Abstract
Background
Evaluation of health-related quality of life (HR-QoL) among cancer patients has gained an increasing importance and is now a key determinant of anticancer treatments’ value. HR-QoL has been assessed in trials testing cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) in breast cancer (BC), using various questionnaires at different timepoints. HR-QoL reports from BC patients treated with CDK4/6i in the real-world setting are also available.
Methods
We systematically reviewed the literature, searching for full-length articles, and selected conference abstracts reporting data on HR-QoL in BC patients at any stage and of any molecular subtype treated with abemaciclib, palbociclib or ribociclib.
Results
A total of 533 full-length articles and 143 abstracts were retrieved. After screening for eligibility, 38 records were included (31 clinical trials; 7 real-world reports). Assessment methods were heterogeneous across studies in terms of questionnaires, evaluation timepoints and endpoints.
Overall, adding CDK4/6i to endocrine therapy did not worsen patients’ HR-QoL, with a positive trend towards pain improvement. Gastrointestinal scores (diarrhea, nausea and appetite loss) statistically favored the control arm among metastatic BC patients receiving abemaciclib, whereas they were superimposable in the early setting. The combination of palbociclib and endocrine therapy showed similar HR-QoL outcomes compared with endocrine therapy alone, but determined better scores compared with chemotherapy. HR-QoL was specifically assessed in premenopausal patients treated with ribociclib, showing similar scores compared with postmenopausal patients.
Conclusions
Despite methodological heterogeneity does not allow a proper comparison, HR-QoL was generally maintained with CDK4/6i. However, differences between abemaciclib, palbociclib and ribociclib exist and mainly rely on the distinct safety profiles of the compounds. These differences should be acknowledged and taken into account in the clinical practice.
Key words: health-related quality of life, cyclin-dependent 4/6 inhibitors, abemaciclib, palbociclib, ribociclib
Highlights
-
•
HR-QoL of BC patients treated with abemaciclib, palbociclib or ribociclib has been reported.
-
•
In these patients, HR-QoL has been evaluated using heterogeneous tools and at different timepoints.
-
•
Overall the addiction of any CDK4/6i to endocrine therapy does not worsen patient HR-QoL, with a positive trend towards pain improvement.
-
•
Gastrointestinal toxicities influenced HR-QoL of patients treated with abemaciclib with better results in the early setting.
-
•
An effort is needed to provide uniform HR-QoL evaluation methods and incorporate them in clinical practice.
Introduction
The combination of a cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) with endocrine therapy (ET) represents the current standard of care for hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) breast cancer (BC) patients.1 In phase III trials the CDK4/6i abemaciclib, palbociclib and ribociclib determined a consistent progression-free survival (PFS) benefit when combined with an aromatase inhibitor or fulvestrant in the first or subsequent line of treatment. Additionally, a significant overall survival advantage emerged with abemaciclib plus fulvestrant and with ribociclib in every disease setting.2,3 These drugs have also been tested in the early neoadjuvant and adjuvant phase with inconsistent results and abemaciclib is already approved in this setting.4,5
Preserving health-related quality of life (HR-QoL) of BC patients represents a goal as important as extending their survival, especially in the advanced setting.6, 7, 8 Recently, the European Society of Medical Oncology (ESMO) included HR-QoL among the determinants of anticancer therapies’ value, by developing the Magnitude of Clinical Benefit Scale (MCBS).9
HR-QoL is a subjective and composite item, which relies on patients’ perception of inner and outer environment. As such, it must be reported directly by patients through validated tools, representing the so-called patient-reported outcomes (PROs).10,11 QoL assessment has routinely been included in clinical trials testing CDK4/6i, using heterogeneous tools and evaluation timepoints. The most frequently employed questionnaires were the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30), its BC-specific companion (EORTC QLQ-BR23), the Functional Assessment of Cancer Therapy-Breast (FACT-B) and EuroQoL five dimensional (EQ-5D). Forms addressing specific items have also been employed, such as the Brief Pain Inventory (BPI) and its short form (BPI-sf). Despite their similar aims, all these tools have different structures and scoring systems, and their cross-comparison is not straightforward.12
Here we systematically review the literature to report the available evidence about HR-QoL of BC patients receiving CDK4/6i both in clinical studies and in a real-world setting, looking at the potential impact of abemaciclib, palbociclib and ribociclib on HR-QoL.
Materials and methods
We carried out a systematic review of the published literature according to the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.13 We included only publications in English reporting information on HR-QoL in BC patients at any stage and of any molecular subtype treated with abemaciclib, palbociclib or ribociclib. Full-length articles and published conference abstracts, including clinical trial results, real-world analyses, case series and case reports, were considered. Reviews, editorials and commentaries were excluded. Literature search for full-length articles was carried out in PubMed and Scopus with no temporal limits, whereas abstracts were retrieved in American Society of Clinical Oncology (ASCO), ESMO, San Antonio Breast Cancer Symposium and St. Gallen International Breast Cancer Congress websites from 2016 to 2021. The following terms were combined for full-length articles search: breast cancer, breast tumor, CDK4/6 inhibitor, cyclin dependent kinase 4/6 inhibitor, abemaciclib, palbociclib, ribociclib, quality of life, QoL, health related quality of life, patient reported outcome. For the conference abstracts an individual search was carried out for each approved CDK4/6i. All the searches were carried out up to 27 February 2022. Two authors screened the selected records for eligibility (FM and VDL) and controversies were resolved by a third author (GB). Data about HR-QoL, including the type of questionnaires employed and the reported outcomes, were extracted from each article and abstract. The risk of bias for the QoL outcome was assessed in the included randomized trials, using the RoB2 tool criteria (Supplementary Figures S1 and S2, available at https://doi.org/10.1016/j.esmoop.2022.100629).14
Results
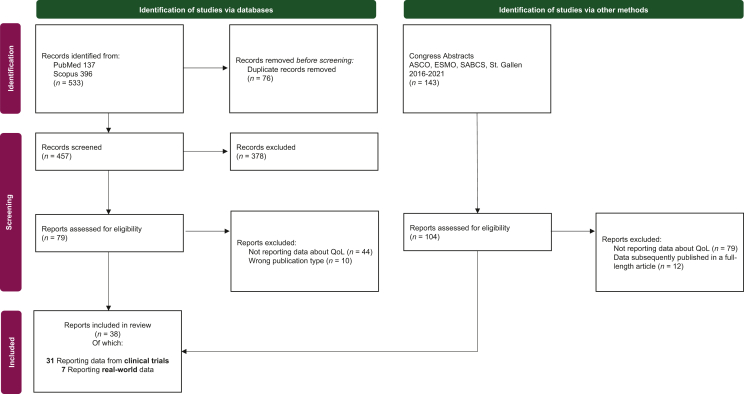
A total of 533 full-length articles and 143 abstracts satisfied the terms of our search. After duplicates removal and full eligibility assessment 38 records were included (Figure 1).
Figure 1.
Flow diagram of included reports according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
ASCO, American Society of Clinical Oncology; ESMO, European Society of Medical Oncology; QoL, quality of life; SABCS, San Antonio Breast Cancer Symposium.
HR-QoL in clinical trials
HR-QoL has been extensively investigated in clinical trials testing CDK4/6i in BC patients. The main characteristics and PROs from these studies are shown in Table 1, whereas HR-QoL outcomes from randomized/multi-arm clinical trials testing CDK4/6i are reported in Table 2.
Table 1.
