Abstract
Background
Malignant pleural mesothelioma (MPM) is a cancer with a high mortality rate and few therapeutic options. After platinum–pemetrexed combination, no further promising drug seems to be effective. Immune checkpoint inhibitors may have some activity in pretreated patients and no data are available in this population about durvalumab.
Materials and methods
DIADEM was a multicenter, open-label, single-arm, phase II trial aimed at evaluating the efficacy and safety of durvalumab. Patients with locally advanced/metastatic MPM who progressed after platinum–pemetrexed chemotherapy were enrolled to receive durvalumab (1500 mg, intravenously Q4W) for 12 months or until evidence of disease progression or unacceptable toxicity. The primary endpoint was the proportion of patients alive and free from progression at 16 weeks (PFS16wks) calculated from treatment initiation. Secondary endpoints were progression-free survival, overall survival, overall response rate, and safety.
Results
Sixty-nine patients with a median age of 69 years (range 44-82 years) were enrolled; 62 patients (89.9%) had epithelioid histotype. As first-line treatment, all patients received platinum derivatives–pemetrexed combination (60.9% with carboplatin and 39.1% with cisplatin). As of March 2021, the median follow-up was 9.2 months (interquartile range 5.2-11.1 months). Six patients (8.7%) completed the 12-month treatment; 60 patients discontinued, of whom 42 for progressive disease, and 4 died. Seventeen patients (28.3%; 95% confidence interval 17.5% to 41.4%) were alive or free from progression at 16 weeks. Eleven patients (18.6%) had a grade 3 or 4 treatment-related adverse event (AE), and one (1.4%) had a grade ≥3 immune-related, treatment-related AE. There was one drug-related death.
Conclusion
Durvalumab alone in pretreated non-selected MPM did not reach a meaningful clinical activity, showing any new major safety issue signals.
Key words: malignant pleural mesothelioma, second line, durvalumab, immune checkpoint inhibitors
Highlights
-
•
This phase II single-arm clinical trial failed to demonstrate durvalumab efficacy in terms of PFS16wks in pretreated MPM.
-
•
The study failed to reach the primary endpoint (proportion of patients alive or free from progression at 16 weeks).
-
•
Median PFS was 1.9 months and median OS 7.3 months, after a median follow-up of 9.2 months.
-
•
Clinical benefit of durvalumab is in line with other immune checkpoint inhibitors in a similar setting.
-
•
No new safety signal emerged with durvalumab.
Introduction
Pleural mesothelioma is still considered an aggressive cancer with increasing incidence and dismal prognosis, due to the complexity in pathological diagnosis and staging and the lack of therapeutic innovation in the last 30 years.1 First-line systemic treatment with a platinum-based doublet plus pemetrexed has been the gold standard since the early 2000s,2 while the use of second-line chemotherapy has been mostly supported by low levels of evidence and uncertain clinical benefit.3 Adding bevacizumab to chemotherapy doublets, as reported in the MAPS trial, did not lead to an impact in clinical practice, despite the statistically significant median overall survival (mOS) improvement.4
Surgery may be considered an option only in selected patients within a hospital with a multidisciplinary group used to managing a high number of malignant pleural mesothelioma (MPM) patients/year; however, some uncertainties of its role remain regarding procedural extension (extended extrapleural pneumonectomy versus pleurectomy/decortication), timing with respect to systemic treatment (‘neo/adjuvant’), histology subgroup indication (epithelioid), and OS impact on clinical disease course.
After a decade of relatively poor results derived from the use of second- or third-line chemotherapy agents, immune checkpoint inhibitors (ICIs), initially explored as monotherapy in several non-randomized phase I and II trials, showed some promising results in terms of activity.5, 6, 7
At the time of DIADEM design, no established role of these compounds was defined, and no clinical results were available regarding the use of monoclonal antibodies directed against programmed death-ligand 1 (PD-L1) in MPM.
After discouraging final results of the PROMISE-meso study, a phase III randomized clinical trial comparing pembrolizumab versus investigator’s choice single-agent chemotherapy for advanced pretreated MPM,8 the CONFIRM trial showed that nivolumab significantly improved progression-free survival (PFS) and OS compared to placebo. These results have opened the way to the use of this drug for heavily pretreated MPM patients.9
More recently, the double blockade of programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4)10 has been translated in the first-line setting placing ipilimumab plus nivolumab as a new standard of care in alternative to platinum–pemetrexed chemotherapy in PD-L1 unselected mesothelioma patients,11 leaving open the new way of the therapeutic path in the second and further lines of treatment.
Durvalumab is an anti-PD-L1 that blocks the interaction of PD-L1 with PD-1 and CD80 (B7.1), without inducing antibody-dependent cell-mediated cytotoxicity. Data from clinical trials are currently available for the combination of durvalumab and tremelimumab in naive and pretreated patients12 and the combination with chemotherapy (cisplatin and pemetrexed) in the first-line setting.13 Both combinations appeared to be active and showed a favorable safety profile.
Based on the first evidence on ICIs on pretreated patients and before data of immunotherapy combinations are available, we designed a phase II study aimed at evaluating the efficacy and safety of durvalumab in patients previously treated with pemetrexed plus platinum derivatives.
Materials and methods
Study design and participants
DIADEM was a phase II, single-arm, multicenter study aimed at exploring the activity of durvalumab in patients with MPM, relapsing after first-line platinum-based treatment. Participants were adults aged 18 years or older, with a histologically confirmed diagnosis of advanced unresectable MPM, previously treated with pemetrexed plus platinum derivative. They were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1; at least one measurable lesion according to the modified Response Evaluation Criteria for Solid Tumours (mRECIST) for mesothelioma14; adequate hepatic, renal, and hematological functions; and archival tumor sample for explorative analysis. Patients with a history of autoimmune disease, brain or leptomeningeal tumor involvement, or concurrent or previous malignancy (except for in situ cervical cancer and basal cell carcinoma of the skin, adequately treated), unless there was no evidence of disease for at least 3 years, were excluded. Full inclusion and exclusion criteria are reported in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100644.
The study complied with the Declaration of Helsinki and was conducted as per Good Clinical Practice guidelines; it was approved by the ethics committees of all study sites. All patients provided written informed consent before enrolment. This trial is registered in ClinicalTrials.gov, NCT04115111.
Procedures
Patients received durvalumab 1500 mg, infused intravenously over ∼60 min, on day 1 every 4 weeks and for a maximum of 12 months. Treatment was discontinued before 12 months in case of confirmed disease progression (unless the investigator considered the patient still benefiting from treatment continuation), unacceptable toxicity, or withdrawal of consent.
