Abstract
Background
Sex differences in cancer have gained attention in recent years. The role of sex as a prognostic factor in gastrointestinal stromal tumours (GIST) has not been well established. The aim of this research was to elucidate potential sex differences in GIST patients and the influence of sex on disease-specific survival (DSS).
Methods
A review of the literature was carried out to obtain an overview of all literature with sex as a covariate on GIST survival analyses. Furthermore, in the Dutch GIST Registry, GIST characteristics between males and females were compared and the influence of sex on DSS was analysed.
Results
A total of 118 articles from the review of the literature met our selection criteria; 58% of the articles found no sex difference in survival and 42% did find a sex difference. All differences favoured female patients, although there was substantial overlap of individual patients in the various reported groups. The Dutch GIST Registry cohort consisted of 1425 patients (46% female). Compared with female patients, male patients had larger tumours (mean 9.0 cm versus 7.9 cm) and higher mitotic rates (34.4% versus 28.0% >5 mitoses/5 mm2). GIST in males was more often metastasized at diagnosis (21.3% versus 13.7%) and incurable (38.5% versus 31.0%). Male patients less often received surgery of the primary tumour (71.7% versus 78.9%), but did experience more tumour ruptures (18.2% versus 13.3%). Male patients had a worse DSS than females. This was not statistically significant when corrected for differences in GIST characteristics.
Conclusions
In case of sex differences in GIST in the literature, male patients have a worse outcome. In our Dutch GIST cohort a similar finding was made, but sex was shown not to be an independent factor. Male patients more often had aggressive GISTs, with larger tumours, higher mitotic rates, more tumour ruptures, and metastases, which could explain the sex differences in DSS.
Key words: GIST, sex differences, sex factors, gender, disease-specific survival
Highlights
-
•
Sex differences in GIST patients are not well established.
-
•
In the literature, several articles found a sex difference in GIST survival, all favouring female patients.
-
•
In the current cohort study, sex was not an independent prognostic factor for GIST-specific survival.
-
•
One should be aware that male GIST patients more often have aggressive GIST characteristics.
Introduction
Sex and gender are important determinants in medicine. Although sex and gender are often used interchangeably in practice, both terms have a different meaning. Gender considers socially constructed roles and behaviours that influence self-identity and self-expression and is influenced by social, environmental, cultural, and behavioural factors.1 Sex refers specifically to biological differences, such as sex chromosomes, sexual hormone levels, and reproductive anatomy.1 Sex differences in cancer patients have gained attention in recent years.2,3 In cancer in non-reproductive organs, the presentation, incidence, and prognosis can differ considerably between males and females. Female patients are more often diagnosed with breast cancer and tumours of the endocrine system, but the cancer incidence rate in the United States was higher for male patients in the majority of other cancer sites.4 Furthermore, survival of Kaposi sarcoma patients was worse for female patients, but for all other major cancer site groupings (e.g. digestive system, soft tissue, endocrine system, lymphoma) age-adjusted survival was worse for male patients.4 These sex differences seem to be caused by behavioural and environmental factors or by physiological differences. A report from a European Society of Medical Oncology (ESMO) workshop on sex medicine and oncology in 2019 summarizes that clinically relevant physiological sex differences in oncology include differences in tumour biology, immune system activity, body composition, and drug pharmacokinetics and pharmacodynamics.3
Gastrointestinal stromal tumours (GISTs) arise throughout the gastrointestinal tract, originate from the interstitial cells of Cajal in the gastrointestinal tract,5 and are therefore classified as soft tissue sarcoma. Although it is the most common gastrointestinal sarcoma subtype, the estimated incidence of GIST is only 10-15 patients per million per year.6 Median age at diagnosis is 60-65 years and there is a slightly higher incidence in males.7 Oncogenic driver mutations in GIST are mostly KIT (75%) or PDGFRA mutations (10%-15%).8, 9, 10 Systemic therapy targeting these mutations with tyrosine kinase inhibitors (TKIs), such as imatinib, sunitinib, and regorafenib, have considerably improved overall survival (OS).11 Important prognostic factors for GIST include the mitotic rate, tumour size, and tumour site.12 Perioperative tumour rupture is also recognized as an adverse prognostic factor.7,13 The role of sex as a prognostic factor, however, has not yet been well established.
A recent paper by Rong et al.14 compared the presentation of gastric GIST between males and females and their OS. Male sex was an adverse prognostic factor for OS according to their multivariable Cox regression analysis. Although sex quite often has been included as a covariate on multivariable survival analyses, no articles compared male and female GIST characteristics in all GIST patients. Besides, the studies that did include sex as a covariate on multivariable analyses did not provide an unequivocal conclusion.
Therefore, the present paper aims to clarify the issue of possible sex differences in GIST patients, focussing on potential differences in tumour and treatment characteristics, and disease-specific survival (DSS). First, a review of the literature on sex as a prognostic factor in GIST patients will be carried out and discussed. Second, tumour and patient characteristics and DSS of male and female GIST patients will be compared in a large Dutch GIST cohort.
Methods
Review of the literature on sex differences in GIST survival
Two searches were carried out in PubMed on 28 October 2021. We used both MeSH terms and searched in the title, abstract, and keywords. In the first search, we searched for GIST in combination with ‘sex factors’. To expand the search strategy, we added the results from a second search for GIST in combination with ‘gender’ or ‘male and female’ and ‘overall survival or disease specific survival’. We applied no limits for publication date. All available articles written in English with >100 GIST patients and sex as a factor in the survival analysis were eligible.