HR-QoL evaluation methods in clinical trials testing CDK4/6i in BC patients
| Trial name | Phase | Setting | Intervention arms (N) | Evaluation tools | Evaluation timepoints |
|---|---|---|---|---|---|
| MONARCH-3 | III | Advanced HR+/HER2− |
- Abemaciclib + NSAI (328) - Placebo + NSAI (165) |
- EQ-5D-5L - EORTC QLQ-C30 - EORTC QLQ-BR23 |
Baseline, q2 cycles C2-19, q3 cycles C>19, 1 follow-up |
| MONARCH-2 | III | Advanced HR+/HER2− |
- Abemaciclib + fulvestrant (446) - Placebo + fulvestrant (223) |
- EQ-5D-5L - EORTC QLQ-C30 - EORTC QLQ-BR23 - BPI-sf |
Baseline, q2 cycles C2-13, q3 cycles C>13, 1 follow-up |
| monarcHER | II | Advanced HR+/HER2+ |
- Abemaciclib + trastuzumab + fulvestrant (79) - Abemaciclib + trastuzumab (79) - SoC (79) |
- EQ-5D-5L - EORTC QLQ-C30 - mBPI-sf |
Every cycle, 1 follow-up |
| monarchE | III | Adjuvant HR+/HER2− |
- Abemaciclib + standard ET (2808) - Placebo + standard ET (2829) |
- FACT-B - FACT-ES - FACIT-Fatigue - FACT-GP5 |
Baseline, 3/6/12/18 months after randomization, 1 follow-up |
| PALOMA-1 | I/II | Advanced HR+/HER2− |
- Palbociclib + letrozole (84) - Letrozole (81) |
- BPI | Every cycle, EoT |
| PALOMA-2 | III | Advanced HR+/HER2− |
- Palbociclib + letrozole (444) - Placebo + letrozole (222) |
- EQ-5D-5L - FACT-B |
C1-3, q2 cycles from C5 |
| PALOMA-3 | III | Advanced HR+/HER2− |
- Palbociclib + fulvestrant (347) - Placebo + fulvestrant (174) |
- EQ-5D - EORTC QLQ-C30 - EORTC QLQ-BR23 |
Every cycle until C4, q2 cycles from C6, EoT |
| PEARL | III | Advanced HR+/HER2− |
- Palbociclib + exemestane or fulvestrant (302) - Capecitabine (299) |
- EQ-5D-3L - EORTC QLQ-C30 - EORTC QLQ-BR23 |
Baseline, q2 cycles until C7, q3 cycles from C10, 1 follow-up |
| YOUNG PEARL | II | Advanced HR+/HER2− |
- Palbociclib + exemestane +OFS (92) - Capecitabine (92) |
- EORTC QLQ-C30 | Baseline, q6 weeks, EoT |
| A5481010 | I/II | Advanced HR+/HER2− |
- Palbociclib + letrozole (42) | - FACT-G - FACT-B - TOI |
C1-3, q2 cycles from C5 |
| PALLAS | III | Adjuvant HR+/HER2− |
- Palbociclib + standard ET (2883) - Placebo + standard ET (2887) |
- EORTC QLQ-C30 - EORTC QLQ-BR23 - BFI - mBPI - BCPT |
C1-3, q3 cycles until C24, 1 during the third year |
| PENELOPE-B | III | Adjuvant HR+/HER2− |
- Palbociclib +standard ET (628) - Placebo + standard ET (616) |
- EORTC QLQ-C30 - EORTC QLQ-BR23 - EORTC QLQ-FA13 |
Baseline, q2 cycles until C11, then q6 months, EoT |
| MONALEESA-2 | III | Advanced HR+/HER2− |
- Ribociclib + letrozole (334) - Placebo + letrozole (334) |
- EQ-5D-5L - EORTC QLQ-C30 - EORTC QLQ-BR23 |
Baseline, q2 cycles for 18 months, then q3 months, EoT |
| MONALEESA-3 | III | Advanced HR+/HER2− |
- Ribociclib + fulvestrant (484) - Placebo + fulvestrant (242) |
- EQ-5D-5L - EORTC QLQ-C30 - BPI-sf |
Baseline, q2 cycles for 18 months, then q3 months, EoT |
| MONALEESA-7 | III | Advanced HR+/HER2− |
- Ribociclib + ET (335) - Placebo + ET (337) |
- EQ-5D-5L - EORTC QLQ-C30 - EORTC QLQ-BR23 |
Each study visit |
| CompLEEment-1 | IV | Advanced HR+/HER2− |
- Ribociclib + letrozole (1230) | - FACT-B | C1-6, C8-10-12, then q3 cycles |
| CORALLEEN | II | Neoadjuvant HR+/HER2− |
- Ribociclib + letrozole (52) - Chemotherapy (54) |
- EORTC QLQ-C30 - EORTC QLQ-BR23 |
Baseline, every cycle, before surgery |
BCPT, Breast Cancer Prevention Trial; BPI-sf, Brief Pain Inventory-short form; C, cycle; EORTC QLQ-BR23, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Breast 23; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; EORTC QLQ-FA13, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Fatigue 13; EoT, end of treatment; EQ-5D, EuroQoL five dimensional; EQ-5D-3L, EuroQoL five dimensional three levels; EQ-5D-5L, EuroQoL five dimensional five levels; ET, endocrine therapy; FACIT, Functional Assessment of Chronic Illness Therapy Library; FACT-B, Functional Assessment of Cancer Therapy-Breast; FACT-ES, Functional Assessment of Cancer Therapy-Endocrine Symptoms; FACT-G, Functional Assessment of Cancer Therapy-General; FACT-GP5, Functional Assessment of Cancer Therapy-General Population 5; HER2−, human epidermal growth factor receptor negative; HR+, hormone receptor positive; HR-QoL, health-related quality of life; mBPI, modified Brief Pain Inventory; mBPI-sf, modified Brief Pain Inventory-short form; NSAI, non-steroidal aromatase inhibitor; OFS, ovarian function suppression; SoC, standard of care; TOI, trial outcome index.
Table 2.
HR-QoL outcomes in randomized/multi-arm clinical trials testing CDK4/6i in BC patients
| Trial name | Population | Questionnaires compliance rate | Outcomes better in CDK4/6i arm | Outcomes comparable in CDK4/6i and control arm | Outcomes better in control arm | Ref |
|---|---|---|---|---|---|---|
| MONARCH-3 | Total population | ≥ 96% At baseline, ≥88% during treatment, ≥ 70% at follow-up |
GHS | Diarrhea scores Nausea/vomiting Appetite loss |
18 | |
| Japanese subpopulation | NR | Financial difficulties | GHS | Diarrhea | 21 | |
| MONARCH-2 | Total population | ≥ 95% At baseline, ≥85% during treatment, ≥ 77% at follow-up |
TTD of pain scores | GHS | Diarrhea scores Nausea/vomiting Appetite loss |
19 |
| Japanese subpopulation | NR | Role functioning | GHS | Diarrhea | 22 | |
| monarcHER | Total population | 100% At baseline, ≥90% during treatment, ≥ 70% at follow-up |
TTD in physical and emotional functioning | GHS | Diarrhea | 17 |
| monarchE | Total population | ≥90% Both arms | GHS/QoL | 23 | ||
| PALOMA-1 | Total population | 95% | BPI GHS/QoL | 29 | ||
| PALOMA-2 | Total population | 96%-100% both arms | Pain scores; TTD of GHS |
GHS/QoL | 25,26 | |
| Asian subpopulation | 90%-100% both arms | FACT-B/FACT-G total scores EQ-5D GHS/QoL |
27 | |||
| PALOMA-3 | Total population | >96.9 Exp arm >95.8 control arm |
Pain scores GHS/QoL TTD |
Hair loss | 32 | |
| Asian subpopulation | >90% Both arms | Dyspnea | GHS/QoL | 33 | ||
| PALOMA-2, 3 | Pooled ≥65 years subpopulation |
NR | Pain scores | GHS/QoL | 28 | |
| PEARL | Total population | >82% Both arms | TTD | GHS/QoL | 36 | |
| YOUNG PEARL | Total population | NR | Appetite loss | EORTC QLQ-C30 global health status/QoL | Insomnia | 37 |
| PALLAS | Total population | NR | GHS/QoL | 40 | ||
| PENELOPE-B | Total population | 73.9% | Physical, cognitive and emotional fatigue GHS/QoL |
41 | ||
| MONALEESA-2 | Total population | 97.0% Exp arm 97.9% control arm |
Pain scores | EORTC QLQ-C30 GHS/QoL TTD by ≥10% in overall HR-QoL |
46,47 | |
| US subpopulation | 99% Exp arm 98% control arm |
Pain scores | EORTC QLQ-C30 GHS/QoL TTD by ≥10% in overall HR-QoL |
49 | ||
| ≥65 Years subpopulation | NR | Pain scores | EORTC QLQ-C30 GHS/QoL TTD by ≥10% in overall HR-QoL |
48 | ||
| MONALEESA-3 | Total population | 92.1% Exp arm 93.4% control arm |
TTD ≥10% in overall HR-QoL TTD ≥10% in EORTC QLQ-C30 functioning scores |
EORTC QLQ-C30 GHS/QoL | 54 | |
| MONALEESA-7 | Total population | >90% Exp arm >83% control arm |
TTD ≥10% in overall HR-QoL EORTC QLQ-C30 subdomains (pain fatigue, physical, emotional, social functioning) |
Work productivity | 50,52,53 | |
| NSAI subpopulation | NR | TTD ≥10% in overall HR-QoL EORTC QLQ-C30 subdomains (pain fatigue, physical, emotional, social functioning) |
Work productivity | 51 | ||
| MONALEESA-3, 7 | Pooleda | NR | GHS (greater benefit in patients living longer) | 55 | ||
| MONALEESA-2, 3, 7 | Pooledb | NR | TTD ≥10% in GHS, pain, emotional functioning scores | GHS/QoL | 56 | |
| CORALLEEN | Total population | 100% Ribociclib 98% chemotherapy |
EORTC QLQ-C30 GHS | 57 |
BC, breast cancer; BPI, Brief Pain Inventory; C, cycle; CDK4/6i, cyclin-dependent kinase 4/6 inhibitors; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; EQ-5D, EuroQoL five dimensional; FACT-B, Functional Assessment of Cancer Therapy-Breast; FACT-G, Functional Assessment of Cancer Therapy-General; GHS, global health status; HR, Health Related; NR, not reported; NSAI, non-steroidal aromatase inhibitor; QoL, quality of life; TTD, time to deterioration; US, United States.