Patients who achieved and maintained disease control [i.e. complete response (CR), partial response (PR), or stable disease (SD)] through to the end of the 12-month durvalumab treatment period could restart the treatment with durvalumab upon evidence of progressive disease (PD) and for up to 12 months with the same treatment schedule.
Patients were followed up every 4 weeks during the treatment phase. Data on physical examination, performance status, adverse events (AEs), concomitant medications, complete blood cell count, and chemistry tests were collected at each study visit. Tumor assessments were carried out 28 days before study entry. After treatment initiation, complete tumor assessments were carried out on a calendar schedule every 8 weeks (approximately every two cycles). If disease progression did not occur at treatment termination, then tumor assessments were continued every 2 months until the evidence of disease progression or death. Progressing patients were followed up for survival only. A local radiologist at each study center assessed the response according to mRECIST criteria for MPM.12 To confirm the accuracy, a centralized review of computed tomography (CT) scans at predefined time points was retrospectively carried out by a blinded radiologist.
Since durvalumab leads to T-cell activation and proliferation, potential immune-related AEs (irAEs) were expected with its administration. Potential AEs of special interest (AESI) including colitis, pneumonitis, dermatitis, hepatitis, and endocrinopathies were collected. Participants were monitored for signs and symptoms of irAEs/AESI and interruption of durvalumab administration was predefined to allow management of all AEs. All AEs were recorded and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.03.
To explore the activity of durvalumab with respect to some biological features of MPM, PD-L1 immunohistochemistry (IHC) expression on archival tissue available was measured with the validated Ventana SP263 IHC assay optimized for use on the automated BenchMark UTRA platform (Ventana Medical Systems, Tucson, AZ). The PD-L1 IHC expression was tested on tumor and tumor-infiltrating lymphocyte. Greater than 100 tumor cells were required to determine PD-L1 status and it was considered positive with a cut-off of >1% of the sample analyzed.
Statistical methods
The primary endpoint was the PFS at 16 weeks (PFS16wks), defined as the proportion of patients who were alive and progression free at the second CT scan assessment, carried out 16 weeks after treatment start. Since clinical worsening could anticipate radiological findings, clinical progression, defined as a significant increase in pain, cough or dyspnea, or general deterioration of clinical conditions, was considered as an event for PFS16wks. PFS16wks was assessed in the per-protocol (PP) population that included all patients with no major violations of eligibility criteria who had received at least two treatment cycles. Patients who did not progress or die within 16 weeks from treatment start and without a disease evaluation at 16 weeks (±3 days) were considered as not assessable for the analysis unless the absence of PD was confirmed in the disease evaluations after the 16th week.
Secondary endpoints were objective response rate (ORR), PFS, OS, and safety profile. ORR was defined as the proportion of assessable patients achieving a CR or PR, based on mRECIST criteria for MPM. PFS was defined as the time from the date of treatment start to the date of disease progression or death from any cause, whichever occurred first. Patients who had not progressed or died while on study or lost to follow-up were censored at their last disease evaluation date. OS was defined as the time from the date of treatment start to the date of death from any cause. Patients not reported as having died at the end of the study were censored at the last date they were known to be alive. Secondary endpoints were analyzed including all patients enrolled with no major violations of eligibility criteria. The PD-L1 expression was considered for the subgroup analysis on ORR with an explorative purpose.
The study followed a Fleming single-stage design, according to A’Hern’s approach. Assuming a PFS16wks of 20% (p0) or less as of no therapeutic interest, and a PFS16wks of 40% (p1) required to consider the results as highly relevant, 47 assessable patients were needed to carry out the analysis of the primary endpoint according to a PP approach, with a type I error equal to 5% (one-sided) and a power equal to 90%. The minimum number of patients alive and progression free at 16 weeks to recommend durvalumab for further investigation was 15. Expecting 20% of patients not assessable for the primary endpoint, the planned sample size included 59 patients.
Statistical analyses
Continuous variables were expressed as medians with their interquartile range (IQR). PFS16wks rate was provided with its 95% confidence interval (CI). Univariable and multivariable Cox proportional hazards models were used to analyze the impact of the demographical and clinical characteristics of patients on survival outcomes. The results were provided as hazard ratios (HRs) and 95% CIs. Statistical models included as covariates age, sex, ECOG performance status, smoking history, histotype, and the previous chemotherapy treatment. Survival curves were estimated by the Kaplan–Meier (KM) method.
The safety profile was evaluated in the safety population that included all patients without major violations of the eligibility criteria who received at least one treatment dose. After the database lock, each AE was re-coded according to the MedDRA dictionary, version 23.1. For patients experiencing the same AE more than once, the most extreme grade experienced by each subject was reported.
All analyses were done with SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
From November 2018 to May 2019, 69 patients were enrolled in seven Italian centers.
The main demographics, tumor characteristics, and previous treatments are summarized in Table 1. The median age was 69.9 years (IQR 65.0-76.3 years), 25 (36.2%) patients were female, and 43 (62.3%) patients had an ECOG performance status equal to 0. Epithelioid and non-epithelioid MPM were diagnosed in 62 (89.9%) and 7 (10.1%) patients, respectively. Concerning the first-line treatment, 42 (60.9%) patients received carboplatin/pemetrexed and 27 (39.1%) cisplatin/pemetrexed.
Table 1.
