Abstract
Background
Trifluridine/tipiracil (FTD/TPI) showed clinical benefit, including improved survival and manageable safety in previously treated patients with metastatic colorectal (mCRC) or gastric/gastroesophageal junction (mGC/GEJC) cancer in the phase III RECOURSE and TAGS trials, respectively. A pooled analysis was conducted to further characterize FTD/TPI safety, including management of haematologic toxicities and use in patients with renal or hepatic impairment.
Patients and methods
Adults with ≥2 prior regimens for advanced mGC/GEJC or mCRC were randomized (2 : 1) to FTD/TPI [35 mg/m2 twice daily days 1-5 and 8-12 (28-day cycle); same dosage in both trials] or placebo plus best supportive care. Adverse events (AEs) were summarized in the safety population (patients who received ≥1 dose) and analysed by renal/hepatic function.
Results
TAGS and RECOURSE included 335 and 533 FTD/TPI-treated and 168 and 265 placebo-treated patients, respectively. Overall safety of FTD/TPI was similar in TAGS and RECOURSE. Haematologic (neutropenia, anaemia) and gastrointestinal (nausea, diarrhoea) AEs were most commonly observed. Laboratory-assessed grade 3-4 neutropenia occurred in 37% (TAGS)/38% (RECOURSE) of FTD/TPI-treated patients (median onset: 29 days/55 days), and 96% (TAGS)/97% (RECOURSE) of cases resolved regardless of renal/hepatic function. Supportive medications for neutropenia were received by 17% (TAGS) and 9% (RECOURSE); febrile neutropenia was reported in 2% and 4%, respectively. Overall grade ≥3 AEs were more frequent in patients with moderate renal impairment [81% (TAGS); 85% (RECOURSE)] versus normal renal function (74%; 67%); anaemia and neutropenia were more common in patients with renal impairment. FTD/TPI safety (including haematologic AEs) was consistent across patients with normal and mildly impaired hepatic function.
Conclusions
These results support FTD/TPI as a well-tolerated treatment in patients with mGC/GEJC or mCRC, with a consistent safety profile. Safety was largely similar in patients with normal or mildly impaired renal/hepatic function; however, patients with renal impairment should be monitored for haematologic toxicities.
Key words: trifluridine/tipiracil, safety, metastatic gastric cancer, metastatic colorectal cancer, neutropenia, renal impairment
Highlights
-
•
This pooled safety analysis shows a consistent safety profile of FTD/TPI in patients with mCRC and mGC/GEJC.
-
•
Haematologic and gastrointestinal adverse events (AEs) were the most common types of AEs with FTD/TPI treatment.
-
•
FTD/TPI was well tolerated; AEs were managed well with dosing modifications and supportive medications.
-
•
The FTD/TPI safety profile was similar in patients with normal or mildly impaired renal or hepatic function.
-
•
Patients with renal impairment should be carefully monitored for haematologic toxicities.
Introduction
Trifluridine/tipiracil (FTD/TPI; TAS-102) is an oral cytotoxic chemotherapy consisting of trifluridine (trifluorothymidine), a thymidine analogue, and tipiracil, a thymidine phosphorylase inhibitor that prevents metabolic degradation of trifluridine.1,2 Preclinical evidence suggested that this antimetabolite is non-cross-resistant with 5-fluorouracil, leading to testing of FTD/TPI in patients with extensive prior fluoropyrimidine therapy.1,3 FTD/TPI was approved for the treatment of patients with previously treated metastatic colorectal cancer (mCRC) in 2015 and of those with previously treated metastatic gastric or gastroesophageal junction cancer (mGC/GEJC) in 2019 based on the survival benefit observed in two global phase III randomized trials, TAGS and RECOURSE.4, 5, 6 In both studies, FTD/TPI was associated with a manageable safety profile. The most common adverse events (AEs) were haematologic, such as neutropenia and anaemia, and gastrointestinal, such as nausea and decreased appetite.5,6
Using data from the large population of patients across the phase III TAGS and RECOURSE trials, we aimed to further characterize the safety of FTD/TPI in patients with previously treated mGC/GEJC or mCRC. Our analysis builds on previous findings by evaluating the incidence and management of AEs, including the use of concomitant medications for neutropenia. Additionally, we assessed AE toxicity and management in patients with renal or hepatic impairment in both studies, given that these comorbidities are common in patients undergoing treatment with chemotherapy and can impact the pharmacokinetics and toxicity profiles of anticancer agents.7, 8, 9, 10, 11
Methods
Study design and patients
Study designs of the TAGS and RECOURSE trials have been described previously.5,6 Briefly, eligible patients had mGC/GEJC (TAGS) or mCRC (RECOURSE), were aged ≥18 years with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, and had disease progression after two or more prior regimens for advanced disease. Prior regimens in TAGS (mGC/GEJC) included a fluoropyrimidine, a platinum agent, and a taxane or irinotecan, or both, as well as anti-human epidermal growth factor receptor 2 (HER2) therapy (for HER2-positive tumours). Prior regimens in RECOURSE (mCRC) included a fluoropyrimidine, oxaliplatin, irinotecan, and bevacizumab, as well as cetuximab or panitumumab in patients with wild-type KRAS tumours.
Patients were randomized (2 : 1) to receive FTD/TPI (35 mg/m2 twice daily on days 1-5 and 8-12 of a 28-day cycle) plus best supportive care (BSC) or placebo plus BSC until disease progression, intolerability, or patient withdrawal. Criteria for dose delays and modifications have been reported previously.5,6 For neutropenia, in general, doses were delayed in patients with an absolute neutrophil count (ANC) <500/mm3 until counts returned to at least 1500/mm3. Dose reductions were made for grade 4 neutropenia that required a delay of >1 week, in which case the dose was reduced by 5 mg/m2 to a minimum allowed dose of 20 mg/m2.
Safety assessments
AEs were recorded from the start of treatment until 30 days after the last dose and were classified and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 4.03. All patients who received at least one dose of study drug (safety population) were included in the pooled safety analysis. Data were summarized separately for the TAGS and RECOURSE trials due to difference in the study populations, particularly with respect to the tumour types and treatment history. Safety data were summarized using descriptive statistics.
