Abstract
Colorectal cancer is one of the most common malignant neoplasms worldwide. Overall mortality is 33%. Synchronous colorectal cancer refers to more than one malignant tumor detected in different segments of the colon, simultaneously or within 6 months of initial diagnosis. The development of colorectal cancer is a multistep process that originates with a genetic mutation leading to a malignant phenotype and generating a growth advantage. Colorectal cancer presents up to 16% of hypermutations, of which 75% are characterized by microsatellite instability which in turn leads to poorer cell differentiation. Patients with synchronous tumors appear to have a higher proportion of microsatellite instability than patients with single tumors. The clinical case of a 35-year-old man with a perforated left colon tumor and a locally advanced synchronous tumor of the right colon and signs of acute abdomen is presented. The treatment should be based on the location of the synchronous tumors, stage at the time of approach, and the patient’s condition. However, when faced with a complication secondary to colonic cancer, adhering to the principles of oncological surgery can be overcome by the nature of the emergency.
Keywords: Colorectal cancer, Synchronous tumor, Perforated cancer, Microsatellite instability
Introduction
Colorectal cancer is one of the main malignant neoplasms worldwide; it ranks second in men and third in women likewise and is the third and fourth leading cause of cancer death, respectively. The estimated mortality per year is close to 33% [1]. The global incidence is estimated to be close to 4.3% [1]. From the embryological point of view, colonic cancer is subdivided into proximal or right when it originates from proximal areas to the splenic flexure (cecum, ascending and transverse colon), while the distal or left refers to the descending and sigmoid colon. It is considered rectal tumor when it is developed 15 cm proximal to the anal sphincter, it has a great capacity as spreading to the liver tissue [1].
The progress in the molecular pathogenesis of colorectal cancer has allowed the definition of two pathways of cellular/molecular development of colorectal cancer, starting from two different precursor lesions: adenoma-carcinoma pathway and the serrated pathway, characterized by different genetic lesions [1]. Most of the colonic malignant tumors histologically are adenocarcinoma; these neoplasms are divided into high-grade and low-grade tumors. Some rare histological types are mucinous adenocarcinoma, adenosquamous carcinoma, signet ring adenocarcinoma cells, and medullary carcinoma. The relation between the histological grade and the long-term survival forecast is not clear [1].
The model of the progressive stepwise accumulation of genetic and epigenetic events required first for the development of adenoma and then of adenocarcinoma was instrumental for the identification of driver somatic mutations occurring at the level of some tumor suppressor genes (APC, TP53, SMAD4) and oncogenes (KRAS and PI3KCA). The nonrandom accumulation of these genetic alterations leads to deregulation of cell proliferation, survival/apoptosis, and differentiation [1].
A classic, conventional pathway is initiated by APC mutations and progresses through the sequential accumulation of genetic mutations and chromosomal instability, causing microsatellite stable (MSS) tumors. The germline mutation pathway is related to germline mutation of mismatch repair (MMR) genes, seen in Lynch syndrome (hereditary nonpolyposis coli), and leads to microsatellite instability (MSI-H). The sessile-serrated-methylation pathway is heterogeneous: a traditional serrated pathway, related to KRAS/BRAF mutations, leading to MSS tumors, with a variable CpG island methylator phenotype [1].
MSI-H is characterized by mutation of the MMR genes such as MLH1, MSH2, MSH6, and PMS2, which are found in 2–5% of all colorectal cancers and occur in Lynch syndrome, characterized by high penetrance and early cancer development with an increased risk of extraintestinal cancers [1]. Multiple primary malignant tumors (MPMT) are defined as two or more different malignant tumors that arise in the same patient. According to the Warren and Gates criteria, proposed in 1931, several conditions are required to qualify as MPMT, among which are: (1) the diagnosed tumors must be malignant in nature; (2) histologically distinct; and (3) has to be excluded that one is a metastasis of the other [2–4]. MPMT can be classified according to the temporality of appearance as synchronous if they are detected simultaneously or up to 6 months after the diagnosis of the first primary tumor, or metachronous if they are detected after 6 months in fact from the diagnosis of the first tumor [4].