Demographics and tumor characteristics—all enrolled patients
| Overall n = 69 | |
|---|---|
| Age (years)—median (Q1-Q3) | 69.9 (65.0-76.3) |
| Min-max | 44.5-82.8 |
| Female sex | 25 (36.2) |
| ECOG performance status | |
| 0 | 43 (62.3) |
| 1 | 26 (37.7) |
| Body mass index (kg/m2)—median (Q1-Q3) | 25.0 (22.4-27.4) |
| Min-max | 17.8-38.6 |
| Comorbidities | 58 (85.3) |
| Missing | 1 |
| Concomitant medications for comorbidities | 46 (90.2) |
| Missing | 7 |
| Concomitant medication for MPM | 28 (40.6) |
| ECG findings | |
| Clinically relevant findings | 3 (4.3) |
| No clinically relevant findings | 66 (95.7) |
| Asbestos exposure | 39 (57.4) |
| Missing | 1 |
| Smoking history | 31 (45.6) |
| Tumor sites at first diagnosis | |
| Pleura | 42 (62.7) |
| Pleura, lymph node | 13 (19.4) |
| Pleura, lung | 4 (6.0) |
| Pleura, lung, lymph node | 2 (3.0) |
| Lung | 1 (1.5) |
| Pleura, adrenal glands, other (mediastinum) | 1 (1.5) |
| Pleura, bone, lymph node | 1 (1.5) |
| Pleura, other (pleural effusion) | 1 (1.5) |
| Pleura, other (mediastinal) | 1 (1.5) |
| Pleura, other (peritoneal carcinomatosis) | 1 (1.5) |
| Missing | 2 |
| Histotype | |
| Epithelioid | 62 (89.9) |
| Biphasic | 4 (5.8) |
| Sarcomatoid | 3 (4.3) |
| Anatomical stage group | |
| I | 1 (2.2) |
| II | 4 (8.7) |
| III | 11 (23.9) |
| IV | 30 (65.2) |
| Missing | 23 |
| First-line chemotherapy type | |
| Carboplatin/pemetrexed | 42 (60.9) |
| Cisplatin/pemetrexed | 27 (39.1) |
| Number of cycles of first-line chemotherapy | |
| 2 | 2 (2.9) |
| 3 | 7 (10.1) |
| 4 | 17 (24.6) |
| 5 | 4 (5.8) |
| 6 | 39 (56.5) |
| Best tumor response of first-line chemotherapy | |
| SD | 36 (53.7) |
| PR | 18 (26.9) |
| PD | 13 (19.4) |
| Missing | 2 |
| Surgery carried out | |
| No | 44 (68.8) |
| Yes | 20 (31.3) |
| Missing | 5 |
| Surgery type | |
| Pleural decortication/pleurectomy (PD) | 10 (50.0) |
| Other (diagnostic video-assisted thoracoscopy) | 2 (10.0) |
| Other (VATS) | 2 (10.0) |
| Extrapleural pneumonectomy (EPP) | 1 (5.0) |
| Other (diagnostic video-assisted thoracoscopy) | 1 (5.0) |
| Other (videothoracoscopy) | 1 (5.0) |
| Other (diagnostic video-assisted thoracoscopy) | 1 (5.0) |
| Other (removal of tumor and ribs X-XI-XII) | 1 (5.0) |
| Other (thoracotomy) | 1 (5.0) |
| Talc pleurodesis | |
| No | 7 (38.9) |
| Yes | 11 (61.1) |
| Missing | 2 |
ECG, electrocardiogram; ECOG, Eastern Cooperative Oncology Group; MPM, malignant pleural mesothelioma; PD, progressive disease; PR, partial response; Q1-Q3, first and third quartile; SD, stable disease; VATS, video-assisted thoracoscopic surgery.
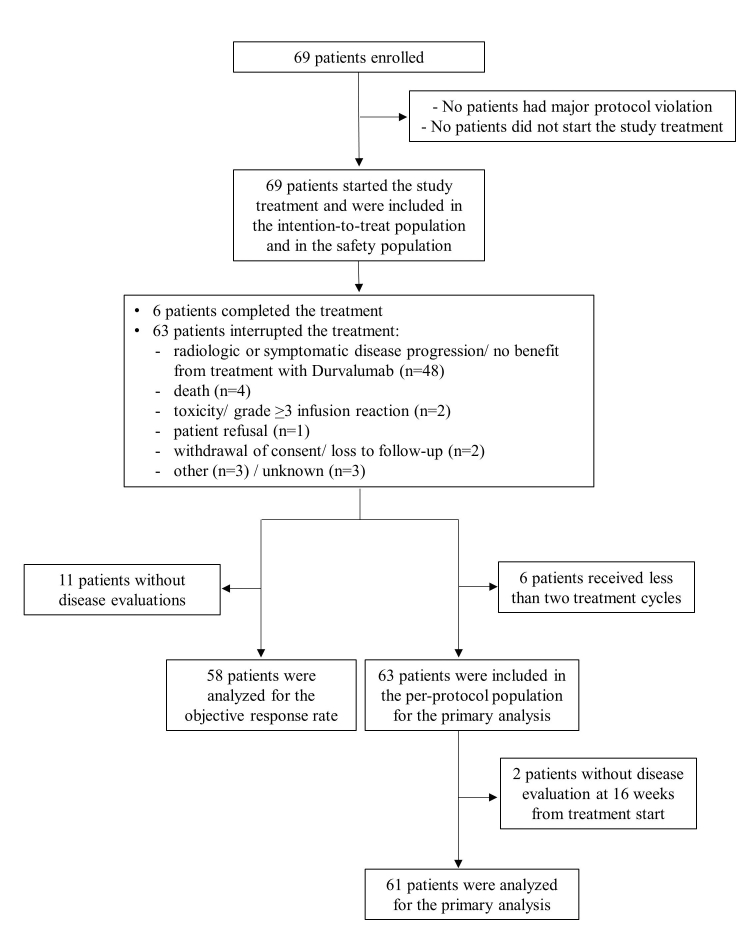
No major protocol violations were detected. All patients received at least one dose of study treatment and six (8.7%) completed the planned treatment, assuming durvalumab for 13 cycles for at least 11 months. The main reasons for treatment discontinuation were radiological or symptomatic disease progression (48 patients, 80.0%), followed by death (4 patients, 6.7%). The median number of cycles was 3 (IQR 2-6). Six patients interrupted the treatment after only one cycle and were excluded from the PP population. Of 63 patients, 2 did not carry out a radiological disease assessment at 16 weeks, therefore 61 patients were assessable for the analysis of the primary endpoint. The patients’ disposition is described in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100644.
The primary endpoint was not met. The proportion of patients alive and without progression at 16 weeks was 27.9% (17 patients, 90% CI 18.6% to 38.8%, 95% CI 17.1% to 40.8%). As per the planned study design and sample size, considering only the first 47 patients assessable for the primary analysis, the PFS16wks rate was 23.4% (11 patients, 90% CI 13.7% to 35.8%, 95% CI 12.3% to 38.0%). In both cases, the CI included the PFS16wks of 20% set as the null hypothesis to denote a treatment efficacy of no therapeutic interest.
Fifty-eight patients (84.1%) had at least one disease evaluation: 6 patients achieved a PR (ORR 10.3%, 95% CI 3.9% to 21.2%) and 27 (46.6%) had SD, thus achieving a disease control rate of 56.9%. The protocol provided for centralized review of CT images: concordance was observed in 53/71 assessments (74.6%); among discordant radiological findings, a worse evaluation was reported in 11 cases (61.1%) and a better evaluation in 7 (38.9%). Out of the six patients reaching a PR, three had a disease progression after 4.4, 7.6, and 12.6 months from the first PR observation; the remaining three patients were censored after 1 day, 1.8, and 6.4 months from the first PR observation.
PD-L1 expression was available in 48 patients. No differences were observed in terms of ORR between patients with different PD-L1 expressions (PD-L1 negative: ORR 3/27 patients, 11.1%; PD-L1 >1%: ORR 2/21 patients, 9.5%).
