Abstract
Despite the continued importance of tuberculosis as a world-wide threat to public health, little is known about the mechanisms used by human lymphocytes to contain and kill the intracellular pathogen Mycobacterium tuberculosis. We previously described an in vitro model of infection of human monocytes (MN) with virulent M. tuberculosis strain H37Rv in which the ability of peripheral blood lymphocytes to limit intracellular growth of the organism could be measured. In the current study, we determined that lymphocyte-mediated killing of intracellular M. tuberculosis occurs within the first 24 h of coculture with infected MN. Natural killer (NK) cells isolated from both purified protein derivative (PPD)-positive and PPD-negative subjects were capable of mediating this early killing of intracellular H37Rv. NK cell-mediated killing of intracellular M. tuberculosis was not associated with the production of gamma interferon. Transferred supernatants of cocultured NK cells and M. tuberculosis-infected MN could not mediate the killing of intracellular M. tuberculosis, and Transwell studies indicated that direct cell-to-cell contact was required for NK cells to mediate the killing of the organism. Killing was not dependent upon exocytosis of NK cell cytotoxic granules. NK cells induced apoptosis of mycobacterium-infected MN, but neither killing of intracellular M. tuberculosis by NK cells nor NK cell-induced apoptosis of infected MN was inhibited by blocking the interaction of FasL and Fas. Thus, human NK cells may mediate killing of intracellular M. tuberculosis via alternative apoptotic pathways.
Tuberculosis remains a major international health problem which is likely to become even more significant in coming years because of the high prevalence of human immunodeficiency virus (HIV) disease in regions where infection with the intracellular pathogen Mycobacterium tuberculosis is endemic. Although it is estimated that one-third of the world's population is currently infected with M. tuberculosis (40), the great majority of these individuals never develop active disease, indicating the ability of human immune responses to contain the organism. Despite the importance of M. tuberculosis infection, little is known about the mechanisms that serve to contain this pathogen. Lymphocytes are thought to activate M. tuberculosis-infected mononuclear phagocytes to mediate the killing of intracellular bacteria, but the killing of M. tuberculosis has not been widely studied as an indicator of protective immunity. Although CD4, CD8, and γδ lymphocytes exhibit proliferative, cytokine, and cytotoxic responses following stimulation by whole M. tuberculosis or its antigens (4, 8, 12, 41, 42), these responses have generally not been correlated with bacterial killing. On the other hand, killing of intracellular Mycobacterium bovis BCG and M. tuberculosis has been assessed in response to various chemical mediators of cell death. These studies have indicated that necrotic death of infected monocytes does not result in killing of intracellular M. tuberculosis, whereas apoptotic cell death does result in bacterial killing (21, 25). However, with the exception of one report involving cell lines restricted to the unusual CD1 antigen-presenting pathway (39), these cytotoxic mechanisms have not been investigated in the context of specific lymphocyte populations capable of mediating killing of intracellular M. tuberculosis.
We previously described an in vitro model of reproducible infection of human monocytes (MN) with virulent M. tuberculosis strain H37Rv in which the ability of peripheral blood lymphocytes (PBL) to limit intracellular growth of the organism could be demonstrated (36). In the current study, we determined that killing of intracellular M. tuberculosis occurred within the first 24 h of coculture of infected MN with unstimulated PBL, which implied a role for innate immune responses in containment of intracellular M. tuberculosis. Natural killer (NK) cells represent a population of lymphocytes which could mediate innate protection against M. tuberculosis. NK cells have been implicated in early immune responses to viruses and to a variety of intracellular pathogens and are capable of rapidly producing gamma interferon (IFN-γ) as well as lysing specific target cells in the absence of prior activation (2, 6, 31, 32). More recently, NK cells also have been shown to express several surface ligands capable of initiating apoptosis (9, 29, 46). We therefore investigated the ability of NK cells to mediate the killing of intracellular M. tuberculosis following 24 h of coculture with infected MN, and we sought to correlate this killing with the production of IFN-γ, granule-mediated lysis of infected MN, and the induction of MN apoptosis.
MATERIALS AND METHODS
Donors.
Subjects were paid healthy volunteers between the ages of 22 and 50. For some studies, purified protein derivative (PPD)-positive subjects were specifically recruited. All protocols were approved by the Institutional Review Board of University Hospitals of Cleveland. Informed consent was obtained from each subject.
Cultivation and processing of mycobacteria.
Broth cultures of M. tuberculosis strain H37Rv and the attenuated vaccine strain M. bovis BCG Pasteur (strains 25618 and 35734, respectively, American Tissue Type Collection [ATCC], Rockville, Md.), were grown in sterile Middlebrook 7H9 medium with 10% Middlebrook ADC enrichment and 0.2% glycerol. Plated cultures were grown on Middlebrook 7H10 agar with 10% Middlebrook OADC enrichment (Difco, Detroit, Mich.) and 0.5% glycerol.
H37Rv and BCG were initially grown to mid-log phase as broth cultures in 1.7-liter rolling bottles (no. 2528-1700; Corning, Corning, N.Y.) at 37°C. Cultures were divided into aliquots and immediately stored at −70°C. These aliquots were used to inoculate all subsequent roller bottle cultures for use in the infection of MN so that all infections were performed with organisms which had undergone only one previous laboratory passage. In preparation for the infection of MN, mycobacteria were processed using a series of mechanical disruptions and centrifugations based on the methods of Schlesinger (33) and previously described in detail (36), which served to minimize clumping and to provide for accurate quantification of the inoculum.
Isolation of blood MN and lymphocytes.
Peripheral blood was obtained by venipuncture from healthy individuals and, mononuclear cells were isolated by density sedimentation using Ficoll-Hypaque (Ficoll-Paque, Pharmacia, Uppsala, Sweden) and washed three times in RPMI 1640 (BioWhittaker, Walkersville, Md.). Peripheral blood mononuclear cells (PBMC) were incubated in tissue culture-grade 100-mm polystyrene petri dishes (Falcon 3003; Becton Dickinson Labware, Lincoln, N.J.) to separate adherent MN and nonadherent PBL populations as previously described (36).
Isolation of human NK cells.
NK cells were isolated from blood lymphocytes using a two-step method. First, CD56+ cells were positively selected using anti-CD56 antibodies in a MACS magnetic column separation system (Miltenyi Biotech, Auburn, Calif.). Each portion of 108 PBL was resuspended in 800 μl of MACS buffer (phosphate-buffered saline with 0.5% bovine serum albumin and 2 mM EDTA) and incubated with 200 μl of MACS CD56 MicroBeads (no. 504-01) for 15 min at 4°C. Cells were washed with 10 to 20 ml of MACS buffer and resuspended in 1 ml of buffer. Blood lymphocytes were then added to a MACS MS separation column (no. 422-01) on a MiniMACS magnet that had been precooled for 20 min at 4°C and primed with 500 μl of buffer. Lymphocytes were allowed to run through the column, which was then washed three times with 500 μl of buffer. The column was then removed from the magnet. After the addition of 1 ml of buffer, a plunger was used to expel positively selected cells into 5-ml polystyrene tubes.
The selected population of CD56+ lymphocytes was further purified by removing residual CD3+ cells using Dynabeads M-450 CD3 (no. 111.13; Dynal). Beads were washed with medium and added to the CD56+ population for a 45-min incubation on a rotating platform at 4°C. CD3+ cells were removed using a Dynal magnetic particle separator. Two-color fluorescence-activated cell sorter analysis using CD3-fluorescein isothiocyanate (FITC) and CD56-Phycoerythrin (PE) (no. 340542 and no. 347747, respectively; Becton Dickinson) confirmed that the resulting cell populations consistently contained >95% CD3− CD56+ NK cells.
Infection of human MN with M. tuberculosis and assessment of intracellular growth.