Synchronous colorectal cancer refers to more than one malignant tumor detected in different segments of the colon, simultaneously or within 6 months of initial diagnosis. The reported incidence of these tumors ranges from 2.3% to 12.4% [5, 6]. By diagnosing the presence of synchronous colorectal cancer, it is possible to avoid the development of advanced-stage metachronous colorectal cancer that requires re-surgical interventions [6].
One-third of the patients with colonic cancer will present to the emergency room with a complicated tumor; this is a high mortality situation that also implies a worse long-term prognosis. These represent 60–85% of the patients undergoing emergency surgery because of colonic disease [7].
Perforation is a much less common complication, it affects between 2% and 12% of the patients with colonic cancer. It is the second leading cause of peritonitis due to colonic disease after acute perforated diverticulitis and represents 38% of cases [7]. Although perforation can occur diastatically (proximal to the tumor) as a complication of its occlusion; the most frequent form of perforation is in the tumor itself, due to necrosis of the neoplastic tissue; representing 65% of the cases. Having a preoperative diagnosis is in turn essential to determine treatment options and plan the type of surgical resection [6, 8].
Objective
The clinical case of a patient with perforated left colon cancer (splenic flexure) with synchronous locally advanced right colonic tumor (para-aortic chain invasion) is presented.
Case Report
A 35-year-old male with no significant chronic degenerative history; surgical history of autograft in the left upper limb, left lateral region of the neck, and left lateral aspect of the thorax secondary to a third-degree burn. Radius and ulna fracture treated with a splint without sequelae. There is a family history of hypertension, type 2 diabetes mellitus, and unspecified gastric carcinoma; all of them in maternal branch.
Current Condition
Starts in October 2019 with intermittent crampy abdominal pain predominantly in the hypogastrium, which progressed to diffuse abdominal pain, associated with weight loss of approximately 10.5 kg in 3 months (80 kg–69.5 kg), and in addition to stools decreased in consistency and mane. Study protocol (external hospital) begins in May 2020 with an abdominal ultrasound which reports multiple heterogeneous liver lesions and splenomegaly, carcinoembryonic antigen of 197.31 ng/mL (nanograms per milliliter), and CA 19–9 antigen of 10,386 ng/mL; the computerized axial tomography of the abdomen revealed metastatic lesions in the liver, thickening of the hepatic angle of the colon, as well as a tumor of the descending colon scheduled for outpatient colonoscopy; however, he went to the General Hospital of Mexico on June 13, 2020, with several abdominal pain (10/10 according to the visual-analog scale) and unquantified fever. Upon admission with unstable vital signs, a tendency to hypotension, tachycardia, and respiratory distress, cardiopulmonary without apparent compromise, abdominal distension, generalized pain, with involuntary muscular resistance and positive rebound in all quadrants; bowel sounds of metallic hue.
Laboratory studies upon admission: leukocytes 15.10 × 10e3/μL, neutrophils 14.6 × 10e3/μL, hemoglobin 6.7 g/dL, platelets 623.00 × 10e3/μL, glucose 108 mg/dL, urea 95.3 mg/dL, creatinine 2.26 mg/dL, uric acid 11.3 mg/dL, total cholesterol 67 mg/dL, triglycerides 135 mg/dL, direct bilirubin 1.27 mg/dL, indirect bilirubin 0.58 mg/dL, aspartate aminotransferase 1442 U/L, alanine aminotransferase 714 U/L, albumin 2.49 g/dL, total proteins 5 g/dL, lactic dehydrogenase 1592 U/L, sodium 123 mEq/L, chlorine 84 mEq/L, potassium 5.7 mEq/L, calcium 7.83 mg/dL, magnesium 2.8 mg/dL, phosphorus 7.2 mg/dL, prothrombin 20.6 s, INR 1.9. activated partial thromboplastin 40.9 s, fibrinogen 628 mg/dL, procalcitonin 43.5 ng/dL, pH 7.3, pCO2 22.8 mm Hg, pO2 17.9 mm Hg, HCO3 11.1 mmol/L, BE 15.2 mmol/L, lactate 15.92 mmol/L.