Pooled analysis included overall population of the MONALEESA-3 trial and patients treated with ribociclib/placebo plus an NSAI in the MONALEESA-7.
Pooled analysis included overall population of the MONALEESA-2 study, in patients receiving ribociclib or placebo plus fulvestrant as first-line treatment in the MONALEESA-3 trial and in patients treated with ribociclib/placebo plus an NSAI in the MONALEESA-7 trial.
Abemaciclib
QoL evaluation was a secondary endpoint in two phase III trials testing abemaciclib plus ET in advanced HR+/HER2− BC (MONARCH-3 and MONARCH-2), in one phase II trial testing abemaciclib plus ET and trastuzumab in HR+/HER2+ advanced BC (aBC) (monarcHER) and in one phase III trial evaluating adjuvant abemaciclib plus ET in HR+/HER2− early BC (monarchE).5,15, 16, 17
Advanced setting
In the MONARCH-3 trial, postmenopausal endocrine-sensitive patients were randomized to abemaciclib or placebo plus a nonsteroidal aromatase inhibitor (NSAI), while in the MONARCH-2 trial premenopausal or postmenopausal endocrine-resistant patients were treated with abemaciclib or placebo plus fulvestrant.15,16 In both studies, HR-QoL was measured using the EORTC QLQ-C30, EORTC QLQ-BR23 and the EQ-5D 5 levels (5L) questionnaires, but results from the latter are not available.18,19 Additionally, in the MONARCH-2 trial, the modified BPI-sf (mBPI-sf) was employed.19 PROs were collected at baseline, every 2 cycles until cycle 19, every 3 cycles thereafter and at treatment discontinuation in the MONARCH-3 trial, whereas in the MONARCH-2 study, QoL was assessed at baseline, at cycle 2, every 2 cycles from cycle 3 to 13, every 3 cycles thereafter and at discontinuation. Questionnaire completion rates were high in both trials, ranging from ≥95% at baseline to ≥70% at the follow-up visit. QoL results were similar in these studies. No difference emerged between the experimental and the control group in terms of changes from baseline for symptoms and functioning scores, with the exception of gastrointestinal items. Indeed, >80% of patients treated with abemaciclib in these trials experienced any-grade diarrhea, with 10%-13% grade ≥3 events.20 Hence, changes from baseline in the EORTC QLQ-C30 symptom scales for diarrhea favored the control arm in both trials (24.64 ± 1.56; P < 0.001 in MONARCH-2 and 18.68 ± 1.80; P < 0.001 in MONARCH-3). Score changes in nausea/vomiting and appetite loss were also better with placebo. None of these differences, apart from diarrhea, however, met the ≥10-point predefined threshold for clinically meaningful decline.18,19
In the MONARCH-2 trial, pain evaluation by mBPI-sf revealed a numerically longer time to deterioration (TTD) among patients in the experimental arm [16.8 versus 11.9 months; hazard ratio (HR), 0.900; P = 0.400]. Median time to sustained deterioration of pain, measured by the EORTC QLQ-C30 pain item, also favored the abemaciclib arm over the control arm [HR 0.62; 95% confidence interval (CI) 0.48-0.79].19 According to subsequent analyses, HR-QoL reported by the Japanese subpopulations of the MONARCH-3 and MONARCH-2 trial did not significantly differ from the overall population’s results.21,22
In the phase II monarcHER study, patients with HR+/HER2+ aBC were treated with abemaciclib, trastuzumab and fulvestrant (group A), abemaciclib and trastuzumab (group B) or standard chemotherapy and trastuzumab (group C). QoL and pain were assessed with the EORTC QLQ-C30 and the mBPI-sf, completed at baseline, at the beginning of every cycle and at the first post-treatment follow-up. The EQ-5D-5L was also employed but its results have not been published yet. Completion rates spanned from 100% at baseline to 70% at the follow-up visit. In this study, the only significant and clinically meaningful difference emerged for the EORTC QLQ-C30 diarrhea item, which was worse in group A compared with group C. Patients in group A, however, experienced a significantly longer TTD in terms of physical and emotional functioning.17
Early setting
In the non-metastatic setting, PROs from the monarchE trial have been recently presented. This open-label phase III trial randomized patients with high-risk, early-stage BC to standard adjuvant ET with or without abemaciclib for up to 2 years.5 Patients had to complete the FACT-B questionnaire, the FACT endocrine symptoms (FACT-ES) subscale, the Functional Assessment of Chronic Illness Therapy (FACIT) library cognitive, bladder and fatigue symptom items and the EQ-5D-5L tool at baseline, after 3, 6, 12, 18 and 24 months and at follow-up. The reported compliance rate was ≥90%. Scores were similar between treatment arms, with patients receiving abemaciclib experiencing diarrhea ‘a little bit’ or ‘somewhat’. Fewer than 10% of patients, however, were bothered ‘quite a bit’ or ‘very much’ by treatment side-effects.23
Palbociclib
Results of HR-QoL evaluation are available from eight phase II and III trials testing palbociclib plus ET in HR+/HER2− BC patients, either in the advanced or early setting.