All haematology measurements were done within 24 h before the start of study treatment from cycle 2 onwards and within 7 days before day 1 of cycle 1 or day 15 of cycle 1. For selected haematological AEs, incidence was investigated by combining related preferred terms. The combined term for neutropenia comprised the preferred terms of neutropenia and decreased neutrophil count. Combined terms for anaemia (consisting of anaemia and decreased haemoglobin concentration), leukopenia (leukopenia and decreased leucocyte count), and thrombocytopenia (thrombocytopenia and decreased platelet count) were also investigated. All other AEs were reported as preferred terms. Neutropenia that was evaluated based on laboratory assessment (ANC) rather than AE reporting during the study period is referred to as chemotherapy-induced anaemia or CIN in this manuscript; CIN grading was classified according to NCI-CTCAE v4.03.
In a post hoc analysis to investigate safety by renal or hepatic function, patients were classified into subgroups based on laboratory measurements at baseline. Renal function subgroups were defined as follows: normal renal function [creatinine clearance (CrCl) ≥90 ml/min], mild renal impairment (CrCl 60-89 ml/min), and moderate renal impairment (CrCl 30-59 ml/min). Patients were classified into two subgroups based on hepatic function: normal hepatic function [total bilirubin and AST ≤ upper limit of normal (ULN)] and mild impairment (total bilirubin between ULN and 1.5× ULN or AST > ULN). As they typically did not meet inclusion criteria, very few patients with severe renal impairment (CrCl <30 ml/min), moderate hepatic impairment (total bilirubin between 1.5× and 3× ULN and any AST), or severe hepatic impairment (total bilirubin >3× ULN and any AST) were included in the study, and, therefore, these subgroups were not part of the renal and hepatic function analyses.
As renal and hepatic function subgroups were defined post hoc, these subanalyses were not powered for statistical significance and no formal statistical comparisons were made between the renal and hepatic function subgroups.
Results
Patients
The safety population in the TAGS trial included 335 patients who received FTD/TPI and 168 who received placebo.6 In the RECOURSE trial, the safety population included 533 and 265 patients who received FTD/TPI and placebo, respectively.5 In each trial, patient demographics and baseline characteristics were balanced between the FTD/TPI and placebo treatment groups and reflected characteristics of the disease populations (Table 1). In the pooled patient population across both trials, 66% of patients were male, 62% were White, and 44% were aged ≥65 years; 75% of all patients had received ≥3 prior systemic regimens.
Table 1.
Baseline characteristics of the trial safety populations
| TAGS |
RECOURSE |
|||
|---|---|---|---|---|
| FTD/TPI (n = 335) | Placebo (n = 168) | FTD/TPI (n = 533) | Placebo (n = 265) | |
| Age, years | ||||
| Median (range) | 64 (24–89) | 62 (32‒82) | 63 (27‒82) | 63 (27‒82) |
| Age category, n (%) | ||||
| <65 years | 182 (54) | 96 (57) | 299 (56) | 147 (55) |
| 65 to <75 years | 103 (31) | 55 (33) | 198 (37) | 94 (35) |
| ≥75 years | 50 (15) | 17 (10) | 36 (7) | 24 (9) |
| Sex, n (%) | ||||
| Male | 250 (75) | 116 (69) | 326 (61) | 164 (62) |
| Ethnicity, n (%) | ||||
| White | 242 (72) | 112 (67) | 305 (57) | 154 (58) |
| Asian | 51 (15) | 29 (17) | 184 (35) | 94 (35) |
| Othera | 4 (1) | 4 (2) | 4 (1) | 5 (2) |
| Missing | 38 (11) | 23 (14) | 40 (8) | 12 (5) |
| Geographic region, n (%) | ||||
| USA, Europe, or Australia | 289 (86) | 141 (84) | 355 (67) | 177 (67) |
| Japan | 46 (14) | 27 (16) | 178 (33) | 88 (33) |
| Primary cancer type, n (%) | ||||
| Gastric | 238 (71) | 120 (71) | 0 | 0 |
| GEJ | 97 (29) | 46 (27) | 0 | 0 |
| Gastric and GEJ | 0 | 2 (1) | 0 | 0 |
| Colon | 0 | 0 | 337 (63) | 160 (60) |
| Rectum | 0 | 0 | 196 (37) | 105 (40) |
| ECOG PS at baseline, n (%) | ||||
| 0 | 123 (37) | 68 (40) | 301 (56) | 147 (55) |
| 1 | 212 (63) | 100 (60) | 232 (44) | 118 (45) |
| Prior number of systemic therapies, n (%) | ||||
| 1-2 | 124 (37) | 63 (38) | 94 (18) | 45 (17) |
| ≥3 | 211 (63) | 105 (63) | 439 (82) | 220 (83) |
| Time since diagnosis of metastasis,bn (%) | ||||
| <18 months | 184 (55) | 102 (61) | 110 (21) | 55 (21) |
| ≥18 months | 151 (45) | 66 (39) | 423 (79) | 210 (79) |
| Renal function at baseline, n (%) | ||||
| Normal (CrCl≥90 ml/min) | 145 (43) | 75 (45) | 306 (57) | 146 (55) |
| Mild impairment (CrCl 60-89 ml/min) | 136 (41) | 70 (42) | 178 (33) | 90 (34) |
| Moderate impairment (CrCl 30-59 ml/min) | 52 (16) | 23 (14) | 47 (9) | 26 (10) |
| Missing | 2 (1) | 0 | 2 (<1) | 3 (1) |
| Hepatic function at baseline,cn (%) | ||||
| Normal | 249 (74) | 132 (79) | 325 (61) | 157 (59) |
| Mild impairment | 84 (25) | 33 (20) | 204 (38) | 100 (38) |
| Moderate impairment | 2 (1) | 1 (1) | 1 (<1) | 4 (2) |
| Severe impairment | 0 | 1 (1) | 0 | 0 |
| Missing | 0 | 1 (1) | 3 (1) | 4 (2) |
AST, aspartate transaminase; CrCL, creatinine clearance; ECOG PS, Eastern Cooperative Oncology Group performance status; FTD/TPI, trifluridine/tipiracil; GEJ, gastroesophageal junction; ULN, upper level of normal.
Includes Black/African Americans.
Calculated using the date of randomization for TAGS and first dose date for RECOURSE.
Normal: total bilirubin and AST ≤ ULN; mild impairment: total bilirubin between ULN and 1.5× ULN or AST > ULN; moderate impairment: total bilirubin between 1.5× and 3× ULN and any AST; severe impairment: total bilirubin >3× ULN and any AST.