Cohort study
All patients registered in the Dutch GIST Registry (DGR), who were diagnosed with GIST between January 2009 and June 2021, were included in the cohort study. The DGR is a database containing data of adult GIST patients treated in one of the five Dutch GIST centres (LUMC Leiden, Erasmus MC Rotterdam, UMC Groningen, Radboudumc Nijmegen, and the Netherlands Cancer Institute Amsterdam). Data on patient characteristics, tumour characteristics, treatment and side-effects, laboratory results, and outcome are prospectively maintained in the DGR. The DGR was approved by the local independent ethics committee (IRBd20-212). For the cohort study, patient and tumour characteristics, including molecular pathology reports, and treatment and follow-up data were collected from the DGR. Tumour status at diagnosis was considered as localized disease for patients with a local or locally advanced GIST, and as metastasized for patients with distant metastases. The required total count of mitoses in GIST is per 5 mm2. With the use of older microscope models, 50 high power fields (HPF) was used, which we considered semi-equivalent to 5 mm2. Low mitotic rate was defined as ≤5 mitoses/5 mm2 and high as >5 mitoses/5 mm2. Patients were subdivided into risk groups based upon the risk classification as specified by Miettinen’s criteria.12 Response evaluation and follow-up of GIST patients treated in Dutch GIST centres is done according to national guidelines that are based on the most recent ESMO guidelines.7 Considered as patients in the palliative setting are patients with incurable, advanced GIST (mostly patients with distant metastases or sometimes locally advanced inoperable GIST).
Statistical analyses
Differences between males and females were investigated using Fisher’s exact test for categorical variables and Mann–Whitney U tests for numeric variables. DSS was calculated from the date of diagnosis to the date of death from GIST or last follow-up. Follow-up time was estimated using the reverse Kaplan–Meier method. The Kaplan–Meier method was used to estimate DSS, censoring deaths from other causes. For a separate analysis, DSS after the start of palliative treatment was calculated in the subgroup of patients with systemic palliative treatment. DSS was compared between males and females using the log-rank test, and multivariable Cox regression analysis for DSS was done to adjust for potential differences between males and females at baseline. At first, separate variables were included in a univariable analysis. Thereafter, sex and variables with a P value <0.10 in the univariable analysis were included in the multivariable model. Variables with a P value <0.05 in the multivariable model were considered significant. Additionally, a propensity score was estimated using factors that differed between males at baseline, were identified in the DSS analysis, or found in the literature review. The propensities given these factors were then used as regression weights (one divided by the propensity of the actual sex) in a proportional hazards model to analyse DSS by sex. Multiple imputation was carried out (with 20 imputations) to account for missing values in factors used for the propensity score. Statistical analyses were carried out in SPSS Statistics Version 25 and R version 4.4.4 (R Project, Vienna, Austria) with the multiple-imputation algorithm from package mice version 3.13.
Results
Review of the literature on sex differences in GIST patients’ survival
The search strategy resulted in a total of 819 articles. Based on the abstract, 598 articles were excluded (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100649). The three most common reasons for exclusion were <100 included patients (n = 367), no GIST (n = 76), or were not available in English (n = 76). Thereafter, another 103 articles were excluded after full read, mostly because sex was not incorporated as a variable in the survival model (n = 75).
Finally, 118 articles met the selection criteria. Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100649, contains an overview of these 118 articles. Within this selection, several publications included patients who had an overlapping presence in different groups. For example, 24 articles described patient groups from the Surveillance, Epidemiology, and End Results (SEER) program, 5 articles patient groups from the National Cancer Database (NCDB), and 4 articles patient groups from the Memorial Sloan Kettering Cancer Center (MSKCC). The SEER program publishes population-based cancer incidence and survival data from different states in the United States (US), covering ∼48% of the US population.15 The SEER program includes the New York State Cancer Registry16 and since the MSKCC is located in New York, patients registered in the MSKCC database are most likely also present in the SEER program. The NCDB is a non-population-based cancer registry sourced from hospital registry data covering ∼70% of all newly diagnosed cancer cases in the US.17 Substantial overlap of patients within the SEER program with the NCDB is therefore expected.
From all 118 articles, 68 articles (58%) found no sex difference for survival on univariable analysis (n = 46) or multivariable analysis (n = 22). In contrast, 50 articles (42%) did find a sex difference on their survival analyses: 8 on univariable analyses [outcome measures: OS, n = 3; disease-free survival (DFS), n = 1; recurrence-free survival (RFS), n = 1; risk of mortality, n = 1; relative survival, n = 1; DSS, n = 1] and 42 on multivariable analyses (outcome measures: OS, n = 25; DSS, n = 13; RFS, n = 2; DFS, n = 1; progression-free survival, n = 1).
In all the articles that found a significant sex difference for survival, female patients had a better outcome. On the multivariable analysis, female hazard ratio (HR) varied from 0.56 to 0.79 for DSS and from 0.18 to 0.83 for OS.
Of note, at least 26 articles (53%) that found a sex difference for survival conceivably used overlapping datasets, partially reporting on the same patients (SEER database n = 19, NCD n = 5, MSCKCC n = 2). From a total of 13 articles with a better DSS for female patients on multivariable analyses, 11 articles were SEER cohorts and 2 articles were MSKCC cohorts, which obviously would introduce bias if included in a classical meta-analysis. Besides, from the 13 articles that found a better DSS for female GIST patients, only 2 articles did correct for three other established prognostic factors (i.e. tumour size at diagnosis, stage at diagnosis, and mitotic rate at baseline) in the multivariate model (both SEER cohorts, one gastric only and one all patients).
