Abstract
Sepsis is a critical syndrome and DIC often develops in severe septicemia. However, cares for severe patients are limited in remote hospitals. In addition, bad weather often makes medical evacuation difficult in such areas. A 66‐year‐old man had urinary tract infection by Escherichia coli, followed by septic shock and DIC rapidly just 2 days after the onset. He recovered in 3 weeks without any massive bleeding. Immediate insertion of the central venous catheter to maintain stable hemodynamics and Gram‐staining for selecting antibiotics were considered essential techniques for the survival of sepsis patients even in remote hospitals.
Keywords: DIC, gram‐staining, remote area, sepsis, shock, thrombocytopenia
A 66‐year‐old male had urinary tract infection by Escherichia coli, followed by septic shock and DIC rapidly just two days after the onset. He was treated and recovered in three weeks without any massive bleeding at a remote‐island‐hospital. Gram‐staining and immediate insertion of CV are essential techniques to care severe infections even in remote hospitals, and significant restrictions should always be aware such as the difficulties of emergency medical transport and the blood components transfusions.
1. INTRODUCTION
Sepsis is a clinical syndrome defined as a systemic response toward infection. Disseminated intravascular coagulation (DIC) is reported that developed in about 35% of severe septicemia, 1 and ultimately causes organ dysfunction and severe bleeding. Early intensive care is needed for improved sepsis mortality, 2 but this care is often limited in remote hospitals like us. In addition, medical transport is often difficult because of bad weather in such areas. 3 This article presents a case report from a remote island hospital of sepsis with severe thrombocytopenia by DIC and discussed the medical setting of remote island hospitals related to critical cares.
2. CASE PRESENTATION
A 66‐year‐old man with the history of diabetes mellitus type 2 and paralysis of the lower body because of an accidental 12th thoracic vertebral fracture and cord injury at the age of 38‐year‐old was admitted to our hospital with a symptom of fever of 38.8 degrees Celsius with shivering. His fever began 2 days before his hospital visit. He had a clear consciousness (Glasgow Coma Scale; E4V5M6). The blood pressure, heart rate, and respiratory rate were 136/110 mmHg, 110/min, and 16/min, respectively. His quick sequential organ failure assessment (qSOFA) score 4 was 0 at the time of his admission. Laboratory data (Table 1) showed purulent urine, leukocytosis with increased segmented leukocyte, and mild decrease in platelet count (Plts). From the SOFA score 4 with the addition of laboratory data (Table 1), sepsis was suspected at this point. 5 Gram‐staining detected gram‐negative rod in the urine. Immediate blood culture was performed, followed by treatment with piperacillin (PIPC) for sever urinary tract infection; two grams of PIPC was given intravenously every 8 h. Six hours after admission, the patient rapidly ran into warm shock; the blood pressure dropped to 70/50 mmHg. An immediate central venous access was taken via right jugular vein; through which administrations of norepinephrine (NE), dopamine (DOA), and bolus infusion of lactate ringer solution (3500 ~ 3000 ml/day) were started. NE of approximately 0.9 μg/kg/min and DOA of 5 μg/kg/min were required until his systolic blood pressure reached 90 mmHg and stabilized (Figure 1). He was diagnosed as septic shock at this point.
TABLE 1.
Laboratory data (reference values)
| Day | 1 | 2 | 3 | 4 | 6 | 7 | 8 | 9 | 10 | 11 | 14 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC/μl (3500 ~ 9700) | 5910 | 25,230 | 33,610 | 27,910 | 13,000 | 13,990 | 15,820 | 13,140 | 20,910 | 12,120 | 11,280 | 7660 |
| SEG (27 ~ 72%) | 96.4 | 97.9 | 95.9 | 94.5 | 87.8 | 89.5 | 97.6 | 89.4 | 88.1 | 83 | ||
| Plts ×104/μl (14 ~ 37.9) | 8.5 | 1.4 | 0.4 a | 0.1 | 1.0 | 1.2 | 3.2 | 4.8 | 6.2 | 9.6 | 11.7 | 15.4 |
| Hb g/dl (13.6 ~ 18.3) | 14.7 | 11.8 | 13.5 | 13.3 | 13.4 | 14.3 | 12.9 | 10.7 | 10.7 | 10.0 | 9.1 | |
| Alb g/dl (3.7 ~ 5.2) | 2.8 | 2.2 | 2.3 | 2.3 | 2.5 | 2.6 | 2.6 | 2.5 | 2.4 | 2.2 | ||
| CRP mg/dl (<0.45) | 26.73 | 21.42 | 24.68 | 15.56 | 2.30 | 4.31 | 3.62 | 6.75 | 8.45 | 4.37 | ||
| AST IU/L (10–40) | 31 | 62 | 32 | 15 | 8 | 6 | 11 | 10 | 9 | 7 | ||
| ALT IU/L (5–45) | 22 | 22 | 23 | 17 | 10 | 10 | 11 | 9 | 8 | 6 | ||
| Cre mg/dl (0.65 ~ 1.09) | 1.09 | 1.41 | 0.61 | 0.58 | 0.6 | 0.74 | 0.63 | 0.59 | 0.56 | |||
| UN mg/dl (8 ~ 20) | 40.7 | 45.8 | 28.5 | 22.5 | 18.5 | 17.1 | 14.2 | 9.4 | 6.7 | |||
| PT s (10.0 ~ 13.0) | 13.2 | 14.5 a | 12.0 | 12.0 | 11.1 | |||||||
| aPTT s (26.0 ~ 38.0) | 37 | 46.8 a | 24.7 | 28.3 | 30.5 | |||||||
| FIBG mg/dl (170 ~ 410) | 557 a | 230 | 233 | |||||||||
| d‐dimer μg/ml (<1.0) | 6.08 | 10.1 a | 3.66 | 2.15 | 2.12 | 1.83 | 1.04 | |||||
| FDP μg/ml (<5) | 24 a | 9 | 7 | |||||||||
| AT‐3 activity (80 ~ 130%) | 51 | 89 | ||||||||||
| qSOFA/SIRS b | 0/2 | |||||||||||
| SOFA | 2→6 | 10 | 9 | 8 | 7 | 7 | 5 | 5 | 4 | 4 | 1 | 0 |
International DIC score was 5 (Overt DIC ≧5), and Japanese DIC score was 7 (DIC ≧7).
