Abstract
Background
Consumption of herbs, food used as medicine and dietary supplements (HFDSs) is common in cancer patients. Herbs and food-drug interactions (HFDIs) can lead to serious adverse effects and can be prevented. We previously reviewed cytochrome P-450 (CYP)-mediated HFDI for 261 HFDSs and we classified the risk of CYP inhibition and induction on a level of evidence scale from 1 (high evidence, supported by several clinical studies) to 5 (low evidence, only limited preclinical data).
Patients and methods
We conducted a prospective, non-interventional study (NCT04128865) to assess whether self-assessment of patients could detect HFDI classified as ‘probable’ (i.e. level 1, 2 or 3 of the scale) in a population of cancer patients. Patients were invited through a tablet application to report their consumption of herbs, regular CYP-interacting food consumption and dietary supplements, as well as some clinical data and cancer treatments. The patient’s completion of the survey could be supervised by a health care professional or not. A prespecified threshold of 5% of HFDIs classified as ‘probable’ detected with the application was deemed relevant.
Results
Between 29 March 2018 and 22 June 2018, 143 patients completed the survey. Ninety-five patients (66%) reported at least one current systemic cancer treatment and were included in the analyses. Seventy-four patients reported an intake of at least one HFDS (77.9%), while 21 patients reported no HFDS (22.1%). Twenty-two HFDIs classified as ‘probable’ were found in 16 patients (16.8%) with the application, which was significantly superior to the prespecified threshold (P = 0.02). The interactions were reported with food (n = 19, 86%) more frequently than with herbs (n = 3, 14%) or with dietary supplements (no interaction reported).
Conclusions
Self-assessment of HFDS interaction with cancer treatment with an application is feasible and should be considered in daily routine. Prospective interventional studies should be conducted to better assess the clinical benefits of this approach.
Key words: connected devices, patient-reported measures, herb–drug interaction, food–drug interaction, anticancer drugs, cytochromes
Abbreviations: HFDS: Herbs, Food and Dietary Supplements - HFDI: Herbs and Food and Drug Interactions - PK: PharmacoKinetics
Highlights
-
•
HFDSs are commonly used by cancer patients and can interact with treatments.
-
•
This clinical study aimed to demonstrate that self-reporting with a tablet application could detect potential interactions.
-
•
By self-reporting with a tablet we could detect 16.8% of patients with potentially clinically relevant HFDS–drug interactions.
Introduction
The use of herbs, food used as medicine and dietary supplements (HFDSs) is increasing in Western countries. HFDSs also represent the core of medical treatments in Asia, Africa and South America where access to pharmaceutical drugs is limited. Between 1997 and 2015, the prevalence of herbal medicine use increased from 12% to one-third of the population in the United States,1 and an estimated proportion of 40%-60% of cancer patients are taking HFDSs.1, 2, 3 HFDSs are also used in phase I trials4,5 where up to 93% of patients report the use of a pharmacologically active substance, mostly vitamins and mineral preparations.
HFDSs contain xenobiotics that are absorbed to exert biological activity. Herbs and food-drug interactions (HFDIs) share the same mechanisms as drug–drug interactions, which have been extensively described in oncology.6, 7, 8 Two main types of drug interactions exist: (i) pharmacokinetic (PK) interactions, where one of the substances affects the concentration of others; and (ii) pharmacodynamic (PD) interactions when the drugs have an additive, synergistic or antagonistic effect or have the same target or pathway. Some HFDIs have been extensively described, such as the induction of cytochrome P-450 (CYP) 3A4 by patients consuming extracts of St. John’s wort (Hypericum perforatum), or its inhibition by grapefruit juice (Citrus paradisi). Bush et al.9 prospectively investigated potential HFDIs in 804 patients and found that 15% of patients (n = 122) were using traditional medicines. Forty-nine patients (40%) were exposed to a potential plant–drug interaction, and eight interactions were actually observed clinically (7% of plant users). While there is growing evidence that HFDI can lead to serious and even fatal adverse effects such as transplant rejection or cardiovascular shock,10 HFDS may still be perceived as harmless by (cancer) patients, notably because they are non-prescription drugs.
HFDI might lead to more severe adverse effects in cancer patients than in other patients, given the narrow therapeutic index of anticancer treatments. Hence, slight changes in clearance or absorption could have a major impact on efficacy or toxicity.11 Engdal et al.12 found 47 potential interactions involving CYPs and P-glycoprotein transport in 42 patients receiving cancer treatments and phytotherapy.
In a preliminary work,13 we systematically reviewed clinical and preclinical in vitro and in vivo data for 189 herbal medicines and food and 72 dietary supplements commonly used or likely to be responsible for interactions. We classified the level of evidence of the interaction between the HFDS and cytochromes from 1 (very likely) to 5 (unlikely), and HFDIs classified from 1 to 3 were considered as clinically proven or ‘probable’ (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100650). Twenty-one HFDSs were found to significantly inhibit or induce CYP3A4, such as grapefruit, pomelo, green tea or cranberry in clinical studies.
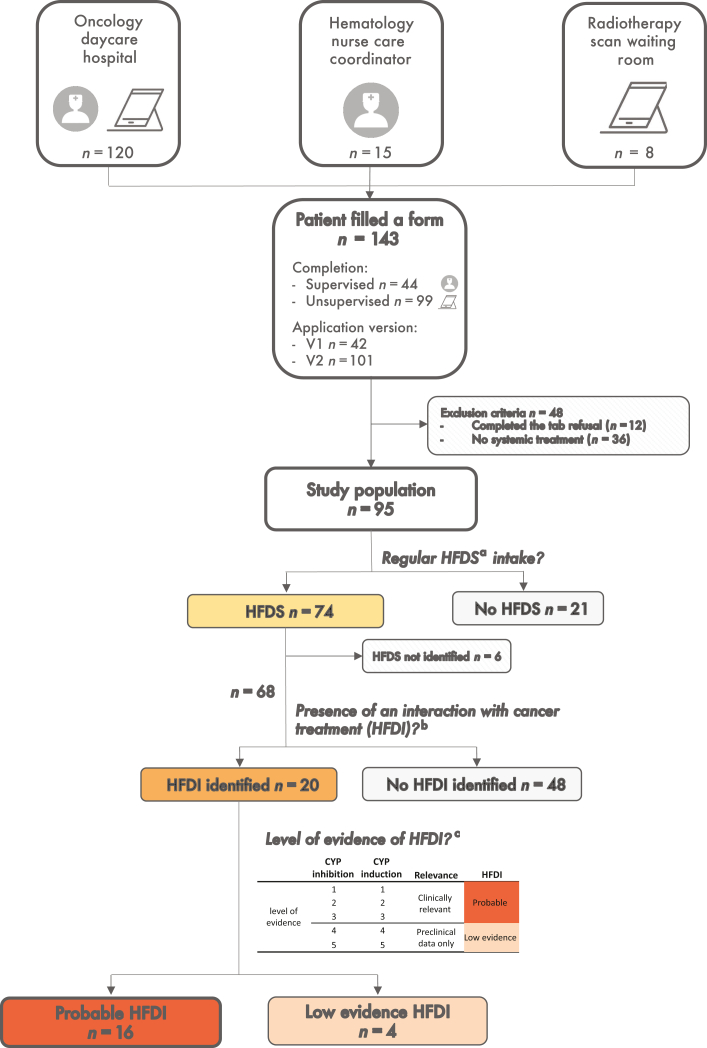
Connected devices, with electronically reported outcome platforms or follow-up applications, can increase patients’ quality of life and overall survival.14, 15, 16 Different strategies could be used depending on their technical knowledge, equipment and background. So far, such device has not been previously tested as a self-assessment tool to detect HFDI. PRINCESSE (PRevention of INteractions Between Phytotherapies and CancEr Treatments by a SmartphonE/Tablet Automated SurvEy) is a prospective observational study (ClinicalTrials.gov—NCT04128865). The primary objective of this study was to assess the percentage of patients with a probable cytochrome-mediated HFDI classified as level 1-3 (clinical evidence), using self-reporting through a tablet application (Figure 1).
