Abstract
Background
A growing body of evidence suggests that non-viral hepatocellular carcinoma (HCC) might benefit less from immunotherapy.
Materials and methods
We carried out a retrospective analysis of prospectively collected data from consecutive patients with non-viral advanced HCC, treated with atezolizumab plus bevacizumab, lenvatinib, or sorafenib, in 36 centers in 4 countries (Italy, Japan, Republic of Korea, and UK). The primary endpoint was overall survival (OS) with atezolizumab plus bevacizumab versus lenvatinib. Secondary endpoints were progression-free survival (PFS) with atezolizumab plus bevacizumab versus lenvatinib, and OS and PFS with atezolizumab plus bevacizumab versus sorafenib. For the primary and secondary endpoints, we carried out the analysis on the whole population first, and then we divided the cohort into two groups: non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) population and non-NAFLD/NASH population.
Results
One hundred and ninety patients received atezolizumab plus bevacizumab, 569 patients received lenvatinib, and 210 patients received sorafenib. In the whole population, multivariate analysis showed that treatment with lenvatinib was associated with a longer OS [hazard ratio (HR) 0.65; 95% confidence interval (CI) 0.44-0.95; P = 0.0268] and PFS (HR 0.67; 95% CI 0.51-0.86; P = 0.002) compared to atezolizumab plus bevacizumab. In the NAFLD/NASH population, multivariate analysis confirmed that lenvatinib treatment was associated with a longer OS (HR 0.46; 95% CI 0.26-0.84; P = 0.0110) and PFS (HR 0.55; 95% CI 0.38-0.82; P = 0.031) compared to atezolizumab plus bevacizumab. In the subgroup of non-NAFLD/NASH patients, no difference in OS or PFS was observed between patients treated with lenvatinib and those treated with atezolizumab plus bevacizumab. All these results were confirmed following propensity score matching analysis. By comparing patients receiving atezolizumab plus bevacizumab versus sorafenib, no statistically significant difference in survival was observed.
Conclusions
The present analysis conducted on a large number of advanced non-viral HCC patients showed for the first time that treatment with lenvatinib is associated with a significant survival benefit compared to atezolizumab plus bevacizumab, in particular in patients with NAFLD/NASH-related HCC.
Key words: advanced HCC, NASH, NAFLD, lenvatinib, sorafenib, atezolizumab, bevacizumab
Highlights
-
•
Recent evidences suggest that non-viral HCCs could be less responsive to immunotherapy.
-
•
Lenvatinib performs better compared to atezolizumab plus bevacizumab in non-viral HCC.
-
•
Sorafenib performs similarly to atezolizumab plus bevacizumab in non-viral HCC.
Introduction
Hepatocellular carcinoma (HCC) represents the sixth most common cancer worldwide and, with 830 180 deaths in 2020, it ranked third among cancer-related deaths.1 Despite recent advances, HCC treatment still constitutes a big challenge, due to the complexity of its pathogenesis and the heterogeneity of etiology. In the last 10 years, a number of therapeutic strategies have been investigated for patients with HCC unsuitable for locoregional approaches.2, 3, 4, 5, 6 Sorafenib was the first multikinase inhibitor (MKI) approved as first-line treatment for unresectable/advanced disease.2,3 Subsequently, the REFLECT trial demonstrated the non-inferiority of lenvatinib, another MKI, compared to sorafenib as first-line treatment,7 which has been confirmed by a number of real-world studies.8, 9, 10, 11, 12, 13 Beyond MKIs, immunotherapy has been recently investigated in the advanced HCC setting.14, 15, 16 Practice-changing results came from the combination therapy trials. The phase III IMbrave150 trial demonstrated a statistically significant improvement in overall survival (OS) and progression-free survival (PFS) with the combination of the anti-programmed cell death-ligand 1 (PD-L1) atezolizumab and the anti-vascular endothelial growth factor (VEGF) bevacizumab, compared to sorafenib. This trial led to the approval of this combination as the new first-line standard of care for unresectable HCC.17 Recently, the phase III HIMALAYA trial demonstrated a statistically significant improvement in OS with the combination of the anti-cytotoxic T-lymphocyte antigen 4 tremelimumab plus the anti-PD-L1 durvalumab compared to sorafenib.18 In addition, the phase III COSMIC-312 trial showed a PFS benefit with the combination of cabozantinib and atezolizumab compared to sorafenib, even though no significant advantage was reported in terms of OS at the pre-planned interim analysis.19 Interestingly, non-preplanned subgroup analyses suggested different efficacy depending on several factors, including etiology. The IMbrave150 trial showed no difference in terms of OS and PFS between atezolizumab plus bevacizumab and sorafenib in patients with non-viral etiology.17 The HIMALAYA trial