Just one paper compared GIST characteristics between male and female patients as a primary objective. In the study by Rong et al.,14 1050 gastric GIST patients (51% females) at all stages were selected from the SEER program. Male patients underwent surgery less often (95.9% versus 98.1% for females, P = 0.032), had relatively larger tumours (>5 cm in 49.6% versus 39.4% for females, P = 0.001), and were significantly more often married. Mitotic index appeared to be lower in female patients (≤5/50 HPF in 82.9% versus 77.0% for males, P = 0.044). After propensity score matching, the OS was worse for male patients with an HR of 1.7 [95% confidence interval (CI) 1.2-2.4, P = 0.007] on multivariate analysis.
Cohort study
Patient and tumour characteristics of males and females with GIST from the DGR
From the 1425 GIST patients who were included, 766 patients were male (53.8%) and 659 female (46.2%). Mean age at diagnosis was 63.4 years and did not differ between males and females (Table 1). The most common primary tumour location for both female and male GIST patients was gastric and no sex difference was observed in the incidence of second malignancies. Male GIST patients, however, did have significantly larger tumours at baseline compared with female patients (9.0 cm versus 7.9 cm, respectively, P = 0.02). In addition, male patients had considerably more often metastatic disease at time of diagnosis (21.3% versus 13.7% for females, P < 0.001).
Table 1.
Baseline characteristics of males and females with GIST in the DGR
| Total (%) N = 1425 |
Males (%) N = 766 (53.8) |
Females (%) N = 659 (46.2) |
P valuea | |
|---|---|---|---|---|
| Mean age at diagnosis (SD) | 63.4 (12.9) | 63.3 (12.7) | 63.5 (13.2) | 0.59 |
| Mean tumour size at diagnosis in cm (SD) | 8.5 (6.2) | 9.0 (6.7) | 7.9 (5.6) | 0.02∗ |
| Primary tumour site | 0.28 | |||
| Stomach | 868 (61.8) | 457 (60.5) | 411 (63.2) | |
| Small intestine | 389 (27.7) | 217 (28.7) | 172 (26.5) | |
| Rectum | 73 (5.2) | 45 (6.0) | 28 (4.3) | |
| Otherb | 75 (5.3) | 36 (4.8) | 39 (6.0) | |
| Unknown | 20 | 11 | 9 | |
| Tumour status at diagnosis | <0.001∗ | |||
| Localized disease | 1172 (82.2) | 603 (78.7) | 569 (86.3) | |
| Metastasized disease | 253 (17.8) | 163 (21.3) | 90 (13.7) | |
| Second malignancy | 0.85 | |||
| No | 1088 (76.4) | 583 (76.1) | 505 (76.6) | |
| Yes | 337 (23.6) | 183 (23.9) | 154 (23.4) | |
| Histology | 0.11 | |||
| Spindle cell | 1023 (77.6) | 529 (75.4) | 494 (80.2) | |
| Epithelioid | 140 (10.6) | 81 (11.5) | 59 (9.6) | |
| Mixed type | 155 (11.8) | 92 (13.1) | 63 (10.2) | |
| Unknown | 107 | 64 | 43 | |
| Baseline mitotic rate | 0.02∗ | |||
| Low (≤5/5 mm2) | 772 (68.7) | 386 (65.6) | 386 (72.0) | |
| High (>5/5 mm2) | 352 (31.3) | 202 (34.4) | 150 (28.0) | |
| Unknown | 301 | 178 | 123 | |
| Baseline risk according to Miettinen | 0.06 | |||
| None | 91 (9.6) | 44 (9.2) | 47 (10.1) | |
| Very low | 150 (15.9) | 73 (15.3) | 77 (16.6) | |
| Low | 228 (24.1) | 111 (23.1) | 117 (25.2) | |
| Moderate | 180 (19.0) | 81 (16.9) | 99 (21.3) | |
| High | 296 (31.3) | 171 (35.6) | 125 (26.9) | |
| Unknown | 227 | 123 | 104 | |
| Not applicable (M1 at diagnosis) | 253 | 163 | 90 | |
| GIST driver mutation | 0.59 | |||
| KIT | 892 (62.6) | 481 (62.8) | 411 (62.4) | |
| PDGFR | 154 (10.8) | 86 (11.2) | 68 (10.3) | |
| NF1 associated | 28 (2.0) | 11 (1.4) | 17 (2.6) | |
| SDH deficiency | 17 (1.2) | 7 (0.9) | 10 (1.5) | |
| Other (e.g. BRAF, NTRK, unclassified) | 91 (6.4) | 50 (6.5) | 41 (6.2) | |
| No mutation analysis carried out | 243 (17.1) | 131 (17.1) | 112 (17.0) |
DGR, Dutch GIST Registry; GIST, gastrointestinal stromal tumour; SD, standard deviation.
Fisher’s exact test for categorical variables and Mann–Whitney U tests for numeric variables.
Other tumour locations such as colon, oesophagus, liver, multifocal, peritoneum, or small pelvis not further specified.
P value <0.05 was considered significant.