In both trials, categorization of baseline renal and hepatic function was similar between the FTD/TPI and placebo groups. Most patients had normal renal function (44% in TAGS and 57% in RECOURSE) or mild renal impairment (41% and 34%, respectively). The majority of patients had normal hepatic function (76% in TAGS and 60% in RECOURSE versus mild hepatic impairment in 23% and 38%, respectively).
Exposure to FTD/TPI was comparable in TAGS and RECOURSE. Mean [standard deviation (SD)] dose intensity was 148.2 (26.8) and 155.0 (20.0) mg/m2/week in the TAGS and RECOURSE trials, respectively, and mean (SD) treatment duration was 12.1 (11.5) and 12.7 (12.0) weeks, respectively.5,6
Overall safety
The overall safety profile of FTD/TPI was comparable across the two trials. Most patients in the FTD/TPI and placebo groups experienced an AE of any grade, including 97% (326/335) and 93% (157/168) in the FTD/TPI and placebo groups, respectively, in TAGS, and 98% (524/533) and 93% (247/265), respectively, in RECOURSE.5,6 Grade ≥3 AEs of any cause were observed in 80% (267/335) and 69% (370/533) of FTD/TPI-treated patients in TAGS and RECOURSE, respectively, and 58% (97/168) and 52% (137/265) of placebo-treated patients.5,6
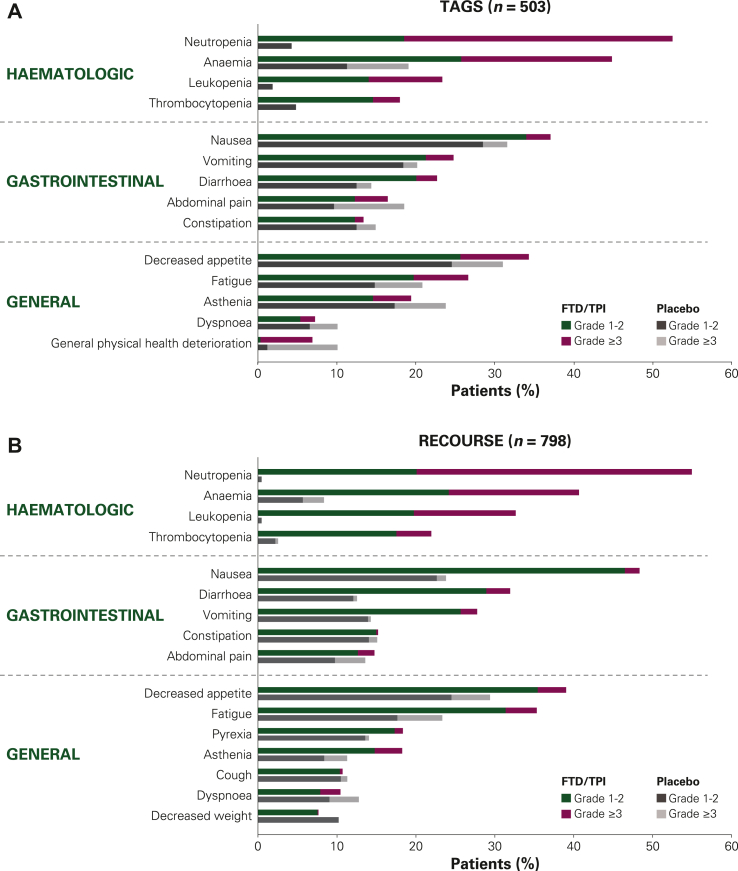
The most common AEs observed with FTD/TPI treatment were haematologic (neutropenia and anaemia) followed by gastrointestinal (nausea, diarrhoea), and general AEs (decreased appetite, fatigue; Figure 1). Haematologic AEs (including grade ≥3 events) occurred much more frequently in FTD/TPI-treated patients than in placebo-treated patients. The incidences of several non-haematologic AEs (abdominal pain, constipation, asthenia, dyspnoea, general deterioration), however, were similar with FTD/TPI and placebo, with some being higher among placebo-treated patients. In TAGS, the majority of most common (≥10%) non-haematologic AEs generally occurred at similar rates among placebo-treated patients and FTD/TPI-treated patients (Table 2). In RECOURSE, higher rates were observed for most non-haematologic AEs in FTD/TPI-treated patients compared with placebo-treated patients.
Figure 1.
Most common AEs of any cause in (A) TAGS and (B) RECOURSE. Haematologic AEs reported as combined preferred terms. All other AEs reported as preferred terms. AE, adverse event; FTD/TPI, trifluridine/tipiracil.
Table 2.
Most common (≥10%) adverse events of any grade in any treatment group
| AEa | AEs, n (%) |
|||
|---|---|---|---|---|
| TAGS |
RECOURSE |
|||
| FTD/TPI (n = 335) | Placebo (n = 168) | FTD/TPI (n = 533) | Placebo (n = 265) | |
| Neutropenia | 176 (53) | 7 (4) | 293 (55) | 1 (<1) |
| Anaemia | 150 (45) | 32 (19) | 217 (41) | 22 (8) |
| Nausea | 124 (37) | 53 (32) | 258 (48) | 63 (24) |
| Decreased appetite | 115 (34) | 52 (31) | 208 (39) | 78 (29) |
| Fatigue | 89 (27) | 35 (21) | 188 (35) | 62 (23) |
| Vomiting | 83 (25) | 34 (20) | 148 (28) | 38 (14) |
| Leukopenia | 78 (23) | 3 (2) | 174 (33) | 1 (<1) |
| Diarrhoea | 76 (23) | 24 (14) | 170 (32) | 33 (12) |
| Asthenia | 65 (19) | 40 (24) | 97 (18) | 30 (11) |
| Thrombocytopenia | 60 (18) | 8 (5) | 117 (22) | 7 (3) |
| Abdominal pain | 55 (16) | 31 (18) | 79 (15) | 36 (14) |
| Constipation | 45 (13) | 25 (15) | 81 (15) | 40 (15) |
| Pyrexia | 25 (7) | 8 (5) | 98 (18) | 37 (14) |
| Dyspnoea | 24 (7) | 17 (10) | 56 (11) | 34 (13) |
| General physical health deterioration | 23 (7) | 17 (10) | 21 (4) | 15 (6) |
| Weight decreased | 20 (6) | 12 (7) | 41 (8) | 27 (10) |
| Cough | 11 (3) | 6 (4) | 57 (11) | 30 (11) |
AE, adverse event; FTD/TPI, trifluridine/tipiracil.