Advanced setting
The phase III PALOMA-2 study recruited postmenopausal women who had not received prior systemic therapy for HR+/HER2− aBC and randomized them to palbociclib or placebo plus letrozole.24 In this study the FACT-B and EQ-5D questionnaires were used. They were administered at baseline, at the beginning of cycles 2 and 3, then every other cycle until treatment discontinuation. Compliance rates were high in both treatment arms throughout the study period (≥95% until cycle 37, with a single 80% in the control arm at cycle 33). Overall scores from the FACT-B at baseline questionnaire were comparable between the experimental and the control group (101.5 ± 19.1 versus 103.2 ± 18.7) as well as their subsequent changes (−0.11, 95% CI 1.42-1.21 in the palbociclib arm versus 0.22, 95% CI, −1.68 to 2.12 in the placebo arm; P = 0.782), with a not significant trend towards longer TTD among patients receiving palbociclib.25,26 Of note, TTD of global health status (GHS) was significantly delayed in patients without disease progression and/or with an objective response, irrespectively of the treatment arm. In the two study groups there were no statistically significant differences in baseline EQ-5D scores observed during treatment [0.74, 95% CI 0.72-0.75 in the experimental arm versus 0.71, 95% CI 0.69-0.73 in the control arm; P = 0.093).25 These results were confirmed in specific subpopulations such as Asian patients and elderly women (≥65 years).27,28
Pain evaluation was a key secondary endpoint of the open-label randomized phase I/II PALOMA-1 trial. In this study, postmenopausal women receiving palbociclib plus letrozole or letrozole alone as first-line treatment of HR+/HER2− aBC were asked to complete the BPI at baseline, at the beginning of each cycle and at treatment discontinuation. Questionnaire’s compliance was ≥95% throughout the cycles. No significant differences in terms of pain severity and pain interference emerged at baseline and post-baseline assessments between the treatment arms. These data, however, were not adjusted for the concomitant use of painkillers.29
HR-QoL results are also available from a single-arm phase II study evaluating palbociclib plus letrozole in Japanese postmenopausal HR+/HER2− aBC patients. PROs were registered using the FACT-G, FACT-B and Treatment Outcome Index questionnaires at baseline, at the beginning of cycles 2 and 3 and then every other cycle. No significant changes emerged in HR-QoL overall scores and in the different subscales throughout the treatment period, with an estimated TTD of 43.0 months according to the FACT-B questionnaire.30
The PALOMA-3 study included women with HR+/HER2− endocrine-resistant aBC who could have received one prior chemotherapy line in the advanced setting.31 Patient-reported HR-QoL was assessed with the EORTC QLQ-C30, EORTC QLQ-BR23 and EQ-5D questionnaires (data not available for the latter instrument), on cycles 1-4, every other cycle from cycle 6 and at the end of treatment. After 14 cycles, ≥93% of patients completed at least one questionnaire. According to the EORTC QLQ-C30 results, GHS at baseline was superimposable in the two study groups, but the addition of palbociclib to fulvestrant granted a significant improvement in global HR-QoL scores [66.1 (95% CI 64.5-67.7) versus 63.0 (95% CI 60.6-65.3); P = 0.0313] and determined a greater delay in TTD of GHS compared with placebo plus fulvestrant. Additionally, patients in the experimental arm experienced an improvement from baseline in emotional functioning and pain scores. A decline in role functioning emerged in the palbociclib arm, however, although cognitive scores decreased in both arms during treatment. No significant differences emerged in the cancer-related symptom subscales of the EORTC QLQ-BR23, with the exception of hair loss, which was worse in the experimental arm.32 In the Asian subpopulation of the study, HR-QoL remained stable during treatment in both arms, except for a greater deterioration of dyspnea in the control group.33 Another subgroup analysis in patients aged ≥65 years demonstrated a delay in pain deterioration among women aged 65-74 years treated with palbociclib plus fulvestrant, but not among those older than 75 years.28
Palbociclib HR-QoL was also evaluated in comparison to chemotherapy. In the phase III PEARL and the phase II Young-PEARL trials, palbociclib plus ET was compared with capecitabine in postmenopausal and premenopausal HR+/HER2− aBC patients, respectively.34,35 In the PEARL trial, patient-reported HR-QoL was evaluated with the EORTC QLQ-C30, EORTC QLQ-BR23 and EQ-5D-3L questionnaires, completed at the baseline, every 2 cycles until cycle 7, every 3 cycles subsequently and at the post-treatment visit. The registered compliance until cycle 13 rate was ≥82%. Patients treated with palbociclib and ET experienced an improvement in GHS from baseline to cycle 3 compared with those treated with capecitabine (+2.9 in the experimental arm versus −2.1 in the control arm, P = 0.007), along with a significant delay in TTD (8.3 months in the experimental arm versus 5.3 months in the control arm; HR 0.70; 95% CI 0.55-0.89; P = 0.003). Better scores were registered in the palbociclib arm for fatigue, pain, dyspnea, insomnia, constipation and diarrhea, whereas patients reported lower hair loss and better sexual enjoyment in the capecitabine arm.36 In the Young-PEARL study, the EORTC QLQ-C30 questionnaire was administered at baseline, every 6 weeks and at the end of treatment with a completion rate of at least one timepoint 100% in the experimental arm and 94.2% in the control arm after 84 weeks. Changes in GHS/QoL scores and TTD were similar between the two treatment arms. A significant delay in TTD was observed, however, in physical functioning, emesis and diarrhea scores among women receiving palbociclib plus ET.37
Early setting
Recently, palbociclib in combination with ET has been evaluated in the adjuvant setting in two phase III trials.38,39 In the open-label PALLAS study, stage II-III HR+/HER− BC patients were randomized to 2 years of palbociclib plus standard endocrine adjuvant therapy or standard endocrine adjuvant therapy alone.38 QoL was measured using the EORTC QLQ-C30 and EORTC QoL-BR23 questionnaires on cycles 1-3, then every 3 cycles and once during the third year. Pain, fatigue, hot flushes, vaginal problems and musculoskeletal symptoms were also assessed at the same timepoints by the mBPI, the Brief Fatigue Inventory (BFI) and the Breast Cancer Prevention Trial (BCPT) symptom scales, with a completion rate ≥89%. No significant differences emerged over time in GHS and in any subscale.40 The PENELOPE-B trial evaluated the addition of 1 year of palbociclib to standard ET among HR+/HER2− women without a pathological complete response after neoadjuvant therapy.39 Patients were asked to complete the EORTC QLQ-C30, EORTC QLQ-BR23 and EORTC QLQ-Fatigue13 (FA13) questionnaires at screening, every other cycle for the first year and every 6 months until the end of treatment thereafter, with an overall 73% compliance. Even in this case, HR-QoL remained comparable between treatment arms throughout the treatment period, but higher fatigue scores were reported among patients receiving palbociclib.41
Ribociclib
QoL represented a secondary endpoint in three phase III (MONALEESA-2, MONALEESA-3, MONNALESA-7), one phase IIIb (CompLEEment-1) and one phase II (CORRALEEN) trials testing ribociclib and ET in BC patients.3,42, 43, 44, 45
Advanced setting
In the MONALEESA-2 study, postmenopausal endocrine-sensitive HR+/HER2− aBC patients were randomized to receive ribociclib or placebo in combination with letrozole.3 HR-QoL was assessed using EORTC QLQ-C30 and BR23 questionnaires along with the EQ-5D-5L questionnaire at screening, every 8 weeks for the first 18 months of treatment, every 12 weeks thereafter and at the time of study discontinuation. Results from the EQ-5D-5L questionnaire have not been published, determining a potential reporting bias. Questionnaire completion rates ranged from >97% in both arms at baseline to 75% at cycle 25. During the treatment period, GHS and QoL scores remained stable and resulted similar in the two arms (TTD ≥10% 27.7 months in the ribociclib arm versus 26.7 months in the placebo arm; HR 0.944, 95% CI 0.720-1.237). A significant TTD delay was observed, however, in patients not experiencing a progression event compared with those whose disease progressed.46 Additionally, pain reduction at 8 weeks was greater in the experimental arm compared with the placebo arm (26% and 15%, respectively), with a clinically meaningful (>5 points) decrease in pain scores maintained up to cycle 15 among patients receiving ribociclib.47 Similar results in terms of GHS and pain scores emerged from two subgroup analyses carried out in elderly patients (≥65 years) and in the subpopulation from the United States enrolled in the trial.48,49
