Abstract
The rapid emergence of highly transmissible SARS-CoV-2 variants poses serious threat to the efficacy of vaccines and neutralizing antibodies. Thus, there is an urgent need to develop new and effective inhibitors against SARS-CoV-2 and future outbreaks. Here, we have identified a series of glycopeptide antibiotics teicoplanin derivatives that bind to the SARS-CoV-2 spike (S) protein, interrupt its interaction with ACE2 receptor and selectively inhibit viral entry mediated by S protein. Computation modeling predicts that these compounds interact with the residues in the receptor binding domain. More importantly, these teicoplanin derivatives inhibit the entry of both pseudotyped SARS-CoV-2 Delta and Omicron variants. Our study demonstrates the feasibility of developing small molecule entry inhibitors by targeting the interaction of viral S protein and ACE2. Together, considering the proven safety and pharmacokinetics of teicoplanin as a glycopeptide antibiotic, the teicoplanin derivatives hold great promise of being repurposed as pan-SARS-CoV-2 inhibitors.
Keywords: SARS-CoV-2, Spike protein, Angiotensin converting enzyme 2, Teicoplanin, Entry inhibitor
Graphical Abstract
1. Introduction
Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), a novel betacoronavirus and the most recent one of the seven coronaviruses (CoVs) known to infect humans, causes the COVID-19 pandemic [1]. Coronaviruses are enveloped, non-segmented, positive-strand RNA viruses that are broadly distributed among humans, animals, and birds, causing acute and chronic infections [1], [2]. While four CoVs (HCoV 229E, OC43, NL63, and HKU1) are responsible for about one third of the common cold cases in humans [1], [2], [3], SARS-CoV [2], [4], [5], MERS-CoV [2], [6], and now SARS-CoV-2 [1], [2] have caused recent epidemics associated with considerable mortality. SARS-CoV-2 has spread rapidly worldwide and led to over 412 million confirmed cases with a ∼1.2 % case fatality rate as of Feb 16, 2022 (World Health Organization).
There are several possible targets in the coronavirus life cycle for therapeutic interventions, including virus attachment and entry, uncoating, genomic RNA replication, translation in the endoplasmic reticulum (ER) and Golgi, assembly, and virion release [2], [7]. Viral attachment and entry are particularly promising as they are the first steps in viral replication cycle [7], [8]. CoVs use their glycosylated spike (S) protein to bind to their cognate cell surface receptors, and initiate membrane fusion and virus entry [8]. For both SARS-CoV and SARS-CoV-2, the S protein mediates entry into cells by binding to angiotensin converting enzyme 2 (ACE2) via the receptor binding domain (RBD), followed by proteolytic activation by human proteases [9]. The cellular transmembrane protease serine 2 (TMPRSS2) and cathepsin (Cat)B/L are critical for priming the SARS-CoV-2 S protein to enhance ACE2-mediated viral entry [10], [11], [12]. Because of its presence at the surface of virus particles and its central role in SARS-CoV-2 entry, the S protein is a major target for vaccines, neutralizing antibodies, and small molecule entry inhibitors [13], [14]. Indeed, in addition to multiple types of SARS-CoV-2 vaccines approved for clinical use, several neutralizing antibodies have also been developed as therapeutics. For example, a recent study reported that REGN-COV2, a cocktail of two potent neutralizing antibodies (REGN10987 + REGN10933), targets nonoverlapping epitopes on SARS-CoV-2 spike protein and inhibits SARS-CoV-2 infection [15], [16].
The efficacy of vaccines and antiviral therapeutics is challenged by the quick emergence and rapid spread of new SARS-CoV-2 variants including the most recent omicron strain [17], [18]. Development of broad-spectrum SARS-CoV-2 inhibitors is thus urgently needed to control the currently circulating and future variants. Small molecules have been shown to inhibit SARS-CoV-2 attachment, priming by the host’s proteases, or viral fusion. For example, Arbidol (ARB)/umifenovir is an indole-derivative molecule that functions as a virus-host cell fusion inhibitor targeting the S protein/ACE2 interaction to prevent viral entry into host cells [19], [20], which is now in clinical trials. A recent study in Japan has reported that nafamostat mesylate can inhibit the membrane fusion triggered by TMPRESS2 [21]. Furthermore, nelfinavir can inhibit cellular proteases that are required for SARS-CoV-2 fusion activation, including TMPRSS2 [22], [23].
Repurposing of the existing approved drugs is an effective strategy to quickly discover and approve drugs for treating COVID-19 patients, such as remdesivir and dexamethasone. These drugs and their derivatives hold great potential in clinical application for treating COVID-19 patients given their proven safety [24]. Glycopeptide antibiotics, such as teicoplanin and vancomycin, are widely used in the treatment of infections caused by Gram-positive bacteria [25], [26]. Teicoplanin has been reported to be active against SARS-CoV and used to treat COVID-19 [27], [28]. In addition, this drug has also been shown to be effective against a wide range of viruses including Ebola virus, influenza virus, flavivirus, hepatitis C virus, HIV [29], [30], [31], [32]. Mechanism study suggests that teicoplanin inhibits the early stages of virus infection, likely through blocking the cleavage of viral spike protein by cathepsin L in the late endosomes [32].
Given the reported antiviral activity of teicoplanin, we screened derivatives of teicoplanin for the activities against SARS-CoV-2. Several teicoplanin derivatives were showed to potently inhibit the replication of SARS-CoV-2 spike pseudovirus, impair viral attachment, and exhibited high affinity to the RBD of S protein. More importantly, these teicoplanin derivatives inhibit the entry function of S protein from both Delta and Omicron strains. All together, our results suggested that the teicoplanin derivatives hold the potential as anti-SARS-CoV-2 agents.
2. Materials and methods
2.1. Compounds
All compounds investigated in these studies were prepared in the laboratory of Shanghai Laiyi Center for Biopharmaceutical R&D, and the purity of all compounds analyzed by HPLC is more than 95 %.