Regarding tumour characteristics, male GIST patients more often had a high mitotic rate compared with females (34.4% versus 28.0%, P = 0.02). No difference in the histology of GIST was found for males and females, with mostly spindle cell type GIST (77.6% in all patients). GIST driver mutations did not differ significantly between males and females, with KIT (62.6%) and PDGFR (10.8%) mutations accounting for the majority of all mutations in both sexes. Male patients had a baseline high-risk GIST in 35.6% of cases, while female patients had a high-risk GIST in 26.9% of cases (P = 0.06).
Treatment of males and females with GIST
Overall, 875 patients were treated with imatinib at any time point during their course of treatment (Table 2). Neoadjuvant imatinib was given in 24.5% of all patients, whereas adjuvant imatinib was prescribed to 27.2% of the patients after surgery. Overall, males more often received imatinib (65.3% versus 56.9%, P = 0.001), but this difference did not remain significant for the neoadjuvant, adjuvant, and palliative imatinib treatments separately. Female patients more frequently underwent surgery of the primary tumour (78.9% versus 71.7%, P = 0.002), which might be explained by the finding that more males had metastatic disease at diagnosis and therefore were no longer eligible for primary surgery. From the patients that had surgery of the primary GIST (n = 1069), 159 patients had perioperative tumour rupture, being more common in male than in female patients (18.2% versus 13.3%, respectively, P = 0.04).
Table 2.
Treatment characteristics and metastatic patterns of males and females with GIST in the DGR
| Total (%) N = 1425 |
Males (%) N = 766 (53.8) |
Females (%) N = 659 (46.2) |
P valuea | |
|---|---|---|---|---|
| Imatinib in any setting | 0.001∗ | |||
| No | 550 (38.6) | 266 (34.7) | 284 (43.1) | |
| Yes | 875 (61.4) | 500 (65.3) | 375 (56.9) | |
| Neoadjuvant imatinib | 0.62 | |||
| No | 1076 (75.5) | 574 (74.9) | 502 (76.2) | |
| Yes | 349 (24.5) | 192 (25.1) | 157 (23.8) | |
| Surgery of primary tumour | 0.002∗ | |||
| No | 356 (25.0) | 217 (28.3) | 139 (21.1) | |
| Yes | 1069 (75.0) | 549 (71.7) | 520 (78.9) | |
| Perioperative tumour rupture | 0.04∗ | |||
| No | 812 (84.2) | 400 (81.8) | 412 (86.7) | |
| Yes | 152 (15.8) | 89 (18.2) | 63 (13.3) | |
| Unknown | 105 | 60 | 45 | |
| Adjuvant imatinib | 0.09 | |||
| No | 778 (72.8) | 387 (70.5) | 391 (75.2) | |
| Yes | 291 (27.2) | 162 (29.5) | 129 (24.8) | |
| Palliative setting anytime during follow-upb | 0.003∗ | |||
| No | 926 (65.0) | 471 (61.5) | 455 (69.0) | |
| Yes | 499 (35.0) | 295 (38.5) | 204 (31.0) | |
| Palliative imatinib | 0.36 | |||
| No | 49 (9.8) | 26 (8.8) | 23 (11.3) | |
| Yes | 450 (90.2) | 269 (91.2) | 181 (88.7) | |
| Palliative sunitinib | 0.23 | |||
| No | 304 (60.9) | 173 (58.6) | 131 (64.2) | |
| Yes | 195 (39.1) | 122 (41.4) | 73 (35.8) | |
| Palliative regorafenib | 0.19 | |||
| No | 411 (82.4) | 237 (80.3) | 174 (85.3) | |
| Yes | 88 (17.6) | 58 (19.7) | 30 (14.7) | |
| Other palliative systemic treatmentc | 0.008∗ | |||
| No | 430 (86.2) | 244 (82.7) | 186 (91.2) | |
| Yes | 69 (13.8) | 51 (17.3) | 18 (8.8) | |
| Local treatment of metastases | 0.90 | |||
| No | 428 (85.8) | 252 (85.4) | 176 (86.3) | |
| Yes | 71 (14.2) | 43 (14.6) | 28 (13.7) | |
| Patients with metastasesd | 482 (100) | 286 (59.3) | 196 (40.7) | |
| Liver metastases | 0.15 | |||
| No | 182 (37.8) | 100 (35.0) | 82 (41.8) | |
| Yes | 300 (62.2) | 186 (65.0) | 114 (58.2) | |
| Peritoneal metastases | 0.04∗ | |||
| No | 185 (38.4) | 121 (42.3) | 64 (32.7) | |
| Yes | 297 (61.6) | 165 (57.7) | 132 (67.3) | |
| Non-liver/non-peritoneal metastases | 0.28 | |||
| No | 417 (86.5) | 243 (85.0) | 174 (88.8) | |
| Yes | 65 (13.5) | 43 (15.0) | 22 (11.2) | |
| Lymph node metastases | 0.03∗ | |||
| No | 460 (95.4) | 268 (93.7) | 192 (98.0) | |
| Yes | 22 (4.6) | 18 (6.3) | 4 (2.0) |
DGR, Dutch GIST Registry; GIST, gastrointestinal stromal tumour.
Fisher’s exact test for categorical variables and Mann–Whitney U tests for numeric variables.
Considered as patients in the palliative setting are patients with incurable, advanced GIST (mostly patients with distant metastases or sometimes locally advanced inoperable GIST).