Haematologic AEs reported as combined preferred terms. All other AEs reported as preferred terms.
Serious AEs were reported in similar percentages of FTD/TPI and placebo-treated patients in TAGS [43% (143/335) and 42% (70/168), respectively] and RECOURSE [30% (158/533) and 34% (89/265); Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100633].5,6 Serious haematologic-related AEs occurred more frequently among FTD/TPI-treated patients compared with placebo; of note, among patients treated with FTD/TPI, serious febrile neutropenia occurred in 4 patients (1%) in TAGS and 14 patients (3%) in RECOURSE compared with no patients in the placebo group of either trial. There were no meaningful differences in the incidences of serious hepatic- or renal-related AEs between FTD/TPI- and placebo-treated patients. Serious grade ≥3 cardiac disorders were relatively rare with FTD/TPI treatment [1% (5/335) and 1% (4/553) in TAGS and RECOURSE, respectively, compared with 1% (1/168) and 1% (3/265) with placebo]. Furthermore, cardiac disorders of any grade were reported infrequently (6% and 4% of FTD/TPI-treated patients in TAGS and RECOURSE, respectively) and with similar frequency to the placebo group (5% and 5%, respectively). Palpitations [n = 6 (2%) and n = 4 (1%), respectively] and sinus tachycardia [0 and n = 5 (1%), respectively] were the only cardiac disorders reported in more than two FTD/TPI-treated patients; cardiac-related AEs such as acute coronary syndrome, acute myocardial infarction, arrhythmia, myocardial infarction, and other cardiac disorders were reported in two or fewer FTD/TPI-treated patients. Treatment-related deaths were reported in one FTD/TPI-treated patient each in TAGS (due to cardiopulmonary arrest) and RECOURSE (due to septic shock).
Haematologic AEs: incidence and onset
The incidence of haematologic AEs among FTD/TPI-treated patients was consistent across TAGS and RECOURSE (Figure 1). The most common haematologic AE with FTD/TPI treatment was neutropenia (or decreased neutrophil count), which occurred in 53% (176/335) and 55% (293/533) of FTD/TPI-treated patients in TAGS and RECOURSE, respectively [4% (7/168) and <1% (1/265) with placebo, respectively].5,6 More than half of all neutropenia AEs reported were grade 3-4 in severity [34% (114/335) and 35% (186/533) in TAGS and RECOURSE, respectively]. Few patients experienced febrile neutropenia, which was reported in 2% (n = 6) and 4% (n = 20) of FTD/TPI-treated patients in TAGS and RECOURSE, respectively (all grade ≥3); there were no cases of febrile neutropenia in placebo-treated patients.
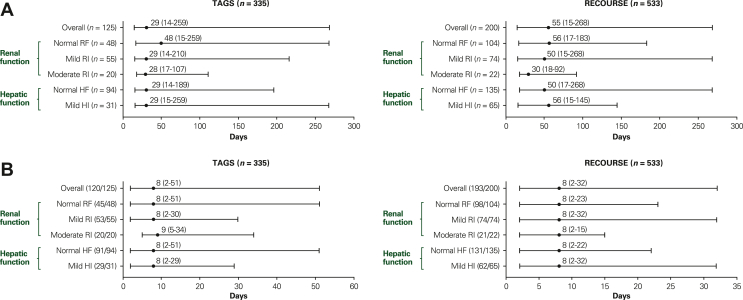
Grade 3-4 CIN (evaluated by laboratory data) occurred in 37% (125/335) and 38% (200/533) of FTD/TPI-treated patients in TAGS and RECOURSE, respectively. Most grade 3-4 CIN events occurred within the first two cycles of FTD/TPI [86% (107/125) and 81% (161/200) of all grade 3-4 CIN in TAGS and RECOURSE, respectively], with 54% (67/125) and 38% (75/200) of events, respectively, occurring in cycle 1. The median time to onset of grade 3-4 CIN among FTD/TPI-treated patients was 29 days (range, 14-259) in TAGS and 55 days (range, 15-268) in RECOURSE (Figure 2A).
Figure 2.
Time to onset and time to resolution of grade 3-4 chemotherapy-induced neutropenia in FTD/TPI-treated patients. (A) Median time to onset (range) is shown with the number of patients in each subpopulation with events. Time to onset was defined as days to first grade 3-4 neutropenia laboratory value that worsened from baseline by at least one grade. (B) Median time to resolution (range) is shown with the number of patients who recovered/number of patients with events for each subpopulation. Time to resolution was defined as recovery from first grade 3-4 neutropenia laboratory value that worsened from baseline by at least one grade; patients who recovered had at least one measurement recorded after the nadir that was grade <2 or the baseline grade or lower. All haematology measurements were carried out within 24 h before the start of study treatment from cycle 2 onwards and within 7 days before day 1 of cycle 1 or day 15 of cycle 1. In TAGS, two patients were not included in the analysis by renal function due to missing baseline data, and two patients were not included in the analysis by hepatic function due to moderate hepatic impairment at baseline. In RECOURSE, two patients were not included in the analysis by renal function due to missing baseline data; for the analysis by hepatic function, one patient was not included due to moderate hepatic impairment at baseline, and three patients were not included due to missing data at baseline. Renal function subgroups were defined as follows: normal renal function [creatinine clearance (CrCl) ≥90 ml/min], mild renal impairment (CrCl 60-89 ml/min), and moderate renal impairment (CrCl 30-59 ml/min). Hepatic function subgroups were defined as follows: normal hepatic function (total bilirubin and AST ≤ ULN) and mild impairment (total bilirubin between ULN and 1.5× ULN or AST > ULN). AST, aspartate transaminase; CrCl, creatinine clearance; FTD/TPI, trifluridine/tipiracil; HF, hepatic function; HI, hepatic impairment; RF, renal function; RI, renal impairment; ULN, upper limit of normal.
Haematologic AEs were rare among placebo-treated patients, except for anaemia of any grade (or decreased haemoglobin concentration), which occurred in 19% (32/168) and 8% (22/265) of placebo-treated patients in TAGS and RECOURSE, respectively. The corresponding incidence of anaemia in FTD/TPI-treated patients was 45% (150/335) and 41% (217/533), respectively.