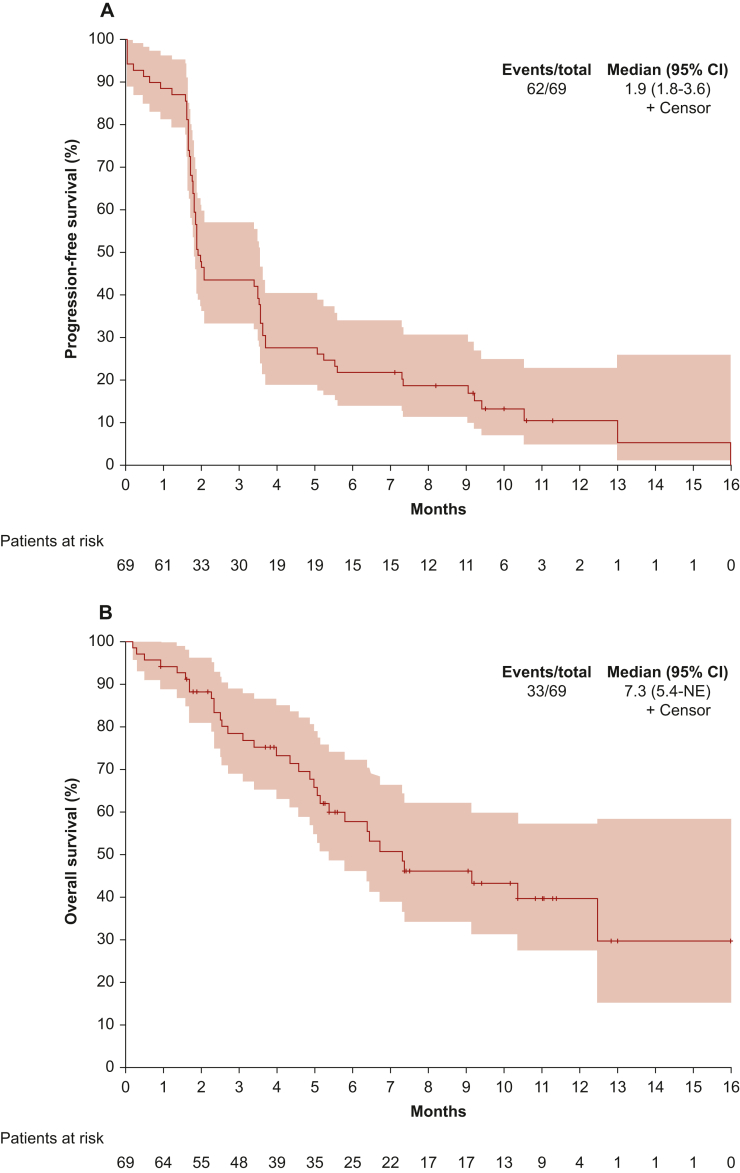
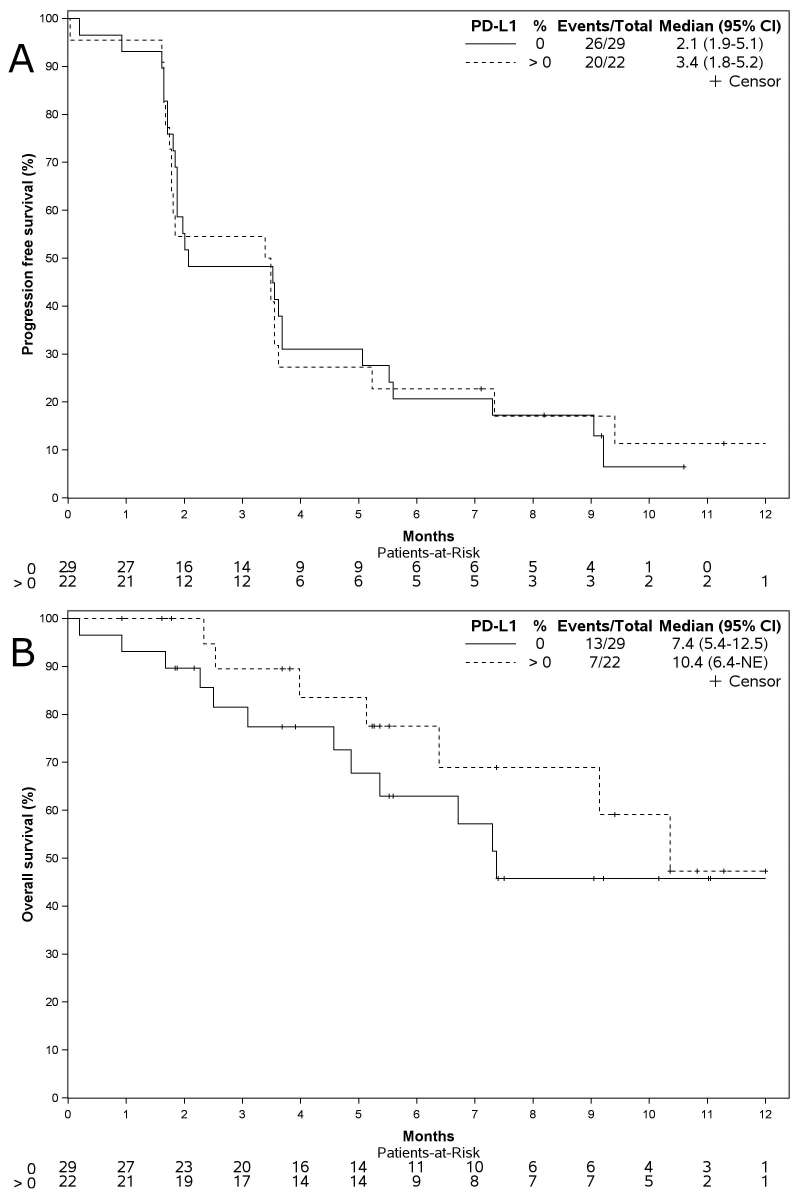
Overall, 60 patients (87.0%) progressed and 33 (47.8%) died during the study. Death was due to the disease progression for 30 (90.9%) patients. At a median follow-up of 9.2 months (IQR 5.2-11.1 months), the median PFS was 1.9 months (IQR 1.6-5.2 months; Figure 1A). The mOS was 7.3 months (first quartile 4.0 months, third quartile not reached; Figure 1B). The univariable Cox proportional hazards analyses on PFS and OS are summarized in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100644. At multivariable analysis, patients with a smoking habit showed a significantly longer PFS (HR 0.52, 95% CI 0.27-0.98, P = 0.044), whereas patients with a non-epithelioid MPM showed a worse PFS (HR 4.43, 95% CI 1.60-12.2, P = 0.004) and OS (HR 5.24, 95% CI 1.38-19.9, P = 0.015) compared to those with an epithelioid MPM (Table 2). The KM survival curves of PFS and OS according to PD-L1 expression are provided in Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100644.