Figure 1.
Principle of data collection and HFDI identification in the study. Data are collected through the ‘Kenko’ application completion by patients. Patients could have filled the first version of the app or the second version that included auto-completion and with or without the supervision of a health care professional. The risk of CYP-mediated drug interaction is automatically assessed according to previous published work.13 CYP, cytochrome P-450; HFDI, herbs and food-drug interaction; HFDS, herb, food and dietary supplements. aHDFS: regular intakes of herb, CYP-interacting food or dietary supplements. bHFDI: herb or food–drug interaction; evaluation based on Gougis et al.13cAccording to literature review.
Patients and methods
Study design
PRINCESSE is a single-center, prospective, observational study. The study was approved by an institutional review and ethical committee [Comité Local d’éthique pour les publications de l’hôpital Cochin (CLEP), n° AAA-2018-08002]. The database was registered (Commission Nationale Informatique et Liberté, n° Wa12515027K). The trial was registered on ClinicalTrials.gov (NCT04128865). All patients enrolled in the study provided electronic informed consent.
Study population
Patients with the following characteristics were eligible for inclusion: (i) history of cancer, (ii) age ≥18 years at diagnosis, (iii) fluency in French and (iv) ability to provide electronic informed consent. Patients with the following characteristics were excluded: (i) no current systemic anticancer treatment (either chemotherapy, targeted therapy, hormone therapy, immunotherapy or any combination).
Patients’ enrollment
Between 29 March 2018 and 22 June 2018, patients were recruited from three sites of Pitié-Salpêtrière Hospital, Paris: (i) the daycare hospital of the oncology department, (ii) coordinating nurse visits in the hematology department and (iii) the waiting room of the scanner of the radiotherapy department. Patients were either encouraged to fill the questionnaire on a tablet by the receptionist or one of the staff members (site 1), by the nurse care coordinator (site 2) or indirectly through advertisements (site 3).
Data collection
Three tablets (Galaxy tab S2, Samsung©, Seoul, South Korea) were dedicated to the study. On sites 1 and 3, the tablet was fixed on a stand. Before 16 April, patients on site 1 were supervised to fill out the questionnaire. After this date, on site 1 and on site 3, tablets were used in an unsupervised manner. On site 2, the tablet was proposed by the nurse care coordinator who invited patients to participate and supervised filling of forms. Forms were filled on the ‘Kenko’ application (Figure 1). The ‘Kenko’ application was updated on 3 May 2018 to allow auto-completion of a prespecified list of HFDSs and anticancer drugs. This updated version of the application also included specific food questionnaires.
Form completion
The form included 16 questions, including sociodemographics [age (years), gender]; cancer history (cancer localization, time since diagnosis), disease stage (metastasis or localized); cancer treatment (surgery, radiotherapy, systemic anticancer treatments).
HFDS intake
HFDS intake was defined as regular use of phytotherapy, dietary supplements, essential oils taken orally, infusions or consumption of over 3 times a week of CYP-interacting food (>2 cups a day for coffee) identified in the previous review.13
Data were collected on the use of alternative therapies (general use of oral therapy, phytotherapy, dietary supplements, essential oils, infusions, homeopathy), the specific name of HFDS intakes (free text fields) and use of other alternative medicines. In the second version of the application, auto-completion was added to fill cancer treatments and HFDS intakes. From 3 May 2018, a specific food questionnaire was added, asking for the daily use of each CYP-interacting food: green tea, cranberry (berries or juice), grapefruit, pomelo, bitter orange (Seville orange), licorice, soy (roots or extracts) and coffee (>2 cups a day).
Definition and classification of HFDIs
The definition and classification of HFDIs were based on a preliminary work in which we systematically reviewed clinical and preclinical in vitro and in vivo data for 189 herbal medicines and food and 72 dietary supplements commonly used or likely to be responsible for interactions.13 HFDS–drug interactions where the HFDS was the victim were not considered.
The level of evidence was classified based on (i) the type of evidence of CYP-mediated HFDI (based on clinical trials versus based on in vitro or preclinical data); (ii) the similarity of the CYP metabolizing anticancer treatment and HFDS (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100650).
HFDI was defined as regular intake of an HFDS interacting with a CYP involved in the metabolism of one of the current anticancer treatments, based on the review mentioned previously.
HFDI was further classified as: (i) ‘probable’ in case of clinical evidence of HFDS interaction with a cytochrome involved in anticancer treatment (i.e. level 1-3 of the classification); (ii) ‘low evidence’ when the evidence of interaction was based on in vitro or preclinical data (i.e. level 4 or 5 of the classification).
Study objectives
The main endpoint was the proportion of HFDIs classified as ‘probable’. We aimed to show that self-reports from a tablet application could identify at least 5% of patients with a probable HFDI in the study population.
Power consideration and data analysis
The prespecified hypothesis was that we could detect 5% of probable HFDIs in the population. The number of probable interactions existing in the population has been estimated at 10%.9 For an α risk of 0.05 and a 1 − β power of 80%, we estimated that a population of 150 individuals was needed (one-sided test) using an exact binomial test. Since this study was set for hypothesis generating purposes, no Bonferroni adjustment was made as per exploratory analysis. Standard quality controls include basic descriptive statistics (including mean, median and range) and outliers will be studied for coherence.