2.2. Cells, viruses, plasmids and reagents
HEK293T cells were obtained from the American Type Culture Collection. HEK293T-ACE2 cells stably expressing ACE2 were generated in our laboratory with puromycin-resistant. All cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM; GBICO) supplemented with 10 % fetal bovine serum (FBS; GBICO) and penicillin/streptomycin. The spike coding sequences for SARS-CoV-2, SARS-CoV, MERS-CoV were codon optimized for human cell usage, and inserted into the pCAGGS vector. Spike-D614G were generated with the Muta-Direct Site-Directed Mutagenesis Kit (SBS genetech Co., Ltd) according to the manufacturer’s instructions. Delta and Omicron SARS-CoV-2 spike variant sequences were obtained from GENEWIZ, Inc, and engineered into pCAGGS vector.
2.3. SARS-CoV-2-lenti-luc pseudovirus preparation
HEK293T cells in six well plates were co-transfected with plasmids pLenti-GFP/luc-Puro, psPAX2 and SARS-CoV-2-spike with a mass ratio of 1:1.5:1.5 (1.0 μg, 1.5 μg, and 1.5 μg, respectively) using Lipofectamine 2000 (Life Technologies). The supernatant was collected at day 2 post transfection and stored at − 80 °C. HIV-1 p24 levels of the pseudovirus particles were determined using the HIV-1 p24 quantitation ELISA Kit (WANTAI BioPham Co., Ltd) according to the manufacturer’s directions.
2.4. Infectivity assay
Effect of compounds was measured using a single-round lentivirus-based pseudovirus infection of HEK293T-ACE2 cell line. The analysis method is as previously described [33]. Firefly luciferase activities were determined using a firefly Luciferase Assay System (Promega). Equal volumes of DMSO were added into the culture medium, which contain different concentrations of the compounds, in order to keep constant final DMSO concentration as 1 % (v/v).
IC50 was calculated from the inhibition curves determined using Graphpad Prism Software.
2.5. HTRF (homogeneous time resolved fluorescence) assay
The HTRF SARS-CoV-2 Spike/ACE2 Binding assay (Cisbio) is designed to measure the interaction between SARS-CoV-2 Spike protein RBD and human ACE2 proteins using anti-Tag1-Europium (HTRF donor) and anti-Tag2-d2(HTRF acceptor). This assay was performed according to the manufacturer’s instructions. Luminescence was measured using VICTORTM X5 2030 Multilable Reader (PerkinElmer).
2.6. Anti-coronavirus activity assay
HCT-8 cells and LLC-MK2 cells were infected with HCoV-OC43 and HCoV-NL63 at MOIs of 0.1 and 0.01, respectively, containing 2 % FBS and each compound. Cells were then incubated at 33 °C for 5 days in a 5 % CO2 incubator. The anti-coronavirus activity of the different strains was measured by using MTS Cell Proliferation Colorimetric assay kits (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The absorbance at 490 nm was measured using the Enspire 2300 Multiable reader (PekinElmer).
2.7. Cell counting kit-8 (CCK-8) assay
Cell viability was examined with Cell Counting kit-8 (CCK8, Beyotime). 1 μL of each tested compound was added to HEK293T cells and incubated for 24 h. Then 10 μL of CCk-8 reagent was added into each well and incubated for 90 min at 37◦C and 5 % CO2. The cell viability was determined by measuring the optical density (OD) absorbance at the wavelength of 450 nm using the Enspire 2300 Multiable reader (PekinElmer).
2.8. Determination of dissociation equilibrium constant (KD) by SPR
Compound solutions with a series of increasing concentrations were applied to all four channels at a 30 μL/min flow rate. RBD-mFc was immobilized on a CM5 sensor chip using standard amine-coupling with running buffer HBS-EP +using a Biacore T200 instrument. The RBD-mFc was immobilized to flow channels 2, and immobilization levelswas ∼3800 RU. The resulting data were fit to a 1:1 binding model using Biacore T200 evaluation software 2.0.
2.9. Molecular docking
the AutoDock4.2 software was utilized to build the systems of RBD complexed with four glycopeptides, RBD-LYSC45, RBD-LYSC70, RBD-LYSC72 and RBD-LYSC97. According to the binding position of RBD and ACE2, the map of grid points was set and calculated. Two hundred conformations were searched. Finally, the results were ranked by docking energy. The top conformation for each system with the lowest binding energy was extracted to carry out further classical MD simulations.
The crystal structure of SARS-CoV-2 WT S-RBD (PDB ID 6M0J), Delta S-RBD (PDB ID 7WBQ) and Omicron S-RBD (PDB ID 7WBP) were chosen as targets for molecular docking studies of compound LYSC72. Protein structures were prepared for docking using AutoDockTools (ADT, version: 1.5.6) by removing co-crystalized water molecules, adding polar hydrogens, and merging Gasteiger charges. The active site of S-RBD was defined as a box of 30 Å × 30 Å × 30 Å, centered near the ACE2 binding region (x = −36.952 Å, y = 38.054 Å, z = 3.595 Å). The grid step size for each docking volume was set to 1 Å. Ligand LYSC72 was prepared using Open Babel toolkit version. Auto Dock Vina software package was used for docking as previously described [34] .
2.10. Molecular dynamic (MD) simulations
The MD simulations with four parallel 30 ns trajectories were carried out for each system including RBD-LYSC45, RBD-LYSC70, RBD-LYSC72 and RBD-LYSC97 with ff14SB force field via AMBER 18 program suite. Each system underwent a two-step energy minimization for water molecules and the rest of the overall system sequentially, followed by the heating step where the system was heated gradually from 0 K to 300 K in 50 ps. Next, the system was equilibrated for 50 ps under the isothermal−isobaric ensemble (300 K and a bar) to obtain an appropriate initial conformation for the subsequent six replicas of 50 ns MD simulations in each system. During MD simulations, the Particle Mesh Ewald (PME) method and the SHAKE algorithm were utilized to describe long-range electrostatic interactions and correct bond lengths. The non-bonded cutoff was set at 10.00 Å. Besides, each MD simulation trajectory was confirmed to reach the stable state in the light of root mean square deviation (RMSD) fluctuations. The cpptraj module of AMBER 18 was used to analyze these MD simulation trajectories.
2.11. Quantification and statistical analysis
Data are presented as the means ± SD from at least three independent experiments and were analyzed with GraphPad Prism. Results were analyzed using nonlinear repression curve fitting.