Figure 1.
Survival curves. Progression-free survival (A) and overall survival (B). Kaplan–Meier curve—all enrolled patients. CI, confidence interval; NE, not estimable.
Table 2.
Impact of demographic and tumor characteristics on progression-free survival and overall survival
| Progression-free survival |
Overall survival |
|||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (1-year increase) | 1.01 (0.98-1.04) | 0.607 | 1.00 (0.96-1.05) | 0.887 |
| Female sex | 0.79 (0.42-1.49) | 0.464 | 0.97 (0.42-2.23) | 0.944 |
| ECOG performance status (1 versus 0) | 1.17 (0.57-2.38) | 0.673 | 1.95 (0.76-4.95) | 0.162 |
| Asbestos exposure | 1.42 (0.75-2.69) | 0.281 | 1.65 (0.74-3.68) | 0.223 |
| Smoking history | 0.50 (0.26-0.94) | 0.032 | 0.40 (0.16-0.99) | 0.047 |
| Histotype (non-epithelioid versus epithelioid) | 4.95 (1.73-14.2) | 0.003 | 5.74 (1.46-22.6) | 0.013 |
| First-line chemotherapy type (cisplatin/pemetrexed versus carboplatin/pemetrexed) | 1.09 (0.59-2.02) | 0.778 | 0.99 (0.41-2.37) | 0.980 |
| Best response to first-line chemotherapy (CR/PR versus SD/PD) | 0.83 (0.43-1.62) | 0.586 | 0.63 (0.22-1.80) | 0.387 |
Multivariable Cox proportional hazards models—all enrolled patients.
CI, confidence interval; CR, complete response; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; PD, progressive disease; PR, partial response; SD, stable disease.
Safety
At least one AE of any grade occurred in 51 (73.9%) patients and 11 (15.9%) patients had at least one grade ≥3 AE. Details on the AEs are provided in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100644.
Table 3 summarizes the frequency of adverse drug reactions (ADRs), assessed as treatment-related by the investigators. Adverse reactions occurred in 27 (39.1%) patients; six events were graded 3 or higher (nausea, muscular weakness, sudden death, amylase increased, lipase increased, infusion-related reaction). irAEs occurred in 15 (21.7%) patients, and only 1 (infusion-related reaction) had grade 3. Four ADRs led to permanent discontinuation of durvalumab in three patients (amylase and lipase increased, infusion-related reaction, hypothyroidism).
Table 3.
Adverse reactions
| n = 69 | G0 n (%) | G1 n (%) | G2 n (%) | G3 n (%) | G4 n (%) | G5 n (%) | G3 + G4 + G5 n (%) |
|---|---|---|---|---|---|---|---|
| Overall | 42 (60.9) | 14 (20.3) | 9 (13.0) | 2 (2.9) | 1 (1.4) | 1 (1.4) | 4 (5.8) |
| Cardiac disorders | 68 (98.6) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Atrial fibrillation | 68 (98.6) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Endocrine disorders | 67 (97.1) | 0 (0.0) | 2 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hyperthyroidism | 68 (98.6) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypothyroidism | 68 (98.6) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal disorders | 61 (88.4) | 5 (7.2) | 2 (2.9) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (1.4) |
| Ischemic colitis | 68 (98.6) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 67 (97.1) | 2 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Flatulence | 68 (98.6) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nausea | 65 (94.2) | 2 (2.9) | 1 (1.4) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (1.4) |
| Vomiting | 68 (98.6) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| General disorders and administration site conditions | 59 (85.5) | 7 (10.1) | 1 (1.4) | 1 (1.4) | 0 (0.0) | 1 (1.4) | 2 (2.9) |
| Sudden death | 68 (98.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 1 (1.4) |
| Fatigue | 64 (92.8) | 4 (5.8) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Infusion-related reaction | 68 (98.6) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (1.4) |
| General disorder or administration site condition NOS | 68 (98.6) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pyrexia | 66 (95.7) | 3 (4.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Infections and infestations | 67 (97.1) | 0 (0.0) | 2 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mucosal infection | 68 (98.6) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rash pustular | 68 (98.6) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Investigations | 66 (95.7) | 1 (1.4) | 1 (1.4) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 1 (1.4) |
| Alanine aminotransferase increased | 67 (97.1) | 1 (1.4) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Aspartate aminotransferase increased | 68 (98.6) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| γ-Glutamyltransferase increased | 68 (98.6) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lipase increased | 68 (98.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 1 (1.4) |
| Serum amylase increased | 68 (98.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 1 (1.4) |
| Metabolism and nutrition disorders | 65 (94.2) | 3 (4.3) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Decreased appetite | 66 (95.7) | 3 (4.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diabetes mellitus | 68 (98.6) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Musculoskeletal and connective tissue disorders | 66 (95.7) | 1 (1.4) | 1 (1.4) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (1.4) |
| Muscular weakness | 66 (95.7) | 1 (1.4) | 1 (1.4) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (1.4) |
| Respiratory, thoracic, and mediastinal disorders | 67 (97.1) | 1 (1.4) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dyspnea | 68 (98.6) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pneumonitis | 68 (98.6) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Skin and subcutaneous tissue disorders | 63 (91.3) | 5 (7.2) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dermatitis acneiform | 68 (98.6) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dry skin | 67 (97.1) | 2 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pruritus | 66 (95.7) | 3 (4.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Skin disorder NOS | 67 (97.1) | 2 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Maximum grade occurred—safety analysis set.
G, grade; n, number of patients; NOS, not otherwise specified.
Thirty-one serious adverse events (SAEs) occurred in 19 (27.5%) patients; 6 (19.4%) were judged as possibly treatment-related (ischemic colitis, sudden death, amylase increased, lipase increased, diabetes mellitus, infusion-related reaction). Six SAEs (29.0%) had a fatal outcome and one of these (sudden death) was assessed as possibly related to both the investigational drug and disease under study. Since an autopsy was not carried out, it was not possible to establish the cause of the sudden death and investigator assessed this event as possibly related to the investigational drug, conservatively. The remaining five SAEs were related to the disease under study and the causes of death were intestinal obstruction, acute cardiac event, acute respiratory distress syndrome with respiratory acidosis and atrial fibrillation, pericardial effusion, and cachexia with dyspnea.
Discussion
To our knowledge, this is the first phase II study to evaluate the efficacy and safety of durvalumab as a single agent in pretreated MPM patients. The primary endpoint—the proportion of patients without progression and alive at 16 weeks—was not met and mPFS and mOS were 1.9 and 7.3 months, respectively, after a median follow-up of 9.2 months. No new safety signal emerged with the regimen: treatment-related AEs and their immune relationship were easily manageable, with a few events leading to permanent treatment discontinuation.