Isolated MN were resuspended at a density of 106/ml in Iscove's modified Dulbecco's medium with NaHCO3, 25 mM HEPES, 1% l-glutamine (subsequently referred to here as IMDM; BioWhitakker 12-722F) with 5% fresh autologous serum (without antibiotics), and 100 μl (105 monocyctes) were divided into aliquots in triplicate wells of round-bottomed 96-well plates (Corning) for CFU assessment for each time point to be studied. Following overnight incubation to allow for readherence, the supernatants were removed and H37Rv was added, in a 1:1 bacterium-to-MN infecting ratio, in 100 μl of IMDM per well with 30% autologous serum. Plates were returned to a 37°C incubator for 1 h, at which time the supernatants were aspirated, and each well was washed three times with RPMI 1640 and 10% fetal calf serum and 1% HEPES to remove noningested mycobacteria. Wells were then refilled with 200 μl of IMDM and 10% non-heat-inactivated autologous serum.
At selected time points, supernatants from the appropriate triplicate wells were aspirated and saved. Cell pellets were lysed with 0.067% sodium dodecyl sulfate (SDS), pooled, and diluted for plating onto 7H10-OADC plates for CFU determination as previously described in detail (36). The results were expressed as CFU per milliliter of lysate, which corresponded to CFU/106 cultured MN.
Addition of blood lymphocytes and NK cells to infected MN.
PBL were resuspended in IMDM containing 10% fresh autologous serum and stored in a 37°C CO2 incubator during the readherence of MN into microtiter wells. The next morning, the lymphocytes were washed and resuspended at a density of 5 × 106/ml in IMDM with 10% autologous serum. Lymphocytes were then added to autologous MN cultures immediately following the rinsing of nonphagocytosed bacteria. The addition of 200 μl of PBL (for a 10:1 PBL/monocyte ratio) was utilized in order to reconstitute the approximate composition of the PBMC. Various ratios of NK cells were added to autologous infected MN to determine dose-response effects as described in Results.
M. tuberculosis-induced production of IFN-γ by lymphocyte populations and blocking of IFN-γ.
Supernatants were collected from cocultured M. tuberculosis-infected human MN and PBL or NK cells after 24 and 96 h of culture. Samples were frozen at −70°C until use, at which time they were thawed and filtered through 0.22-μm (pore-size) filters to remove residual organisms. IFN-γ concentrations in supernatants were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (EH-IFNγ; Endogen, Cambridge, Mass.). ELISA plates were read using an automated plate reader (Molecular Devices Corp., Menlo Park, Calif.) and a dual-wavelength reading at λ = 450/570. In blocking studies, neutralizing polyclonal antibody to human IFN-γ (Endogen P-700) was added to cultures at a concentration of 10 μg/ml.
Supernatant transfer studies.
Supernatants were collected from 96-well microtiter cocultures of NK cell, and M. tuberculosis-infected human monocytes were established in the fashion described above. Supernatants removed after 6, 18, and 24 h of coculture were filtered to remove residual organisms and added to freshly infected MN from the same subjects. The CFU counts were determined at 24 h of culture, and the supernatant-mediated reduction in intracellular M. tuberculosis was compared to that observed in cocultures of M. tuberculosis-infected MN cocultured with NK cells.
Assessment of the requirement for direct contact between NK cells and M. tuberculosis-infected MN in killing of intracellular bacilli.
Dual-chamber studies were established using 24-well Transwell plates (no. 3413; Costar, Cambridge, Mass.). A total of 6 × 105 MN was added to the lower chamber of each well and infected with M. tuberculosis using a 1:1 bacterium-to-cell ratio as described above. For each subject, 3 × 106 NK cells were then added to the upper chamber of one well (separated from the infected MN by a 0.4-μm [pore size] membrane) and directly to infected MN in the lower chamber of another well. After 24 h of coculture, lower-chamber cells were lysed to allow for CFU determination. Intracellular M. tuberculosis levels in cultures of MN alone were compared to those within cultures in which NK cells were added in direct contact with infected MN and to wells in which NK cells were separated by membrane filters from the MN.
Assessment of NK cell-mediated cytotoxicity.
NK cell activity was measured using the standard target K562 human leukemia cell line (no. 45506; ATCC) in a chromium release assay using radioactive 51Cr. K562 cells were labeled with 100 μCi of 51Cr (1 mCi/ml; ICN Radiochemicals, Irvine, Calif.) for 2 h at 37°C, washed four times, and resuspended in 10% fetal calf serum at 105 cells/ml, of which 3,000 to 5,000 cell aliquots were plated into 96-well round-bottomed plates. NK cells were added to the wells in various effector/-target ratios (1:1, 5:1, and 20:1). Plates were centrifuged at 200 × g for 30 s and incubated at 37°C for 4 h. Supernatants were harvested and 51Cr release measured by using a gamma counter. The spontaneous release of 51Cr was measured in wells containing target cells alone. The maximum release was determined from target cells which had been lysed with 3% SDS. The percent specific 51Cr release was calculated for each experimental group by using the following equation: percent specific release = [(cpm experimental − cpm spontaneous release)/(cpm maximum release − cpm spontaneous release)] × 100.
Inhibition of exocytosis of NK cell cytotoxic granules.
For some studies, NK cell granule release was inhibited using the calcium chelator EGTA (E 0396; Sigma, St. Louis, Mo.). For cytotoxicity studies, NK cells were resuspended in medium containing 5 mM EGTA prior to the addition to K562 cells, and the specific 51Cr release determined as described above. In CFU studies, NK cells were resuspended in IMDM containing 5 mM EGTA prior to the addition to M. tuberculosis-infected MN. Control studies adding IMDM with EGTA to infected MN alone were also performed.
Measurement of NK cell-induced MN apoptosis.
NK-cell mediated apoptosis of mycobacterium-infected MN was measured using FITC labeling of DNA strand breaks with a commercially available TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) assay (In Situ Cell Death Detection Kit 1684795; Boehringer-Mannheim, Indianapolis, Ind.). In order to accurately quantify apoptosis in a mixed cell system, MN were also labeled using CD14-PE (no. 347497; Becton Dickinson) and analyzed by two-color flow cytometry. Because of the need to perform flow cytometry outside of a biosafety level 3 facility, MN were infected with M. bovis BCG for these studies. MN were placed in polypropylene tubes and incubated for 1 h with BCG in IMDM with 30% autologous serum in a 10:1 bacterium-to-cell ratio. Infected MN were then pelleted by centrifugation at 480 × g and resuspended in IMDM with 10% serum. NK cells were added to infected autologous MN in a 5:1 ratio and incubated for 24 h. Uninfected MN, BCG-infected MN, and uninfected MN cocultured with NK cells were incubated as well for use as controls. Cells were labeled with anti-CD14 and fixed with 2% paraformaldehyde prior to permeabilization and labeling for apoptosis with the TUNEL reagent according to the manufacturer's protocol.
To assess apoptosis within MN cultures infected with virulent M. tuberculosis, in situ TUNEL assays were performed using three-color fluorescent microscopy. To allow for recognition of infected cells stained with both CD14-PE and TUNEL-FITC, H37Rv was labeled with the amine-reactive fluorescent blue dye AlexaFluor 350 carboxylic acid, succinimidyl ester (A10168; Molecular Probes, Eugene, Oreg.). Bacilli were washed and resuspended in PBS (pH 8.7; adjusted by the addition of 1 M sodium bicarbonate buffer). AlexaFluor 350 solution was prepared according to the manufacturer's recommendations by dissolving 5 g of the dye in 500 μl of dimethyl sulfoxide. Then, 25 μl of this solution was added to 450 μl of H37Rv. Following a 30-min incubation at room temperature, H37Rv was washed three times and diluted to the appropriate concentration for infection of MN in the same manner as that described for BCG above. NK cells were then added to cultures in a 5:1 ratio relative to the number of infected MN. Following 24 h of coculture, this preparation was incubated with CD14-PE, washed, and fixed by overnight incubation with 2% paraformaldehyde. The next day, cells were counted, and cytospin slides were prepared using 50,000 cells per slide. In situ TUNEL assessment of apoptosis was the performed according to the manufacturer's protocol (Boehringer-Mannheim). Fluorescent micrograph images using appropriate filters for each color were obtained using a digital camera (Spot Digital Camera; Diagnostic Instruments, Sterling Heights, Mich.), and composite three-color micrographs were assembled using Spot Advanced Software (Diagnostic Instruments).
Blocking of Fas-FasL interaction.