The MONALEESA-7 trial was designed to specifically test ribociclib plus ET (NSAI or tamoxifen and ovarian function suppression) in a premenopausal population with advanced HR+/HER2− BC.43 In this study, PROs were registered using the EORTC QLQ-C30, QLQ-BR23, EQ-5D-5L and the Work Productivity and Activity Impairment-General Health (WPAI-GH) questionnaires, collected at the beginning of each visit. A lower percentage of patients in the control group (≥83%) completed the questionnaires at >1 baseline assessment compared with the experimental group (≥90%). In this study, TTD in GHS was significantly delayed in the ribociclib arm compared with the placebo arm (median 35.8 versus 23.3 months, respectively; HR 0.67, 95% CI 0.52-0.86). As reported in the MONALEESA-2 and -3 trials, patients without disease progression experienced a greater delay in TTD of GHS compared with patients with disease progression. Additionally, ribociclib led to a longer maintenance in key subdomains (pain, fatigue, physical, emotional and social functioning) of the EORTC QLQ-C30 questionnaire, compared with placebo.50 When considering only the NSAI cohort, results were similar to those reported for the overall population.51 According to WPAI, work productivity was maintained in both arms.52 Moreover, a post hoc analysis demonstrated that higher fatigue and pain and lower overall HR-QoL, physical, role, social and emotional functioning levels were associated with a greater productivity loss.53
The CompLEEment-1 study was a single-arm phase IIIb trial, investigating first-line ribociclib in combination with letrozole.44 QoL was scored with the FACT-B questionnaire on day 1 of cycle 1-6, every 2 cycles until cycle 12 and every 3 cycles thereafter. In this study, the median delay to first occurrence of a clinically relevant deterioration (≥7-point decrease) in overall FACT-B score was not reached, implying that QoL was maintained while on treatment. Similarly, individual FACT-B domain scores for emotional and functional well-being did not decrease below baseline level throughout the treatment.44
The MONALEESA-3 trial randomized men and postmenopausal women to ribociclib plus fulvestrant or placebo plus fulvestrant.42 PROs were collected using the EORTC QLQ-C30 questionnaire along with the Brief Pain Inventory-short form (BPI-sf) and the EQ-5D-5L tool, at the same timepoints reported for the MONALEESA-2 trial. The questionnaire compliance rate remained ≥80% during the first 12 months of treatment. Compared with baseline, GHS persistently improved until the end of study in both arms. Even in this trial, a TTD delay in GHS was observed among patients without disease progression.54 Consistently, a pooled analysis including the overall population of the MONALEESA-3 trial and patients treated with ribociclib/placebo plus an NSAI in the MONALEESA-7 trial demonstrated a greater GHS improvement in patients with longer overall survival, especially among subjects receiving ribociclib.55 Another pooled analysis of HR-QoL results was conducted in the whole population of the MONALEESA-2 study, in patients receiving ribociclib plus fulvestrant or placebo plus fulvestrant as first-line treatment in the MONALEESA-3 trial and in patients treated with ribociclib/placebo plus an NSAI in the MONALEESA-7 trial. In line with the result of the single studies, GHS and QoL were maintained throughout the treatment period to decline at the time of study discontinuation in both arms, whereas TTD of GHS, pain and emotional functioning was delayed in the ribociclib group.56
Early setting
CORRALEEN was a phase II neoadjuvant trial comparing ribociclib plus letrozole with chemotherapy in women with HR+/HER2− luminal B early-stage BC. Patient-reported HR-QoL was assessed using the EORTC QLQ-C30 and QLQ-BR23 questionnaires at baseline, at each cycle and before surgery. Whereas the EORT QLQ-C30 GHS scores were similar at baseline between the two groups, they considerably decreased before surgery in the chemotherapy arm compared with the ribociclib arm. Notably, 38% of patients in the ribociclib group and 68% of patients in the chemotherapy group reported a clinically meaningful deterioration in HR-QoL by the end of treatment.57
HR-QoL in the real-world setting
Limited data are available about HR-QoL among subjects treated with CDK4/6i in the real-world scenario. Most of the evidence in this setting regards patients receiving palbociclib, with a single study including subjects treated with any CDK4/6i (Table 3).
Table 3.
HR-QoL from CDK4/6 inhibitors real-world experiences in BC patients
| Author (year) | Intervention | Setting | N | Evaluation tools | Evaluation timepoints | HR-QoL outcomes | Ref |
|---|---|---|---|---|---|---|---|
| Loi (2021) | Palbociclib + letrozole | Advanced | 252 | EQ-5D | Baseline, every cycle, EoT | No significant changes in GHS from baseline | 59 |
| Rahman (2022) | Palbociclib + letrozole | Advanced | 88 | FACIT-F | Screening, baseline, third week for 6 cycles | 24% New-onset fatigue during treatment; 15% severe fatigue | 63 |
| Richardson (2021) | Palbociclib + ET | Advanced | 139 | SF-12, CES-D-10, targeted questions |
Baseline, different evaluations on daily, weekly and monthly base for 6 months | No significant changes in GHS from baseline; low depression incidence | 62 |
| Rocque (2020) | Palbociclib + ET | Advanced | 522 | EORTC QLQ-C30 | Baseline, q3 months, EoT | No significant changes in GHS status from baseline; trend towards pain improvement | 60 |
| Rocque (2021)a | Palbociclib + ET | Advanced | 233 | EORTC QLQ-C30 | Baseline, q3 months, EoT | No significant changes in GHS from baseline | 61 |
| Stearns (2018) | Palbociclib + letrozole | Advanced | 334 | EQ-5D | Baseline, every cycle, EoT | No significant changes in GHS from baseline | 58 |
| Oswald (2021) | CDK4/6 inhibitor + ET | Advanced | 20 | FACT-B, FACT-G, FACIT-F, BCPT, NEQ |
1-Time evaluation | HR-QoL scores 104.8 (SD 18.4) with the FACT-B and 80.3 (SD 12.5) with the FACT-G; mean fatigue score 33.8 (SD 12.1); patients slightly to moderately bothered at the BCPT symptoms scale; 2.8 average unmet needs at the NEQ |
64 |
BC, breast cancer; BCPT, Breast Cancer Prevention Trial; BPI-sf, Brief Pain Inventory-short form; CDK4/6, cyclin-dependent kinase 4/6; CES-D-10, Center for Epidemiological Studies Depression Scale; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; EoT, end of treatment; EQ-5D, EuroQoL five dimensional; ET, endocrine treatment; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; FACT-B, Functional Assessment of Cancer Therapy-Breast; FACT-G, Functional Assessment of Cancer Therapy-General; HR-QoL, health-related quality of life; GHS, global health status; NEQ, Needs Evaluation Questionnaire; SD, standard deviation; SF-12, Short Form-12.
Black, indigenous and people of color subpopulation of the POLARIS study (interim analysis of total population reported by Rocque et al.60 in 2020).
Data about HR-QoL have been reported for the Canadian and Australian cohort of the palbociclib plus letrozole expanded access program. For this purpose, the EQ-5D questionnaire was employed on day 1 of each cycle and at the time of treatment discontinuation. In both cohorts, questionnaire completion rates were high (>90%) and general health status remained stable over the observation period.58,59 The prospective, observational multicenter POLARIS study evaluated HR-QoL in patients with HR+/HER2− aBC treated with palbociclib plus ET.60,61 The EORTC QLQ-C30 questionnaire was administered at baseline, monthly until cycle 3 and then every 3 months. According to preliminary results on the first 522 enrolled patients, HR-QoL remained stable during the first 6 months of treatment, with a trend toward pain improvement.60 A longer follow-up is available for the black, indigenous and people of color (BIPOC) study subpopulation, with 112 patients who completed 12 months of treatment. Even in this subgroup, HR-QoL and symptom scores did not significantly change from baseline.61 In another prospective observational study, PROs of 139 individuals treated with palbociclib plus ET were registered using a mobile application. Patients were trained to complete different questionnaires at various timepoints for 6 months. Daily assessments included pain, mood and fatigue whereas family/social life, productivity, physical activities, energy and overall health and QoL were evaluated weekly. The Short Form-12 (SF-12) and the Center for Epidemiological Studies Depression Scale (CES-D-10) were administered on a monthly base. Overall, general health status did not decline through the observation period, whereas depression incidence remained low.62 Patient-reported fatigue was specifically assessed by the FACIT-F scale among 88 subjects treated with palbociclib and ET in a real-world context. Patients completed the questionnaire at baseline and on the third week of 6 consecutive cycles. Twenty-four percent of patients experienced the onset of fatigue with a 15% incidence of severe symptoms.63 Oswald and colleagues64 reported on the HR-QoL of a small cohort of patients treated with any CDK4/6i and ET, using multiple questionnaires [FACT-B, FACT-G, FACIT-F, BCPT Symptom Scales and the Needs Evaluation Questionnaire (NEQ)] administered at a single timepoint. Of the 20 patients enrolled, 14 were receiving palbociclib, 3 abemaciclib and 3 ribociclib. The average HR-QoL scores obtained were 104.8/148 [standard deviation (SD) 18.4] with FACT-B and 80.3/108 (SD 12.5) with FACT-G. Mean fatigue score was 33.8 (SD 12.1), thus exceeding the ≤34 threshold for fatigue severity. Lastly, according to the BCPT symptoms scale, patients reported to be slightly to moderately bothered by symptoms and declared a mean of 2.8 unmet needs at the NEQ.64
Discussion
The combination of a CDK4/6i with ET represents the current standard of care for HR+/HER2− aBC.2 Hence, many patients are currently receiving a CDK4/6i worldwide, and their number is expected to increase in the future, making the impact of these drugs on HR-QoL a relevant issue. To our knowledge this is the first systematic review about HR-QoL in ER+/HER2− BC patients treated with CDK4/6i.