Although the study failed to reach the primary endpoint, the magnitude of clinical benefit achieved with durvalumab is in line with other ICIs in a similar setting.
ICIs in a pretreated MPM population demonstrated a mixed result and a wide difference in efficacy, probably due to the heterogeneity of clinical trials in terms of inclusion criteria, study design, and population sample size. As summarized in Table 4, four ICIs have been tested as single agent against relapsed MPM. Nivolumab confirmed its efficacy in a phase III trial and had been approved as the standard of care in pretreated MPM9; other ICIs did not achieve favorable results in terms of OS and PFS.5,8,15, 16, 17 The DIADEM study is unique in that it evaluated durvalumab as a single agent in pretreated MPM, and showed a clinical activity comparable to that of other ICIs in this setting. To further improve the efficacy of immunotherapy, combinations of anti-PD-1 and anti-CTLA-4 were tested and showed an increased disease control compared to the single agents, in pretreated patients, disease control with respect to single agent even if the only phase II trial published to date was not comparative in its nature.18 These results were the background for exploring the frontline combination in the phase III CheckMate 743 study.11
Table 4.
Clinical trials investigating immune checkpoint inhibitors in malignant pleural mesothelioma pretreated population
| Study | Phase | Agent(s) | n | ORR (%) | mPFS (months) | mOS (months) |
|---|---|---|---|---|---|---|
| KN028 | Ib | Pembrolizumab | 25 (PD-L1+) | 20 | 5.4 | 18 |
| JAVELIN | Ib | Avelumab | 53 | 9 | 4.1 | 10.7 |
| Treme (Italy) | II | Tremelimumab | 29 | 14 | 6.2 | 11.3 |
| MERIT | II | Nivolumab | 34 | 29 | 6.1 | 17.3 |
| Pembrolizumab (Chicago) | II | Pembrolizumab | 64 | 22 | 4.1 | 11.5 |
| NIBIT-Meso1 | II | Tremelimumab/durvalumab | 40 (30% 1L) | 28 | 5.7 | 16.6 |
| INITIATE | II | Nivolumab/ipilimumab | 34 | 29 | NR (>6.2) | NR (>12.7) |
| MAPS2 | II | Nivolumab versus nivolumab/ipilimumab | 63 and 62 | 19 and 28 | 4 and 5.6 | 11.9 and 15.9 |
| DETERMINE | IIb | Tremelimumab versus placebo | 571 | 4.5 | 2.8 | 7.7 |
| PROMISE-Meso | III | Pembrolizumab versus chemotherapy | 144 | 22 | 2.5 | 10.7 |
| CONFIRM | III | Nivolumab versus placebo | 332 | 10.4 | 3 | 9.2 |
| DIADEM | II | Durvalumab | 69 | 10.3 | 2 | 7.3 |
1L, first line; mOS, median overall survival; mPFS, median progression-free survival; n, number of patients enrolled; NR, not reached; ORR, objective response rate.
Also durvalumab was tested in combination with other agents: in the phase II NIBIT-MESO-1 study, durvalumab plus tremelimumab achieved an immune-related objective response in 28% of patients; as in this trial only 30% of patients were treatment-naive while the majority received a maximum of one line of platinum-based therapy, it is difficult to perceive the real benefit of the combination in the naive population.12
Another strategy that appears more promising is the combination of chemotherapy and immunotherapy in the first-line setting, as studied in the phase II, single-arm DREAM trial. In this case, durvalumab was combined with cisplatin and pemetrexed and showed a PFS at 6 months of 57% and an ORR of 48%.13
Combining durvalumab and chemotherapy doublet confirmed further its efficacy as reported in the phase II, single-arm PrE0505 trial, where mOS of the combination reached in a naive population was 20.4 months suggesting a possible additive synergy with respect to chemotherapy treatment alone demonstrated in historical controls.19
Despite the promising results of immunotherapy in MPM, the identification of patients who could have the maximal clinical benefit from these therapies is still challenging for several reasons.
Almost half of the patients with MPM treated with ICIs showed a progression at the first radiological evaluation, in a pattern likely similar to the hyperprogression observed in non-small-cell lung cancer.20,21 Similarly, in our study, we observed a rate of PD of 42% with the higher probability happening in the first 2 months of treatment. Although in MPM it has not been ascertained that this phenomenon is a clear hyperprogression, caution should be exercised in the clinical selection of candidates to immunotherapy.
Furthermore, predictive markers of response that can maximize the effect of the therapy are lacking. At present, there are no tissue/serological markers that can be used in clinical practice and the results regarding the tissue expression of PD-L1 rather than the sarcomatoid/biphasic histological subtype appear discordant depending on the line of therapy and the combination of ICI studied.22
In a multivariate analysis, we tried to describe in which subgroup of patients durvalumab could gain the best clinical benefit. Histology is a recognized prognostic factor; therefore, we firstly stratified the cohort according to epithelioid or non-epithelioid subtype. In our study, the epithelioid subtype had an impact in PFS and OS compared with the non-epithelioid subtype but, as in other clinical trials, the sarcomatoid/biphasic MPMs were under-represented (10%). Similarly, in the CONFIRM study, the mOS was 10.2 months with nivolumab versus 6.9 months with placebo (HR 0.69, P = 0.009), and the 12-month OS was 40% and 26.7%, respectively, in patients with the epithelioid subtype; in both cases, these results were better than those achieved in patients with the non-epithelioid subtype (OS 5.9 and 6.7 months, and 12-month OS 34.6% and 30.8%, respectively; HR 0.79, P = 0.572).9 The lack of benefit in the non-epithelioid subtype might be due to the small number of patients in this group. However, data emerging from the CheckMate743 study indicated that OS was improved with nivolumab plus ipilimumab versus chemotherapy regardless of histology, with evidence of higher treatment effect in patients with non-epithelioid histology [HR 0.48 (95% CI 0.34-0.69)] than in those with the epithelioid subtype [0.85 (0.69-1.04)]. mOS with nivolumab plus ipilimumab was similar between non-epithelioid and epithelioid subtypes [18.1 months (95% CI 12.2-22.8 months) versus 18.2 months (16.9-21.9 months)], as were 3-year OS rates (22% versus 24%).11,23
Available data so far suggest a possible role of ICIs in the first-line setting of sarcomatoid/biphasic histotype, while the role of ICI in combination with or after first-line chemotherapy may deserve a role in the more heterogenous epithelioid subtype.