The population with an identified probable HFDI was compared to the population without any interaction. Proportions were compared using Fisher’s exact test, and quantitative variables with a Wilcoxon test. Statistical analyses were carried out with R software version 4.1.2 (R Core Team - Alcatel-Lucent, NJ).
Results
Patients’ characteristics and treatments
One hundred and forty-three patients completed the form. Twelve patients declined participation through the ‘refusal tab’ of the application. Thirty-six patients did not fill any systemic treatment and were not included in the analysis (exclusion criteria) leaving 95 patients for the analysis (Figure 1). Most patients were recruited from the oncology department (n = 82), while a minority was recruited in the hematology (n = 12) or in the radiotherapy department (n = 1). Thirty-four patients (36%) were supervised by a health care professional during the application completion. The population for which data acquisition was unsupervised was different regarding cancer types with more patients having breast cancer (34% versus 21%) and less patients with hematologic diseases (3% versus 35%) (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100650).
The study population is detailed in Table 1. The median age was 57 years, and gender was well balanced (women/men 55%/45%, respectively). The repartition of cancer by localization was as follows: breast cancer (n = 28, 30%), lung cancer (n = 16, 17%), hematologic cancer (n = 14, 15%) and gynecological cancer (n = 10, 10%). Half of the patients (n = 49) had no surgery or no planned surgery. Thirty-five patients (37%) had no radiotherapy or no planned radiotherapy. Eighteen percent of patients were all tobacco smokers, and 3% were cannabis users. The repartition of patient’s characteristics and cancer localizations were compatible with the local distribution of patients in daycare hospital and the hematology department.
Table 1.
Population characteristics of patients with one or more herb, CYP-interacting food or dietary supplement (HFDS group) and patients with no HFDS intakes
| HFDS |
|||||
|---|---|---|---|---|---|
| Overall | No | Yes | P value | ||
| n | 95 | 21 (22.1) | 74 (77.9) | ||
| Population characteristics | |||||
| Age (years) | Median (IQR) | 55.8 (16.1) | 54.2 (19.6) | 56.1 (15.5) | 0.704 |
| Gender | Female | 43 (55.1) | 8 (66.7) | 35 (53.0) | 0.531 |
| Male | 35 (44.9) | 4 (33.3) | 31 (47.0) | ||
| Data collection modalities | |||||
| Supervision of a health care | Supervised | 34 (35.8) | 11 (32.4) | 23 (67.6) | 0.12 |
| professional | Unsupervised | 61 (64.2) | 10 (16.4) | 51 (83.6) | |
| Version of the application | Version 1 | 26 (27.4) | 11 (42.3) | 15 (57.7) | 0.006 |
| Version 2 | 69 (72.6) | 10 (14.5) | 59 (85.5) | ||
| Site | 1—Oncology—daycare hospital | 82 (86.3) | 20 (24.4) | 62 (75.6) | 0.445 |
| 2—Hematology—nurse | 12 (12.6) | 1 (8.3) | 11 (91.7) | ||
| 3—Radiotherapy—scanner | 1 (1.1) | 0 (0) | 1 (100) | ||
| Cancer and treatments | |||||
| Number of anticancer treatments Median (IQR) | 1.8 (1.0) | 2.0 (1.0) | 1.8 (1.0) | 0.420 | |
| Systemic cancer treatments | Chemotherapy | 58 (61.1) | 12 (20.7) | 46 (79.3) | 0.801 |
| IV targeted therapy | 30 (31.6) | 8 (26.7) | 22 (73.3) | 0.595 | |
| Oral targeted therapy | 12 (12.6) | 0 (0) | 12 (100) | 0.062 | |
| Hormone therapy | 6 (6.3) | 2 (33.3) | 4 (66.7) | 0.611 | |
| Immunotherapy | 8 (8.4) | 3 (37.5) | 5 (62.5) | 0.369 | |
| Not identified | 12 (12.6) | 2 (16.7) | 10 (83.3) | 1 | |
| Cancer type | Breast | 28 (29.5) | 8 (28.6) | 20 (71.4) | 0.67 |
| Gastrointestinal | 6 (6.3) | 2 (33.3) | 4 (66.7) | ||
| Gynecological | 10 (10.5) | 1 (10) | 9 (90) | ||
| Hematologic | 14 (14.7) | 1 (7.1) | 13 (92.9) | ||
| Head and neck | 8 (8.4) | 3 (37.5) | 5 (62.5) | ||
| Lung | 16 (16.8) | 3 (18.8) | 13 (81.2) | ||
| Urological | 4 (4.2) | 1 (25) | 3 (75) | ||
| Other | 8 (8.4) | 2 (25) | 6 (75) | ||
| Unknown | 1 (1.1) | 0 (0) | 1 (100) | ||
| Stage | Localized cancer | 28 (34.6) | 8 (28.6) | 20 (71.4) | 0.115 |
| Metastasized cancer | 48 (59.3) | 9 (18.8) | 39 (81.2) | ||
| Patient does not know | 5 (6.2) | 3 (60) | 2 (40) | ||
| Time since diagnosis | <6 months | 13 (20.0) | 1 (7.7) | 12 (92.3) | 0.806 |
| 6 months to 1 year | 15 (23.1) | 3 (20) | 12 (80) | ||
| 1 to 2 years | 11 (16.9) | 2 (18.2) | 9 (81.8) | ||
| 2 to 5 years | 14 (21.5) | 1 (7.1) | 13 (92.9) | ||
| >5 years | 12 (18.5) | 2 (16.7) | 10 (83.3) | ||
| Surgery | No | 49 (51.6) | 10 (20.4) | 39 (79.6) | 0.91 |
| Planned | 6 (6.3) | 1 (16.7) | 5 (83.3) | ||
| Yes (<1 year) | 19 (20.0) | 4 (21.1) | 15 (78.9) | ||
| Yes (>1 year) | 9 (9.5) | 3 (33.3) | 6 (66.7) | ||
| Patient does not know | 12 (12.6) | 3 (25) | 9 (75) | ||
| Radiotherapy | No | 35 (36.8) | 9 (25.7) | 26 (74.3) | 0.813 |
| Ongoing | 6 (6.3) | 0 (0) | 6 (100) | ||
| Planned | 12 (12.6) | 3 (25) | 9 (75) | ||
| Yes (<1 year) | 23 (24.2) | 4 (17.4) | 19 (82.6) | ||
| Yes (>1 year) | 8 (8.4) | 2 (25) | 6 (75) | ||
| Patient does not know | 11 (11.6) | 3 (27.3) | 8 (72.7) | ||
| Smoking habits | Tobacco | 14 (18.2) | 2 (14.3) | 12 (85.7) | 1 |