Overall, the results show that the addiction of a CDK4/6i to ET does not worsen patient HR-QoL, with a positive trend towards pain improvement. Nonetheless, some differences among different CDK4/6i may be highlighted.
In clinical trials testing abemaciclib, HR-QoL was mainly affected by the relevant incidence of diarrhea.20 Indeed, in the MONARCH-2, MONARCH-3 and monarcHER trials, gastrointestinal items (diarrhea, nausea/vomiting, appetite loss) were the only EORTC QLQ-C30 parameters which statistically favored the control arm.17, 18, 19 These findings were confirmed by a matching adjusted indirect comparison analysis of QoL outcomes from the MONARCH-3 and MONALEESA-2 trials.65 Conversely, gastrointestinal scores were similar between experimental and control arms of the monarchE trial, with <10% of patients severely bothered by side-effects.23 Of note, the rate of G3 diarrhea in this trial was lower (7%) compared with that reported in the advanced setting studies (∼15%).20 An improvement over time in the management of this symptom may represent a potential explanation for the different toxicity rate and HR-QoL observed in the advanced and early disease.
Palbociclib was the first CDK4/6i approved by regulatory agencies and the one displaying more HR-QoL evidence from clinical trials. Even though palbociclib has a favorable toxicity profile, it has not shown a QoL improvement in trials evaluating its addition to ET.26,31 In the PEARL and Young-PEARL studies, however, palbociclib plus ET showed better HR-QoL outcomes and a better safety profile compared with capecitabine.36,37
Ribociclib determined more satisfactory results, particularly among premenopausal patients.43 Preserving HR-QoL in this subgroup of patients is challenging, since younger women usually present worse HR-QoL and lower social and emotional scores compared with older patients.66 Hence, the maintenance of overall QoL and work productivity along with the delay in TTD of GHS reported in the MONALEESA-7 trial are particularly relevant in clinical practice. Given these results, ribociclib and letrozole in premenopausal patients represents the only CDK4/6i combination, and more in general the only regimen, awarded with the top score of the MCBS in the non-curative BC setting (i.e. 5/5).67
Data about HR-QoL of BC patients treated with CDK4/6i outside clinical trials are scarce and most of them have been registered in patients treated with palbociclib. The main limitations of PROs collection in the real-world setting include small sample size and haphazard approaches to therapeutic regimens and questionnaires employed. Overall, the available evidence seems to confirm HR-QoL results obtained in clinical trials, without major discordance or new observations.
Given methodological heterogeneity in HR-QoL evaluations, it is difficult to derive definitive conclusions and to perform direct comparisons (i.e. a meta-analysis) between the three CDK4/6i. Still, a striking and consistent correlation exists between disease outcomes and PROs. In other words, patients whose disease responds to treatment display better QoL regardless of the therapy received. Vice versa, a pooled analysis of the MONARCH 1, 2 and 3 trials demonstrated that PROs are independently associated with treatment efficacy, since patients with low physical function scores displayed shorter PFS compared with patients with higher scores.68 These results reinforce the assumption that objective outcomes, reported by the investigators, and subjective outcomes, reported by patients, are tightly correlated and should both be considered to judge the overall value of a treatment. In this light, CDK4/6i provided an unprecedented advancement in the management of BC patients by conjugating efficacy and QoL preservation. To capture the overarching benefit of these therapies, the introduction of novel composite outcome measures, like the overall treatment utility, can be useful in the future.69 The often prolonged patients’ exposure to CDK4/6i could determine long-term effects (e.g. cognitive impairment, functioning deterioration), however, which can be currently underestimated and will deserve further investigation.70 Still, the available data about the impact of CDK4/6i on QoL support their use in every patient subpopulation, including younger and elderly patients.50,71
In the future, a concerted effort should be made to uniform HR-QoL evaluation in clinical trials testing CDK4/6i, to produce scientific data which could allow direct comparison between different drugs and to inform patient-centered therapeutic choices. An even more substantial effort will be required to incorporate PROs evaluation in the clinical practice. To this end, innovative digital tools are being developed, with the ultimate goal of improving the quality of care for cancer patients.72
Funding
None declared.
Disclosure
VDL received speakers and travel grants from Roche, Novartis, Lilly, Pfizer, Gilead, Seagen, Gentili, Takeda and Exact Sciences. GB received speakers grants from Novartis and travel grants from Lilly, Novartis and Pfizer. FM received speakers grants from Istituto Gentili, Lilly, Novartis, Pfizer; advisory grant from Amgen; travel grants from Gilead and Roche. MM received speakers grant from Lilly, Pfizer and Novartis. GA received consultant grants from Roche, AstraZeneca, Eli Lilly, Pfizer, Novartis and speakers grant from Roche, Sandoz, Takeda, Pfizer, Eli Lilly and Novartis. LDM received honoraria and non-financial support from Roche, Novartis, Pfizer, Merck Sharp & Dohme (MSD), Genomic Health, Takeda, Ipsen, Eisai, Eli Lilly, Celgene, Pierre Fabre, Seagen, Daiichi Sankyo, Exact Sciences and Amgen. MG received speaker’s bureau from Lilly, Novartis, Pfizer, Roche, Eisai, Seagen, AstraZeneca, MSD. FP received consultant grants from Amgen, AstraZeneca, Daiichi Sankyo, Eisai, Eli Lilly, MSD, Novartis, Pierre-Fabre, Roche, Seagen and research funding from AstraZeneca, Eisai, Roche, Celgene, GlaxoSmithKline, other. MDL received honoraria from Roche, Novartis, Pfizer, Lilly, Amgen, Pierre Fabre, AstraZeneca, MSD, Seattle Genetics, Gilead Sciences, Takeda, Ipsen; consulting or advisory grant from Roche, Novartis, Pfizer, Lilly, Amgen, AstraZeneca, MSD, Pierre Fabre, Seattle Genetics, Gilead Sciences, Ipsen, Takeda; speaker’s bureau from Novartis; research funding from Novartis, Roche, Lilly, Puma Biotechnology, Pfizer, Daiichi Sankyo, MSD, MacroGenics, Bristol Myers Squibb.
All other authors have declared no conflicts of interest.
Supplementary data
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across the included randomized controlled trials.
Risk of bias summary: review authors’ judgements about each risk of bias item for each randomized controlled trial included.