Sparse data, the limited sample size of many clinical trials, and population heterogeneity led to a non-univocal conclusion of ICI activity and efficacy in chemotherapy-refractory population. Two recent meta-analyses indicate a possible opportunity for this ‘orphan drug’ population even if ICI predictive factors may be claimed to increase the risk–benefit ratio of this therapeutic solution.24,25
Further studies are warranted to better elucidate the impact of the combination of ICI + chemotherapy regarding the different histotype sensitivity and the outcomes of MPM treatment and data from IND227 NCT02784171 and DREAM3R NCT04334759 studies would probably contribute to addressing this point.
In order to study some putative predictive factors, as an explorative analysis, we evaluated a possible role of PD-L1 expression. We did not observe any evidence of a different activity of durvalumab in respect of positive or negative PD-L1 expression. This observation is consistent with data obtained from other studies evaluating the efficacy of durvalumab in MPM. In the DREAM study, there was no apparent association between tumor expression of PD-L1 and PFS, with an mPFS of 6.3 months (95% CI 5.3-10.4 months) for patients with PD-L1-negative tumors, and 6.6 months (5.5-9.0 months) for patients with PD-L1-positive tumors.13 In the NIBIT-MESO-1 study, no significant association was observed between baseline PD-L1 expression, measured as a continuous variable, and the endpoints of immune-related objective response (P = 0.92), immune-related disease control (P = 0.55), immune-related PFS (P = 0.75), and 1-year OS (P = 0.54).12 In other studies, however, some difference in the response to immunotherapy was highlighted. In the Javelin trial, ORR was 19% (3 of 16) in PD-L1-positive and 7% (2 of 27) in PD-L1-negative tumors, considering a ≥5% PD-L1 cut-off.16 In the CheckMate 743 study, OS benefit by tumor PD-L1 expression level for nivolumab plus ipilimumab versus chemotherapy was greater in patients with tumor expression of PD-L1 of ≥1% [HR 0.69 (95% CI 0.55-0.87)] than in patients with expression of <1% [0.94 (0.62-1.40)].11 The role of PD-L1 expression as a predictive biomarker in MPM remains an important issue to be resolved, as extensively reviewed.26
Lastly, the smoking habit seems to have a favorable impact on durvalumab treatment in terms of PFS; however, the relatively small number of patients, the lack of an impact on OS and the lack of information on asbestos exposure, tumor mutational burden, and tumoral microenvironment27 relegate this to possible speculation.
This trial has several limitations: firstly, lack of control arm reducing robustness of the final results on the experimental drug; secondly, the central review of CT scan unveiled a moderate discrepancy between the centralized and the site evaluations. Since the evaluation of the primary endpoint was done on response evaluated by investigators, this discrepancy could cause a limitation in the validity of the study results. Moreover, independent evaluation was available for a subgroup of patients.
A further limitation was the choice of the primary endpoint justified at the time of the study design and validated as a surrogate for OS in clinical trials using chemotherapeutic agents: the validity of the PFS rate as an endpoint especially in a single-arm trial is strictly connected to the reproducibility of the CT evaluations and the variability in the timing of the disease evaluations that can affect the accuracy on the treatment effect evaluation.
Conclusions
The present study did not reach the primary endpoint and the monotherapy with durvalumab failed to demonstrate a promising activity in MPM patients, pretreated with platinum/derivatives–pemetrexed. The treatment was confirmed as safe.
Considering the current therapeutic paradigm, it is possible to conclude that in the future more physicians could choose the combination of ICIs in first-line treatment, in particular the single-agent anti-PD-1 for the non-epithelioid histology, although there is a room for single agent in the second line of a sequential strategy in patients with epithelioid MPM, despite the lack of data from randomized trials about therapeutic sequential strategy which can support this approach.
Despite the emerging data from the present study, which leaves an open debate about the role of single-agent immunotherapy in pretreated patients, durvalumab may deserve a role in association with chemotherapy in naive mesothelioma patients.
Acknowledgements
The authors thank Elisa Sala, PhD, medical writer, for her helpful contribution in drafting the manuscript, and Davide Poli and Walter Torri for their contribution in conducting the study.
Funding
The work was supported by AstraZeneca (no grant number).
Disclosure
PAZ declares payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events and for the participation on a Data Safety Monitoring Board or Advisory Board from Merck Sharp and Dohme, Astellas, Janssen, Sanofi, Ipsen, Pfizer, Novartis, Bristol Meyer Squibb, Amgen, AstraZeneca, Roche, and Bayer; FG declares payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Astra Zeneca and Eli Lilly; ER, FG, IDS, and LC declare that funding was given to Istituto di Ricerche Farmacologiche Mario Negri IRCCS to partially support the study; DLC declares payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events and for the participation on a Data Safety Monitoring Board or Advisory Board from BMS, MSD, Astra Zeneca, Boehringer Ingelheim, Lilly, Amgen, Roche, and Novartis. All other authors have declared no conflicts of interest.
Contributor Information
D.L. Cortinovis, Email: d.cortinovis@asst-monza.it.
DIADEM groupD:
D. Cortinovis, S. Canova, F. Colonese, M.I. Abbate, L. Sala, E. Sala, M. Perez Gila, F. Bono, F. Pagni, G.L. Ceresoli, A. D’Aveni, M. Bonomi, F. Grosso, A. De Angelis, F. Ugo, M. Belletti, P.A. Zucali, M. Perrino, F. De Vincenzo, A. Santoro, F. Gelsomino, A. Ardizzoni, G. Pasello, S. Frega, M. Mencoboni, L. Carlucci, I. De Simone, M. D’Incalci, F. Galli, D. Poli, E. Rulli, and V. Torri
Appendix 1
The DIADEM Group
List of participating institutions and co-authors
ASST Ospedale San Gerardo, Monza
D. Cortinovis, S. Canova, F. Colonese, M. I. Abbate, L. Sala, E. Sala, M. Perez Gila, F. Bono, P. Bidoli, F. Pagni
Cliniche Humanitas Gavazzeni, Bergamo
G. L. Ceresoli, A. D’Aveni, M. Bonomi
Azienda Ospedaliera SS Antonio e Biagio e Cesare Arrigo, Dipartimento Attività Integrate Ricerca e Innovazione (DAIRI),
F. Grosso, A. De Angelis, F. Ugo, M. Belletti
Istituto Clinico Humanitas—IRCCS, Milano
P. A. Zucali, M. Perrino, F. De Vincenzo, A. Santoro
Sant’Orsola-Malpighi Hospital, Bologna
F. Gelsomino, A. Ardizzoni
Istituto Oncologico Veneto IRCCS, Padova
G. Pasello, S. Frega
ASL 3 Genovese-Ospedale Villa Scassi, Genova, Italy
M. Mencoboni
Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Milano
L. Carlucci, I. De Simone, M. D’Incalci, F. Galli, D. Poli, E. Rulli, V. Torri
Supplementary data
Supplementary Fig S1.