Imaging studies: abdominal X-ray in two positions shows dilation of intestinal loops of up to 12 cm, inter-loop edema, chest radiograph with the presence of subdiaphragmatic free air (shown in Fig. 1a–b). Because of the clinical picture of an acute abdomen and perforation of the hollow viscus, an emergency exploratory laparotomy was decided on June 13, 2020, with the following findings: 5 × 3cm perforation in a tumor of the left colon (splenic angle); tumor measuring approximately 12 × 10 × 8cm in the proximal third of the ascending colon locally advanced to the para-aortic ganglionic chain with evidence of necrosis, free intestinal fluid in the cavity approximately 600 mL (shown in Fig. 2a–b), multiple firms and lax loop-loop adhesions, multiple liver metastases, and abscesses. Radical total colectomy was performed with resection at the level of the R2 ileocolic artery and formation of a Brooke-type terminal ileostomy (shown in Fig. 3a–c). The duration of the surgical procedure was 1 h 16 min, with an anesthetic time of 2 h and total bleeding estimated at 500 mL. The patient was transferred to the intensive care unit due to hemodynamic instability after the surgical event, where he remained under management for septic shock and multiple organ failure. He presented adequate ileostomy functioning after 12 h after surgery. After 48 h, he presented refractory septic shock and cardiorespiratory arrest without response to advanced maneuvers, for which he died.
Fig. 1.
a Chest X-ray PACS system, the General Hospital of Mexico. b Abdominal X-ray PACS system, the General Hospital of Mexico.
Fig. 2.
a Surgical specimen (colon). General Surgery Service, the General Hospital of Mexico. b Surgical specimen indicating perforation site. General Surgery Service, the General Hospital of Mexico.
Fig. 3.
a Surgical specimen, macroscopic view. Pathology Unit, the General Hospital of Mexico. b Surgical specimen macroscopic view of the right colon. Pathology Unit, the General Hospital of Mexico. c Surgical specimen macroscopic view of the left colon. Pathology Unit, the General Hospital of Mexico.
Histopathological report: in the histological sections, a malignant neoplastic lesion of epithelial lineage with a glandular and in loose nests pattern is observed, which replaces the mucosa and infiltrates the lamina propria, muscularis mucosas, submucosa, muscularis propria, and the subserosa. Loss of continuity and perforation are observed in the wall of lesion 3. In lesion 1 and 3, there are neoplastic cells, with hyperplasia or mesothelial inflammation. Less than 50% of the lesion is made up with intestinal phenotype glands of different sizes, lined by pseudostratified simple columnar epithelium, with a nucleus: cytoplasm ratio of 2–3:1, clear cytoplasm, round nuclei with moderate atypia, membrane reinforcement nuclear, “salt and pepper” chromatin, evident nucleolus, and few mitosis; between the glands, there is fibrovascular tissue and segmented mononuclear inflammatory infiltrate (shown in Fig. 4).
Fig. 4.
a Normal and neoplastic colonic mucosa transition. Complete loss of epithelial architecture is observed, which is replaced by neoplastic glands (H&E-stained photomicrograph ×4 objective). b Neoplastic glands are observed arranged on a villloglandular pattern, with cylindrical cells, with hyperchromatic, elongated nuclei, with loss of polarity. Apical cytoplasmic vacuoles are seen in some cells (H&E-stained photomicrograph ×10 objective). c, d In an approach to these neoplastic glands, some fused; we can identify the neoplastic morphology of the cells that lose the nucleus cytoplasm relationship, with hyperchromatic nuclei, with moderate to severe atypia. Inflammatory infiltrate is also observed (H&E-stained photomicrograph ×40 objective). d An approach to the neoplastic glands where the loss of polarity is clearly observed, the nuclei are hyperchromatic, and an apical cytoplasmic vacuole is observed (H&E-stained photomicrograph. ×40 objective).