References
- 1.Burstein H.J. Systemic therapy for estrogen receptor-positive, HER2-negative breast cancer. N Engl J Med. 2020;383:2557–2570. doi: 10.1056/NEJMra1307118. [DOI] [PubMed] [Google Scholar]
- 2.Spring L.M., Wander S.A., Andre F., Moy B., Turner N.C., Bardia A. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet. 2020;395:817–827. doi: 10.1016/S0140-6736(20)30165-3. [DOI] [PubMed] [Google Scholar]
- 3.Hortobagyi G.N., Stemmer S.M., Burris H.A., et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386:942–950. doi: 10.1056/NEJMoa2114663. [DOI] [PubMed] [Google Scholar]
- 4.Loibl S., Furlanetto J. Integrating CDK4/6 inhibitors in the treatment of patients with early breast cancer. Breast. 2022;62(Suppl 1):S70–S79. doi: 10.1016/j.breast.2021.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston S.R.D., Harbeck N., Hegg R., et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE) J Clin Oncol. 2020;38:3987–3998. doi: 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vodicka E., Kim K., Devine E.B., Gnanasakthy A., Scoggins J.F., Patrick D.L. Inclusion of patient-reported outcome measures in registered clinical trials: evidence from ClinicalTrials.gov (2007-2013) Contemp Clin Trials. 2015;43:1–9. doi: 10.1016/j.cct.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Bottomley A., Reijneveld J.C., Koller M., et al. Current state of quality of life and patient-reported outcomes research. Eur J Cancer. 2019;121:55–63. doi: 10.1016/j.ejca.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso F., Paluch-Shimon S., Senkus E., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31:1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherny N.I., Sullivan R., Dafni U., et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) Ann Oncol. 2015;26:1547–1573. doi: 10.1093/annonc/mdv249. [DOI] [PubMed] [Google Scholar]
- 10.Karimi M., Brazier J. Health, health-related quality of life, and quality of life: what is the difference? Pharmacoeconomics. 2016;34:645–649. doi: 10.1007/s40273-016-0389-9. [DOI] [PubMed] [Google Scholar]
- 11.Gordon B.E., Chen R.C. Patient-reported outcomes in cancer survivorship. Acta Oncol. 2017;56:166–173. doi: 10.1080/0284186X.2016.1268265. [DOI] [PubMed] [Google Scholar]
- 12.Cardoso F., Cella D., Velikova G., et al. Quality-of-life methodology in hormone receptor-positive advanced breast cancer: current tools and perspectives for the future. Cancer Treat Rev. 2022;102 doi: 10.1016/j.ctrv.2021.102321. [DOI] [PubMed] [Google Scholar]
- 13.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne J.A.C., Savovic J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 15.Goetz M.P., Toi M., Campone M., et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 16.Sledge G.W., Toi M., Neven P., et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 17.Tolaney S.M., Wardley A.M., Zambelli S., et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21:763–775. doi: 10.1016/S1470-2045(20)30112-1. [DOI] [PubMed] [Google Scholar]
- 18.Goetz M.P., Martin M., Tokunaga E., et al. Health-related quality of life in MONARCH 3: abemaciclib plus an aromatase inhibitor as initial therapy in HR+, HER2- advanced breast cancer. Oncologist. 2020;25:e1346–e1354. doi: 10.1634/theoncologist.2020-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman P.A., Toi M., Neven P., et al. Health-related quality of life in MONARCH 2: abemaciclib plus fulvestrant in hormone receptor-positive, HER2-negative advanced breast cancer after endocrine therapy. Oncologist. 2020;25:e243–e251. doi: 10.1634/theoncologist.2019-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rugo H.S., Huober J., Garcia-Saenz J.A., et al. Management of abemaciclib-associated adverse events in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: safety analysis of MONARCH 2 and MONARCH 3. Oncologist. 2021;26:e53–e65. doi: 10.1002/onco.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi M., Tokunaga E., Mori J., et al. Japanese subgroup analysis of the phase 3 MONARCH 3 study of abemaciclib as initial therapy for patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. Breast Cancer. 2022;29:174–184. doi: 10.1007/s12282-021-01295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue K., Masuda N., Iwata H., et al. Japanese subpopulation analysis of MONARCH 2: phase 3 study of abemaciclib plus fulvestrant for treatment of hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer that progressed on endocrine therapy. Breast Cancer. 2021;28:1038–1050. doi: 10.1007/s12282-021-01239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tolaney S., Blancas I., Im Y.H., et al. Patients’ quality of life and side effect perceptions in monarchE, a study of abemaciclib plus endocrine therapy in adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer. Breast. 2021;56:S20–S21. [Google Scholar]
- 24.Finn R.S., Martin M., Rugo H.S., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 25.Rugo H.S., Diéras V., Gelmon K.A., et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: results from the PALOMA-2 trial. Ann Oncol. 2018;29:888–894. doi: 10.1093/annonc/mdy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rugo H.S., Finn R.S., Diéras V., et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174:719–729. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Im S.A., Mukai H., Park I.H., et al. Palbociclib plus letrozole as first-line therapy in postmenopausal Asian women with metastatic breast cancer: results from the phase III, randomized PALOMA-2 study. J Global Oncol. 2019;5:1–19. doi: 10.1200/JGO.18.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rugo H.S., Turner N.C., Finn R.S., et al. Palbociclib plus endocrine therapy in older women with HR+/HER2– advanced breast cancer: a pooled analysis of randomised PALOMA clinical studies. Eur J Cancer. 2018;101:123–133. doi: 10.1016/j.ejca.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Bell T., Crown J.P., Lang I., et al. Impact of palbociclib plus letrozole on pain severity and pain interference with daily activities in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer as first-line treatment. Curr Med Res Opin. 2016;32:959–965. doi: 10.1185/03007995.2016.1157060. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi M., Masuda N., Nishimura R., et al. Palbociclib-letrozole as first-line treatment for advanced breast cancer: updated results from a Japanese phase 2 study. Cancer Med. 2020;9:4929–4940. doi: 10.1002/cam4.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner N.C., Slamon D.J., Ro J., et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 32.Harbeck N., Iyer S., Turner N., et al. Quality of life with palbociclib plus fulvestrant in previously treated hormone receptor-positive, HER2-negative metastatic breast cancer: patient-reported outcomes from the PALOMA-3 trial. Ann Oncol. 2016;27:1047–1054. doi: 10.1093/annonc/mdw139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwata H., Im S.A., Masuda N., et al. PALOMA-3: phase III trial of fulvestrant with or without palbociclib in premenopausal and postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer that progressed on prior endocrine therapy-safety and efficacy in Asian patients. J Glob Oncol. 2017;3:289–303. doi: 10.1200/JGO.2016.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin M., Zielinski C., Ruiz-Borrego M., et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: a phase III randomised controlled trial-PEARL. Ann Oncol. 2021;32:488–499. doi: 10.1016/j.annonc.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Park Y.H., Kim T.Y., Kim G.M., et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019;20:1750–1759. doi: 10.1016/S1470-2045(19)30565-0. [DOI] [PubMed] [Google Scholar]
- 36.Kahan Z., Gil-Gil M., Ruiz-Borrego M., et al. Health-related quality of life with palbociclib plus endocrine therapy versus capecitabine in postmenopausal patients with hormone receptor-positive metastatic breast cancer: patient-reported outcomes in the PEARL study. Eur J Cancer. 2021;156:70–82. doi: 10.1016/j.ejca.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Lee S., Im S.A., Kim G.M., et al. Patient-reported outcomes of palbociclib plus exemestane with GnRH agonist versus capecitabine in premenopausal women with hormone receptor-positive metastatic breast cancer: a prospective, open-label, randomized phase ll trial (KCSG-BR 15-10) Cancers (Basel) 2020;12:3265. doi: 10.3390/cancers12113265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gnant M., Dueck A.C., Frantal S., et al. Adjuvant palbociclib for early breast cancer: the PALLAS trial results (ABCSG-42/AFT-05/BIG-14-03) J Clin Oncol. 2022;40:282–293. doi: 10.1200/JCO.21.02554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loibl S., Marme F., Martin M., et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer-the Penelope-B trial. J Clin Oncol. 2021;39:1518–1530. doi: 10.1200/JCO.20.03639. [DOI] [PubMed] [Google Scholar]
- 40.Naughton M.J., Zahrieh D., Gnant M., et al. Abstract P4-10-01: quality of life and symptom severity in the PALLAS randomized trial of palbociclib with adjuvant endocrine therapy in early breast cancer (AFT-05) Cancer Res. 2022;82 P4-10-01-P14-10-01. [Google Scholar]