Blocking anti-FasL monoclonal antibody NOK1 (Pharmingen, San Diego, Calif.) was added in final concentration of 10 μg/ml to assays of both NK cell-mediated killing of intracellular M. tuberculosis H37Rv and NK cell-induced apoptosis of BCG-infected MN. Results were determined by CFU assay and TUNEL assay, respectively, as described above. As a control for the efficacy of blocking of FasL by NOK1, the antibody was also added to cultures in which a previously described FasL-expressing transfectant KFL9 (35) was incubated with Jurkat target cells. The ability of KFL9 to induce apoptosis of Jurkat cells in the presence of medium alone and with the addition of 10 μg of NOK1 per ml was assessed by the TUNEL method. Cells were incubated with anti-CD54-PE prior to the TUNEL procedure in order to distinguish the two cell populations. Apoptosis of Jurkat was calculated as percentage of CD54-negative cells that stained positively with the TUNEL reagent.
Assessment of the effects of blocking multiple NK cell effector pathways simultaneously.
To evaluate the possibility that multiple effector functions of NK cells function cooperatively to result in the killing of intracellular M. tuberculosis, we assessed the results of simultaneously blocking IFN-γ with the antibody P-700 (again at concentration 10 μg/ml), granule release with 5 mM EGTA, and Fas-FasL interactions with the anti-FasL antibody NOK1 (10 μg/ml). The effects of each pair of these inhibitors upon NK cell-mediated killing of intracellular M. tuberculosis was determined at 24 h of coculture, as was the effect of simultaneous addition of all three inhibitors to the cultures.
Statistics.
All statistical comparisons were made using paired t tests and calculated on Prism software (GraphPad Software, San Diego, Calif.).
RESULTS
PBL mediate killing of intracellular M. tuberculosis within the first 24 h of coculture.
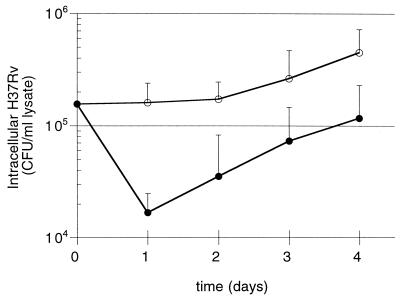
Standard assays of intracellular growth of M. tuberculosis have measured the CFU at days 4 and 7 following infection. Because our previous study suggested PBL-mediated killing of intracellular M. tuberculosis within human MN occurred prior to the day 4 time point, we sought to clarify the kinetics of early PBL-mediated limitation of growth of M. tuberculosis. The CFU of intracellular M. tuberculosis was determined on a daily basis during days 0 to 4 of cultures of infected MN alone and cocultures of infected MN plus PBL. Figure 1 shows the results for studies of five subjects and indicates consistent reduction of intracellular M. tuberculosis within the first 24 h after the addition of PBL to infected MN (P = 0.006 compared to CFU within MN alone). Following this 9.6-fold early reduction, growth of M. tuberculosis both within MN alone and within MN cocultured with PBL were essentially the same through days 2 to 4 (2.6-fold growth versus 3.3-fold growth, respectively). The finding of killing within 24 h of the addition of unprimed lymphocytes suggested a role for innate immune responses such as those mediated by NK cells in the early inhibition of intracellular M. tuberculosis.
FIG. 1.
PBL-mediated reduction in intracellular M. tuberculosis occurs within the first 24 h of coculture with infected MN. The CFU count of intracellular M. tuberculosis H37Rv within infected MN was determined at 24 h intervals for cultures of MN alone (○) and infected MN cocultured with PBL in a 10:1 PBL MN ratio (●). As illustrated, PBL mediated a drop in intracellular M. tuberculosis of approximately 1 log within the first 24 h of coculture, whereas in days 2 to 4 the rates of intracellular growth of the organism in the two culture conditions were comparable. The results represent the mean and the standard deviation (SD) from five donors.
Isolated human NK cells can mediate early killing of intracellular M. tuberculosis.
Flow cytometry confirmed that the two-step enrichment procedure described above consistently yielded a cell population composed of greater that 95% CD3− CD56+ lymphocytes, which are subsequently referred to as NK cells.
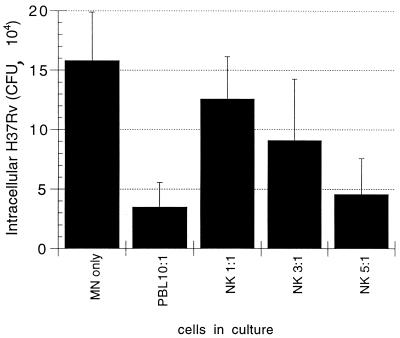
In initial studies, NK cells were added in ratios of 1:1, 3:1, and 5:1 to M. tuberculosis-infected MN. As illustrated in Fig. 2, NK cells were able to mediate killing of intracellular M. tuberculosis within 24 h of coculture, and the magnitude of this effect increased with increasing ratios of NK cells relative to M. tuberculosis-infected MN. The reductions in intracellular M. tuberculosis-mediated by NK cells in both 3:1 and 5:1 ratios were statistically significant (P = 0.006 and P = 0.003, respectively). In order to maximize our ability to assess the mechanisms involved in this killing, the 5:1 ratio of NK cells to infected MN was used in all subsequent studies. Donor-to-donor variability can be substantial in human studies of mycobacteria, as indicated by large standard deviations, particularly of CFU of infected MN alone. Nevertheless, we found that relatively low numbers of subjects were required to demonstrate statistically significant effects of NK cells on the viability of intracellular M. tuberculosis in the various studies described.
FIG. 2.
NK cells mediate the killing of intracellular M. tuberculosis within 24 h of coculture with infected MN. CFU of intracellular M. tuberculosis was assessed in MN alone and following the addition of PBL (in a 10:1 ratio relative to MN) and of isolated CD3− CD56+ NK cells (in ratios of 1:1, 3:1, and 5:1 relative to infected MN). The results indicate the mean and SD for four subjects studied. As illustrated, NK cells were capable of killing intracellular M. tuberculosis. The magnitude of this effect increased with the use of higher ratios of NK to infected MN and was statistically significant following the addition of NK cells in both a 3:1 ratio (P = 0.006) and a 5:1 ratio (P = 0.003).
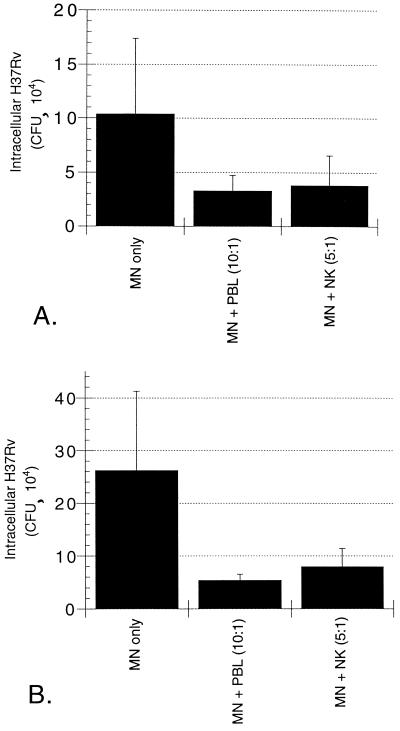
To confirm that our NK cell-enriched cell populations were effectively depleted of antigen-specific immune responses to M. tuberculosis, we compared the effects of the addition of NK cells obtained from PPD-positive and PPD-negative donors to M. tuberculosis-infected MN. As shown in Fig. 3, NK cells from both subject groups mediated killing of intracellular H37Rv. The magnitude of M. tuberculosis killing following the addition of NK cells from the two groups was similar, since NK cells of PPD-positive subjects mediated 63% reduction in intracellular M. tuberculosis compared to CFU within infected MN alone (P = 0.036, Fig. 3A), whereas NK cells from PPD-negative subjects mediated 70% reduction in CFU (P = 0.013, Fig. 3B).
FIG. 3.