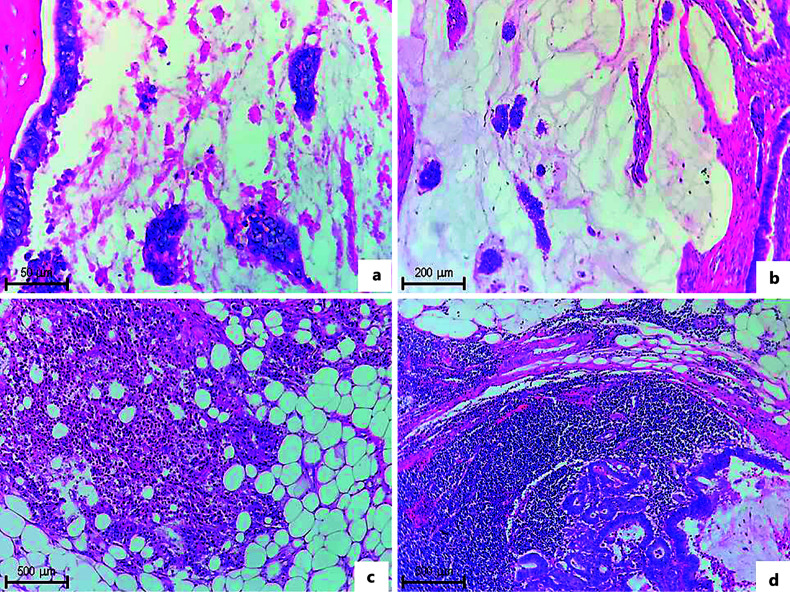
The rest of the lesion is made up of mucus lakes and few nests of neoplastic cells, some arranged in glands of different sizes, lined by simple cuboidal epithelium, with a nucleus: cytoplasm ratio of 2–3:1, clear cytoplasm, round nuclei with moderate to severe atypia, reinforcement of the nuclear membrane, “salt and pepper” chromatin, evident nucleolus, and few mitosis. In the distal mucosa adjacent to the lesion, a polyp is identified, consisting entirely of finger-like projections with vascular stems and lined by simple pseudostratified columnar epithelium, with a nucleus: cytoplasm ratio of 2–3: 1, vacuolated cytoplasm, nuclei with loss of the polarity with moderate atypia, reinforcement of the nuclear membrane, chromatin in “salt and pepper”, and evident nucleolus, without mitosis. On the lymph nodes, replacement of the parenchyma by nests of neoplastic cells is observed (shown in Fig. 5–7).
Fig. 5.
a, b Groups of neoplastic cells are observed, immersed in a clear eosinophilic material that corresponds to mucin and exceeds more than 50% of the neoplasia (H&E-stained photomicrograph. ×5 objective). c Loose neoplastic cells are identified, reaching the serous layer of the intestine, mixed with a predominantly mononuclear inflammatory infiltrate (H&E-stained photomicrograph ×5 objective). d A lymph node is identified in the subserosal adipose tissue, with infiltration by neoplastic glands with mucinous material (H&E-stained photomicrograph ×5 objective).
Fig. 6.
In the serous layer up to the pericolonic adipose tissue, acute and chronic abscessed inflammation is observed with scattered neoplastic cells, arranged in small nests, in addition to perineural invasion (H&E-stained photomicrograph ×5 objective).
Fig. 7.
Immunohistochemical study. We can observe nuclear positivity of the four markers in the nucleus of neoplastic cells at the sites of the lesion with an intestinal pattern, with moderate intensity, with the exception of PMS2, and with mild intensity. The study ruled out MSI-H (polymeric peroxidase-binding complex ×10 objective lens and ×40 objective lens to insertions).
According to the surgical findings and pathological report, it is classified as stage IVc (T4c, N2b, and M1c) according to the TNM classification of the American Joint Committee on Cancer (JACC), eighth edition, 2017 [9]. The CARE Checklist has been completed by the authors for this case report, attached as supplementary material.