Other than imatinib, sunitinib, or regorafenib.
Patients can have multiple distant metastases locations (e.g. liver and peritoneum or liver and lymph node).
P value <0.05 was considered significant.
Not only did more males have metastatic disease at diagnosis, male GIST patients also developed metastases more often during follow-up in this cohort (male 38.5% versus female 31.0%, median follow-up 3.43 years for males versus 3.39 years for females). Once in the palliative treatment setting, no differences were found between males and females regarding percentage of patients receiving palliative imatinib, sunitinib, or regorafenib treatment. Furthermore, local treatment of metastases occurred both in males and females with GIST.
Metastatic patterns among males and females with GIST
From the patients with GIST metastases, several sex differences were found regarding the location of GIST metastases (Table 2). Firstly, females more often had peritoneal metastases (67.3% versus 57.7% for males, P = 0.04). By contrast, males significantly more often had lymph node metastases, although the number of patients with lymph node metastases was low (6.3% males versus 2.0% females, P = 0.03). No significant sex differences were found in the frequency of liver metastases or metastases outside of the liver and peritoneum.
Survival of males and females with GIST
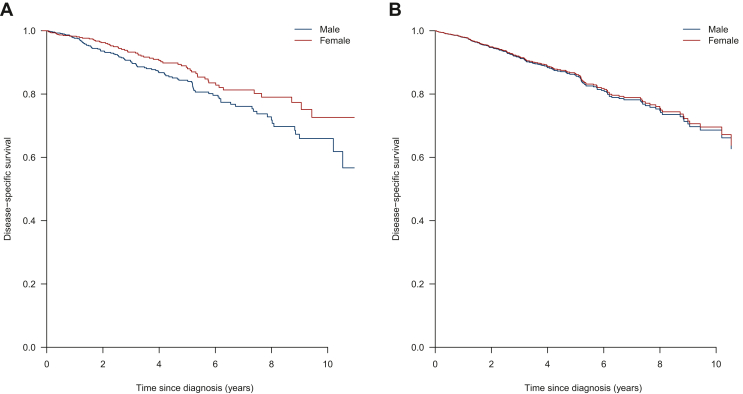
The median follow-up time in the cohort was 3.4 years (interquartile range 1.1-6.1 years). Within the follow-up time, 158 patients died due to disease progression. Using the Kaplan–Meier method, the DSS was significantly longer for female GIST patients compared with male GIST patients (P = 0.04, median not reached) (Figure 1A).
Figure 1.
(A) Kaplan–Meier estimated disease-specific survival. (B) Predicted disease-specific survival curves after weighting and multiple imputation.
Multiple imputation was carried out for missing values of likely confounders (percentage missing values: number of mitoses 21.1%, KIT exon 11 mutation status 17.1%, histology 7.5%, tumour rupture 7.4%, baseline primary tumour size 4.1%, location primary tumour 1.4%). After multiple imputation, a Cox regression analysis was carried out. Using the P value threshold of 0.05, higher age, larger baseline tumour size, metastatic disease at diagnosis, and no surgery of the primary tumour were associated with worse DSS on multivariable Cox regression analysis (Table 3). Sex was not a significant prognostic factor for DSS on multivariable Cox regression analysis after multiple imputation (female HR 0.86, 95% CI 0.61-1.22, P = 0.41, Figure 1B), nor in a propensity-score weighted Cox model after multiple imputation (female HR 0.96, 95% CI 0.69-1.33, P = 0.82).
Table 3.
Multivariable model of disease-specific survival in entire DGR cohort after multiple imputation (n = 1425, 158 events)
| Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Sex | ||||||
| Male | Reference | Reference | ||||
| Female | 0.71 | 0.52-0.98 | 0.04∗ | 0.86 | 0.61-1.22 | 0.41 |
| Age at diagnosis (years) | 1.03 | 1.01-1.04 | <0.001∗ | 1.02 | 1.01-1.03 | 0.01∗∗ |
| Tumour size at diagnosis (cm) | 1.08 | 1.05-1.10 | <0.001∗ | 1.05 | 1.02-1.07 | <0.001∗∗ |
| Primary site | <0.001∗ (3 df) | 0.43 (3 df) | ||||
| Stomach | Reference | Reference | ||||
| Small intestine | 0.96 | 0.66-1.38 | 0.82 | 1.05 | 0.71-1.54 | 0.82 |
| Rectum | 0.60 | 0.26-1.38 | 0.23 | 0.71 | 0.31-1.64 | 0.42 |
| Other | 3.31 | 1.97-5.57 | <0.001 | 1.48 | 0.84-2.61 | 0.18 |
| Tumour status at diagnosis | ||||||
| Local disease | Reference | Reference | ||||
| Metastatic disease | 7.37 | 5.36-10.14 | <0.001∗ | 2.99 | 1.96-4.54 | <0.001∗∗ |
| Baseline mitotic rate | ||||||
| Low (≤5/5 mm2) | Reference | Reference | ||||
| High (>5/5 mm2) | 1.93 | 1.33-2.79 | 0.001∗ | 1.50 | 1.05-2.13 | 0.03 |
| KIT status | ||||||
| KIT exon 11 | Reference | |||||
| Non-KIT exon 11 | 1.26 | 0.90-1.77 | 0.18 | |||
| Surgery of primary tumour | ||||||
| Yes | Reference | Reference | ||||
| No | 6.96 | 5.07-9.55 | <0.001∗ | 2.96 | 1.97-4.44 | <0.001∗∗ |
| Perioperative tumour rupture | ||||||
| Yes | 0.82 | 0.49-1.37 | 0.45 | |||
| No | Reference | |||||
CI, confidence interval; df, degrees of freedom; DGR, Dutch GIST Registry; HR, hazard ratio.