3. Results
3.1. Teicoplanin derivatives inhibit the cell entry of SARS-CoV-2
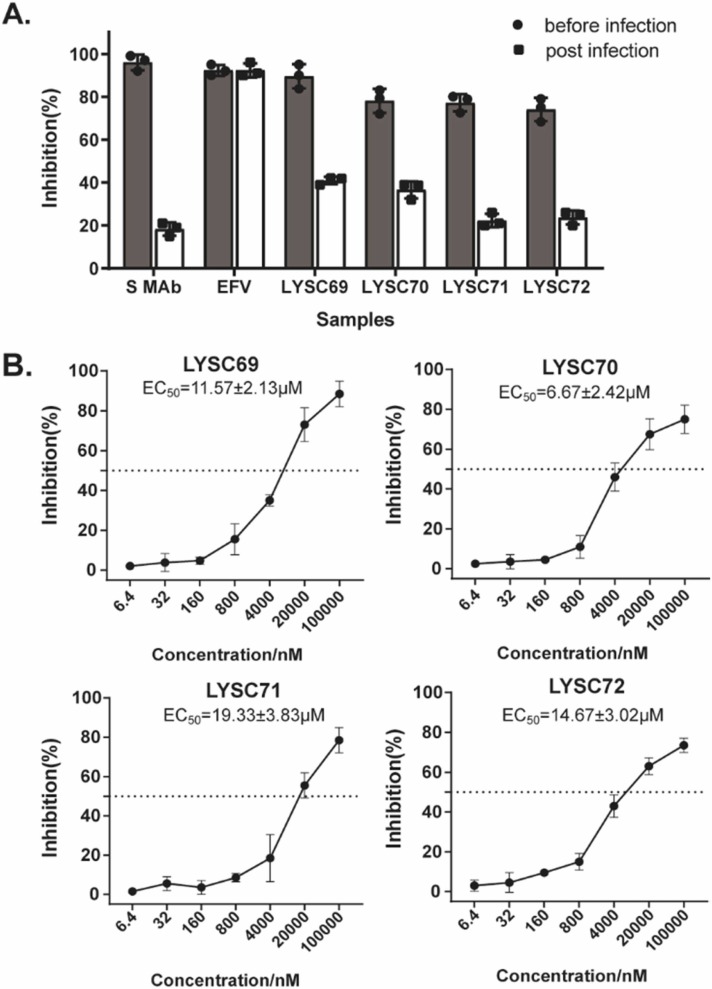
To investigate if teicoplanin and its derivatives inhibit SARS-CoV-2 entry, we firstly established a SARS-CoV-2 pseudovirus assay that uses pseudotyped lentiviral particles containing SARS-CoV-2 spike protein to mimic SARS-CoV-2 cell entry via binding to its ACE2 receptor. The SARS-CoV-2 spike pseudovirus particles encode firefly luciferase in the lentiviral vector. The firefly luciferase is expressed after the SARS-CoV-2 pseudovirus enters ACE2-expressing cells, and the luciferase activity reflects the efficacy of S protein-mediated viral entry. Next, we infected HEK293T-ACE2 cells with the SARS-CoV-2 pseudovirus in the presence of each compound at the concentration of 10 μM, followed by the quantification of luciferase activity at 48 hr post-infection. The results showed that among the 36 teicoplanin derivatives ( Fig. 1A), more than half of them exhibited an inhibitory effect on luciferase activity, suggesting their potential activity against the entry of SARS-CoV-2. Six compounds including LYSC69, LYSC70, LYSC71, LYSC72, LYSC100 and LYSC101, showed more than 60 % inhibition of luciferase activity, which are more potent than that of either teicoplanin or vancomycin (Fig. 1B and Table S1). We thus selected these compounds for further investigation.
Fig. 1.
Teicoplanin derivatives inhibit the cell entry of SARS-Cov-2. (A) The molecular structures of the compounds. (B and C) Compounds indicated above were diluted in culture medium mixed with equal volume of pseudovirus, and then the mixture was added to pre-seeded HEK293T-ACE2 for infection. After 48 h incubation, levels of luciferase activity in the infected cells were measured to evaluate the inhibitory effect of compounds. Data were presented as mean ± standard deviations (SD). One representative result from at least three independent experiments was shown. (D) HEK293T-ACE2 cells were treated with these inhibitors as indicated. The CC50 values were measured with the cell counting kit-8. Results shown are the average of three independent experiments and error bars indicate SD.
Next, we examined the dose response and selectivity of these teicoplanin derivatives. The result revealed that these six compounds inhibited the infection of SARS-CoV-2 pseudovirus in a dose-dependent manner, with EC50 values ranging from 0.80 to 2.00 μM (Fig. 1C and Table 1). Meanwhile, we also measured their inhibitory effect on the infectivity of VSV-G pseudotyped lentivirus as a control, and found that LYSC69, LYSC70, LYSC71 and LYSC72 exhibited much more potency against SARS-CoV-2 pseudovirus than the VSV-G pseudovirus (Fig. 1C and Table 1), whereas LYSC100 and LYSC101 showed a similarly activity for both SARS-CoV-2 spike and VSV-G pseudotyped viruses. These results suggest a selective inhibition of SARS-CoV-2 entry by LYSC69, LYSC70, LYSC71 and LYSC72.
Table 1.
Inhibitory effect of teicoplanin derivatives.
| Sample | EC50 (S)/μΜa | EC50 (VSVG)/μΜ | CC50/μΜb | TI (CC50/EC50)c |
|---|---|---|---|---|
| LYSC69 | 0.82 | 3.69 | 32.78 | 39.98 |
| LYSC70 | 0.91 | 20.89 | 67.21 | 73.86 |
| LYSC71 | 2.67 | > 100 | 50.00 | 18.73 |
| LYSC72 | 2.52 | 93.65 | 25.00 | 9.92 |
| LYSC100 | 0.84 | 0.91 | 31.54 | 37.55 |
| LYSC101 | 6.94 | 15.25 | > 200 | / |
| Vancomycin hydrochloride | > 100 | > 100 | > 200 | / |
| Teicoplanin | > 100 | > 100 | > 200 | / |
EC50: median effect concentration.
CC50: concentration cytotoxicity 50%.
TI: therapeutic index, CC50/EC50.