systemic inflammatory response syndrome (SIRS) score.
FIGURE 1.
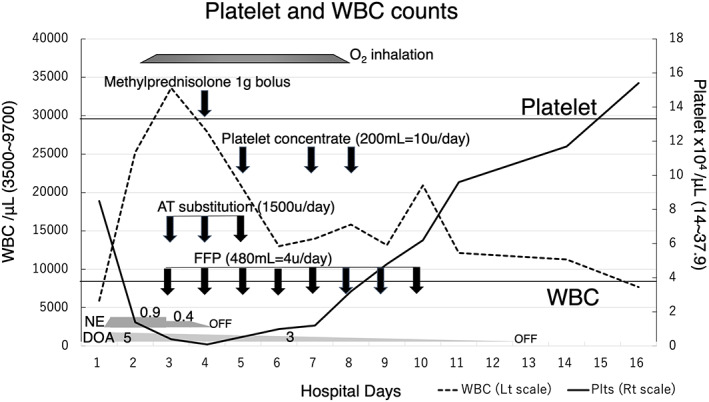
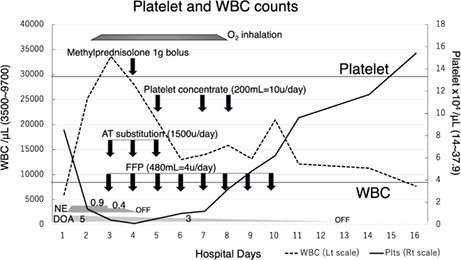
The time course of platelet (Plts) and white blood cell (WBC) counts is shown. Plts continued to decline drastically from Day2 to Day4. WBC extremely increased until Day3. Then they started to improve. Each arrow indicates the timing of administration of methylprednisolone, platelet concentrate, AT substitution, and FFP. Also, each trapezoid or polygon shows the duration of oxygen inhalation (2–4 litters/min), the dose of norepinephrine (μg/kg/min), and the dose of dopamine (μg/kg/min). The upper and lower horizontal lines indicate normal values for Plts (lower bound) and WBC (upper limit).
On the next hospital day (Day2), his Plts stepped down to 1.4 × 104/μl, and white blood cell jumped up to 25,230/μl. Septic DIC appeared to be progressing rapidly (Table 1, Figure 1), yet no massive hemorrhage was observed. Oxygen inhalation was required to maintain oxygen saturation of peripheral artery (SpO2) above 95% from Day2 to Day7. Transfusion of fresh frozen plasma (FFP) from Day3 to Day10, and treatment with antithrombin (AT) substitution from Day3 to Day5 was performed (Figure 1).
According to the urine and blood culture outcomes reported on Day3, Escherichia coli was detected in blood culture as well as in urine and was susceptible to PIPC, sulbactam/cefoperazone, meropenem, and several cephem antibiotics.
The high‐grade febrile over 38–39 degrees Celsius and marked leukocytosis continued on Day4, so bolus administration of 1000 mg of methylprednisolone was carried out under the unavoidable circumstances of persistent inflammation. And after that, the patient became afebrile and stable. Regardless of intensive treatments for DIC, Plts eventually reached to 0.1 × 104/μl, but remained stable without massive bleeding. Finally, on Day5, we started platelet concentrate (PC) transfusions (Figure 1). He continued to get well and was able to begin peroral intake on Day8, and Plts reached normal levels on Day16 (Figure 1, Table 1). He started rehabilitation for independent living in a wheelchair on Day16 and was discharged on Day46.