NK cells from both PPD-positive (A) and PPD-negative (B) subjects mediate a significant reduction in intracellular M. tuberculosis at 24 h. The addition of NK cells from PPD-positive donors to M. tuberculosis-infected MN resulted in a 63% decrease in intracellular H37Rv at 24 h, whereas the addition of NK cells from PPD-negative donors mediated a 70% reduction in the organism. The NK cell-mediated reduction in intracellular M. tuberculosis compared to CFU within MN alone was significant for both groups of subjects (P = 0.036 for PPD-positive subjects; P = 0.013 for PPD-negative subjects). The graphs illustrate the mean and SD for five subjects in each group.
Killing of intracellular M. tuberculosis by NK cells is not associated with IFN-γ production.
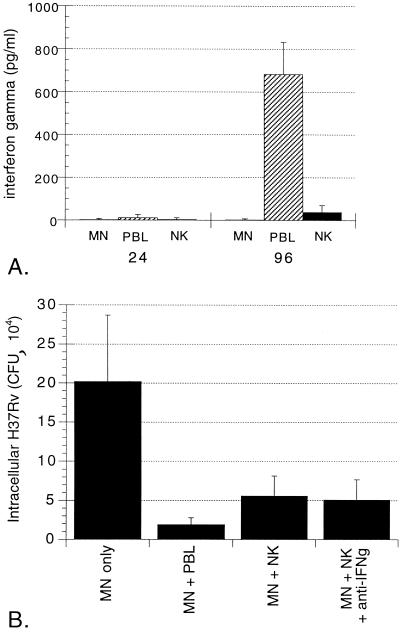
Because early production of IFN-γ has been demonstrated to be essential for NK cell-mediated protection against other pathogens, we sought to determine whether IFN-γ played a role in the ability of these cells to mediate killing of intracellular M. tuberculosis. IFN-γ was measured in supernatants collected at 24 and 96 h from cultures of M. tuberculosis-infected MN alone and of infected MN cocultured with either PBL or isolated NK cells. As illustrated in Fig. 4A, none of the cell populations studied produced detectable quantities of IFN-γ following 24 h of coculture with M. tuberculosis-infected MN. NK cells also produced only minimal amounts of IFN-γ at 96 h of coculture with M. tuberculosis-infected MN (37 ± 36 pg/ml), whereas PBL produced substantial amounts of the cytokine (683 ± 149 pg/ml, P = 0.0002 compared to infected MN alone).
FIG. 4.
Killing of intracellular M. tuberculosis by NK cells is not mediated by IFN-γ. (A) Results of the measurement of IFN-γ within 24- and 96-h culture supernatants of M. tuberculosis-infected MN alone and infected MN cocultured with PBL or NK cells. As illustrated, none of the 24-h supernatants contained appreciable amounts of IFN-γ. At 96 h, PBL had produced significant amounts of IFN-γ in response to M. tuberculosis-infected MN. IFN-γ production by NK cells remained minimal, however, and was not significantly greater than within supernatants of infected MN alone. The data represent the mean and SD values for five subjects studied. (B) Effects of addition of blocking anti-IFN-γ polyclonal antibody (10 μg/ml) to cultures. As shown, blocking antibody had no effect on the NK cell-mediated killing of intracellular M. tuberculosis NK cells in medium alone induced a 72% reduction in intracellular M. tuberculosis, whereas in the presence of anti-IFN-γ, NK cells mediated a 75% reduction in intracellular H37Rv (P = 0.252 compared to NK cells in medium alone). The results represent the mean and SD of studies of cells obtained from five subjects.
To rule out the possibility that NK-cell mediated killing of intracellular M. tuberculosis could be mediated by local IFN-γ production not detectable by ELISA, we added anti-IFN-γ blocking antibody to cultures. As indicated in Fig. 4B, the addition of anti-IFN-γ had no impact on the ability of NK cells to mediate the killing of intracellular M. tuberculosis. Killing by NK cells both in medium alone and in the presence of anti-IFN-γ was statistically significant (P = 0.011 and 0.012, respectively, compared to CFU within MN alone)
Transferred supernatants of cocultured NK cells and M. tuberculosis-infected MN do not have the capacity to mediate killing of intracellular M. tuberculosis.
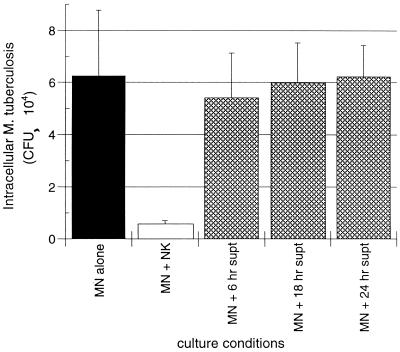
To assess the possibility that NK cell-mediated killing of intracellular M. tuberculosis results from the actions of cytokines other than IFN-γ or of combinations of cytokines, we studied the ability of supernatants collected from cocultured NK cells and M. tuberculosis-infected MN to alter intracellular growth of M. tuberculosis within freshly infected MN. The CFU levels of intracellular M. tuberculosis were assessed 24 h after the addition of the supernatants and compared to CFU within MN alone and within MN to which NK cells were added. As illustrated in Fig. 5, NK cells mediated significant killing of intracellular M. tuberculosis (P = 0.012) at 24 h. In contrast, the addition of supernatants collected at 6, 18, and 24 h of coculture did not result in significant reduction of intracellular M. tuberculosis compared to that observed in infected MN alone (P = 0.136, P = 0.410, and P = 0.471, respectively).
FIG. 5.
Supernatants of cocultured NK cells and M. tuberculosis-infected MN alone cannot mediate the killing of intracellular M. tuberculosis. As illustrated, the 92% reduction in intracellular M. tuberculosis mediated by the addition of NK cells (compared to the CFU level within infected MN alone) was much greater than that mediated by the addition of supernatants of cocultured NK cells and infected MN. Supernatants collected after 6, 18, and 24 h of coculture resulted in reductions in intracellular M. tuberculosis of only 13, 4, and 1.2%, respectively. The results represent the mean and SD studies from four subjects.
NK cell-mediated killing of intracellular M. tuberculosis requires direct contact between NK cells and M. tuberculosis-infected MN.
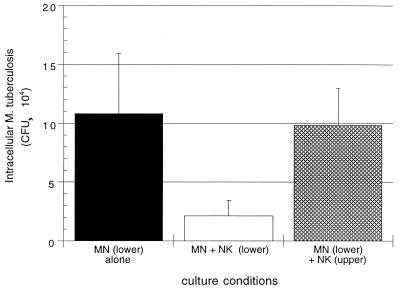
To further assess the role of soluble factors as opposed to direct cell-to-cell contact in killing of M. tuberculosis by NK cells, we established cultures of NK cells and M. tuberculosis-infected MN within a dual-chamber culture system. Comparison was made between the CFU levels of intracellular M. tuberculosis in cultures in which NK cells were added to infected MN within the same chamber of Transwell culture plates and those in which the same number of NK cells were placed in an upper chamber separated from infected MN by a 4-μm (pore-size) filter. As shown in Fig. 6, NK cells directly cocultured with infected MN mediated a significant reduction in the intracellular M. tuberculosis compared to CFU levels at 24 h within MN alone (P = 0.011). In contrast, NK cells placed within the upper chamber did not significantly alter CFU of M. tuberculosis within MN on the other side of the filter (P = 0.248).
FIG. 6.
NK cell-mediated killing of intracellular M. tuberculosis requires direct contact between NK cells and infected MN. The killing assay was modified to fit a 24-well dual-chamber Transwell plate format. The addition of NK cells directly to M. tuberculosis-infected MN within the lower chamber resulted in an 81% reduction in intracellular M. tuberculosis compared to the CFU count within MN alone. In contrast, the addition of NK cells to the top chamber of the Transwell plates, in which these effector cells were separated from infected MN by a 4-μm membrane, resulted in a nonsignificant 9.5% reduction in intracellular M. tuberculosis. The results represent the mean and SD of studies of four subjects.
Inhibition of granule release does not affect the ability of human NK cells to kill intracellular M. tuberculosis.