We further determined the in vitro 50 % cytotoxic concentration (CC50) of these teicoplanin derivatives using the CCK-8 assay, to determine the therapeutic index (TI) (CC50/EC50). Overall, all the compounds (LYSC69, LYSC70, LYSC71, LYSC72, LYSC100, LYSC101) display relatively low toxicity toward the 293 T-ACE2 cells with the CC50 > 25 μM (Fig. 1D and Table 1), resulting in the TI value of 39.98, 73.86, 18.73, and 9.92, respectively (Table 1). These results indicate that the inhibition of SARS-CoV-2 pseudovirus infection is not due to the cytotoxicity of the tested compounds. Taken together, these data suggest that LYSC69, LYSC70, LYSC71 and LYSC72 act as selective entry inhibitors of SARS-CoV-2.
3.2. Teicoplanin derivatives inhibit viral attachment
To further demonstrate that these four teicoplanin derivatives selectively suppress viral entry of SARS-CoV-2, we performed the time-of-addition experiment using SARS-CoV-2 pseudovirus. LYSC69, LYSC70, LYSC71 and LYSC72 were added before or after pseudovirus infection. SARS-CoV-2 neutralizing antibody (spike Mab, Cat: 40150-D001) and HIV-1 reverse transcriptase inhibitor efavirenz were used as controls, which blocked viral entry specifically and viral replication by inhibiting reverse transcriptase, respectively. The results showed a much more potent inhibition of pseudovirus infection when the four teicoplanin derivatives were added before virus infection than added after virus infection, an observation similar to the inhibition by SARS-CoV-2 neutralizing antibody. In contrast, efavirenz showed similar inhibition at both time points of drug addition ( Fig. 2A). These data support that the four teicoplanin derivatives inhibit an early step of SARS-CoV-2 pseudovirus infection, as an entry inhibitor does. In addition, we tested different doses of these compounds in an infectivity assay where compounds were added during virus attachment on ice and then removed before cell culture was returned to 37 ℃. As shown in Fig. 2B, addition of the compounds before viral attachment potently inhibited viral infection in a dose-dependent manner, with the EC50 ranging from 6 μM to 20 μM. Furthermore, we performed the time-of-addition experiment using SARS-CoV-2 pseudovirus in Calu-3 cells that were widely used in SARS-CoV-2 research, and found that all the compounds blocked the cellular attachment of lentiviral particles pseudotyped to the Calu-3 cell line with similar activity, respectively (Fig. S3), providing further evidence to support the antiviral activity of these compounds.
Fig. 2.
Teicoplanin derivatives inhibit viral attachment. (A) HEK293T-ACE2 cells were re-seeded in 96-well plates (104/well). Compounds indicated as above were added before and after pseudovirus infection, respectively. (B) HEK293T-ACE2 cells were re-seeded in 96-well plates (104/well). Compounds were added during virus attachment on ice for 1 h and then removed before cells were cultured at 37 ℃. After 48 h incubation, levels of luciferase activity in the infected cells were measured to evaluate the inhibitory effect of compounds. Results shown are the average of three independent experiments and error bars indicate SD.
3.3. Teicoplanin derivatives inhibit binding of SARS-CoV-2 RBD to ACE2 in vitro
The S proteins of both SARS-CoV and SARS-CoV-2 bind to ACE2 via their RBD domain [35], [36]. Therefore, we next evaluated the effect of our compounds on RBD binding to ACE2 using a homogeneous time resolved fluorescence assay (HTRF). In this assay, a specific HTRF signal is directly proportional to the degree of RBD/ACE2 interaction. The results showed that compared with teicoplanin and vancomycin, LYSC69, LYSC70, LYSC71 and LYSC72 inhibited the RBD/ACE2 interaction with the IC50 from 50 μM to 100 μM ( Fig. 3), suggesting that these teicoplanin derivatives interfere with the binding of SARS-CoV-2 RBD to ACE2, and thereby the virus attachment. We noted that the IC50 values of these compounds to inhibit the RBD/ACE2 interaction are higher than those of inhibiting virus infection (Table 1 and Fig. 2B). This discrepancy is most likely due to the higher concentrations of RBD and/or ACE2 used in the HTRF assay. For example, at least 10-fold higher concentration of SARS-CoV-2 neutralizing antibody was required to inhibit the RBD/ACE2 interaction than that to block viral attachment (Fig. S4).
Fig. 3.
Teicoplanin derivatives inhibit binding of SARS-CoV-2 RBD to ACE2 in vitro by HTRF. RBD and human ACE2 proteins were labeled by anti-Tag1-Europium(HTRF donor) and anti-Tag2-d2(HTRF acceptor). A series of diluted compounds were added to the reaction buffer. After incubated for 3 h at room temperature, luminescence was measured using VICTORTM X5 2030 Multilable Reader. The ratio of the acceptor and donor emission signals (Signal 665 nm/Signal 620 nm) was calculated to evaluate the binding efficiency. Results shown are the average of three independent experiments. The error bars indicate SD.
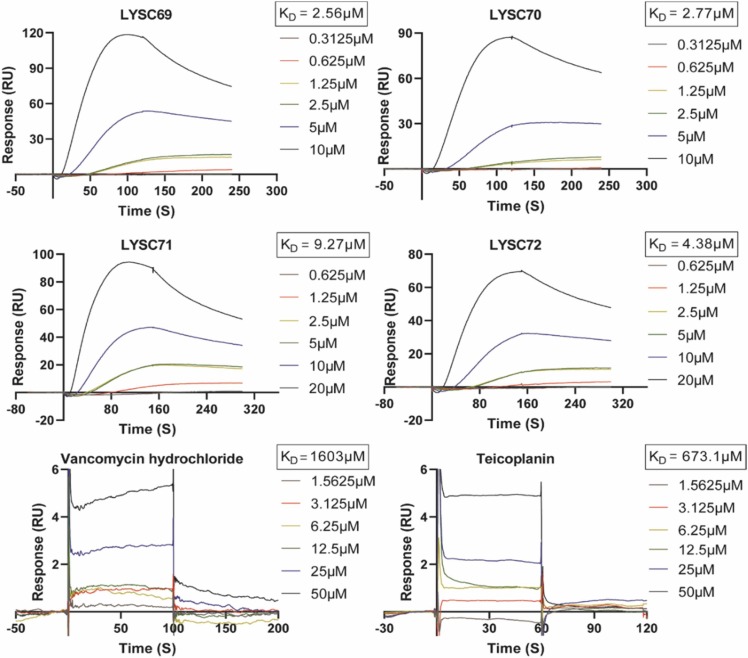
3.4. Teicoplanin derivatives bind to RBD with high affinity
We next measured the binding affinity of the teicoplanin derivatives with the RBD domain of S protein using the SPR assay. We hypothesized that these compounds may bind to the RBD domain and interrupt the interaction between the S protein and ACE2. First, the RBD fragment was immobilized on a CM5 chip, then compounds flew across the surface. We found that the teicoplanin derivatives bound to the RBD domain efficiently, with KD (equilibrium dissociation constant) ranging from 2.56 μM to 9.27 μM ( Fig. 4). In contrast, vancomycin and teicoplanin exhibited much lower binding affinity with predicted KD of 1603 μM and 673.1 μM, respectively. In line with it, vancomycin and teicoplanin were showed to fail to block binding of SARS-CoV-2 RBD to ACE2 in vitro (Fig. 3). These results provide evidence to support that the teicoplanin derivatives directly bind to the RBD domain of S protein, leading to their antiviral activity.