3. DISCUSSIONS
The cares for critically ill patients are limited by the range of basic critical care skills available from the medical stuff and equipment. Our facility is a single critical access hospital with 54 beds on the remote island with one operation room capable for a general anesthesia, laboratory room with bacteriological examination, computed tomography device, and two ventilators, but no dialysis unit. We have an emergency medical transport system with doctor‐helicopters and their operation is limited from dawn to dusk and is often difficult because of bad weather in our area; navigation rate was reported as 63.4%. 3
This patient chose to continue receiving treatment in our hospital, because he was having chronic medical condition care at our hospital. Therefore, we had his long‐term medical record, such as being prone to urinary tract infections because of urination disorder owing to lower body paralysis and diabetes mellitus, which was an advantage of a remote hospital not found in tertiary care centers. Conversely, dialysis is not available in our facilities, so if hemodialysis was required during treatment, we would have had to transfer this patient to a tertiary care center. However, this procedure may have been difficult because of the rapid progression of illness. It was a significant restriction of the treatment of critically ill patients in remote hospitals.
Platelet concentrate transfusion is also one of the most vulnerable points in remote island hospitals. PC transfusions are recommended to maintain a platelet count of at least 5.0 × 104/μl for patients with active bleeding. 6 Despite fairly lower level of platelet count, that was much lower than threshold recommended of PC transfusion in the literature, there was no symptomatic bleeding. However, we should have started PC transfusion earlier since the theory that administration of blood components might exacerbate disseminated coagulation activation has never been proven in clinical or experimental studies. 7 One of the factors that delayed the transfusion of PC is that our hospital is on a remote island; PCs are transported via aircraft from the mainland, so it usually takes a few days after ordering.
We chose the first‐line antibiotics based on the result of Gram‐staining, and blood and urine culture revealed that appropriate antibiotics administration was started before septic shock. Liu et al. reported that a delay in antibiotic administration significantly increased the adjusted in‐hospital mortality with an odds ratio (OR) of 1.09 (1.05–1.13) for each hour of delay. 8 Gram‐staining fortunately led to the initiation of appropriate antibiotics administration before septic shock in this case. Thus, Gram‐staining method is a simple and useful technique that can be performed even in a remote hospital.
Immediate insertion of the central venous catheter (CV) is necessary not only for the administration of vasopressors, but also for bolus fluid infusion as the first‐line therapy for septic shock patients. 2 If the insertion of CV was not available in our hospital, we must not have actually been able to save this patient. The immediate insertion of the CV is considered to be an essential capability in remote hospitals.
About corticosteroid use in sepsis, steroid pulse for septic shock is not recommended in the latest guidelines. 9 In our case, we should have considered using low‐dose steroids instead of a pulse for ongoing requirement as a dose of norepinephrine ≧0.25 μg/kg/min to maintain the target blood pressure. 10
4. CONCLUSIONS
Gram‐staining and immediate insertion of CV are essential techniques to care severe infectious disease even in remote hospitals, and significant restrictions should always be aware such as the difficulties of emergency medical transport and the blood components transfusions.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
INFORMED CONSENT
Written informed consent was obtained from the patient for publication of this case report.
Takeshita K, Izumisato T, Takane T, Nishihara K. A case of disseminated intravascular coagulation of sepsis that caused extreme‐thrombocytopenia treated at a remote‐island‐hospital. J Gen Fam Med. 2023;24:50–53. 10.1002/jgf2.578
REFERENCES
- 1. Okamoto K, Tamura T, Sawatsubashi Y. Sepsis and disseminated intravascular coagulation. J Intensive Care. 2016;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gavelli F, Castello LM, Avanzi GC. Management of sepsis and septic shock in the emergency department. Intern Emerg Med. 2021;16(6):1649–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asai Y. The present conditions and the future about emergency transportation in the southern Hokkaido area. J Jpn Counc Traffic Sci. 2015;14(3):42–6. [Google Scholar]
- 4. Singer M, Deutschman CS, Seymour CW, Shankar‐Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta A, Khalid A, Swaroop M. Sepsis and septic shock. Clinical Management of Shock ‐ The Science and Art of Physiological Restoration; 2020.
- 6. Wada H, Matsumoto T, Yamashita Y. Diagnosis and treatment of disseminated intravascular coagulation (DIC) according to four DIC guidelines. J Intensive Care. 2014;2(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papageorgiou C, Jourdi G, Adjambri E, Walborn A, Patel P, Fareed J, et al. Disseminated intravascular coagulation: an update on pathogenesis, diagnosis, and therapeutic strategies. Clin Appl Thromb Hemost. 2018;24(9_suppl):8S–28S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu VX, Fielding‐Singh V, Greene JD, Baker JM, Iwashyna TJ, Bhattacharya J, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196(7):856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishida O, Ogura H, Egi M, Fujishima S, Hayashi Y, Iba T, et al. The Japanese clinical practice guidelines for Management of Sepsis and Septic Shock 2016 (J‐SSCG 2016). Acute Med Surg. 2018;5(1):3–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–247. [DOI] [PMC free article] [PubMed] [Google Scholar]