Variables with P < 0.10 in univariable analysis were included in multivariable analysis.
P < 0.05 is considered significant in multivariable analysis.
DSS was also analysed for the 470 patients treated with systemic treatment in the palliative setting (281 males and 189 females). No multiple imputation or weighting was carried out in this subgroup. In this analysis, 109 patients were excluded for analysis due to missing data and therefore 361 patients were included in the model, 97 of who died from the disease (Table 4). Females did not have a significantly better DSS than males on univariable analysis (HR 1.18, 95% CI 0.85-1.65 compared with males) or in a multivariable model (HR 1.13, 95% CI 0.73-1.77) after start of palliative systemic therapy. Patients without surgery of the primary tumour (HR 1.78) and patients without KIT exon 11 mutation (HR 2.10) had a significantly higher risk for death from disease.
Table 4.
Disease specific survival in GIST patients of the DGR treated with palliative systemic treatment (n = 361 patients / 97 events in multivariable analysis)
| Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Sex | ||||||
| Male | Reference | Reference | ||||
| Female | 1.18 | 0.85-1.65 | 0.32 | 1.13 | 0.73-1.77 | 0.58 |
| Age at diagnosis (years) | 1.01 | 1.00-1.03 | 0.04∗ | 1.01 | 0.99-1.03 | 0.29 |
| Tumour size at diagnosis (cm) | 1.03 | 1.00-1.05 | 0.06∗ | 1.02 | 0.99-1.05 | 0.30 |
| Primary site | 0.004∗ (3 df) | 0.15 (3 df) | ||||
| Stomach | Reference | Reference | ||||
| Small intestine | 0.56 | 0.38-0.83 | 0.004∗ | 0.68 | 0.42-1.09 | 0.11 |
| Rectum | 0.47 | 0.19-1.15 | 0.10∗ | 0.59 | 0.22-1.59 | 0.30 |
| Other | 1.24 | 0.72-2.12 | 0.44 | 1.41 | 0.72-2.74 | 0.31 |
| Tumour status at diagnosis | ||||||
| Local disease | Reference | Reference | ||||
| Metastatic disease | 1.40 | 1.00-1.96 | 0.05∗ | 0.83 | 0.51-1.35 | 0.45 |
| Baseline mitotic rate | ||||||
| Low (≤5/5 mm2) | Reference | |||||
| High (>5/5 mm2) | 1.31 | 0.86-1.98 | 0.21 | |||
| KIT status | ||||||
| KIT exon 11 | Reference | Reference | ||||
| Non-KIT exon 11 | 1.89 | 1.33-2.69 | <0.001∗ | 2.10 | 1.38-3.19 | <0.001∗∗ |
| Surgery of primary tumour | ||||||
| Yes | Reference | Reference | ||||
| No | 1.83 | 1.32-2.53 | <0.001∗ | 1.78 | 1.07-2.94 | 0.03∗∗ |
| Perioperative tumour rupture | ||||||
| No | Reference | Reference | ||||
| Yes | 0.48 | 0.27-0.83 | 0.009∗ | 0.63 | 0.32-1.22 | 0.17 |
| Peritoneal metastases | ||||||
| No | Reference | |||||
| Yes | 1.18 | 0.84-1.65 | 0.35 | |||
| Lymph node metastases | ||||||
| No | Reference | |||||
| Yes | 1.13 | 0.56-2.31 | 0.73 | |||
CI, confidence interval; df, degrees of freedom; DGR, Dutch GIST Registry; GIST, gastrointestinal stromal tumour; HR, hazard ratio.
P < 0.10 in univariable analysis was included in multivariable analysis.
P < 0.05 is considered significant in multivariable analysis.
Discussion
Although some previous studies suggest that female GIST patients have a better outcome, and female patients also had a better outcome in a large Dutch GIST cohort on univariable analysis, sex was not an ‘independent’ prognostic variable for DSS in a multivariable analysis, nor when using the propensity score. The reason why sex seems prognostic in our univariable analyses is possibly because males have more aggressive GIST characteristics at baseline (i.e. larger tumours, with high mitotic rate, and more often ruptured) and are more likely to have metastatic GIST. Once started with palliative systemic treatment, no association of sex with DSS was found, not even in univariable analysis.
This is the first study specifically looking at sex differences in GIST at all locations and comparing GIST-specific survival in male and female GIST patients. As mentioned before, Rong et al.14 recently published a paper on sex differences in gastric GIST patients. As outcome measure, they used OS. Because females have a longer life expectancy in the general population worldwide,18 however, DSS seems a more appropriate outcome measure to assess the prognostic value of sex. Another statistical limitation in the analysis of Rong et al.14 is that they did not correct for stage of disease in their multivariable model. This could have affected the results since we know survival is worse for patients with metastatic disease compared with those with local disease only.19 In a sensitivity analysis on our dataset, the difference in DSS between males and females disappeared even in a model with sex and disease stage without any further covariates (results not shown), although removal of disease stage from the multivariable presented in Table 3 did not change much in the estimated HR of sex.