Fig. 4.
Kinetic and equilibrium binding analysis of teicoplanin derivatives binding to RBD. RBD-mFc was immobilized on a CM5 sensor chip using the Biacore T200 instrument. The association and dissociation curves of these compounds were shown. Compounds were dissolved with a series of increasing concentrations, and KD values were acquired from fitting to a 1:1 binding model using Biacore T200 evaluation software 2.0. KD: equilibrium dissociation constant.
3.5. Teicoplanin derivatives selectively inhibit coronaviruses
SARS-CoV-2 belongs to the beta-coronavirus according to the sequence released. Evolutionary analyses have shown that the 2019-nCoV shares 79 % homology with SARS-CoV and 50 % with MERS-CoV [1], [2], [3]. SARS-CoV-2 S protein acquired the D614G mutation early in the pandemic, which confers greater infectivity and has been preserved in almost all the viral variants [37]. We therefore tested the teicoplanin derivatives against S proteins from SARS-CoV, MERS-CoV and the variants of concern (VOCs) of SARS-CoV-2 D614G.Pseudoviruses were produced using the S protein either containing D614G mutation, as well as SARS-CoV and MERS-CoV. As shown in Fig. 5A, the teicoplanin derivatives inhibited the infection of pseudoviruses carrying S proteins of SARS-CoV-2 and SARS-CoV by approximate 70 % at 10 μM but not MERS-CoV.
Fig. 5.
Evaluation of antiviral activity of teicoplanin derivatives against human coronavirus. (A) HEK293T-ACE2 were infected with pseudoviruses (SARS-CoV-2, D614G, SARS-CoV and MERS), and treated with 10 μM teicoplanin derivatives. After 48 h, levels of luciferase activity in the infected cells were measured to evaluate the inhibitory effect of the compounds. Data were presented as mean ± standard deviations (SD). One representative result from at least three independent experiments was shown. (B and C) HCT-8 and LLC-MK2 cells were infected with HCoV-OC43 (B) or HCoV-NL63 (C) at a MOI of 0.1 and 0.01, respectively, and treated with serial dilutions of teicoplanin derivatives. The impact of treatment on cell viability was measured by MTS assay after 120 h post infection. The inhibition ratio was calculated by measuring the absorbance at 490 nm. Results shown are the average of three independent experiments. Error bars indicate SD.
It is known that the S proteins of SARS-CoV-2 and SARS-CoV bind to the receptor ACE2, whereas MERS-CoV S protein recognizes host dipeptidyl-peptidase 4 and sialic acid [38]. The results in Fig. 5A suggest that the teicoplanin derivatives recognize a common motif that the S proteins of SARS-CoV and SARS-CoV variants use to bind ACE2. In agreement with the hypothesis, we found that these compounds also potently inhibited the infection of CoV-NL63 that uses ACE2 as the receptor, with an EC50 value below 0.4 μM (Fig. 5B). In contrast, OC43 that uses 9-O-acetylsialic acids as the receptor, was much less sensitive to the teicoplanin derivatives (Fig. 5C).
3.6. Predicted binding site of teicoplanin derivatives on spike protein RBD
To better understand the antiviral mechanism of the teicoplanin derivatives, we next performed computer modeling to predict the binding interface between the compounds and the RBD domain. According to different antiviral activities of these glycopeptides, the representative glycopeptides LYSC45, LYSC70, LYSC72 and LYSC97 were selected for modeling. Firstly, substrate docking was performed with AutoDockTool 1.5.6, and the appropriate conformation with low energy was chosen to construct the systems of RBD in complex with these four glycopeptides (RBD-LYSC45, RBD-LYSC70, RBD-LYSC72 and RBD-LYSC97) (Fig. S1). Then classical molecular dynamic simulations with four parallel 30 ns trajectories were carried out for each system. The RMSD fluctuation along with the simulation time indicated that each system was in a rather stable state (Fig. S2). The representative structures from the major cluster of MD simulations ( Fig. 6) showed that LYSC72 and LYSC70 had more hydrogen bond interactions with RBD than LYSC45 and LYSC97, which was consistent with their differential antiviral activities. LYSC72 also formed abundant intramolecular hydrogen bonds which stabilize its binding conformation. The surrounding residues responsible for hydrogen bond and hydrophobic interactions were highlighted and some of them including Q493, L455, F456 and N501 were reported in recent studies on the binding mode of SARS-CoV-2 with ACE2. Therefore, these results support the interaction of these glycopeptides with the RBD of SARS-CoV-2 S protein.
Fig. 6.
Predicted binding site of teicoplanin derivatives with spike protein RBD. Shown are the representative structures from the major cluster of MD simulations for RBD-LYSC45, RBD-LYSC70, RBD-LYSC72 and RBD-LYSC97 systems. The glycopeptides LYSC45, LYSC70, LYSC72 and LYSC97 were colored in green, blue, pink and orange respectively. And the surrounding residues were colored in purple.