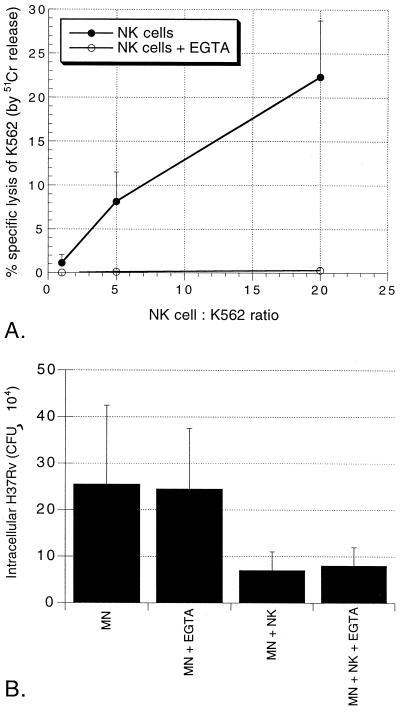
Because a major effector mechanism of NK cells is that of granule-mediated cytotoxicity, we sought to determine whether this pathway was involved in NK cell-mediated killing of intracellular M. tuberculosis The calcium chelator EGTA inhibits granule release from NK cells and was therefore used to investigate the role of granules in the killing of H37Rv. As illustrated in Fig. 7A, CD3− CD56+ NK cells lyse the standard NK target K562 line in a dose-dependent fashion. In the presence of 5 mM EGTA, granule-dependent lysis was completely inhibited, indicating that this concentration of EGTA effectively blocked calcium-dependent granule release and activity. Parallel CFU studies were performed simultaneously using NK cells from the same subjects in order to assess the effects of blocking of granule release on the ability of NK cells to kill intracellular M. tuberculosis H37Rv (Fig. 7B). We found that 5 mM EGTA itself had no direct effect on the intracellular growth of M. tuberculosis, as illustrated. NK cells in medium reduced the CFU level of intracellular H37Rv by 83% compared to the CFU level found within MN alone. In the presence of 5 mM EGTA, NK cells still mediated 82% reduction of the intracellular M. tuberculosis These studies thus indicated that the killing of intracellular M. tuberculosis by NK cells is independent of the release of cytotoxic granules.
FIG. 7.
NK cell-mediated killing of intracellular M. tuberculosis is independent of the release of cytotoxic granules. (A) Purified NK cells in medium alone mediate the lysis of the standard K562 target leukemia cell line in a dose-dependent fashion (●), whereas in the presence of 5 mM EGTA this granule-dependent lysis is completely eliminated (○). The data represent the mean and SD of the percent specific lysis for four subjects studied. (B) Results of parallel studies of the effects of inhibition of granule release on the killing of intracellular M. tuberculosis. The reduction in intracellular M. tuberculosis by NK cells both in medium alone and in the presence of EGTA was statistically significant (P = 0.047 and P = 0.043, respectively). The figure illustrates the mean and SD for four subjects studied.
NK cells induce apoptosis of M. bovis BCG-infected autologous MN.
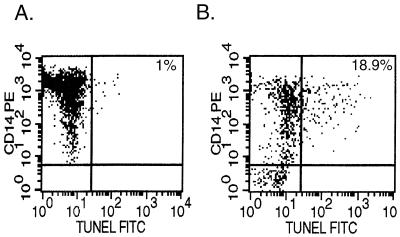
Since previous investigations have demonstrated that chemically induced apoptosis of MN infected with M. bovis BCG and virulent M. tuberculosis results in the killing of intracellular bacteria, we assessed whether NK cells mediate apoptosis of infected MN. MN apoptosis was measured by the TUNEL method with analysis by flow cytometry to determine the percentage of CD14+ MN which were undergoing apoptosis. Because flow cytometry cannot be performed in our biosafety level 3 facility, we infected MN with the attenuated strain M. bovis BCG rather than H37Rv for these studies. Representative results are displayed in Fig. 8. MN apoptosis, represented by dual staining with CD14-PE and TUNEL-FITC, was minimal within BCG-infected MN alone at 24 h after infection (Fig. 8A). The addition of NK cells, however, increased the percentage of apoptotic MN from <1% to 18.9% in this subject (Fig. 8B). Table 1 summarizes the mean results of TUNEL assays from separate experiments using NK cells from four donors. As shown, apoptosis was minimal (1.4%) in uninfected MN and was not significantly increased in BCG-infected MN (3.5%, P = 0.100). The addition of NK cells to uninfected MN significantly increased apoptosis to 11.5% of MN (P = 0.014 compared to MN alone), and apoptosis of BCG-infected MN following the addition of NK cells increased to 21.4% (P = 0.009 compared to BCG-infected MN alone, P = 0.005 compared to uninfected MN incubated with NK cells).
FIG. 8.
Representative TUNEL assay showing ability of NK cells to induce apoptosis of BGC-infected human MN. MN apoptosis is indicated as percentage of CD 14-PE positive cells which are also TUNEL-FITC-positive. As illustrated in this example, BCG-infected MN alone display minimal apoptosis (A), whereas 18.9% of CD14+ cells become apoptotic during 24 hours of co-culture with NK cells (B). (Both dot plots represent data gated for monocytes from FSC/SSC images.)
TABLE 1.
NK cells induce apoptosis of BCG-infected human MN
| Cells in culture | % Apoptosis of CD14+ MNa (SD) |
|---|---|
| Uninfected alone | 1.4 (1.7) |
| BCG-infected MN | 3.5 (1.5) |
| Uninfected MN plus NK cells | 11.5 (5.0)b |
| BCG-infected MN plus NK cells | 21.4 (8.2)c |
Values represent the mean and SD of the percentage of CD14+ monocytes undergoing apoptosis (as measured by the TUNEL assay) for studies of NK cells from four subjects.
P = 0.014 compared to uninfected MN alone as calculated by the paired t test.
P = 0.009 compared to BCG-infected MN alone; P = 0.005 compared to uninfected MN plus NK cells.
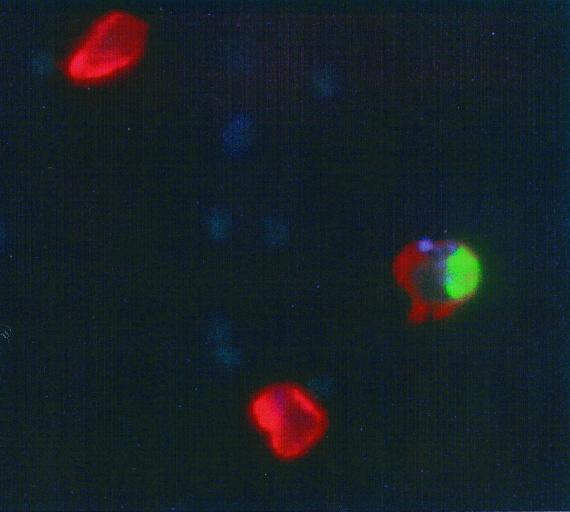
Because it has been reported that virulent M. tuberculosis, in comparison to BCG, renders infected cells resistant to apoptosis (1), we made use of an in situ TUNEL assay to confirm that NK cells also induced the apoptosis of H37Rv-infected MN. As the fluorescent micrograph shown in Fig. 9 illustrates, M. tuberculosis-infected MN did undergo apoptosis following coculture with human NK cells.
FIG. 9.
MN infected with virulent M. tuberculosis H37Rv undergo apoptosis in the presence of NK cells. H37Rv was stained with the fluorescent blue dye AlexaFluor 350 prior to infection. After 24 h of coculture with NK cells, MN were labeled with anti-CD14-PE, and cytospin slides were prepared for the in situ determination of apoptosis by using an FITC-labeled TUNEL reagent. As shown in this composite image, the addition of the smaller, unstained NK cells resulted in apoptosis (green) of the red-stained MN infected with (blue) AlexaFluor 350-stained H37Rv.
NK cell-mediated killing of intracellular M. tuberculosis and apoptosis of infected MN are both independent of FasL-Fas interactions.