Discussion
The development of colorectal cancer depends on genetic and environmental factors. Colorectal cancer is a multistep process that originates with a genetic mutation that is considered to be tumor initiating, leading to a malignant phenotype and generating a growth advantage compared to its normal counterpart. According to the atlas of the cancer genome, colorectal cancer presents up to 16% of hypermutations, of which 75% are characterized by MSI-H, while in colorectal cancers without hypermutations, genetic mutations in APC TP53, SMAD4, KRAS, and P13KCA are found [1].
The prevalence of synchronous colonic tumors is low; the average age of presentation is the seventh decade of life. Patients with inflammatory bowel disease, hereditary nonpolyposis colorectal cancer, familial adenomatous polyposis, hyperplastic polyps, or serrated polyps have been shown to be at an increased risk of developing colorectal carcinoma at younger ages [10, 11]. Hereditary colorectal cancer syndromes present an increased risk of presenting synchronous tumors close to 10%. Most synchronous colon tumors were associated with greater MSI-H than single colorectal tumors [11–13].
In our case, due to the clinical presentation, patient age, and pathological findings, the study of this early synchronous tumor is proposed. The scarcity of polyps in the pathological specimen and two locally advanced tumors influenced our analysis of the most common etiological causes of this form of presentation, and MSI-H was considered as a probable cause.
Colorectal cancers exhibiting MSI-H have different molecular properties compared to the majority of the tumors associated with microsatellite stability. The pathway of carcinogenesis with MSS tumors is responsible for the development of about 85% of colorectal cancers. The remaining 15% of colorectal cancers develop through an alternative pathway of colon carcinogenesis, involving a defective MMR system due to the inactivation of genes such as MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2, determining a high rate of somatic mutations (hypermutation) and MSI-H [1].
Tumor location represents a source of heterogeneity in its biology and clinical phenotype. The right colon has a different embryological origin and blood supply than the left colon and rectum. The distribution of different molecular subtypes of colon cancer suggests that most right colon tumors have mutations in microsatellites, KRAS, and PI3KCA with respect to left colorectal cancers, also having a worse prognosis. Mutations in TP53 and APC were more frequent in the left colon and rectum [1].
Patients with synchronous tumors appear to have a higher proportion of MSI-H than patients with single tumors. Tumors with microsatellite stability have been more common in the left colon, while tumors with high MSI-H are more common in the right colon, which in turn leads to poorer cell differentiation. Analysis of large groups of synchronous colorectal cancers shows that the vast majority have MSI-H and that the tumors present more alterations of inter- and intra-tumoral heterogeneity which should be considered to select the most appropriate therapy and monitor resistance to it [1].
After analyzing the family history of the patient and reviewing the literature in our case, we found insufficient data to be able to establish an association with a hereditary colorectal cancer syndrome, but we decided to continue with the immunohistochemical studies to demonstrate the presence of microsatellites in the tumor due to an important tendency to study microsatellites in locally advanced and synchronous colon tumors; however, the result was negative for MSI-H. This helps us investigate other causes of genetic mutations, for which more analysis of future cases is required, to establish a better diagnostic route from the genetic point of view, since this could condition the establishment of more exact treatments and greater control of disease that increases survival rates.
Nowadays, due to current diagnostic methods, preoperative surgical and therapeutic plans can be established to avoid complications and early recurrence of these tumors. A fundamental component for the diagnosis of colorectal cancer is a colonoscopy since it allows the identification of advanced polyps in up to 15–50% of cases [14].
Different types of resections have been proposed; however, the type of therapy must be individualized. Adequate lymph node resection improves the prognosis of these patients [6, 15].
Treatment variables include extended surgical resection with anastomosis when there are multiple tumors in the colon. It is also possible to perform segmental resections with anastomosis if the case allows [16, 17]. Among the complications of performing segmental resections are ileus and stenosis, while performing an extended resection, complications such as ileus, intestinal leakage, intra-abdominal collections, and mesenteric artery thrombosis can be expected [6, 16].