Currently, five ‘variants of concern’ (VOC) have been identified (Alpha, Beta, Gamma, Delta and Omicron). SARS-CoV-2 variants have accumulated specific mutations in the S protein [39]. The Delta variant, especially the newly emerged Omicron variant, carry a list signature mutations in viral S protein which have promoted their high transmission [40]. We aligned the sequences of SARS-CoV-2 WT S-RBD, Delta S-RBD and Omicron S-RBD using the multiple sequence alignment tool CLUSTALW ( Fig. 7A). The amino acid sequence of Delta S-RBD and Omicron S-RBD shows 99 % and 93 % similarity to that of wild type, respectively. So molecular modeling studies were done using AutoDock Vina to verify the conception and provide insight into their ligand-binding site interactions, exemplified as inhibitors LYSC72(Fig. 7B). Based on the predicted binding model, these mutations most unlikely affect the binding of LYSC72 to RBD variants. Therefore, no difference in susceptibility for compounds is expected. In line with it, we found a similar effect of teicoplanin derivatives on pseudoviruses containing these two variants, compared with that of ancestral virus (Fig. 7C). The result supports the potential of the teicoplanin derivatives as a broad entry inhibitor against different SARS-CoV-2 variants.
Fig. 7.
Predicted binding site of teicoplanin derivatives with spike protein RBD variants. (A)Sequences aligned the SARS-CoV-2 WT S-RBD, Delta S-RBD and Omicron S-RBD using the multiple sequence alignment tool CLUSTALW. (B) Predicted binding sites for LYSC72 bound to SARS-CoV-2 WT S-RBD (green), Delta S-RBD (purple) and Omicron S-RBD (yellow). In the 2D ligand-protein interaction diagrams, residues within 5 Å of LYSC72 are displayed. Hydrogen bond is represented by the arrows. The π-π stacking and salt bridge are shown in green and purple lines, respectively. Here the color code is that dark blue is positive charged, red is negative charged, light blue is polar, and green is hydrophobic. (C) HEK293T-ACE2 were infected with pseudoviruses (Delta-S and Omicron-S), and treated with 10 μM teicoplanin derivatives. After 48 h, levels of luciferase activity in the infected cells were measured to evaluate the inhibitory effect of the compounds. Data were presented as mean ± standard deviations (SD). One representative result from at least three independent experiments was shown.
4. Discussion
In this work, we have identified four teicoplanin derivatives including LYSC69, LYSC70, LYSC71 and LYSC72 as pan-SARS-CoV-2 inhibitors. Mechanism study suggests that the four teicoplanin derivatives selectively inhibit SARS-CoV-2 entry through binding to S protein and interrupting its interaction with ACE2 receptor. The influence of substituents in R1 and R2 positions was investigated for a series of teicoplanin derivatives (Fig. 1B and Table S1). Compounds including LYSC69, LYSC70, LYSC71, LYSC72 showed significantly more potent than teicoplanin in inhibiting the entry of SARS-CoV-2 (Table 1). The structure-activity relationships analysis highlighted that the introduction of Chlorobiphenyl or N-ethyldecan-1-amine group at R1 position of teicoplanin, and introduction of 4-(Fluorophenyl)methanamine or 4-(Aminomethy)benzonitrile group in the R2 position at the same time may improve the antiviral activity of derivatives. These modifications might contribute to enhanced binding affinity with the RBD domain, resulting in an inhibitory effect upon the interaction of viral S protein with ACE2 and thereby viral entry, which awaits further investigation. In addition, teicoplanin was reported to inhibit the cleavage of the viral spike protein by cathepsin L [32], one key step that is required for efficient virus-cell membrane fusion. The four teicoplanin derivatives may have preserved this activity to augment their antiviral function, since we found that the antiviral activity of these compounds is stronger when they are maintained through the infection than when the drug was present only at the attachment step.
The SPR assay data showed that these four teicoplanin derivatives bound to the RBD domain efficiently, providing further evidence to support their functions in blocking the interaction between S and the acceptor ACE2. In line with it, computer modeling suggest that the amino acid residues involving in the interactions with teicoplanin derivatives were also reported to participate in the binding to ACE2 [41]. Importantly, some of the LYSC72-interacting amino acids are highly conserved cross the variants of SARS-CoV-2, suggesting a potential broad activity of this compound. In support of this, we found that the teicoplanin derivatives inhibited the viral infection mediated by S proteins of the Delta and Omicron SARS-CoV-2 variants. Therefore, the teicoplanin derivatives may hold the promise of becoming pan-SARS-CoV-2 inhibitors which are urgently needed to control the continuously evolving SARS-CoV-2.
Teicoplanin is a well-characterized antibiotic, commonly used to treat serious infections caused by multiple antibiotic-resistant Gram-positive bacteria (REF). Teicoplanin has also been shown to inhibit the replication of SARS-CoV-2 at concentrations that were achievable in vivo when used in clinics [42], and thus can be used to treat bacterium and virus co-infection. Teicoplanin itself has been shown to inhibit the cathepsin L protease through the direct interaction of the teicoplanin lipophilic moiety with the enzyme [43]. This inhibition has been reported to extend to inhibit the activity of the SARS-CoV2 main cysteine protease (SARS-CoV2 3CL Pro) [44]. In this work, we found that the teicoplanin derivatives inhibit virus infection by a completely different mechanism, through binding to the RBD of viral spike protein, thus preventing the engagement of viral receptor ACE2, and abrogating virus attachment and entry. This new antiviral mechanism may have rendered these teicoplanin derivatives stronger viral inhibitors than the parental drug teicoplanin, whereas it should be noted that in vivo study is required for further evaluation of these compounds against SARS-CoV-2 infection. The benefit of cautious use of teicoplanin derivatives in treating SARS-CoV-2 infection should outweigh the risk of inducing the development of antibiotic resistance.
Funding
This work was supported by Shanghai Science and Technology Project (20S11900900), the National Natural Science Foundation of China (81903679), CAMS Innovation Fund for Medical Sciences (2021-I2M-1–038, 2021-I2M-1-055, 2021-I2M-1-030 and 2022-I2M-JB-014).
CRediT authorship contribution statement
Ling Ma: Investigation, Methodology, Writing – original draft preparation. Yali Li: Resources, Ting Shi: Software, Writing – original draft preparation. Zhiling Zhu: Methodology. Jianyuan zhao: Data curation, Validation. Yongli Xie: Data curation, Validation. Jiajia Wen: Data curation, Validation. Saisai Guo: Data curation, Validation. Jing Wang: Data curation, Validation. Jiwei Ding: Data curation, Validation. Chen Liang: Writing – review & editing. Guangzhi Shan: Writing – review & editing. Quanjie Li: Software, Supervision. Mei Ge: Conceptualization, Supervision, Writing – review & editing. Shan Cen: Conceptualization, Supervision, Writing – review & editing.