| Cannabis | 2 (2.8) | 0 | 2 (100) | 1 | |
| HFDS and alternative medicines | |||||
| Oral alternative medicine | Phytotherapy—herb extracts | 19 (20.4) | 0 | 19 (100) | — |
| Infusions | 20 (33.9) | 0 | 20 (100) | — | |
| Essential oil (aromatherapy) | 6 (6.5) | 0 | 6 (100) | — | |
| Dietary supplements | 22 (23.7) | 0 | 22 (100) | — | |
| Homeopathy | 12 (12.9) | 1 (8.3) | 11 (91.7) | 0.448 | |
| Other alternative medicine | Acupuncture | 8 (66.7) | 0 | 8 (100) | 0.091 |
| Hypnose | 1 (8.3) | 0 | 1 (100) | ||
| Qi gong | 1 (8.3) | 1 (100) | 0 | ||
| Reiki | 2 (16.7) | 1 (50.0) | 1 (50.0) | ||
| Food habits | Green tea | 17 (26.6) | 0 | 17 (100) | — |
| Coffee (>2 cups a day) | 39 (60.9) | 0 | 39 (100) | — | |
| Licorice (roots or sweets) | 1 (1.6) | 0 | 1 (100) | — | |
| Soy | 3 (4.7) | 0 | 3 (100) | — | |
| Grapefruit | 5 (7.8) | 0 | 5 (100) | — | |
| Pomelo | 3 (4.7) | 0 | 3 (100) | — | |
| Bitter orange (Seville orange) | 10 (15.6) | 0 | 10 (100) | — | |
| Cranberry | 3 (4.7) | 0 | 3 (100) | — | |
| Patient reported at least | Yes | 74 (77.9) | 0 | 74 (100) | — |
| one HFDS | No | 21 (22.1) | 21 (100) | 0 | |
| Probable HFDI identified | Yes | 16 (16.8) | 0 | 16 (100) | — |
| No | 79 (83.2) | 21 (26.6) | 58 (73.4) | ||
The population with HFDS was compared with the population without HFDS using Wilcoxon test for quantitative variables and Fisher’s exact test for categorical variables. Missing values (total n = 95): supervised: 0; version: 0; center: 0; age: 18; gender: 27; cancer type: 0; time since diagnosis: 30; stage: 14; surgery: 0; radiotherapy: 0; tobacco: 18; cannabis: 24; phytotherapy: 2; dietary supplements: 2; aromatherapy: 2; homeopathy: 2; infusions: 36; food habits: 31. P-values under 0.05 are in red and P-values between 0.05 and 0.1 are bold.
HFDS, herb, food and dietary supplements; HFDI, herb and food–drug interaction; IQR, interquartile range; IV, intravenous.
HFDS intakes
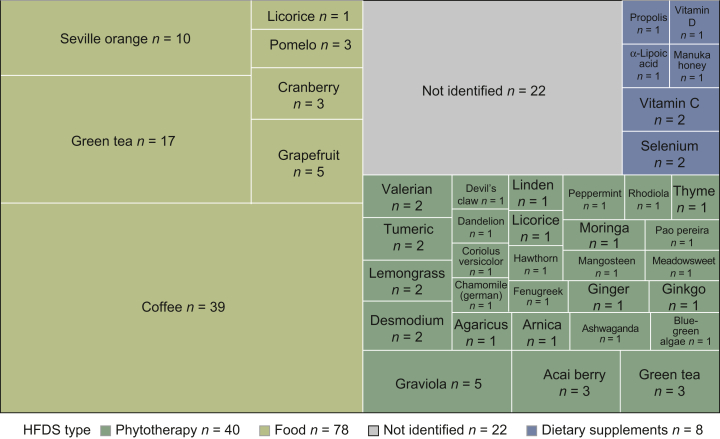
Overall, 74 patients (n = 74/95, 77.9%) reported at least one chronic intake of HFDS, and a total of 148 HFDSs were reported (Figure 2) (phytotherapy n = 40, 27%; CYP-interacting food n = 78, 53%; dietary supplement n = 8, 5%). Twenty-two HFDSs were reported but could not be automatically identified or mapped (15%, Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100650). The most frequently reported HFDS was coffee with 39 patients reporting daily use of over two cups. All 39 patients reported at least one other HFDS. Twelve patients reported the use of other alternative therapies (8/12 acupuncture). Four patients reported the use of 5 or more HFDSs, and one patient reported 10.
Figure 2.
Treemap plot of the 148 HFDSs reported among the 95 patients of the study. Green tea and licorice could have been used as a phytotherapy (intake of herb/root extract) or as food (infusion or roots) through the specific food questionnaire. Phytotherapies are represented in green, food in olive green and dietary supplements in blue. HFDS, herbs, food and dietary supplements.
No significant differences in characteristics were found between the groups of patients reporting HFDS intake and patients who did not (Table 1), except for a higher proportion of patients reporting HFDS intake with version 2 of the application than those with no HFDS reported (80% versus 48%, P = 0.006). Among the 26 patients who filled version 1 of the application, only 15 patients had an HFDS (58%) compared to 59 (86%, P = 0.006) with version 2 (Table 1). Patients and tumor characteristics according to the version of the application are detailed in Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100650.
HFDI identification
Among 74 patients who reported at least one HFDS, 6 patients reported only HFDS that could not be identified on the prespecified list (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100650), leaving 68 patients with potential HFDS–cancer treatment interactions for analysis.
Among 31 HFDIs reported, 22 were classified as probable and 9 had a low level of evidence (Table 2). Sixteen patients (16/95, 16.8%) had at least one HFDI classified as probable, which was significantly superior to the 5% prespecified threshold (P = 0.02). This result remains significant in the unsupervised population (11/61 patients, 18.0%, P = 0.04). Most HFDI classified as probable were identified as related to food products (20/22). Two herbs were identified as interacting: green tea (Camellia sinensis) and peppermint (Mentha piperita). No HFDIs classified as probable were found with dietary supplements. Green tea through the food questionnaire was identified within eight interactions. One patient had three interactions (green tea, pomelo and grapefruit). Four interactions were level 1, 8 were level 2 and 10 interactions were level 3. Six of the 22 food interactions concerned oral drugs [lenvatinib (×2), lapatinib, ibrutinib, dexamethasone and letrozole]. Eighteen of 22 probable interactions were mediated through CYP3A4. Only one cytochrome induction leading to probable HFDI was found (CYP3A4 induction by licorice).
Table 2.