Management of AEs
Similar proportions of FTD/TPI-treated patients had AEs of any cause leading to dosing modifications (delays, interruptions, or reductions) in TAGS (58%; 195/335) and RECOURSE (54%; 289/533). Dosing delays or interruptions were used more frequently (57% and 52% of patients in TAGS and RECOURSE, respectively) than dose reductions (11% and 14%) to manage AEs of any cause in FTD/TPI-treated patients.
Neutropenia of any grade led to dosing delays, dosing interruptions, or dose reductions in 37% (125/335) and 40% (215/533) of FTD/TPI-treated patients in TAGS and RECOURSE, respectively. Supportive medications for neutropenia were used by 17% (58/335) and 9% (50/533) of all FTD/TPI-treated patients in TAGS and RECOURSE, respectively (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100633) and all but 4 of these patients received granulocyte colony-stimulating factor (G-CSF). We found that most cases of neutropenia resolved based on evaluation of laboratory data; grade 3-4 CIN resolved in 96% (120/125) and 97% (193/200) of patients in TAGS and RECOURSE, respectively, and the median time to resolution for these events was 8 days in both trials (Figure 2B).
The rates of permanent treatment discontinuations due to AEs were low in both trials [13% (43/335) in TAGS, 10% (55/533) in RECOURSE]. One patient in each trial discontinued because of grade ≥3 neutropenia.
Renal and hepatic impairment subgroup analysis
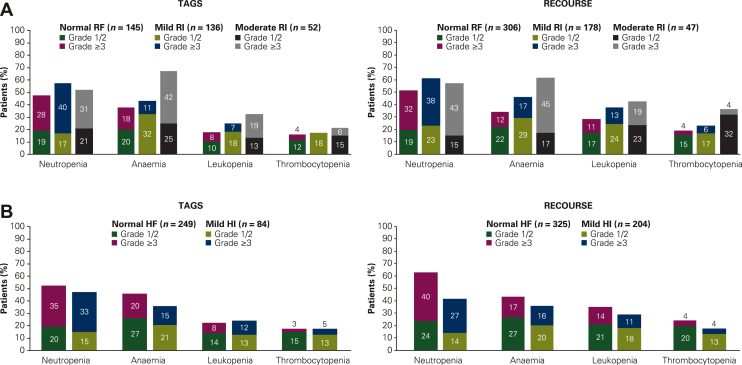
The safety profile of FTD/TPI was generally similar in patients with normal renal function and those with mild renal impairment; however, in both trials, the overall incidence of grade ≥3 AEs was somewhat higher in patients with moderate renal impairment (81% in TAGS; 85% in RECOURSE) compared with those with normal renal function (74% and 67%, respectively). Furthermore, although the incidence of non-haematologic AEs was comparable across the renal function subgroups (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100633), the incidence of haematologic AEs was higher among patients with mild and moderate renal impairment than in patients with normal renal function (Figure 3A). The FTD/TPI safety profile was also consistent across the hepatic function subgroups; incidences of haematologic AEs were similar in patients with normal hepatic function and those with mild hepatic impairment (Figure 3B).
Figure 3.
Incidences of haematologic AEs in FTD/TPI-treated patients by (A) renal function and (B) hepatic function. Haematologic AEs were reported as combined preferred terms. Renal function subgroups were defined as follows: normal renal function [creatinine clearance (CrCl) ≥90 ml/min], mild renal impairment (CrCl 60-89 ml/min), and moderate renal impairment (CrCl 30-59 ml/min). Hepatic function subgroups were defined as follows: normal hepatic function (total bilirubin and AST ≤ ULN) and mild impairment (total bilirubin between ULN and 1.5× ULN or AST > ULN). AE, adverse event; AST, aspartate transaminase; CrCl, creatinine clearance; FTD/TPI, trifluridine/tipiracil; HF, hepatic function; HI, hepatic impairment; RF, renal function; RI, renal impairment; ULN, upper limit of normal.
Among all haematologic AEs, anaemia was most frequent in FTD/TPI-treated patients with moderate renal impairment across both trials (64% compared with 35% in patients with normal renal function). Grade ≥3 anaemia (or decreased haemoglobin) occurred in 42% (22/52; TAGS) and 45% (21/47; RECOURSE) of patients with moderate renal impairment; the corresponding percentages in the normal renal function subgroup were 18% (26/145) and 12% (37/306), respectively.
In both trials, neutropenia was more frequent in patients with mild or moderate renal impairment than in patients with normal renal function (Figure 3A). In patients with moderate renal impairment, the majority of grade 3-4 CIN events occurred during cycle 1 [70% (14/20) and 55% (12/22) in TAGS and RECOURSE, respectively], with a median time to onset of 28 days (range, 17-107) and 30 days (range, 18-92), respectively (Figure 2A). Most grade 3-4 CIN events occurred within the first two cycles in patients with normal [TAGS: 88% (42/48); RECOURSE: 81% (84/104)] or mildly impaired [TAGS: 82% (45/55); RECOURSE: 80% (59/74)] renal function. Median time to onset of grade 3-4 CIN was similar in patients with normal or mildly impaired hepatic function and most commonly occurred within the first two cycles (Figure 2B). The overall number of patients with febrile neutropenia was small, which may limit observations; however, febrile neutropenia did not occur more commonly in patients with renal or hepatic impairment (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100633).
Neutropenia was managed with dosing modifications or supportive medications in patients with renal or hepatic impairment, and nearly all events resolved. In TAGS, the proportion of patients who received supportive medications for neutropenia was similar across renal and hepatic function subgroups and similar to that of the overall population (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100633). In RECOURSE, a slightly higher percentage of patients with moderate renal impairment received G-CSF (23%) compared with the overall population; however, percentages were similar between the overall population and those with normal or mildly impaired renal or hepatic function. In the overall patient populations and across all subgroups in both trials, regardless of baseline renal or hepatic function (Figure 2B), grade 3-4 CIN resolved with a median time to resolution of approximately 8 days.
Discussion
The results of this pooled analysis indicate that FTD/TPI was well tolerated in patients with mCRC or mGC/GEJC, with a consistent safety profile observed for FTD/TPI across the TAGS and RECOURSE trials. The most frequent AEs in the FTD/TPI groups were haematologic and gastrointestinal-related. In both trials, haematologic AEs were more frequent with FTD/TPI than with placebo. Whereas gastrointestinal-related and other non-haematologic AEs were comparable in the FTD/TPI and placebo arms in TAGS, these AEs were more frequent with FTD/TPI than with placebo in RECOURSE. AEs were managed well with dosing modifications and supportive medications. In both trials, similar proportions of FTD/TPI-treated patients required dosing modifications to manage AEs, with dosing delays used more frequently than dose reductions. Altogether, although AEs were common, discontinuation rates due to AEs were low.