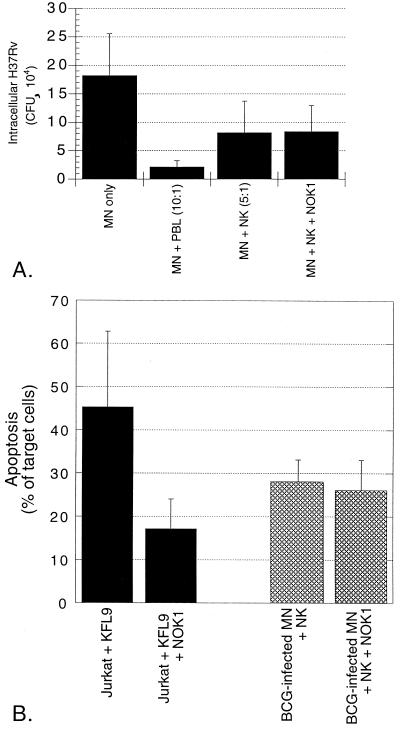
The best characterized of many known pathways of apoptosis induction is that mediated by the interaction of FasL with Fas. Because NK cells can express FasL, we investigated the role of this interaction in NK cell-mediated killing of intracellular M. tuberculosis using the neutralizing anti-FasL antibody NOK1 (10 μg/ml). As shown in Fig. 10A, the addition of NOK1 had no effect on the ability of NK cells to kill intracellular H37Rv. For the studies of the four subjects illustrated, NK cells alone mediated 51.1% reduction in intracellular H37Rv compared to MN alone (P = 0.018). In the presence of NOK1, The CFU of intracellular M. tuberculosis was reduced by 56.2% by NK cells (P = 0.014). Figure 10B illustrates the effects of NOK1 on apoptosis. Induction of apoptosis of Jurkat cells by the FasL transfectant KFL9 was significantly inhibited by the addition of NOK1, since the percentage of TUNEL-positive Jurkat cells decreased from 45.3 to 17.1% (P = 0.039). In contrast, NK cell-mediated apoptosis of BCG-infected MN was not inhibited by NOK1 (27.7% in medium alone versus 25.8% with NOK1; P = 0.227). Thus, both apoptosis of BCG-infected MN and killing of intracellular M. tuberculosis by NK cells are mediated by pathways other than those initiated by the interaction of FasL and Fas.
FIG. 10.
Both NK cell-mediated apoptosis of BCG-infected MN and killing of intracellular M. tuberculosis are independent of Fas-FasL interactions. Anti-FasL blocking antibody NOK1 (10 μg/ml) was added to cultures containing infected MN and NK cells. Panel A shows the mean and the SD for CFU studies of five subjects. As illustrated, the addition of NOK1 had no effect on the ability of NK cells to kill intracellular H37Rv. Likewise, although NOK1 did inhibit the FasL-dependent induction of apoptosis of Jurkat cells by the FasL-transfectant KFL9 cell line, NK cell-mediated apoptosis of MN-infected M. bovis BCG was not affected by the addition of NOK1, as shown in panel B. Jurkat-KFL9 results represent the mean and SD from triplicate experiments. MN-NK data is presented as mean and SD from studies of four subjects.
NK cell-mediated killing of intracellular M. tuberculosis is not blocked by the simultaneous blocking of NK cell granule release, IFN-γ, and Fas-FasL.
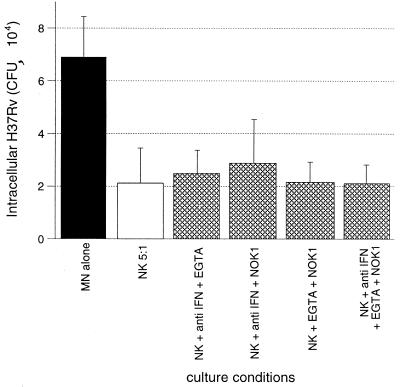
To rule out the possibility that multiple mechanisms contributing to NK cell-mediated killing of M. tuberculosis must be blocked simultaneously in order to inhibit the observed killing, we studied the effects of EGTA treatment and the use of neutralizing antibodies to IFN-γ and FasL in various combinations (Fig. 11). In the presence of the various combinations of two of the three inhibitors, as well as the simultaneous blockage of all three pathways, NK cells retained their ability to mediate significant reduction in intracellular M. tuberculosis compared to CFU within MN alone, as illustrated (P < 0.001 in each case).
FIG. 11.
NK cell-mediated killing of intracellular M. tuberculosis cannot be accounted for by interactions of IFN-γ, cytotoxic granule release, and Fas-FasL interactions. To assess the possibility that the additive effects of these pathways were required in the killing of M. tuberculosis by NK cells, neutralizing anti-IFN-γ monoclonal antibody, EGTA, and NOK1 (anti-FasL) were added to cocultures of NK cells and infected MN in combination. As shown, none of the combinations of two or all three of the inhibitors led to a significant decrease in the ability of NK cells to kill intracellular M. tuberculosis. The results represent the mean and SD for five subjects studied.
DISCUSSION
Human cell-mediated immunity to M. tuberculosis is assumed to involve the ability of lymphocytes to activate infected mononuclear phagocytes, resulting in the killing of intracellular bacilli. Nevertheless, studies of the role of lymphocyte responses to M. tuberculosis and its antigens have generally not addressed the correlation between the various responses of these cells and bacterial killing. In this study, we demonstrated that human NK cells can mediate the early killing of intracellular M. tuberculosis, and we examined the relationship between the various innate immune functions of NK cells and this killing.
Studies of protective human responses to M. tuberculosis have largely focused on healthy PPD-positive individuals who are considered to have developed specific immunity to M. tuberculosis PPD-positive individuals display resistance to reinfection upon subsequent exposure to M. tuberculosis (37), and the remarkable susceptibility of HIV-infected individuals with dysfunction and depletion of CD4+ T cells provides strong support for the concept that antigen-specific responses play a major role in resistance to M. tuberculosis (15, 24). However, a role for innate immune responses in protection against M. tuberculosis has been suggested as well by clinical scenarios in which heavily exposed individuals do not develop PPD reactivity (16) and by the observation that some PPD-negative individuals display in vitro responses to M. tuberculosis (18, 30). Our finding that unprimed human PBL mediated the killing of the intracellular M. tuberculosis within 24 h of coculture with infected MN implied a role for innate immunity in this process. Several effector functions of NK cells may serve to mediate innate immune responses. The “NK activity” which led to the initial identification of this cell population specifically refers to the ability of nonprimed cells to lyse target cells in an HLA-unrestricted manner (31). Furthermore, NK cells can be activated by infected MN independently of specific antigen recognition by cytokines such as interleukin-12 (IL-12), IL-15, and IL-18 (10, 20, 43). Activated NK cells can serve as early sources of IFN-γ (28, 32, 38) and of proinflammatory chemokines such as MIP1α (7).
We observed that unstimulated NK cells could mediate killing of intracellular H37Rv at 24 h of coculture in a dose-dependent fashion. Previous studies have reported that NK cells can inhibit intracellular growth of M. avium (5) and the avirulent M. tuberculosis strain H37Ra (45). Although killing of a recent clinical isolate of M. tuberculosis by IL-12-stimulated NK cells has also been observed (13), the current findings provide the first report of the ability of unstimulated NK cells to mediate the killing of a well-characterized virulent strain of M. tuberculosis. Our subsequent studies were aimed at identifying the mechanisms by which this killing occurred.
The antimicrobial functions of NK cells have been studied in murine models of infection with a wide variety of intracellular pathogens. The production of IFN-γ by NK cells plays an essential role in the containment of murine infection with cytomegalovirus, listeria, and various intracellular parasites (3, 28, 32). In contrast, we observed that the killing of intracellular H37Rv by human NK cells was not associated with the production of IFN-γ. The finding that neutralizing antibodies to IFN-γ had no effect on NK cell-mediated killing of M. tuberculosis further rules out a role for IFN-γ in this process. These observations are consistent with an earlier report that PPD does not induce human NK cells to produce IFN-γ unless activated T cells are present as well (19). Our subsequent studies involving both the transfer of supernatants of cocultured NK cells and M. tuberculosis-infected MN and the assessment of NK cell activity in Transwell cultures further indicate that NK cell-mediated killing of intracellular M. tuberculosis cannot be attributed to the effects of soluble mediators. We therefore investigated the role of the cytotoxic effector functions of human NK cells in the killing of intracellular M. tuberculosis.