Interaction between HFDS and anticancer treatments found in the study with the application
| Patient’s study number | HFDS | Cancer treatment | CYP involved | CYP modification | Level | Supervised | Auto-implementation | Food questionnaire | HFDS type | HFDI category |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | Green tea | Vinorelbine | CYP3A | Inhibition | 3 | Yes | No | No | Herb | Probable |
| 30 | Green tea | Paclitaxel | CYP3A | Inhibition | 3 | No | Yes | Yes | Food | Probable |
| Licorice | Paclitaxel | CYP3A | Induction | 1 | No | Yes | Yes | Food | Probable | |
| 36 | Green tea | Letrozole | CYP3A | Inhibition | 3 | No | Yes | Yes | Food | Probable |
| 38 | Cranberry | Paclitaxel | CYP3A | Inhibition | 2 | No | Yes | Yes | Food | Probable |
| Grapefruit | Paclitaxel | CYP3A | Inhibition | 1 | No | Yes | Yes | Food | Probable | |
| 40 | Coffee | Cyclophosphamide | CYP1A2 | Inhibition | 2 | No | Yes | Yes | Food | Probable |
| 44 | Green tea | Trastuzumab-emtansine | CYP3A | Inhibition | 3 | No | Yes | Yes | Food | Probable |
| 46 | Green tea | Paclitaxel | CYP3A | Inhibition | 3 | No | Yes | Yes | Food | Probable |
| 55 | Coffee | Cyclophosphamide | CYP1A2 | Inhibition | 2 | No | Yes | Yes | Food | Probable |
| 60 | Peppermint | Lenvatinib | CYP3A | Inhibition | 2 | No | Yes | No | Herb | Probable |
| Green tea | Lenvatinib | CYP3A | Inhibition | 2 | No | Yes | Yes | Food | Probable | |
| 71 | Grapefruit | Dexamethasone | CYP3A | Inhibition | 1 | Yes | Yes | Yes | Food | Probable |
| 73 | Cranberry | Lapatinib | CYP3A | Inhibition | 2 | No | Yes | Yes | Food | Probable |
| 78 | Green tea | Ibrutinib | CYP3A | Inhibition | 3 | Yes | Yes | Yes | Food | Probable |
| 79 | Green tea | Vinblastine | CYP3A | Inhibition | 3 | Yes | Yes | Yes | Food | Probable |
| Pomelo | Vinblastine | CYP3A | Inhibition | 3 | Yes | Yes | Yes | Food | Probable | |
| 85 | Coffee | Cyclophosphamide | CYP1A2 | Inhibition | 2 | Yes | Yes | Yes | Food | Probable |
| 87 | Green tea | Vinblastine | CYP3A | Inhibition | 3 | No | Yes | Yes | Food | Probable |
| Pomelo | Vinblastine | CYP3A | Inhibition | 3 | No | Yes | Yes | Food | Probable | |
| Grapefruit | Vinblastine | CYP3A | Inhibition | 1 | No | Yes | Yes | Food | Probable | |
| 89 | Coffee | Cyclophosphamide | CYP1A2 | Inhibition | 2 | No | Yes | Yes | Food | Probable |
The scale for the HFDS level of evidence of interaction with cytochromes is available in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100650, and has been reported in a previous study.13 Version 2 of the application allowed both auto-implementation and the acquisition of CYP-interacting food through a specific food questionnaire. In version 2 of the application, food–drug interactions could be detected either through the general questionnaire or the specific food questionnaire.
CYP, cytochrome P-450; HFDI, herb and food–drug interaction; HFDS, herb, food and dietary supplements.
Differences in the population with an interaction
For most variables tested (Table 3), no significant difference was found between HFDIs classified as probable and those of lower certainty. However, the proportion of patients from the hematology department (10% versus 25%) was higher in the probable HFDI group. Fourteen of the 16 patients with probable HFDI had a breast/gynecological cancer or a hematologic cancer (14/16 versus 38/79, P = 0.005). No significant difference was found between patients who were supervised for the completion (37%, 29/79) and those with no supervision (31%, 5/16, P = 0.9).
Table 3.
Population characteristics of patients with and without one or more identified herb–drug or food–drug interaction (HFDI) classified as probable
| HFDI classified as ‘probable’ |
|||||
|---|---|---|---|---|---|
| Overall | No | Yes | P value | ||
| n | 95 | 79 (83.2) | 16 (16.8) | ||
| Population characteristics | |||||
| Age | Median (IQR) | 55.8 (16.1) | 56.3 (16.8) | 53.9 (13.5) | 0.606 |
| Gender | Female | 43 (55.1) | 32 (74.4) | 11 (25.6) | 0.344 |
| Male | 35 (44.9) | 30 (85.7) | 5 (14.3) | ||
| Data collection modalities | |||||
| Supervision of a health care | Supervised | 34 (35.8) | 29 (85.3) | 5 (14.7) | 0.897 |
| professional | Unsupervised | 61 (64.2) | 50 (82) | 11 (18) | |
| Version of the application | Version 1 | 26 (27.4) | 25 (96.2) | 1 (3.8) | 0.077 |
| Version 2 | 69 (72.6) | 54 (78.3) | 15 (21.7) | ||
| Site | 1—Oncology—daycare hospital | 82 (86.3) | 71 (86.6) | 11 (13.4) | 0.019 |
| 2—Hematology—nurse | 12 (12.6) | 8 (66.7) | 4 (33.3) | ||
| 3—Radiotherapy—scanner | 1 (1.1) | 0 (0) | 1 (100) | ||
| Cancer and treatments | |||||
| Number of anticancer treatments Median (IQR) | 1.8 (1.0) | 1.7 (0.9) | 2.1 (1.1) | 0.144 | |
| Systemic cancer treatments | Chemotherapy | 58 (61.1) | 48 (82.8) | 10 (17.2) | |
| IV targeted therapy | 30 (31.6) | 24 (80) | 6 (20) | ||
| Oral targeted therapy | 12 (12.6) | 8 (66.7) | 4 (33.3) | ||
| Hormone therapy | 6 (6.3) | 5 (83.3) | 1 (16.7) | ||
| Immunotherapy | 8 (8.4) | 8 (100) | 0 (0) | ||
| Not identified | 12 (12.6) | 11 (91.7) | 1 (8.3) | ||
| Cancer type | Breast | 28 (29.5) | 23 (82.1) | 5 (17.9) | 0.103 |
| Gastrointestinal | 6 (6.3) | 6 (100) | 0 (0) | ||
| Gynecological | 10 (10.5) | 7 (70) | 3 (30) | ||
| Hematologic | 14 (14.7) | 8 (57.1) | 6 (42.9) | ||
| Head and neck | 8 (8.4) | 8 (100) | 0 (0) | ||
| Lung | 16 (16.8) | 15 (93.8) | 1 (6.2) | ||
| Urological | 4 (4.2) | 4 (100) | 0 (0) | ||
| Other | 8 (8.4) | 7 (87.5) | 1 (12.5) | ||
| Unknown | 1 (1.1) | 1 (100) | 0 (0) | ||
| Stage | Localized cancer | 28 (34.6) | 24 (85.7) | 4 (14.3) | 0.669 |
| Metastasized cancer | 48 (59.3) | 42 (87.5) | 6 (12.5) | ||
| Patient does not know | 5 (6.2) | 5 (100) | 0 (0) | ||
| Time since diagnosis | <6 months | 13 (20.0) | 9 (69.2) | 4 (30.8) | 0.545 |