Study flowchart – All enrolled patients.
Supplementary Fig S2.
Progression free survival (A) and overall survival (B) according to the PD-L1 expression. Kaplan-Meier curve – All enrolled patients.
References
- 1.Nowak A.K., Jackson A., Sidhu C. Management of advanced pleural mesothelioma-at the crossroads. JCO Oncol Pract. 2022;18(2):116–124. doi: 10.1200/OP.21.00426. [DOI] [PubMed] [Google Scholar]
- 2.Vogelzang N.J., Rusthoven J.J., Symanowski J., et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 3.Manegold C., Symanowski J., Gatzemeier U., et al. Second-line (post-study) chemotherapy received by patients treated in the phase III trial of pemetrexed plus cisplatin versus cisplatin alone in malignant pleural mesothelioma. Ann Oncol. 2005;16(6):923–927. doi: 10.1093/annonc/mdi187. [DOI] [PubMed] [Google Scholar]
- 4.Zalcman G., Mazieres J., Margery J., et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387(10026):1405–1414. doi: 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]
- 5.Alley E.W., Lopez J., Santoro A., et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017;18(5):623–630. doi: 10.1016/S1470-2045(17)30169-9. [DOI] [PubMed] [Google Scholar]
- 6.Okada M., Kijima T., Aoe K., et al. Clinical efficacy and safety of nivolumab: results of a multicenter, open-label, single-arm, Japanese phase II study in malignant pleural mesothelioma (MERIT) Clin Cancer Res. 2019;25(18):5485–5492. doi: 10.1158/1078-0432.CCR-19-0103. [DOI] [PubMed] [Google Scholar]
- 7.Quispel-Janssen J., van der Noort V., de Vries J.F., et al. Programmed death 1 blockade with nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncol. 2018;13(10):1569–1576. doi: 10.1016/j.jtho.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 8.Popat S., Curioni-Fontecedro A., Dafni U., et al. A multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: the European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Ann Oncol. 2020;31(12):1734–1745. doi: 10.1016/j.annonc.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Fennell D.A., Ewings S., Ottensmeier C., et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2021;22(11):1530–1540. doi: 10.1016/S1470-2045(21)00471-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui W., Popat S. Immune checkpoint inhibition for unresectable malignant pleural mesothelioma. Drugs. 2021;81(9):971–984. doi: 10.1007/s40265-021-01506-0. [DOI] [PubMed] [Google Scholar]
- 11.Baas P., Scherpereel A., Nowak A.K., et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397(10272):375–386. doi: 10.1016/S0140-6736(20)32714-8. [DOI] [PubMed] [Google Scholar]
- 12.Calabro L., Morra A., Giannarelli D., et al. Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1): an open-label, non-randomised, phase 2 study. Lancet Respir Med. 2018;6(6):451–460. doi: 10.1016/S2213-2600(18)30151-6. [DOI] [PubMed] [Google Scholar]
- 13.Nowak A.K., Lesterhuis W.J., Kok P.S., et al. Durvalumab with first-line chemotherapy in previously untreated malignant pleural mesothelioma (DREAM): a multicentre, single-arm, phase 2 trial with a safety run-in. Lancet Oncol. 2020;21(9):1213–1223. doi: 10.1016/S1470-2045(20)30462-9. [DOI] [PubMed] [Google Scholar]
- 14.Byrne M.J., Nowak A.K. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15(2):257–260. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 15.Desai A., Karrison T., Rose B., et al. Canada; Toronto: 2018. Phase II trial of pembrolizumab ( NCT02399371) in previously treated malignant mesothelioma: final analysis. Paper presented at the 19th IASLC World Conference on Lung Cancer Toronto. September 23-26. [Google Scholar]
- 16.Hassan R., Thomas A., Nemunaitis J.J., et al. Efficacy and safety of avelumab treatment in patients with advanced unresectable mesothelioma: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5(3):351–357. doi: 10.1001/jamaoncol.2018.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maio M., Scherpereel A., Calabro L., et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017;18(9):1261–1273. doi: 10.1016/S1470-2045(17)30446-1. [DOI] [PubMed] [Google Scholar]
- 18.Scherpereel A., Mazieres J., Greillier L., et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20(2):239–253. doi: 10.1016/S1470-2045(18)30765-4. [DOI] [PubMed] [Google Scholar]
- 19.Forde P.M., Anagnostou V., Sun Z., et al. Durvalumab with platinum-pemetrexed for unresectable pleural mesothelioma: survival, genomic and immunologic analyses from the phase 2 PrE0505 trial. Nat Med. 2021;27(11):1910–1920. doi: 10.1038/s41591-021-01541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrara R., Mezquita L., Texier M., et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543–1552. doi: 10.1001/jamaoncol.2018.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Champiat S., Dercle L., Ammari S., et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–1928. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 22.Ceresoli G.L., Pasello G. Immune checkpoint inhibitors in mesothelioma: a turning point. Lancet. 2021;397(10272):348–349. doi: 10.1016/S0140-6736(21)00147-1. [DOI] [PubMed] [Google Scholar]
- 23.Peters S., Scherpereel A., Cornelissen R., et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol. 2021;32:S1283–S1346. doi: 10.1016/j.annonc.2022.01.074. [DOI] [PubMed] [Google Scholar]
- 24.Banna G.L., Signori A., Curioni-Fontecedro A., et al. Systemic therapy for pre-treated malignant mesothelioma: a systematic review, meta-analysis and network meta-analysis of randomised controlled trials. Eur J Cancer. 2022;166:287–299. doi: 10.1016/j.ejca.2022.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Tagliamento M., Bironzo P., Curcio H., et al. A systematic review and meta-analysis of trials assessing PD-1/PD-L1 immune checkpoint inhibitors activity in pre-treated advanced stage malignant mesothelioma. Crit Rev Oncol Hematol. 2022;172 doi: 10.1016/j.critrevonc.2022.103639. [DOI] [PubMed] [Google Scholar]
- 26.Mansfield A.S., Brown R.J., Sammon C., et al. The predictive and prognostic nature of programmed death-ligand 1 in malignant pleural mesothelioma: a systematic literature review. JTO Clin Res Rep. 2022;3(5) doi: 10.1016/j.jtocrr.2022.100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harber J., Kamata T., Pritchard C., Fennell D. Matter of TIME: the tumor-immune microenvironment of mesothelioma and implications for checkpoint blockade efficacy. J Immunother Cancer. 2021;9(9) doi: 10.1136/jitc-2021-003032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.