Because NK cell cytotoxicity has classically been attributed to the release of cytotoxic granules containing perforin and other effector molecules (17), we next sought to determine whether granule release was essential for NK cell-mediated killing of intracellular M. tuberculosis. Free calcium is required for the exocytosis of NK cell cytotoxic granules, and the calcium chelator EGTA has therefore been utilized to demonstrate granule-independent effects of NK cells (44). The addition of EGTA to our cultures blocked the granule-mediated lysis of K562 targets by NK cells but had no effect on the ability of NK cells to kill intracellular H37Rv. These findings indicate that the killing of M. tuberculosis by NK cells is independent of granule exocytosis. Our observations contrast with those reported in studies of CD1-restricted cytotoxic T-cell lines recognizing lipid and lipoglycan antigens of M. tuberculosis (39). The CD1-restricted lymphocytes were found to comprise two distinct populations that exhibited differing patterns of cytotoxicity. CD4− CD8+ lymphocytes killed infected MN by granule-mediated cytotoxicity that resulted in the killing of intracellular M. tuberculosis, whereas CD4− CD8− lymphocytes killed infected MN via Fas-FasL-induced apoptosis and had no effect on the viability of the organism. The clinical significance of CD1-restricted lymphocytes remains uncertain, however, and it has recently been suggested that the CD1 pathway may in fact be coopted by M. tuberculosis in order to facilitate bacterial survival (34). Our assessment that cytotoxic granules do not play a role in the killing of M. tuberculosis by NK cells is consistent with two studies which reported that the course of M. tuberculosis infection in perforin knockout mice does not differ from that observed in wild-type animals (11, 22), as well as with the observation that chemically induced necrosis of BCG-infected human MN does not result in the killing of intracellular organisms (25). An earlier report by Molloy et al. found that human LAK cells lysed BCG-infected MN but that lysis did not result in the killing of the organism (26). Although a large proportion LAK precursors are CD56+ CD3− NK cells, the authors' observation that the cells with lytic activity specific for mycobacterium-infected cells were CD56− CD3+ emphasizes the fact that an IL-2-stimulated LAK population is not equivalent to the isolated NK cells we studied. In addition, IL-2 stimulation may not enhance the other activities of LAK precursor cells as it does their capacity for cytolysis. The culture conditions used in that study also differed from ours in that LAK cells were added to MN 6 days after infection, at which time the infected cells were reported to contain an average of 10 to 20 bacilli. This level of infection could have resulted in a model system more conducive to cell lysis and less so to bacterial killing than the early, low-level infection model we studied.
The finding that NK cells induce apoptosis of BCG-infected MN is intriguing as a possible mechanism of NK cell-mediated bacterial killing in light of previous studies of cytotoxicity directed at MN infected with both M. bovis BCG (21, 25) and M. tuberculosis (27). These investigations showed that chemical- or cytokine-induced apoptosis of the infected cells was associated with decreased viability of the mycobacteria within the target MN. Although the protocol used in these studies resulted in the infection of 65 to 75% of monocytes, only 21% of monocytes were found to be apoptotic by TUNEL analysis after 24 h of coculture with NK cells. Nevertheless, the addition of NK cells mediated a 60 to 80% reduction in the CFU level of intracellular M. tuberculosis at the same time point. This discrepancy could indicate that the killing of intracellular M. tuberculosis is not tied to NK cell-mediated monocyte apoptosis. Alternatively, it may be that bacterial killing is a more rapid effect of the relevant apoptotic pathways than the DNA splicing which results in positive TUNEL staining. In this case, the timing of our assays could underestimate the ultimate extent of MN apoptosis occurring within the cultures. An additional possibility is that the apoptosis of some cells serves to activate effector functions of nonapoptotic MN, resulting in bacterial killing. This latter mechanism has been described in in vitro studies of MN infection with M. avium (14). Previous studies had indicated that infection with virulent M. tuberculosis increases the resistance of isolated MN to apoptosis and that this resistance is based on increased production of soluble tumor necrosis factor (TNF) receptor by the infected cells (1). Our fluorescent microscopy studies indicate that MN infected with virulent H37Rv are susceptible to NK cell-mediated apoptosis. Because of the apparent specificity of these previous observations for TNF-mediated apoptosis, however, our findings do not necessarily contradict this earlier report.
Because of the possible relationship of the observed NK cell-mediated apoptosis to the killing of intracellular M. tuberculosis, we investigated a possible role of the interaction of FasL and Fas in this killing. Our studies showed that blocking of this apoptotic pathway had no effect on the ability of NK cells to mediate the killing of intracellular M. tuberculosis or upon NK cell-mediated apoptosis of BCG-infected MN. In addition to FasL, however, NK cells have recently been shown to express other apoptosis-inducing surface ligands, including membrane-bound TNF (23), TRAIL (TNF-related apoptosis-inducing ligand) (46), and CD40L (9). Our findings suggest that NK cell-induced apoptosis of infected MN is mediated by these or other as-yet-undescribed pathways and may be linked to the killing of intracellular M. tuberculosis.
In summary, unstimulated NK cells from both PPD-positive and PPD-negative subjects can mediate the early killing of intracellular M. tuberculosis H37Rv. This killing, which takes place within the first 24 h of coculture, is not associated with the production of IFN-γ by NK cells and does not require the release of NK cell cytotoxic granules, but it is associated with the induction of NK cell-mediated MN apoptosis. Both the killing of intracellular M. tuberculosis and the induction of MN apoptosis by NK cells are independent of the interaction of FasL and Fas. Further clarification of the mechanisms by which NK cells mediate the killing of intracellular M. tuberculosis may facilitate the development of strategies for augmenting innate immunity as a means of prevention and treatment of tuberculosis. Such interventions may be particularly relevant for HIV-infected individuals in whom antigen-specific CD4+ T-cell responses to M. tuberculosis are impaired. In addition, understanding of the mechanisms by which unstimulated NK cells mediate the killing of intracellular M. tuberculosis may help illuminate the pathways by which stimulated antigen-specific lymphocyte populations contain this important pathogen.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants HL59851, AI35027, AI27243, AI95383, AI36219, and AI01581 and by American Lung Association Research grant RG-1489-N. R. F. Silver was also supported by a Parker B. Francis Fellowship in Pulmonary Research sponsored by the Francis Families Foundation.
We thank Abhay Patki and Roxana Rojas of Case Western Reserve University for assistance with the protocols for the TUNEL assays and the MACS sorting system, respectively. We also thank Laurie Hall and Eric Pearlman of Case Western Reserve University for critical review of the manuscript.