| 6 months to 1 year | 15 (23.1) | 11 (73.3) | 4 (26.7) | ||
| 1 to 2 years | 11 (16.9) | 10 (90.9) | 1 (9.1) | ||
| 2 to 5 years | 14 (21.5) | 12 (85.7) | 2 (14.3) | ||
| >5 years | 12 (18.5) | 8 (66.7) | 4 (33.3) | ||
| Surgery | No | 49 (51.6) | 41 (83.7) | 8 (16.3) | 0.249 |
| Planned | 6 (6.3) | 5 (83.3) | 1 (16.7) | ||
| Yes (<1 year) | 19 (20.0) | 13 (68.4) | 6 (31.6) | ||
| Yes (>1 year) | 9 (9.5) | 9 (100) | 0 (0) | ||
| Patient does not know | 12 (12.6) | 11 (91.7) | 1 (8.3) | ||
| Radiotherapy | No | 35 (36.8) | 29 (82.9) | 6 (17.1) | 0.355 |
| Ongoing | 6 (6.3) | 4 (66.7) | 2 (33.3) | ||
| Planned | 12 (12.6) | 11 (91.7) | 1 (8.3) | ||
| Yes (<1 year) | 23 (24.2) | 17 (73.9) | 6 (26.1) | ||
| Yes (>1 year) | 8 (8.4) | 7 (87.5) | 1 (12.5) | ||
| Patient does not know | 11 (11.6) | 11 (100) | 0 (0) | ||
| Smoking habits | Tobacco | 14 (18.2) | 11 (78.6) | 3 (21.4) | 1 |
| Cannabis | 2 (2.8) | 1 (50.0) | 1 (50.0) | 0.38 | |
| HFDS and alternative medicines | |||||
| Oral alternative medicine | Phytotherapy—herb extracts | 19 (20.4) | 14 (73.7) | 5 (26.3) | 0.306 |
| Infusions | 20 (33.9) | 16 (80) | 4 (20.0) | 0.424 | |
| Essential oil (aromatherapy) | 6 (6.5) | 3 (50.0) | 3 (50.0) | 0.061 | |
| Dietary supplements | 22 (23.7) | 18 (81.8) | 4 (18.2) | 1 | |
| Homeopathy | 12 (12.9) | 11 (91.7) | 1 (8.3) | 0.684 | |
| Other alternative medicine | Acupuncture | 8 (66.7) | 6 (75) | 2 (25) | 0.721 |
| Hypnose | 1 (8.3) | 1 (100) | 0 (0) | ||
| Qi gong | 1 (8.3) | 1 (100) | 0 (0) | ||
| Reiki | 2 (16.7) | 1 (50) | 1 (50) | ||
| Food habits | Green tea | 17 (26.6) | 9 (52.9) | 8 (47.1) | 0.016 |
| Coffee (>2 cups a day) | 39 (60.9) | 32 (82.1) | 7 (17.9) | 0.235 | |
| Licorice (roots or sweets) | 1 (1.6) | 0 | 1 (100) | 0.234 | |
| Soy | 3 (4.7) | 3 (100) | 0 | 1 | |
| Grapefruit | 5 (7.8) | 2 (40.0) | 3 (60.0) | 0.079 | |
| Pomelo | 3 (4.7) | 1 (33.3) | 2 (66.7) | 0.134 | |
| Bitter orange (Seville orange) | 10 (15.6) | 10 (100) | 0 | 0.1 | |
| Cranberry | 3 (4.7) | 1 (33.3) | 2 (66.7) | 0.134 | |
| Patient reported at least | Yes | 74 (77.9) | 58 (78.4) | 16 (21.6) | 0.045 |
| one HFDS | No | 21 (22.1) | 21 (100) | 0 (0) | |
| Probable HFDI identified | Yes | 16 (16.8) | 0 (0) | 16 (100) | — |
| No | 79 (83.2) | 79 (100) | 0 (0) | ||
The population with an HFDI classified as probable was compared to the population without an HFDI using Wilcoxon test for quantitative values and Fisher’s exact test for categorical variables. Missing values (total n = 95): supervised: 0; version: 0; center: 0; age: 18; gender: 27; cancer type: 0; time since diagnosis: 30; stage: 14; surgery: 0; radiotherapy: 0; tobacco: 18; cannabis: 24; phytotherapy: 2; dietary supplements: 2; aromatherapy: 2; homeopathy: 2; infusions: 36; food habits: 31. P-values under 0.05 are in red and P-values between 0.05 and 0.1 are bold.
HFDI, herb and food–drug interaction; HFDS, herb, food and dietary supplements; IQR, interquartile range; IV, intravenous.
Discussion
In the present study, we showed that a tablet application helped identify interactions between herbs, dietary supplements or food and cancer treatment in the routine care of cancer patients.
Our study gives several insights into the field of oral alternative medicine and cancer care.
Firstly, we found that 77.9% (74/95) of patients reported a regular intake of HFDS. Among these patients 148 HFDSs were identified, mainly CYP-interacting food (n = 78/148, 53%). This proportion is higher than in previous reported studies in cancer patients. Out of 1739 patients with cancer in the US, Rashrash and colleagues1 reported that 43% were using herbal medicine. In the early breast cancer prospective cohort CANTO,3 among 5237 women, 23.0% reported oral alternative medicines, mostly homeopathy (65.4%). Finally, in another cohort from phase I trials, out of 212 patients, 72 (34%) were taking herbs or dietary supplements. As clinically relevant herb–drug or food–drug interactions can occur, leading to increased toxicity or decreased efficacy, identifying such intakes is critical for good care.
Secondly, among 74 patients who reported HFDS intakes, we identified 22 HFDIs classified as probable, i.e. likely to be clinically significant, within 16 patient reports (n = 16/95, 16.8%). In a previous study in 804 patients, Bush et al.9 found that 122 patients (15%) were using traditional medicines, 49 of whom (6% of all patients) had a potential herb–drug interaction. We found a higher rate of HFDI in our study which could be explained by the specific food questionnaire that we used. However, the clinical impact of HFDI we found in our study, even classified as ‘probable’, could in fact be low. We found four patients with an HFDI involving cyclophosphamide and coffee. Cyclophosphamide is an oxazaphosphorine derivative that has a complex pharmacology. It is a prodrug which needs to be activated by several cytochromes, including CYP3A, CYP2B6 and CYP1A213 to exert its activity. Coffee inhibits CYP1A2 and can lead to a clinically significant rise in concentrations of drugs metabolized through this CYP.17 Although the interaction of coffee with CYP1A2 is clinically proven, the role of CYP1A2 in the activation of cyclophosphamide is unlikely to result in a decreased efficacy since most of this activation occurs via CYP3A and CYP2B.18 Similarly, CYP modifications due to HFDS consumption are often mild (<25%). The relevance of these interactions for treatments with large therapeutic indexes, such as letrozole, may be irrelevant. Our study did not take into account the volume, frequency and schedule of HFDSs taken by the patient, although it modifies the intensity of the interaction.13 Subsequent studies should be able to quantify HFDS consumption as well as time between drug and herb intake to better assess HFDI relevance.
