodular meningeal abnormalities almost disappeared after steroid therapy and the symptoms improved after the treatment. These findings and the clinical course suggest that these nodular lesions seen on the Cube FLAIR sequence images explain the presence of aseptic meningitis of irAEs.
In conclusion, our case suggests that Gd‐enhanced Cube FLAIR sequence of cerebral MRI can diagnose immune‐related aseptic meningitis caused by ICIs and enables early appropriate treatment. To our best knowledge, this is the first case reporting cerebral MRI images with a Gd‐enhanced Cube FLAIR sequence obtained during the clinical course of immune‐related aseptic meningitis. However, it is unclear whether the nodular abnormalities seen on MRI with the Gd‐enhanced Cube FLAIR sequence are specific to aseptic meningitis of irAEs. More case reports are required to confirm these findings.
AUTHOR CONTRIBUTIONS
Ryo Kuroda and Hiroaki Nakagawa wrote the initial draft of the manuscript and conducted the literature search. Hiroaki Nakagawa, Yasuki Uchida, Yoko Tsunoda, and Keiko Narumiya edited the manuscript. Tatsuya Oki, Shinnosuke Hiratsuka, and Yukihiro Nagatani prepared the figures. Masafumi Yamaguchi and Yasutaka Nakano reviewed and edited the manuscript. All authors reviewed the manuscript.
CONFLICT OF INTEREST
None declared.
ETHICS STATEMENT
The authors declare written informed consent was obtained for the publication of this manuscript and accompanying images.
ACKNOWLEDGMENT
We would like to thank Editage (www.editage.com) for English language editing.
Kuroda R, Nakagawa H, Uchida Y, Narumiya K, Tsunoda Y, Yamaguchi M, et al. Immune‐related aseptic meningitis diagnosed by Cube FLAIR on enhanced magnetic resonance imaging for a lung cancer patient administered atezolizumab: A case report. Respirology Case Reports. 2023;11:e01076. 10.1002/rcr2.1076
Associate Editor: David Lam
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blackmon JT, Viator TM, Conry RM. Central nervous system toxicities of anti‐cancer immune checkpoint blockade. J Neurol Neuromed. 2016;1:39–45. [Google Scholar]
- 3. Stuby J, Herren T, Naumburger GS, Papet C, Rudiger A. Immune checkpoint inhibitors therapy‐associated encephalitis: a case series and review of the literature. Swiss Med Wkly. 2020;150:w20377. 10.4414/smw.2020.20377 [DOI] [PubMed] [Google Scholar]
- 4. Thouvenin L, Olivier T, Banna G, Addeo A, Friedlaender A. Immune checkpoint inhibitor‐induced aseptic meningitis and encephalitis: a case‐series and narrative review. Ther Adv Drug Saf. 2021;12:20420986211004745. 10.1177/20420986211004745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patzig M, Burke M, Bruckmann H, Fesl G. Comparison of 3D cube FLAIR with 2D FLAIR for multiple sclerosis imaging at 3 tesla. Rofo. 2014;186:484–8. 10.1055/s-0033-1355896 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