The FTD/TPI safety profile observed in this pooled analysis was consistent with reports from the individual phase III trials,5,6 and earlier phase II trials.12,13 Additionally, analyses of real-world populations of patients with mCRC treated with FTD/TPI14, 15, 16 showed similar safety findings, suggesting that the observed FTD/TPI safety profile was consistent across a broad spectrum of patients.
The potential for treatment-limiting toxicities, particularly neutropenia, is an important concern in patients undergoing treatment with chemotherapy. Neutropenia (or decreased neutrophil count) was the most common haematologic AE in patients treated with FTD/TPI, and most grade 3-4 CIN events occurred within the first two treatment cycles. In related analyses from RECOURSE, the onset of any-grade CIN in cycles 1 and 2 was associated with longer overall survival and progression-free survival in FTD/TPI-treated patients.17 FTP/TPI-treated patients who developed grade ≥3 CIN had greater improvements in overall and progression-free survival than those who did not develop CIN. Furthermore, grade ≥3 CIN was strongly predictive of improved overall survival, regardless of time of onset, an observation also reported in other studies of FTD/TPI in colorectal cancer.17, 18, 19 In both RECOURSE and TAGS, neutropenia was managed well with supportive medications and dosing modifications. Grade 3-4 CIN resolved in most patients in a median of 8 days (which correlated with the average timing of the first haematologic measurement after dose holds), and only one patient in each trial discontinued due to neutropenia. The frequency of neutropenia and the survival benefit associated with this AE among patients treated with FTD/TPI underscores the importance of having effective management strategies for patients with neutropenia and other AEs.
In line with the findings of a phase I study of FTD/TPI in patients with advanced solid tumours and varying degrees of hepatic impairment,20 the current pooled analysis showed a similar incidence of AEs, including haematologic AEs, in patients with mild hepatic impairment and those with normal hepatic function. FTD/TPI is not recommended in patients with moderate to severe hepatic impairment based on results of the phase I study,20 and patients with moderate or severe hepatic impairment were generally not enrolled in TAGS and RECOURSE.
While the FTD/TPI safety profile was generally comparable in patients with mild renal impairment and those with normal renal function, the incidence of grade ≥3 AEs and haematologic AEs (anaemia and neutropenia) was marginally higher in patients with mild or moderate renal impairment than in patients with normal renal function (patients with severe renal impairment were not generally enrolled in the studies). Patients with moderate renal impairment also had an earlier onset of grade 3-4 CIN compared with those with normal renal function. A phase I study designed to evaluate FTD/TPI in patients with advanced solid tumours and varying degrees of renal impairment21 reported similar AE patterns, also concluding that FTD/TPI was generally well tolerated in patients with mild to moderate renal impairment. In that study, a lower FTD/TPI dose of 20 mg/m2 was found to be tolerable in patients with severe renal impairment.21
Importantly, in the current pooled analysis, haematologic AEs were well managed in patients with either hepatic or renal impairment using dosing modifications or supportive medications, and grade 3-4 CIN resolved in nearly all patients with either impairment type within the same timeframe (approximately 8 days) as patients with normal function. A limitation of the current subanalyses of hepatic and renal impairment, however, was the retrospective post hoc nature; unlike the phase I studies carried out prospectively in these populations,20,21 neither TAGS nor RECOURSE were designed to evaluate patients with renal or hepatic impairment.
These data also highlight potential advantages of the safety profile of FTD/TPI over that of fluoropyrimidines. While fluoropyrimidines are a cornerstone of combination chemotherapy regimens for mGC and mCRC,22, 23, 24 drug resistance is common and AEs can impact treatment decisions.25, 26, 27 Cardiotoxicity is of particular concern, with incidences ranging from 1% to 19%.28, 29, 30 In contrast, cardiac disorders were infrequent among FTD/TPI-treated patients in TAGS or RECOURSE. Another limitation of fluoropyrimidine treatment is related to use in patients with dihydropyrimidine dehydrogenase (DPD) deficiency, who are at risk for severe and life-threatening side-effects.31 As FTD/TPI metabolism does not involve DPD, patients with DPD deficiency may be treated with FTD/TPI.32
In conclusion, results of this pooled analyses support FTD/TPI as a well-tolerated treatment in patients with mGC/GEJC or mCRC, with a consistent safety profile across these patient populations. AEs were generally well managed with dosing modifications and supportive medications. Based on the large population of patients from TAGS and RECOURSE, we found that grade ≥3 AEs, including anaemia and neutropenia, were somewhat more frequent in patients with moderate renal impairment, indicating that patients with renal impairment should be monitored for these toxicities.
Acknowledgements
We thank the patients and families who made the TAGS and RECOURSE trials possible, the clinical study teams involved in the trials, as well as the data and safety monitoring board members.
Funding
This work was supported by Taiho Oncology, Inc. and Taiho Pharmaceutical (no grant number). This analysis was funded by Taiho Oncology, Inc. Professional medical writing and editorial assistance were provided by Vasupradha Vethantham, Meredith Kalish, and Jennifer L. Robertson at Ashfield MedComms, an Inizio company, funded by Taiho Oncology, Inc.