Thirdly, we found that most interactions were food-mediated rather than herb-mediated. Most published studies focused on oral alternative interactions rather than food–drug interactions.9,12 Though frequent, food–drug interactions are rarely reported or considered, and they may be the tip of the iceberg of HFDI. Some of these interactions may increase the risk of anticancer drug toxicity or inefficacy. Grapefruit is a strong CYP3A4 inhibitor that could be responsible for severe toxicity by decreasing the bioavailability of anticancer drugs metabolized by CYP3A4.13
Finally, we found that a tablet application could successfully be used to collect both HFDS and cancer treatments and ultimately detect HFDI. This finding remains in the population that filled the questionnaire without the presence of a health care professional. This is particularly noteworthy because collecting the use of HFDS is time-consuming with up to 10 HFDSs for a single patient in our cohort. Also, few health care professionals are trained in the field of herb–drug and food–drug interactions and identifying them requires pharmacological expertise. Our work demonstrates that the acquisition of data does not need to be supervised by a health care professional to be relevant when auto-completion and precise questionnaires are provided. An extensive analysis of HFDS to identify probable HFDI could therefore be, at least partially, automated. The collection of these self-reported data is doable in routine care, and the use of such device and applications is expected to grow in oncology in the near future. However, we showed a major role of active encouragement by the staff to fill out the application questionnaire. In the radiotherapy department where simple passive advertising was used, only one patient completed the questionnaire and was included in our study within 3 months. After the end of the data acquisition phase of the study, the tablet was stolen from the stand, despite appropriate locking measures. Passive enrollment failed to contribute to our study and should not be considered as an appropriate option.
Our study has several strengths. To our knowledge, no study to date has examined how self-reported HFDS intakes could identify potential drug interactions. Our work is thus the first to demonstrate the feasibility of such an approach. Of note, we cannot exclude that some interactions failed to be identified, either because patients failed to accurately report data, or because these HFDIs have not been published or identified in the literature review we relied on.13
Our study opens several perspectives. We confirmed the high frequency of HFDS intakes and showed that a substantial proportion of HFDSs interact with anticancer treatment. An automated questionnaire could highlight potential herb–drug and food–drug interaction and help clinicians and clinical pharmacists to detect them and prevent them when relevant.19
Clinically significant PK herb–drug or food–drug interaction could have several consequences. When relevant cytochromes are inhibited, the interaction could lead to a decrease in drug clearance and increased mean plasma concentration of the anticancer drug, thus increasing the risk of drug-induced toxicity.11 One patient with daily grapefruit juice intake, a CYP3A4 inhibitor, had a doubling in docetaxel plasma.20 In this case, the docetaxel dose was low (40 mg/m2), but a doubling of docetaxel exposure could have dramatic consequences with a higher dose.21 In contrast, the induction of cytochromes involved in anticancer drugs’ metabolism could increase drug clearance and decrease drug exposure, leading to reduced efficacy. CYP3A4 induction properties of piperine (with curcumin) have been observed to decrease everolimus Ctrough concentration below drug level targets with clinical consequences22 and likely led to a decreased anticancer efficacy.
Similar questionnaires could also be implemented on a smartphone device. In a breast cancer survivor population, over 70% of the population had access to a smartphone.23 A smartphone application could allow the implementation of patient-oriented questionnaires on a large scale and provide prospective data for future studies. In this proof-of-concept study, we demonstrated that a tablet application could identify and help prevent herb–drug and food–drug interactions. The clinical benefit of such an approach to decrease anticancer drug toxicity or inefficacy remains unknown and should be explored on a larger-scale study focusing on clinical outcomes.
Acknowledgments
Funding
None declared.
Disclosure
OM is an employee and shareholder of Amgen since 1 February 2022. OM has consulting activities for Amgen, Astra-Zeneca, Bayer, Blueprint Medicines, Bristol Myers-Squibb, Eli-Lilly, Incyte, Ipsen, Lundbeck, MSD, Novartis, Pfizer, Roche, Servier, Vifor Pharma; board membership for Amgen, Astra-Zeneca, Bayer, Blueprint Medicines, Bristol Myers-Squibb, Eli-Lilly, Lundbeck, MSD, Novartis, Pfizer, Roche, Servier, Vifor Pharma; speaker bureau of Eli-Lilly, Roche, Servier; stock ownership of Amplitude surgical, Transgene and employee of Gustave Roussy and ESMO (Annals of Oncology). AG, as part of the Drug Development Department (DITEP), is a Principal/sub-Investigator of Clinical Trials for Abbvie, Adaptimmune, Aduro Biotech, Agios Pharmaceuticals, Amgen, Argen-X Bvba, Arno Therapeutics, Astex Pharmaceuticals, Astra Zeneca, Astra Zeneca Ab, Aveo, Bayer Healthcare Ag, Bbb Technologies Bv, Beigene, Bioalliance Pharma, Biontech Ag, Blueprint Medicines, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, Bristol-Myers Squibb International Corporation, Ca, Celgene Corporation, Cephalon, Chugai Pharmaceutical Co., Clovis Oncology, Cullinan-Apollo, Daiichi Sankyo, Debiopharm S.A., Eisai, Eisai Limited, Eli Lilly, Exelixis, Forma Tharapeutics, Gamamabs, Genentech, Gilead Sciences, Glaxosmithkline, Glenmark Pharmaceuticals, H3 Biomedicine, Hoffmann La Roche Ag, Incyte Corporation, Innate Pharma, Institut De Recherche Pierre Fabre, Iris Servier, Janssen Cilag, Janssen Research Foundation, Kura Oncology, Kyowa Kirin Pharm. Dev., Lilly France, Loxo Oncology, Lytix Biopharma As, Medimmune, Menarini