Several limitations are inherent to the retrospective design of this study. For example, we had to use multiple imputation to handle missing baseline characteristics. Nevertheless, this is a large GIST cohort with real-world data from GIST expertise centres with uniform practices, enabling the comparison of GIST characteristics and GIST-specific survival between males and females.
As reported in the ESMO report on gender medicine, differences in drug pharmacokinetics and pharmacodynamics could play a role in why cancer outcome differs for males and females.3 These factors were not taken into account in the current cohort study, nor in the survival analyses described in the current review of the literature. For GIST patients, however, previous studies have shown that the exposure to the TKIs imatinib and sunitinib differed between male and female GIST patients. Male GIST patients more often have a low imatinib exposure,20 probably because of a higher imatinib clearance.21 Also for sunitinib, female sex was identified as a covariate that significantly increases sunitinib exposure in a population pharmacokinetic analysis.22 These differences in exposure might have contributed to the survival difference found in the US cohorts, since adequate exposure to imatinib or sunitinib is correlated with a better outcome.23 In the current Dutch cohort, the majority of patients received therapeutic drug monitoring, where the TKI dose was adjusted based on measured drug levels. It could be that more males in the Dutch cohort did have an adequate TKI exposure after dose increases based on therapeutic drug monitoring. For future research, it would be interesting to include adequate TKI exposure, TKI dose and toxicity in the survival model to establish their role in potential sex differences for GIST survival.
It remains unclear why some historical cohorts did find a sex difference in relation to outcome and in the current Dutch cohort sex was shown not to be an independent prognostic factor. There may be several explanations. First, the sex difference in historical cohorts could be present due to inadequate correction for confounders. Only two cohorts corrected for all three prognostic factors that were found to be different between males and females in the current cohort study: tumour size, mitotic rate at diagnosis, and stage at diagnosis. None of these cohorts did compare male and female GIST characteristics. These two cohorts both concern patients from the SEER program.24,25 Liu et al.24 included only gastric patients in their analysis (diagnosed between 1998 and 2015) and found an HR of 1.3 for male DSS. Song et al.25 included 2841 GIST patients diagnosed between 2004 and 2015 in a training set and found a significant sex difference in DSS as well. Median follow-up time in their SEER cohort was 34 months. Song et al.25 excluded patients with a second malignancy (n = 1390), although these patients were included in the current cohort. Nevertheless, the proportion of patients with a second malignancy was comparable for males and females, so this unlikely causes the conflicting results. Second, another possible source of error could be the smaller sample size of the Dutch cohort. Furthermore, there could be unclarified differences between GIST patients in the US and in the Netherlands, regarding for example prescriptions and compliance explaining the contradictory results. Future studies should focus on comparing characteristics between female and male GIST patients to further establish the role of sex in GIST patients.
A possible interpretation of the results of the current analysis is as follows: on average, male GIST patients have a worse prognosis than female patients. It could be more important, however, to consider other factors than sex, such as tumour size, resectability, and presence of metastases for a good prediction of prognosis. Nevertheless, worse prognosis of males on average may be an indication that there exist problems that are particularly pronounced within the male subgroup. Questions that arise from this hypothesis are among others: does untreated GIST grow or metastasize faster in males than in females? Are males waiting longer to see a doctor than females? Is it more difficult to establish the diagnosis of GIST in males? There could be much to gain if these problems could be identified and then addressed.
In conclusion, unlike what several historical cohorts from the United States suggest, sex was not an ‘independent’ prognostic factor for DSS in the current Dutch GIST cohort.
The reason why sex seems prognostic in our univariable analysis seems to be that male patients more often have aggressive GISTs, characterised by larger tumours, more often ruptured with a high mitotic rate, and are more frequently metastasized. These factors are all associated with a worse outcome. Future research is necessary to understand these sex differences in GIST presentation and biology.
Acknowledgments
Funding
This work was supported by an unrestricted research grant for the Dutch GIST Registry received from Novartis [grant number 3017/13], Pfizer [grant number WI189378], Bayer [grant number 2013-MED-12005], and Deciphera [grant number 4EE9EEC-7F19-484D-86A4-646CFE0950A5]. These funding sources did not have any involvement in the conduction of this research.
Disclosure
RM received research grants from Astellas, Bayer, Cristal Therapeutics, PamGene, Pfizer, Roche, Sanofi, Servier. NS provided consultation or attended advisory boards for Boehringer Ingelheim, Ellipses Pharma, Hengrui Europe Therapeutics; NS received research grants for the institute from AB Science, AbbVie, Actuate Therapeutics, ADC Therapeutics, Amgen, Array, Ascendis Pharma, Astex, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, BridgeBio, Bristol-Myers Squibb, Cantargia, Celgene, CellCentric, Crescendo, Cytovation, Deciphera, Eli Lilly, Exelixis, Genentech, Genmab, Gilead, GlaxoSmithKline, Incyte, InteRNA, Janssen/Johnson & Johnson, Kinate, Merck, Merck Sharp & Dohme, Merus, Molecular Partners, Novartis, Numab, Pfizer, Pierre Fabre, Regeneron, Roche, Sanofi, Seattle Genetics, Servier, Taiho, Takeda (outside the submitted work). WTAvdG received a research grant from Eli Lilly (to the institute); WTAvdG was on advisory boards from SpringWorks, Bayer and PTC Therapeutics (all to the institute); WTAvdG other non-financial interests: president EORTC, board member ECO, Chair Dutch Sarcoma Group, Chair Dutch AYA ‘Young and Cancer’ Care Network, board member (CTOS). All other authors have declared no conflicts of interest.