Disclosure
EVC reports institutional research grants or funding from Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb (BMS), Celgene, Ipsen, Lilly, Merck Sharp & Dohme (MSD), Merck KGaA, Novartis, Roche, and Servier; and participation in advisory boards for AbbVie, Array, Astellas, AstraZeneca, Bayer, Beigene, Biocartis, Boehringer Ingelheim, BMS, Celgene, Daiichi, Halozyme, GlaxoSmithKline, Helsinn, Incyte, Ipsen, Janssen Research, Lilly, MSD, Merck KGaA, Mirati, Novartis, Pierre Fabre, Roche, Seattle Genetics, Servier, Sirtex, Terumo, Taiho, TRIGR, and Zymeworks. HH has received payment for expert testimony from Taiho. KS reports institutional research funding from Amgen, Astellas Pharma, Chugai Pharmaceuticals, Daiichi Sankyo, Eisai, Merck Pharmaceuticals, Medi Science, Ono Pharmaceuticals, and Taiho Pharmaceuticals; participation in an advisory role for AbbVie Inc., Amgen, Boehringer Ingelheim, BMS, Daiichi Sankyo, Eli Lilly and Company, GlaxoSmithKline, Janssen, Merck Pharmaceutical, Novartis, Ono Pharmaceutical, Pfizer Inc., Takeda Pharmaceuticals, and Taiho Pharmaceuticals; and reports honoraria from BMS and Takeda Pharmaceuticals. AO reports support for the present work from Taiho; grants from Chugai Pharma and Takeda Pharmaceuticals; and has participated in an advisory role for Taiho. AF reports institutional grants from AstraZeneca, Bayer, BMS, Eli Lilly, Merck, MSD, Novartis, Roche, Sanofi, and Servier and reports consulting fees from Amgen, Bayer, BMS, Daiichi Sankyo, Incyte, Lilly, Merck, MSD, Pierre Fabre, Roche, and Servier. TY reports institutional research grants or funding from Amgen, Chugai Pharmaceutical Co., Ltd, Daiichi Sankyo, Genomedia Inc., MSD, Nippon Boehringer Ingelheim, Ono Pharmaceutical, Parexel International, Pfizer Japan, Sanofi, Sysmex Corporation, and Taiho Pharmaceutical; reports honoraria from Bayer Yakuhin Ltd, Chugai Pharmaceutical, Eli Lilly Japan, Merck Biopharma, MSD, Ono Pharmaceutical, and Taiho Pharmaceutical. TD reports institutional research funding from AbbVie, BMS, Boehringer Ingelheim, Chugai Pharma, Daiichi Sankyo, Eisai, Janssen Pharma, IQVIA, Lilly, Merck Biopharma, MSD, Novartis, Pfizer, Sumitomo Dainippon, and Taiho; consulting fees from AbbVie, Bayer, Chugai Pharma, Kaken Pharma, Kyowa Kirin, Otsuka Pharma, Rakuten Medical, PRA Health Science, Shionogi, Sumitomo Dainippon, Takeda, and Taiho; honoraria from AstraZeneca, BMS, Daiichi Sankyo, Ono Pharma, and Rakuten Medical; and has participated in an advisory role for AbbVie, Amgen, Astellas Pharma, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Janssen Pharma, MSD, and Novartis. DI reports receiving consulting fees from Amgen, Astellas, AstraZeneca, Bayer, BMS, Daiichi Sankyo, Eli Lilly, MacroGenics, Merck, and Taiho; honoraria from AstraZeneca, Daiichi Sankyo, and Merck; and has participated on a data safety monitoring board for MacroGenics and Merck. HTA has served as an advisor/consultant for Bicycle Therapeutics, Daiichi, Guardant Health, Labgenius, Roche, and Servier; has served on the data monitoring board for Bicycle Therapeutics and Daiichi Sankyo; and reports stock options in Ellipses Pharma. BG has received institutional research funding from Boehringer Ingelheim, GlycoNex, Helix Biopharma, Hutchison MediPharma, Mirati Therapeutics, NGM Biopharma, Roche/Genentech, Sirnaomics, Taiho Oncology, Toray, and Trishula Therapeutics; consulting fees from BMS, Boston Therapeutics, Exelixis, Foundation Medicine, Ipsen, Pfizer, Roche/Genentech, and Taiho Oncology; and honoraria from BMS, Ipsen, and Taiho Oncology; and has participated in a data safety monitoring board for Roche/Genentech. KAB is an employee at Taiho Oncology, and owns stock of Eli Lilly. LM is a consultant statistician for Taiho Oncology, Inc. and is paid by Taiho Oncology Inc. JT reports personal financial interest in form of scientific consultancy role for Array Biopharma, AstraZeneca, Avvinity, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann–La Roche Ltd, Genentech Inc., HalioDx SAS, Hutchison MediPharma International, Ikena Oncology, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, NeoPhore, Novartis, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Scandion Oncology, Servier, Taiho, Tessa Therapeutics, and TheraMyc; educational collaboration with Imedex, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education and Physicians Education Resource (PER); institutional financial interest in form of financial support for clinical trials or contracted research for Amgen Inc., Array Biopharma Inc., AstraZeneca Pharmaceuticals LP, BeiGene, Boehringer Ingelheim, BMS, Celgene, Debiopharm International SA, F. Hoffmann-La Roche Ltd, Genentech Inc., HalioDx SAS, Hutchison MediPharma International, Janssen-Cilag SA, MedImmune, Menarini, Merck Health KGAA, MSD, Merus NV, Mirati, Novartis Farmacéutica SA, Pfizer, PharmaMar, Sanofi Aventis Recherche & Développement, Servier, Taiho Pharma USA Inc., Spanish Association Against Cancer Scientific Foundation, and Cancer Research UK.
RM has declared no conflicts of interest.
Ethics approval
Both the TAGS and RECOURSE studies were approved by the institutional review boards or independent ethics committees at each participating institution before enrolment of patients. Each study was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent.
Acknowledgments
Data sharing
Data generated or analyzed during this study are on file with Taiho Oncology, Inc., and Taiho Pharmaceuticals Co., Ltd, and are not publicly available. Enquiries about data access should be sent to th-datasharing@taiho.co.jp.