The decision to perform an extended resection or multiple resections should be based on the location of the synchronous tumors, stage at the time of approach, and the patient’s condition [6, 17]. However, when faced with perforated colonic cancer, adhering to the principles of oncological surgery can be overcome by the nature of the emergency. Managing shock, controlling sepsis, and source of infection in these unstable patients may make an R0 resection or adequate lymphadenectomy difficult [18, 19].
In this clinical case, an open approach was performed due to the suspicion of perforation of the hollow viscus, and due to the location of both tumors, one on the proximal third of the ascending colon and the other on the splenic flexure, it was decided to perform an extended resection with total colectomy, due to the perforation in one of the tumors, the presence of intestinal material, and contamination of the abdominal cavity, it was decided to perform terminal ileostomy. The incidence of liver metastases in synchronous colon cancer is 11.8%; the possibility of performing resection of synchronous tumors and liver metastases has been described to improve the prognosis of the patients; however, in this clinical case, liver metastases were considered unresectable due to their location, depth, and abscesses presence [15, 20, 21].
Some authors recommend that after the resection of synchronous tumors with unresectable distant metastases, an adjuvant treatment should be chosen, including chemotherapy, selective chemotherapy through arterial infusion, coagulation, radiotherapy, among others. Therapies should be individualized after a specific observation period to rule out hidden metastases [15, 17, 19, 21].
Conclusions
The genetic study of colon cancer requires further investigation in complex cases; the clinical characteristics, degree of invasion, and macroscopic characteristics of the tumor are still insufficient to direct molecular studies of these tumors that allow us to establish directed therapies from the medical and surgical point of view.
Surgical treatment of synchronous tumors should be individualized, and the possibility of resecting the primary tumor and metastases, if any, should be evaluated. Adequate oncological resection can be affected by intraoperative complications, the presence of sepsis, and compromise of the patient’s hemodynamic status. In unresectable cases, consider adjuvant therapy after a period of observation and control of sepsis, as well as improvement of hemodynamic status. Multidisciplinary management in specialized centers is essential for the treatment and timely detection of synchronous malignant colonic tumor complications, therefore, improves long-term patient survival.
Statement of Ethics
This case report was reviewed, and the need for approval was waived by the Investigation Committee of the Hospital General de México. The authors have no ethical conflicts to disclose. Written informed consent was approved by the patient’s next of kin for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There were no funding sources.
Author Contributions
Marco Antonio Robles González, Marcela Pérez Villaseñor, Ana Alfaro Cruz, Sergio Ulises Pérez Escobedo, and Yanetzy Elizabet Corona Flores: acquisition, analysis, and interpretation of data for the work; revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding Statement
There were no funding sources.
Data Availability Statement
All data analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Testa U, Pelosi E, Castelli G. Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci. 2018;6(2):31. 10.3390/medsci6020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Warren S, Gates O. Multiple primary malignant tumors, a survey of the literature and statistical study. Am J Cancer. 1932;16:1358–414. [Google Scholar]
- 3. Ladrón de Guevara D, Quera R, Rozas S, Schacher S, Reyes JM, Pardo C, et al. Cáncer sincrónico y metacrónico detectado con PET/CT en población oncológica. Rev Med Chile. 2017;145(11):1421–8. 10.4067/s0034-98872017001101421. [DOI] [PubMed] [Google Scholar]