Quantification and statistical analysis
Data are presented as the means ± SD from at least three independent experiments and were analyzed with GraphPad Prism. Results were analyzed using nonlinear repression curve fitting.
Author contributions
SC and MG conceptualized the study. LM carried out the virology experiment. YL prepared all the compound. TS and QL performed the computer modeling. ZZ, JZ, YX, JW, SG, JW and JD participated in the data analysis and validation. QL, MG and SC supervised the study. LM and TS wrote the original draft. CL, GS, MG and SC reviewed and edited the manuscript.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the National Infrastructure of Microbial Resources (NIMR-2014-3, China) and the CAMS Collection Center of Pathogenic Micro-organisms (CAMS-CCPM-A, China) for providing valuable reagents.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.biopha.2023.114213.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., Novel A. Coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik Y.A. Properties of coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020;42(1):3–11. [PubMed] [Google Scholar]
- 3.Li S.W., Lin C.W. Human coronaviruses: clinical features and phylogenetic analysis. BioMedicine. 2013;3(1):43–50. doi: 10.1016/j.biomed.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navas-Martin S., Weiss S.R. Vol. 16. SARS: lessons learned from other coronaviruses; 2003. pp. 461–474. (Viral Immunology). [DOI] [PubMed] [Google Scholar]
- 5.Ivan A., Azoicăi D. [SARS (Severe Acute Respiratory Syndrome). Emergent transmissible disease. Rev. Med. -Chir. a Soc. De. Med. si Nat. Din. Iasi. 2003;107(2):250–252. [PubMed] [Google Scholar]
- 6.Geng H., Tan W. A novel human coronavirus: middle East respiratory syndrome human coronavirus. Sci. China Life Sci. 2013;56(8):683–687. doi: 10.1007/s11427-013-4519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng P.K., Wong D.A., Tong L.K., Ip S.M., Lo A.C., Lau C.S., Yeung E.Y., Lim W.W. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363(9422):1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taguchi F., Kubo H., Suzuki H., Yamada Y.K. Localization of neutralizing epitopes and receptor-binding site in murine coronavirus spike protein. Adv. Exp. Med. Biol. 1995;380:359–365. doi: 10.1007/978-1-4615-1899-0_58. [DOI] [PubMed] [Google Scholar]
- 9.Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA. 2005;102(33):11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravaioli S., Tebaldi M. ACE2 and TMPRSS2 potential involvement in genetic susceptibility to SARS-COV-2 in cancer patients. 2020;29 doi: 10.1177/0963689720968749. 963689720968749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte C., Akkaoui J., Ho A., Garcia C., Yamada C., Movila A. Age-dependent effects of the recombinant spike protein/SARS-CoV-2 on the M-CSF- and IL-34-differentiated macrophages in vitro. Biochem Biophys. Res Commun. 2021;546:97–102. doi: 10.1016/j.bbrc.2021.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rensi S., Altman R.B., Liu T., Lo Y.C., McInnes G., Derry A., Keys A. Homology Modeling of TMPRSS2 yields candidate drugs that may inhibit entry of SARS-CoV-2 into human. Cells ChemRxiv: Prepr. Serv. Chem. 2020 [Google Scholar]
- 13.Nie C., Ma L., Luo H., Bao J., Cheng C. Spiky nanostructures for virus inhibition and infection prevention. Smart Mater. Med. 2020;1:48–53. doi: 10.1016/j.smaim.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unni S., Aouti S., Thiyagarajan S., Padmanabhan B. Identification of a repurposed drug as an inhibitor of Spike protein of human coronavirus SARS-CoV-2 by computational methods. J. Biosci. 2020;45:1. doi: 10.1007/s12038-020-00102-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baum A., Ajithdoss D. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. 2020;370(6520):1110–1115. doi: 10.1126/science.abe2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y., Sun H., Yu H., Li S., Zheng Q., Xia N. Neutralizing antibodies against SARS-CoV-2: current understanding, challenge and perspective. Antib. Ther. 2020;3(4):285–299. doi: 10.1093/abt/tbaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kandeel, M.; Mohamed, M.E.M.; Abd El-Lateef, H.M.; Venugopala, K.N.; El-Beltagi, H.S., Omicron variant genome evolution and phylogenetics. 2021. [DOI] [PMC free article] [PubMed]
- 18.Lu, L.; Mok, B.W.; Chen, L.L.; Chan, J.M.; Tsang, O.T.; Lam, B.H.; Chuang, V.W.; Chu, A.W.; Chan, W.M.; Ip, J.D.; Chan, B.P.; Zhang, R.; Yip, C.C.; Cheng, V.C.; Chan, K.H.; Jin, D.Y.; Hung, I.F.; Yuen, K.Y.; Chen, H.; To, K.K., Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 2021. [DOI] [PMC free article] [PubMed]
- 19.Nojomi M., Yassin Z., Keyvani H., Makiani M.J., Roham M., Laali A., Dehghan N., Navaei M., Ranjbar M. Effect of Arbidol (Umifenovir) on COVID-19: a randomized controlled trial. BMC Infect. Dis. 2020;20(1):954. doi: 10.1186/s12879-020-05698-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen F.F., Zhong M., Liu Y., Zhang Y., Zhang K., Su D.Z., Meng X., Zhang Y. The characteristics and outcomes of 681 severe cases with COVID-19 in China. J. Crit. care. 2020;60:32–37. doi: 10.1016/j.jcrc.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann M., Schroeder S., Kleine-Weber H., Müller M.A., Drosten C., Pöhlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob. Agents Chemother. 2020;64:6. doi: 10.1128/AAC.00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohashi H., Watashi K., Saso W., Shionoya K., Iwanami S., Hirokawa T., Shirai T., Kanaya S., Ito Y., Kim K.S., Nomura T., Suzuki T., Nishioka K., Ando S., Ejima K., Koizumi Y., Tanaka T., Aoki S., Kuramochi K., Suzuki T., Hashiguchi T., Maenaka K., Matano T., Muramatsu M., Saijo M., Aihara K., Iwami S., Takeda M., McKeating J.A., Wakita T. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. iScience. 2021;24(4) doi: 10.1016/j.isci.2021.102367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musarrat F., Chouljenko V., Dahal A., Nabi R., Chouljenko T., Jois S.D., Kousoulas K.G. The anti-HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARSCoV-2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID-19 infections. 2020;92(10):2087–2095. doi: 10.1002/jmv.25985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar D., Trivedi N. Disease-drug and drug-drug interaction in COVID-19: Risk and assessment. Biomed. Pharm. 2021;139 doi: 10.1016/j.biopha.2021.111642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood M.J. The comparative efficacy and safety of teicoplanin and vancomycin. J. Antimicrob. Chemother. 1996;37(2):209–222. doi: 10.1093/jac/37.2.209. [DOI] [PubMed] [Google Scholar]
- 26.de Lalla F. Antimicrobial chemotherapy in the control of surgical infectious complications. J. Chemother. 1999;11(6):440–445. doi: 10.1179/joc.1999.11.6.440. [DOI] [PubMed] [Google Scholar]
- 27.Baron S.A., Devaux C., Colson P., Raoult D., Rolain J.M. Teicoplanin: an alternative drug for the treatment of COVID-19? Int. J. Antimicrob. Agents. 2020;55(4) doi: 10.1016/j.ijantimicag.2020.105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceccarelli G., Alessandri F., Oliva A., Borrazzo C., Dell'Isola S., Ialungo A.M., Rastrelli E., Pelli M. Role Teicoplanin Treat. SARS-CoV-2 Infect.: A Retrosp. Study Crit. Ill. COVID-19 Patients (Tei-COVID Study) 2021;93(7):4319–4325. doi: 10.1002/jmv.26925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preobrazhenskaya M.N., Olsufyeva E.N. Polycyclic peptide and glycopeptide antibiotics and their derivatives as inhibitors of HIV entry. Antivir. Res. 2006;71(2–3):227–236. doi: 10.1016/j.antiviral.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sipos A., Török Z., Rőth E., Kiss-Szikszai A., Batta G., Bereczki I., Fejes Z., Borbás A., Ostorházi E., Rozgonyi F., Naesens L., Herczegh P. Synthesis of isoindole and benzoisoindole derivatives of teicoplanin pseudoaglycon with remarkable antibacterial and antiviral activities. Bioorg. Med. Chem. Lett. 2012;22(23):7092–7096. doi: 10.1016/j.bmcl.2012.09.079. [DOI] [PubMed] [Google Scholar]
- 31.Bereczki I., Kicsák M., Dobray L., Borbás A., Batta G., Kéki S., Nikodém É., Ostorházi N., Rozgonyi E., Vanderlinden F., Naesens E., Herczegh L., P Semisynthetic teicoplanin derivatives as new influenza virus binding inhibitors: synthesis and antiviral studies. Bioorg. Med. Chem. Lett. 2014;24(15):3251–3254. doi: 10.1016/j.bmcl.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Zhou N., Pan T., Zhang J., Li Q., Zhang X., Bai C., Huang F., Peng T., Zhang J., Liu C., Tao L., Zhang H. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of ebola virus, middle east respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV) J. Biol. Chem. 2016;291(17):9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu J., Gao Q., He C., Huang A., Tang N., Wang K. Development of cell-based pseudovirus entry assay to identify potential viral entry inhibitors and neutralizing antibodies against SARS-CoV-2. Genes Dis. 2020;7(4):551–557. doi: 10.1016/j.gendis.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q., Yi D., Lei X., Zhao J., Zhang Y., Cui X., Xiao X., Jiao T., Dong X., Zhao X., Zeng H., Liang C., Ren L., Guo F., Li X., Wang J., Cen S. Corilagin inhibits SARS-CoV-2 replication by targeting viral RNA-dependent RNA polymerase. Acta Pharm. Sin. B. 2021;11(6):1555–1567. doi: 10.1016/j.apsb.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakraborti S., Prabakaran P., Xiao X., Dimitrov D.S. The SARS coronavirus S glycoprotein receptor binding domain: fine mapping and functional characterization. Virol. J. 2005;2:73. doi: 10.1186/1743-422X-2-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prabakaran P., Xiao X., Dimitrov D.S. A model of the ACE2 structure and function as a SARS-CoV receptor. Biochem Biophys. Res Commun. 2004;314(1):235–241. doi: 10.1016/j.bbrc.2003.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoehl S., Ciesek S. [The virology of SARS-CoV-2] Der Gastroenterol.: Z. fur Gastroenterol. und Hepatol. 2020;15(6):452–456. doi: 10.1007/s11377-020-00482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qing E., Hantak M., Perlman S. Distinct roles for sialoside and protein receptors in coronavirus. Infection. 2020;11:1. doi: 10.1128/mBio.02764-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park G., Hwang B.H. SARS-CoV-2 variants: mutations and effective changes. Biotechnol. Bioprocess Eng.: BBE. 2021;26(6):859–870. doi: 10.1007/s12257-021-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G., Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J Med Virol 2021. [DOI] [PubMed]
- 41.Jawad B., Adhikari P., Podgornik R., Ching W.Y. Key interacting residues between RBD of SARS-CoV-2 and ACE2 receptor: combination of molecular dynamics simulation and density functional calculation. 2021;61(9):4425–4441. doi: 10.1021/acs.jcim.1c00560. [DOI] [PubMed] [Google Scholar]
- 42.Jean S.S., Lee P.I., Hsueh P.R. Treatment options for COVID-19: The reality and challenges. J. Microbiol., Immunol., Infect. = Wei mian yu gan ran za zhi. 2020;53(3):436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bereczki I., Papp H. Nat. Apocarotenoids Their Synth. Glycopeptide Conjug. Inhib. SARS-CoV-2 Replica. 2021;14:11. [Google Scholar]
- 44.Tripathi P.K., Upadhyay S., Singh M., Raghavendhar S., Bhardwaj M., Sharma P., Patel A.K. Screening and evaluation of approved drugs as inhibitors of main protease of SARS-CoV-2. Int. J. Biol. Macromol. 2020;164:2622–2631. doi: 10.1016/j.ijbiomac.2020.08.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material