Ricerche, Merck Kgaa, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Molecular Partners Ag, Nanobiotix, Nektar Therapeutics, Nerviano Medical Sciences, Novartis Pharma, Octimet Oncology Nv, Oncoethix, Oncomed, Oncopeptides, Onyx Therapeutics, Orion Pharma, Oryzon Genomics, Ose Pharma, Pfizer, Pharma Mar, Philogen S.P.A., Pierre Fabre Medicament, Plexxikon, Rigontec Gmbh, Roche, Sanofi Aventis, Sierra Oncology, Sotio A.S, Syros Pharmaceuticals, Taiho Pharma, Tesaro, Tioma Therapeutics, Wyeth Pharmaceuticals France, Xencor, Y’s Therapeutics; research grants from Astrazeneca, BMS, Boehringer Ingelheim, Janssen Cilag, Merck, Novartis, Pfizer, Roche, Sanofi; and non-financial support (drug supplied) from Astrazeneca, Bayer, BMS, Boringher Ingelheim, Johnson & Johnson, Lilly, Medimmune, Merck, NH TherAGuiX, Pfizer, Roche. SC has consulting activities for Novartis, Gilead, Atara, Pierre Fabre, Janssen, Takeda, Abbvie, Astra Zeneca. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Rashrash M., Schommer J.C., Brown L.M. Prevalence and predictors of herbal medicine use among adults in the United States. J Patient Exp. 2017;4(3):108–113. doi: 10.1177/2374373517706612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hietala M., Henningson M., Ingvar C., Jönsson P.E., Rose C., Jernström H. Natural remedy use in a prospective cohort of breast cancer patients in southern Sweden. Acta Oncol. 2011;50(1):134–143. doi: 10.3109/0284186X.2010.484812. [DOI] [PubMed] [Google Scholar]
- 3.Lapidari P., Djehal N., Havas J., et al. Determinants of use of oral complementary-alternative medicine among women with early breast cancer: a focus on cancer-related fatigue. Breast Cancer Res Treat. 2021;190(3):517–529. doi: 10.1007/s10549-021-06394-2. [DOI] [PubMed] [Google Scholar]
- 4.Hlubocky F.J., Ratain M.J., Wen M., Daugherty C.K. Complementary and alternative medicine among advanced cancer patients enrolled on phase I trials: a study of prognosis, quality of life, and preferences for decision making. J Clin Oncol. 2007;25(5):548–554. doi: 10.1200/JCO.2005.03.9800. [DOI] [PubMed] [Google Scholar]
- 5.Dy G.K., Bekele L., Hanson L.J., et al. Complementary and alternative medicine use by patients enrolled onto phase I clinical trials. J Clin Oncol. 2004;22(23):4810–4815. doi: 10.1200/JCO.2004.03.121. [DOI] [PubMed] [Google Scholar]
- 6.Beijnen J.H., Schellens J.H.M. Drug interactions in oncology. Lancet Oncol. 2004;5(8):489–496. doi: 10.1016/S1470-2045(04)01528-1. [DOI] [PubMed] [Google Scholar]
- 7.Blower P., de Wit R., Goodin S., Aapro M. Drug-drug interactions in oncology: why are they important and can they be minimized? Crit Rev Oncol Hematol. 2005;55(2):117–142. doi: 10.1016/j.critrevonc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Parsad S., Ratain M.J. Drug-drug interactions with oral antineoplastic agents. JAMA Oncol. 2017;3(6):736–738. doi: 10.1001/jamaoncol.2016.3323. [DOI] [PubMed] [Google Scholar]
- 9.Bush T.M., Rayburn K.S., Holloway S.W., et al. Adverse interactions between herbal and dietary substances and prescription medications: a clinical survey. Altern Ther Health Med. 2007;13(2):30–35. [PubMed] [Google Scholar]
- 10.Posadzki P., Watson L., Ernst E. Herb-drug interactions: an overview of systematic reviews. Br J Clin Pharmacol. 2013;75(3):603–618. doi: 10.1111/j.1365-2125.2012.04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gougis P., Palmieri L.J., Funck-Brentano C., et al. Major pitfalls of protein kinase inhibitors prescription: a review of their clinical pharmacology for daily use. Crit Rev Oncol Hematol. 2019;141:112–124. doi: 10.1016/j.critrevonc.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Engdal S., Klepp O., Nilsen O.G. Identification and exploration of herb-drug combinations used by cancer patients. Integr Cancer Ther. 2009;8(1):29–36. doi: 10.1177/1534735408330202. [DOI] [PubMed] [Google Scholar]
- 13.Gougis P., Hilmi M., Geraud A., Mir O., Funck-Brentano C. Potential cytochrome P450-mediated pharmacokinetic interactions between herbs, food, and dietary supplements and cancer treatments. Crit Rev Oncol Hematol. 2021;166 doi: 10.1016/j.critrevonc.2021.103342. [DOI] [PubMed] [Google Scholar]
- 14.Basch E., Deal A.M., Dueck A.C., et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basch E., Deal A.M., Kris M.G., et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denis F. Web-mediated follow-up and prognosis in lung cancer patients. Med Sci (Paris) 2018;34(6-7):590–594. doi: 10.1051/medsci/20183406020. [DOI] [PubMed] [Google Scholar]
- 17.Raaska K., Raitasuo V., Laitila J., Neuvonen P.J. Effect of caffeine-containing versus decaffeinated coffee on serum clozapine concentrations in hospitalised patients. Basic Clin Pharmacol Toxicol. 2004;94(1):13–18. [PubMed] [Google Scholar]
- 18.Chang T.K., Yu L., Maurel P., Waxman D.J. Enhanced cyclophosphamide and ifosfamide activation in primary human hepatocyte cultures: response to cytochrome P-450 inducers and autoinduction by oxazaphosphorines. Cancer Res. 1997;57(10):1946–1954. [PubMed] [Google Scholar]
- 19.Schnipper J.L., Kirwin J.L., Cotugno M.C., et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565–571. doi: 10.1001/archinte.166.5.565. [DOI] [PubMed] [Google Scholar]
- 20.Valenzuela B., Rebollo J., Pérez T., Brugarolas A., Pérez-Ruixo J.J. Effect of grapefruit juice on the pharmacokinetics of docetaxel in cancer patients: a case report. Br J Clin Pharmacol. 2011;72(6):978–981. doi: 10.1111/j.1365-2125.2011.04052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruno R., Hille D., Riva A., et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16(1):187–196. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]
- 22.Mir O., Ropert S., Chamseddine A.N., Paci A. Curcumin dietary supplements and everolimus-based cancer treatment. Ann Oncol. 2018;29(1):287–288. doi: 10.1093/annonc/mdx714. [DOI] [PubMed] [Google Scholar]
- 23.Moon Z., Zuchowski M., Moss-Morris R., Hunter M.S., Norton S., Hughes L.D. Disparities in access to mobile devices and e-health literacy among breast cancer survivors. Support Care Cancer. 2022;30(1):117–126. doi: 10.1007/s00520-021-06407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.