Supplementry data
References
- 1.Hall M., Krishnanandan V.A., Cheung M.C., et al. An evaluation of sex- and gender-based analyses in oncology clinical trials. J Natl Cancer Inst. 2022;114:1186–1191. doi: 10.1093/jnci/djac092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clocchiatti A., Cora E., Zhang Y., Dotto G.P. Sexual dimorphism in cancer. Nat Rev Cancer. 2016;16(5):330–339. doi: 10.1038/nrc.2016.30. [DOI] [PubMed] [Google Scholar]
- 3.Wagner A.D., Oertelt-Prigione S., Adjei A., et al. Gender medicine and oncology: report and consensus of an ESMO workshop. Ann Oncol. 2019;30(12):1914–1924. doi: 10.1093/annonc/mdz414. [DOI] [PubMed] [Google Scholar]
- 4.Dong M., Cioffi G., Wang J., et al. Sex differences in cancer incidence and survival: a pan-cancer analysis. Cancer Epidemiol Biomarkers Prev. 2020;29(7):1389–1397. doi: 10.1158/1055-9965.EPI-20-0036. [DOI] [PubMed] [Google Scholar]
- 5.Joensuu H. Gastrointestinal stromal tumor (GIST) Ann Oncol. 2006;17(suppl 10):x280–x286. doi: 10.1093/annonc/mdl274. [DOI] [PubMed] [Google Scholar]
- 6.Søreide K., Sandvik O.M., Søreide J.A., Giljaca V., Jureckova A., Bulusu V.R. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39–46. doi: 10.1016/j.canep.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 7.Casali P.G., Blay J.Y., Abecassis N., et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:20–33. doi: 10.1016/j.annonc.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich M.C., Corless C.L., Duensing A., et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 9.IJzerman N.S., Drabbe C., den Hollander D., et al. Gastrointestinal stromal tumours (GIST) in young adult (18-40 years) patients: a report from the Dutch GIST registry. Cancers (Basel) 2020;12(3):730. doi: 10.3390/cancers12030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verschoor A.J., Bovée J.V.M.G., Overbeek L.I.H., Hogendoorn P.C.W., Gelderblom H., PALGA-Group The incidence, mutational status, risk classification and referral pattern of gastro-intestinal stromal tumours in the Netherlands: a nationwide pathology registry (PALGA) study. Virchows Arch. 2018;472(2):221–229. doi: 10.1007/s00428-017-2285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanke C.D., Demetri G.D., von Mehren M., et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26(4):620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen M., Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23(2):70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Joensuu H., Vehtari A., Riihimäki J., et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265–274. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 14.Rong J., Chen S., Song C., et al. The prognostic value of gender in gastric gastrointestinal stromal tumors: a propensity score matching analysis. Biol Sex Differ. 2020;11(1):43. doi: 10.1186/s13293-020-00321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.About the Surveillance, Epidemiology, and End Results (SEER) Program: National Cancer Institute (NCI) 2022. https://seer.cancer.gov/about/overview.html Available at.
- 16.New York in Surveillance, Epidemiology, and End Results (SEER) Program: National Cancer Institute (NCI) https://seer.cancer.gov/registries/new_york.html Available at.
- 17.Surgeons ACo. National Cancer Database. https://www.facs.org/quality-programs/cancer/ncdb Available at.
- 18.Max Roser EO-OaHR. Life Expectancy. Published online at OurWorldInData.org. 2013. Available at https://ourworldindata.org/life-expectancy [Online Resource]. Accessed January 18, 2022.
- 19.Güller U., Tarantino I., Cerny T., Schmied B.M., Warschkow R. Population-based SEER trend analysis of overall and cancer-specific survival in 5138 patients with gastrointestinal stromal tumor. BMC Cancer. 2015;15:557. doi: 10.1186/s12885-015-1554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IJzerman N.S., Groenland S.L., Koenen A.M., et al. Therapeutic drug monitoring of imatinib in patients with gastrointestinal stromal tumours - results from daily clinical practice. Eur J Cancer. 2020;136:140–148. doi: 10.1016/j.ejca.2020.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Widmer N., Decosterd L.A., Csajka C., et al. Population pharmacokinetics of imatinib and the role of alpha-acid glycoprotein. Br J Clin Pharmacol. 2006;62(1):97–112. doi: 10.1111/j.1365-2125.2006.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houk B.E., Bello C.L., Kang D., Amantea M. A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clin Cancer Res. 2009;15(7):2497–2506. doi: 10.1158/1078-0432.CCR-08-1893. [DOI] [PubMed] [Google Scholar]
- 23.Groenland S.L., Verheijen R.B., Joerger M., et al. Precision dosing of targeted therapies is ready for prime time. Clin Cancer Res. 2021;27(24):6644–6652. doi: 10.1158/1078-0432.CCR-20-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu M., Song C., Zhang P., et al. A nomogram for predicting cancer-specific survival of patients with gastrointestinal stromal tumors. Med Sci Monit. 2020;26 doi: 10.12659/MSM.922378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song W., Lv C.G., Miao D.L., et al. Development and validation of a nomogram for predicting survival in patients with gastrointestinal stromal tumours. Eur J Surg Oncol. 2018;44(10):1657–1665. doi: 10.1016/j.ejso.2018.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.