- 4. Lv M, Zhang X, Shen Y, Wang F, Yang J, Wang B, et al. Clinical analysis and prognosis of synchronous and metachronous multiple primary malignant tumors. Medicine. 2017 Apr;96(17):e6799. 10.1097/md.0000000000006799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. González SME, González VJA, Llanes DG, et al. Cáncer colorrectal sincrónico. Rev Mex Coloproctol. 2008;14(1):5–9. [Google Scholar]
- 6. Lee BC, Yu CS, Kim J, Lee JL, Kim CW, Yoon YS, et al. Clinicopathological features and surgical options for synchronous colorectal cancer. Medicine. 2017;96(9):e6224. 10.1097/md.0000000000006224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kreisler E, Biondo S, Martí-Ragué J. La cirugía en el cáncer de colon complicado. Cirugía Española. 2006 Jul;80(1):9–15. 10.1016/s0009-739x(06)70909-5. [DOI] [PubMed] [Google Scholar]
- 8. Demandante CGN, Troyer DA, Miles TP. Multiple primary malignant neoplasms. Am J Clin Oncol. 2003 Feb;26(1):79–83. 10.1097/00000421-200302000-00015. [DOI] [PubMed] [Google Scholar]
- 9. Benson AB, Venook AP, Cederquist L, Chan E, Chen Y-J, Cooper HS, et al. Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(3):370–98. 10.6004/jnccn.2017.0036. [DOI] [PubMed] [Google Scholar]
- 10. Kuo Y-H, Hung H-Y, You J-F, Chiang J-M, Chin C-C. Common habitual behaviors and synchronous colorectal cancer risk: a retrospective case-control study. Int J Colorectal Dis. 2019 Jul 5;34(8):1421–30. 10.1007/s00384-019-03326-x. [DOI] [PubMed] [Google Scholar]
- 11. Lam AK-Y. Synchronous colorectal cancer: clinical, pathological and molecular implications. World J Gastroenterol. 2014;20(22):6815. 10.3748/wjg.v20.i22.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu H, Chang DT, Nikiforova MN, Kuan S-F, Pai RK. Clinicopathologic features of synchronous colorectal carcinoma. Am J Surg Pathol. 2013 Nov;37(11):1660–70. 10.1097/pas.0b013e31829623b8. [DOI] [PubMed] [Google Scholar]
- 13. Arakawa K, Hata K, Nozawa H, Kawai K, Tanaka T, Nishikawa T, et al. Prognostic significance and clinicopathological features of synchronous colorectal cancer. Anticancer Res. 2018 Oct;38(10):5889–95. 10.21873/anticanres.12932. [DOI] [PubMed] [Google Scholar]
- 14. Tan WJ, Ng NZP, Chen YD, Chee YHM, Foo FJ, Tang CL, et al. Synchronous polypectomy during endoscopic diagnosis of colorectal cancer – is the risk of tumour implantation at the polypectomy site significant? BMC Gastroenterol. 2018 Aug 29;18(1):133. 10.1186/s12876-018-0861-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20(2):207–39. 10.1007/s10147-015-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Z, Wang D, Wei Y, Liu P, Xu J. Clinical outcomes of laparoscopic-assisted synchronous bowel anastomoses for synchronous colorectal cancer: initial clinical experience. Oncotarget. 2016 Nov 3;8(6):10741–7. 10.18632/oncotarget.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moris D, Tsilimigras DI, Machairas N, Merath K, Cerullo M, Hasemaki N, et al. Laparoscopic synchronous resection of colorectal cancer and liver metastases: a systematic review. J Surg Oncol. 2019;119(1):30–9. 10.1002/jso.25313. [DOI] [PubMed] [Google Scholar]
- 18. Tebala GD, Natili A, Gallucci A, Brachini G, Khan AQ, Tebala D, et al. Emergency treatment of complicated colorectal cancer. Cancer Manag Res. 2018 Apr;10:827–38. 10.2147/cmar.s158335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lisle D, Lee-Kong S. Surgical management of complicated colon cancer. Clin Colon Rectal Surg. 2015 Nov 22;28(04):228–33. 10.1055/s-0035-1564621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montalvo-Javé E, Jiménez Bobadilla B, Espejel Deloiza M, Aguilar Preciado I, Negrete Cervantes L, Diliz-Pérez H. Synchronous resection of colon adenocarcinoma and bisegmentectomy of liver metastases. Case Rep Gastroenterol. 2019 May 16;13(2):238–44. 10.1159/000499423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Augestad KM, Bakaki PM, Rose J, Crawshaw BP, Lindsetmo RO, Dorum LM, et al. Metastatic spread pattern after curative colorectal cancer surgery. A retrospective, longitudinal analysis. Cancer Epidemiol. 2015;39(5):734–44. 10.1016/j.canep.2015.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.