REFERENCES
- 1.Balcewicz-Sablinska M K, Keane J, Kornfeld H, Remold H G. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNFα. J Immunol. 1998;161:2636–2641. [PubMed] [Google Scholar]
- 2.Bancroft G. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- 3.Bancroft G J, Bosma M J, Bosma G C, Unanue E R. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell independent mechanism. J Immunol. 1986;137:4–9. [PubMed] [Google Scholar]
- 4.Barnes P F, Grisso C L, Abranms J S, Band H, Rea T H, Modlin R L. γδ T lymphoyctes in human tuberculosis. J Infect Dis. 1992;165:506–512. doi: 10.1093/infdis/165.3.506. [DOI] [PubMed] [Google Scholar]
- 5.Bermudez L E, Wu M, Young L S. Interleukin-12 stimulated natural killer cells can activate human macrophages to inhibit growth of Mycobacterium avium. Infect Immun. 1995;63:4099–4104. doi: 10.1128/iai.63.10.4099-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biron C A, Nguyen K B, Pien G C, Cousens L P, Salazar-Mather T P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 7.Bluman E M, Bartynski K J, Avalos B R, Caligiuri M A. Human natural killer cells produce abundant macrophage inflammatory protein 1-α in response to monocyte-derived cytokines. J Clin Investig. 1996;97:2722–2727. doi: 10.1172/JCI118726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boom W H, Wallis R S, Chervenak K A. Human Mycobacterium tuberculosis-reactive CD4+ T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phaogocytes. Infect Immun. 1991;59:2737–2743. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carbonne E, Ruggiero G, Terrazzano G, Palonba C, Manzo C, Fontana S, Spits H, Karre K, Zappacosta S. A new mechanism of NK cell cytoxicity activation: The CD40-CD40 ligand interaction. J Exp Med. 1997;185:2053–2060. doi: 10.1084/jem.185.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carson W E, Ross M E, Baiocchi R A, Marien M J, Boiani N, Grabstein K, Caligiuri M A. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-γ by natural killer cells in vitro. J Clin Investig. 1995;96:2578–2582. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper A M, D'Souza C, Frank A A, Orme I M. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997;65:1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Libero G, Flesch I, Kaufmann S H E. Mycobacteria-reactive Lyt-2+ T cell lines. Eur J Immunol. 1988;18:59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- 13.Denis M. Interleukin-12 augments cytolytic activity of natural killer cells toward Mycabacterium tuberculosis-infected human monocytes. Cell Immunol. 1994;156:529–536. doi: 10.1006/cimm.1994.1196. [DOI] [PubMed] [Google Scholar]
- 14.Fratazzi C, Arbeit R D, Carini C, Remold H G. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J Immunol. 1997;158:4320–4327. [PubMed] [Google Scholar]
- 15.Havlir D V, Barnes P F. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1999;340:367–373. doi: 10.1056/NEJM199902043400507. [DOI] [PubMed] [Google Scholar]
- 16.Houk V N, Baker J H, Sorensen K, Kent D C. The epidemiology of tuberculosis infection in a closed environment. Arch Environ Health. 1968;16:26. doi: 10.1080/00039896.1968.10665011. [DOI] [PubMed] [Google Scholar]
- 17.Kagi D, Ledermann B, Burki K, Zinkernagel R M, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 18.Kemp E B, Belshe R B, Hoft D F. Immune responses stimulated by percutaneous and intradermal Bacille Calmette-Guerin. J Infect Dis. 1996;174:113–119. doi: 10.1093/infdis/174.1.113. [DOI] [PubMed] [Google Scholar]
- 19.Kemp K, Hviid L, Kharazmi A, Kemp M. Interferon γ production by human T-cells and natural killer cells in vitro in response to antigens from the two intracellular pathogens Mycobacterium tuberculosis and Leishmania major. Scand J Immunol. 1997;46:495–499. doi: 10.1046/j.1365-3083.1997.d01-154.x. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi M, Fitz L, Ryan M, Hewick R M, Clark S C, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–837. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lammas D A, Stober C, Harvey C J, Kendrick N, Panchalingam S, Kumararatne D S. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 22.Laochumroonvorapong P, Wang J, Liu C-C, Ye W, Moriera A L, Elkon K B, Freedman V H, Kaplan G. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for early control of mycobacterial infection in mice. Infect Immun. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee R K, Spielman J, Zhao D Y, Olsen K J, Podack E R. Perforin, Fas ligand, and tumor necrosis factor are the major cytotoxic molecules used by lymphokine-activated killer cells. J Immunol. 1996;157:1919–1925. [PubMed] [Google Scholar]
- 24.Markowitz N, Hansen N I, Hopewell P C, Glassroth J, Kvale P A, Mangura B T, Wilcosky T C, Wallace J M, Rosen M J, Reichman L B. Incidence of tuberculosis in the United States among HIV-infected persons. Ann Intern Med. 1997;126:123–32. doi: 10.7326/0003-4819-126-2-199701150-00005. [DOI] [PubMed] [Google Scholar]
- 25.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes in coupled with killing of intracellular Bacillus Calmette-Guerin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molloy A, Meyn P A, Smith K D, Kaplan G. Recognition and destruction of Bacillus Calmette-Guerin-infected human monocytes. J Exp Med. 1993;177:1691–1698. doi: 10.1084/jem.177.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oddo M, Renno T, Attinger A, Bakker T, MacDonald H R, Meylan P A R. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol. 1998;160:5448–5454. [PubMed] [Google Scholar]
- 28.Orange J S, Wang B P, Terhorst C, Biron C A. Requirement for natural killer cell-produced interferon γ in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oshimi Y, Oda S, Honda Y, Nagata S, Miyazki S. Involvement of Fas ligand and Fas-mediated pathway in the cytotoxicity of human natural killer cells. J Immunol. 1996;157:2909–2915. [PubMed] [Google Scholar]
- 30.Ravn P, Boesen H, Pedersen B K, Andersen P. Human T cell responses induced by vaccination with Mycobacterium bovis Bacillus Calmette-Guerin. J Immunol. 1997;158:1949–1955. [PubMed] [Google Scholar]
- 31.Robertson M J, Ritz J. Biology and clinical relevance of human natural killer cells. J Am Soc Hematol. 1990;76:2421–2438. [PubMed] [Google Scholar]
- 32.Scharton-Kersten T M, Sher A. Role of natural killer cells in innate resistance to protozoan infections. Curr Opin Immunol. 1997;9:44–51. doi: 10.1016/s0952-7915(97)80157-4. [DOI] [PubMed] [Google Scholar]
- 33.Schlesinger L. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 34.Shinkai K, Locksley R M. CD1, tuberculosis, and the evolution of major histocompatibility complex molecules. J Exp Med. 2000;191:907–914. doi: 10.1084/jem.191.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sieg S, Smith D, Kaplan D. Differential activity of soluble versus cellular Fas ligand: regulation by an accessory molecule. Cell Immunol. 1999;195:89–95. doi: 10.1006/cimm.1999.1530. [DOI] [PubMed] [Google Scholar]
- 36.Silver R F, Li Q, Boom W H, Ellner J J. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T-cells in PPD-positive but not in PPD-negative subjects. J Immunol. 1998;160:2408–2417. [PubMed] [Google Scholar]
- 37.Stead W W. Management of health care workers after inadvertent exposure to tuberculosis: a guide for the use of preventative therapy. Ann Intern Med. 1995;122:906–912. doi: 10.7326/0003-4819-122-12-199506150-00003. [DOI] [PubMed] [Google Scholar]
- 38.Stein-Streilein J, Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increased morbidity and mortality to pulmonary influenza infection. J Virol. 1986;136:1435–1441. [PubMed] [Google Scholar]
- 39.Stenger S, Mazzaccaro R J, Uyemura K, Cho C, Barnes P F, Rosat J-P, Sette A, Brenner M B, Porcelli S A, Bloom B R, Modlin R L. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 40.Sudre P, ten Dam G, Kochi A. Tuberculosis: A global overview of the situation today. Bull W H O. 1992;70:149–159. [PMC free article] [PubMed] [Google Scholar]
- 41.Tan J S, Canaday D H, Boom W H, Balaji K N, Schwander S K, Rich E A. Human alveolar T lymphocyte responses to Mycobacterium tuberculosis antigens: role for CD4+ and CD8+ cytotoxic T cells and relative resistance of alveolar macrophages to lysis. J Immunol. 1997;159:290–297. [PubMed] [Google Scholar]
- 42.Tsukaguchi K, Balaji K, Boom W. CD4 αβ T cell and γδ T-cell responses to Mycobacterium tuberculosis: similarities and differences in Ag recognition, cytoxic effector function, and cytokine production. J Immunol. 1995;154:1786–1796. [PubMed] [Google Scholar]
- 43.Ushio S, Namba M, Okura T, Hattori K, Nukada Y, Akita K, Tanabe F, Konishi K, Micalled M, Fujii M, Torigoe K, Tanimoto T, Fukuda S, Ikeda M, Okamura H, Kurimoto M. Cloning of the cDNA for human IFNγ-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–4279. [PubMed] [Google Scholar]
- 44.Vujanovic N L, Nagashima S, Herberman R B, Whiteside T L. Nonsecretory apoptotic killing by human NK cells. J Immunol. 1996;157:1117–1126. [PubMed] [Google Scholar]
- 45.Yoneda T, Ellner J J. CD4+ T cell and natural killer cell-dependent killing of Mycobacterium tuberculosis by human monocytes. Am J Respir Crit Care Med. 1998;158:395–403. doi: 10.1164/ajrccm.158.2.9707102. [DOI] [PubMed] [Google Scholar]
- 46.Zamai L, Ahman M, Bennett I M, Azzoni L, Alnemri E S, Perussia B. Natural killer cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;188:2375–2380. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]