Supplementary data
References
- 1.Emura T., Suzuki N., Yamaguchi M., Ohshimo H., Fukushima M. A novel combination antimetabolite, TAS-102, exhibits antitumor activity in FU-resistant human cancer cells through a mechanism involving FTD incorporation in DNA. Int J Oncol. 2004;25(3):571–578. [PubMed] [Google Scholar]
- 2.Fukushima M., Suzuki N., Emura T., et al. Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2’-deoxyribonucleosides. Biochem Pharmacol. 2000;59(10):1227–1236. doi: 10.1016/s0006-2952(00)00253-7. [DOI] [PubMed] [Google Scholar]
- 3.Emura T., Murakami Y., Nakagawa F., Fukushima M., Kitazato K. A novel antimetabolite, TAS-102 retains its effect on FU-related resistant cancer cells. Int J Mol Med. 2004;13(4):545–549. [PubMed] [Google Scholar]
- 4.LONSURF® (trifluridine and tipiracil) tablets [prescribing information] Taiho Oncology Inc; Princeton, NJ: 2019. [Google Scholar]
- 5.Mayer R.J., Van Cutsem E., Falcone A., et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 6.Shitara K., Doi T., Dvorkin M., et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–1448. doi: 10.1016/S1470-2045(18)30739-3. [DOI] [PubMed] [Google Scholar]
- 7.Launay-Vacher V. Renal dysfunction has statistically and clinically significant deleterious effects on anticancer drug safety. J Clin Oncol. 2016;34(20):2428. doi: 10.1200/JCO.2015.65.1554. [DOI] [PubMed] [Google Scholar]
- 8.Eklund J.W., Trifilio S., Mulcahy M.F. Chemotherapy dosing in the setting of liver dysfunction. Oncology (Williston Park) 2005;19(8):1057–1063. [PubMed] [Google Scholar]
- 9.Launay-Vacher V., Oudard S., Janus N., et al. Renal Insufficiency and Cancer Medications (IRMA) Study Group. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer. 2007;110(6):1376–1384. doi: 10.1002/cncr.22904. [DOI] [PubMed] [Google Scholar]
- 10.King P.D., Perry M.C. Hepatotoxicity of chemotherapy. Oncologist. 2001;6(2):162–176. doi: 10.1634/theoncologist.6-2-162. [DOI] [PubMed] [Google Scholar]
- 11.Mudd T.W., Guddati A.K. Management of hepatotoxicity of chemotherapy and targeted agents. Am J Cancer Res. 2021;11(7):3461–3474. [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshino T., Mizunuma N., Yamazaki K., et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012;13(10):993–1001. doi: 10.1016/S1470-2045(12)70345-5. [DOI] [PubMed] [Google Scholar]
- 13.Bando H., Doi T., Muro K., et al. A multicenter phase II study of TAS-102 monotherapy in patients with pre-treated advanced gastric cancer (EPOC1201) Eur J Cancer. 2016;62:46–53. doi: 10.1016/j.ejca.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Yoshino T., Uetake H., Fujita N., et al. TAS-102 safety in metastatic colorectal cancer: results from the first postmarketing surveillance study. Clin Colorectal Cancer. 2016;15(4):e205–e211. doi: 10.1016/j.clcc.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Samawi H.H., Brezden-Masley C., Afzal A.R., Cheung W.Y., Dolley A. Real-world use of trifluridine/tipiracil for patients with metastatic colorectal cancer in Canada. Curr Oncol. 2019;26(5):319–329. doi: 10.3747/co.26.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwakman J.J.M., Vink G., Vestjens J.H., et al. Feasibility and effectiveness of trifluridine/tipiracil in metastatic colorectal cancer: real-life data from The Netherlands. Int J Clin Oncol. 2018;23(3):482–489. doi: 10.1007/s10147-017-1220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshino T., Cleary J.M., Van Cutsem E., et al. Neutropenia and survival outcomes in metastatic colorectal cancer patients treated with trifluridine/tipiracil in the RECOURSE and J003 trials. Ann Oncol. 2020;31(1):88–95. doi: 10.1016/j.annonc.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasi P.M., Kotani D., Cecchini M., et al. Chemotherapy induced neutropenia at 1-month mark is a predictor of overall survival in patients receiving TAS-102 for refractory metastatic colorectal cancer: a cohort study. BMC Cancer. 2016;16:467. doi: 10.1186/s12885-016-2491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nose Y., Kagawa Y., Hata T., et al. Neutropenia is an indicator of outcomes in metastatic colorectal cancer patients treated with FTD/TPI plus bevacizumab: a retrospective study. Cancer Chemother Pharmacol. 2020;86(3):427–433. doi: 10.1007/s00280-020-04129-6. [DOI] [PubMed] [Google Scholar]
- 20.Saif M.W., Rosen L., Rudek M.A., et al. Open-label study to evaluate trifluridine/tipiracil safety, tolerability and pharmacokinetics in patients with advanced solid tumours and hepatic impairment. Br J Clin Pharmacol. 2019;85(6):1239–1246. doi: 10.1111/bcp.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saif M.W., Becerra C.R., Fakih M.G., et al. A phase I, open-label study evaluating the safety and pharmacokinetics of trifluridine/tipiracil in patients with advanced solid tumors and varying degrees of renal impairment. Cancer Chemother Pharmacol. 2021;88(3):485–497. doi: 10.1007/s00280-021-04308-z. [DOI] [PubMed] [Google Scholar]
- 22.Van Cutsem E., Cervantes A., Adam R., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 23.Smyth E.C., Verheij M., Allum W., et al. ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 24.Wagner A.D., Syn N.L., Moehler M., et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longley D.B., Harkin D.P., Johnston P.G. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 26.Polk A., Vaage-Nilsen M., Vistisen K., Nielsen D.L. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev. 2013;39(8):974–984. doi: 10.1016/j.ctrv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Peeters M., Cervantes A., Vera S.M., Taieb J. Trifluridine/tipiracil: an emerging strategy for the management of gastrointestinal cancers. Future Oncol. 2018;14(16):1629–1645. doi: 10.2217/fon-2018-0147. [DOI] [PubMed] [Google Scholar]
- 28.Labianca R., Beretta G., Clerici M., Fraschini P., Luporini G. Cardiac toxicity of 5-fluorouracil: a study on 1083 patients. Tumori. 1982;68(6):505–510. doi: 10.1177/030089168206800609. [DOI] [PubMed] [Google Scholar]
- 29.Shiga T., Hiraide M. Cardiotoxicities of 5-fluorouracil and other fluoropyrimidines. Curr Treat Options Oncol. 2020;21(4):27. doi: 10.1007/s11864-020-0719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sara J.D., Kaur J., Khodadadi R., et al. 5-fluorouracil and cardiotoxicity: a review. Ther Adv Med Oncol. 2018;10 doi: 10.1177/1758835918780140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Medicines Agency EMA recommendations on DPD testing prior to treatment with fluorouracil, capecitabine, tegafur and flucytosine. 30 April 2020. https://www.ema.europa.eu/en/documents/press-release/ema-recommendations-dpd-testing-prior-treatment-fluorouracil-capecitabine-tegafur-flucytosine_en.pdf Available at:
- 32.Lenz H.J., Stintzing S., Loupakis F. TAS-102, a novel antitumor agent: a review of the mechanism of action. Cancer Treat Rev. 2015;41(9):777–783. doi: 10.1016/j.ctrv.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.