- 41.García-Saenz J.A., Marmé F., Rugo H.S., et al. 122MO Quality of life from the Penelope-B study on high-risk HR+/HER2- early breast cancer patients treated with endocrine therapy with or without palbociclib. Ann Oncol. 2021;32:S410–S411. [Google Scholar]
- 42.Slamon D.J., Neven P., Chia S., et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol. 2021;32:1015–1024. doi: 10.1016/j.annonc.2021.05.353. [DOI] [PubMed] [Google Scholar]
- 43.Im S.A., Lu Y.S., Bardia A., et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381:307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 44.De Laurentiis M., Borstnar S., Campone M., et al. Full population results from the core phase of CompLEEment-1, a phase 3b study of ribociclib plus letrozole as first-line therapy for advanced breast cancer in an expanded population. Breast Cancer Res Treat. 2021;189:689–699. doi: 10.1007/s10549-021-06334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prat A., Saura C., Pascual T., et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020;21:33–43. doi: 10.1016/S1470-2045(19)30786-7. [DOI] [PubMed] [Google Scholar]
- 46.Verma S., O'Shaughnessy J., Burris H.A., et al. Health-related quality of life of postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treated with ribociclib + letrozole: results from MONALEESA-2. Breast Cancer Res Treat. 2018;170:535–545. doi: 10.1007/s10549-018-4769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janni W., Alba E., Bachelot T., et al. First-line ribociclib plus letrozole in postmenopausal women with HR+, HER2− advanced breast cancer: tumor response and pain reduction in the phase 3 MONALEESA-2 trial. Breast Cancer Res Treat. 2018;169:469–479. doi: 10.1007/s10549-017-4658-x. [DOI] [PubMed] [Google Scholar]
- 48.Burris H.A., Tolaney S.M., Hart L.L., et al. Maintenance of health-related quality of life in elderly patients treated with ribociclib + letrozole in MONALEESA-2. J Clin Oncol. 2018;36 1041. [Google Scholar]
- 49.Yardley D.A., Hart L., Favret A., et al. Efficacy and safety of ribociclib with letrozole in US patients enrolled in the MONALEESA-2 study. Clin Breast Cancer. 2019;19:268–277.e261. doi: 10.1016/j.clbc.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Harbeck N., Franke F., Villanueva-Vazquez R., et al. Health-related quality of life in premenopausal women with hormone-receptor-positive, HER2-negative advanced breast cancer treated with ribociclib plus endocrine therapy: results from a phase III randomized clinical trial (MONALEESA-7) Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920943065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu Y.S., Bardia A., Vázquez R.V., et al. Updated overall survival (OS) and quality of life (QoL) in premenopausal patients (pts) with advanced breast cancer (ABC) who received ribociclib (RIB) or placebo (PBO) plus goserelin and a nonsteroidal aromatase inhibitor (NSAI) in the MONALEESA-7 (ML-7) trial. Ann Oncol. 2019;30:v106. [Google Scholar]
- 52.Harbeck N., Hurvitz S., Bardia A., et al. Abstract P1-19-06: Patient-reported outcomes, including work productivity, from the MONALEESA-7 trial of ribociclib plus endocrine therapy in patients with HR+/HER2− advanced breast cancer. Cancer Res. 2020;80 P1-19-06-P11-19-06. [Google Scholar]
- 53.Tripathy D., Curteis T., Hurvitz S.A., et al. Correlation between work productivity loss (WPL) and European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30) domains from the MONALEESA-7 (ML-7) trial of premenopausal women with HR+/HER2- advanced breast cancer (ABC) J Clin Oncol. 2021;39 1051. [Google Scholar]
- 54.Fasching P.A., Beck J.T., Chan A., et al. Ribociclib plus fulvestrant for advanced breast cancer: health-related quality-of-life analyses from the MONALEESA-3 study. Breast. 2020;54:148–154. doi: 10.1016/j.breast.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fasching P.A., Harbeck N., Jerusalem G., et al. 233P Association of quality of life (QOL) with overall survival (OS) in patients (pts) with HR+/HER2− advanced breast cancer (ABC) treated with ribociclib (RIB) + endocrine therapy (ET) in the MONALEESA-3 (ML-3) and ML-7 trials. Ann Oncol. 2021;32:S460. [Google Scholar]
- 56.Fasching P.A., Bardia A., Nusch A., et al. 276O Pooled analysis of patient (pt)-reported quality of life (QOL) in the MONALEESA (ML)-2, -3, and -7 trials of ribociclib (RIB) plus endocrine therapy (ET) to treat hormone receptor–positive, HER2-negative (HR+/HER2−) advanced breast cancer (ABC) Ann Oncol. 2020;31:S350–S351. [Google Scholar]
- 57.Villacampa G., Paré L., Hernando C., et al. Abstract P4-10-04: Health-related quality of life (HRQoL) in hormone receptor-positive, HER2-negative, luminal B breast cancer patients treated with ribociclib plus letrozole or chemotherapy. Cancer Res. 2022;82 P4-10-04-P14-10-04. [Google Scholar]
- 58.Stearns V., Brufsky A.M., Verma S., et al. Expanded-access study of palbociclib in combination with letrozole for treatment of postmenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer. Clin Breast Cancer. 2018;18:e1239–e1245. doi: 10.1016/j.clbc.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Loi S., Karapetis C.S., McCarthy N., et al. Palbociclib plus letrozole as treatment for postmenopausal women with hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer for whom letrozole therapy is deemed appropriate: an expanded access study in Australia and India. Asia-Pac J Clin Oncol. 2021 doi: 10.1111/ajco.13653. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rocque G., Blum J.L., Montero A., et al. Abstract PD10-03: quality of life in patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer treated with palbociclib in real-world practice settings. Cancer Res. 2020;80 PD10-03-PD10-03. [Google Scholar]
- 61.Rocque G.B., Blum J.L., Montero A., et al. Real-world quality of life (QoL) in black, indigenous and people of color (BIPOC) treated with palbociclib (PAL) and endocrine therapy for hormone receptor–positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC): a subgroup analysis from POLARIS. J Clin Oncol. 2021;39 1071. [Google Scholar]
- 62.Richardson D., Zhan L., Mahtani R., et al. A prospective observational study of patient-reported functioning and quality of life in advanced and metastatic breast cancer utilizing a novel mobile application. Breast Cancer Res Treat. 2021;187:113–124. doi: 10.1007/s10549-020-06082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahman S.A., Mayer E.L., Poort H., et al. Abstract P4-09-01: Incidence of patient-reported fatigue developing in patients receiving palbociclib and endocrine therapy for advanced HR+ HER2- breast cancer. Cancer Res. 2022;82 P4-09-01-P04-09-01. [Google Scholar]
- 64.Oswald L.B., Arredondo B., Kadono M., et al. A mixed-methods study of cyclin-dependent kinase 4 and 6 inhibitor symptom burden and quality of life among metastatic breast cancer patients and providers. Cancer Med. 2021;10:4823–4831. doi: 10.1002/cam4.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rugo H.S., O'Shaughnessy J., Jhaveri K.L., et al. Quality of life (QOL) with ribociclib (RIB) plus aromatase inhibitor (AI) versus abemaciclib (ABE) plus AI as first-line (1L) treatment (tx) of hormone receptor-positive/human epidermal growth factor receptor-negative (HR+/HER2-) advanced breast cancer (ABC), assessed via matching-adjusted indirect comparison (MAIC) J Clin Oncol. 2022;40 1015. [Google Scholar]
- 66.Bardia A., Hurvitz S. Targeted therapy for premenopausal women with HR(+), HER2(-) advanced breast cancer: focus on special considerations and latest advances. Clin Cancer Res. 2018;24:5206–5218. doi: 10.1158/1078-0432.CCR-18-0162. [DOI] [PubMed] [Google Scholar]
- 67.In: https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-scorecards/scorecard-158-1. Accessed April 23, 2022.
- 68.Badaoui S., Kichenadasse G., Rowland A., Sorich M.J., Hopkins A.M. Patient-reported outcomes predict progression-free survival of patients with advanced breast cancer treated with abemaciclib. Oncologist. 2021;26:562–568. doi: 10.1002/onco.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Handforth C., Hall P., Marshall H., Seymour M. Overall treatment utility: a novel outcome measure to convey the balance of benefits and harms from cancer treatment. J Geriatr Oncol. 2013;4:S49. [Google Scholar]
- 70.Kjoe P.R.L.M., van der Wall E., Schagen S.B. Endocrine therapy with or without CDK4/6 inhibitors in women with hormone-receptor positive breast cancer: what do we know about the effects on cognition? Clin Breast Cancer. 2022;22:191–199. doi: 10.1016/j.clbc.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 71.Battisti N.M.L., De Glas N., Sedrak M.S., et al. Use of cyclin-dependent kinase 4/6 (CDK4/6) inhibitors in older patients with ER-positive HER2-negative breast cancer: Young International Society of Geriatric Oncology review paper. Ther Adv Med Oncol. 2018;10 doi: 10.1177/1758835918809610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minvielle E., di Palma M., Mir O., Scotte F. The use of patient-reported outcomes (PROs) in cancer care: a realistic strategy. Ann Oncol. 2022;33:357–359. doi: 10.1016/j.annonc.2021.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across the included randomized controlled trials.
Risk of bias summary: review authors’ judgements about each risk of bias item for each randomized controlled trial included.