did not show a benefit from the combination in patients with hepatitis C virus-related HCC.18 The interim analysis from the COSMIC-312 trial showed a benefit from the combination therapy in patients with viral-related HCC, mainly hepatitis B virus patients, and no benefit in non-viral patients.19 These emerging data are of particular interest and are redirecting the investigation with a special focus on different etiologies underlying the pathogenesis of liver cancer. In fact, it has been hypothesized that viral and/or non-viral etiology could influence the immune contexture of HCC leading to differential response to treatments.20 A number of studies are currently focusing on non-viral etiologies, including non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), associated to metabolic syndrome, obesity, dyslipidemia, and type 2 diabetes.21 NAFLD prevalence has been estimated to be ∼25% in the general population worldwide, and epidemiologic models predict a further increase in NAFLD/NASH prevalence.21 Innate and adaptive immune-cell activation in combination with the endoplasmic reticulum stress and increased release of metabolites in patients with NASH are hypothesized to trigger the necro-inflammation of hepatocytes resulting in fibrotic regeneration and increased risk of HCC.22, 23, 24, 25, 26, 27, 28 An important repercussion of NAFLD and its biologic pathways on the immune system has been hypothesized. Recently, Pfister et al. demonstrated that the increase of hepatic CD8+PD1+ T cells induced by immunotherapy impairs immune surveillance and could trigger hepatocarcinogenesis in the mouse model of NASH.20 Furthermore, Pfister and collaborators carried out a meta-analysis of three phase III immunotherapy studies (CheckMate 459, Imbrave150, and KEYNOTE-240) and analyzed the survival results based on etiology (viral versus non-viral). Notably, in the subset of patients with non-viral HCC, they did not show an improved survival with immunotherapy.14 Based on these results, we recently carried out a multicenter retrospective analysis on a large cohort of patients treated with lenvatinib as first-line treatment for advanced disease and showed that NASH-related etiology is an independent positive prognostic and predictive factor for OS, suggesting a possible role of the etiology in the selection of patients candidate to lenvatinib.29
Moving from these premises, we designed this study with the aim of evaluating survival outcomes in HCC patients with non-viral etiology treated with immunotherapy versus MKIs.
Materials and methods
Study population
The study population derived from the retrospective analysis of prospectively collected patients treated with atezolizumab plus bevacizumab or lenvatinib or sorafenib as first-line treatment for advanced HCC [Barcelona Clinic Liver Cancer stage C (BCLC-C)] or intermediate HCC (BCLC-B) deemed not eligible for locoregional therapies.
The study population is described in the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2022.100591.30
The primary endpoint of the study was OS with atezolizumab plus bevacizumab versus lenvatinib.
Secondary endpoints were PFS with atezolizumab plus bevacizumab versus lenvatinib, and OS and PFS with atezolizumab plus bevacizumab versus sorafenib.
The present study was approved by the ethics committee (EC) at each center, complied with the provisions of the Good Clinical Practice guidelines and the Declaration of Helsinki and local laws, and fulfilled the Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data. In particular, the protocol was firstly approved by the San Raffaele Authority Hospital EC for the coordinating center and subsequently approved by the remaining EC (number DSAN854-A-OS/5). A written Informed consent was obtained according to the EC’s recommendations.
Statistical analysis
Frequency tables were generated for categorical variables. Continuous variables were presented using median and range. OS was defined as the time from the start date of studied treatment to the date of death. PFS was defined as the time from the start date of studied treatment to the date of progression or death or last follow-up whichever occurred first. OS and PFS were reported as median values expressed in months, with 95% confidence interval (CI). Survival curves were estimated using the product-limit method of Kaplan–Meier. The role of stratification factors was analyzed using log-rank tests.
Unadjusted and adjusted hazard ratios (HRs) by baseline characteristics were calculated using the Cox proportional hazards model. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0.31 Then, a propensity score matching analysis was carried out (Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2022.100591).32
A MedCalc package (MedCalc® version 16.8.4) was used for statistical analysis.
Results
Atezolizumab plus bevacizumab versus lenvatinib
Clinical outcomes in the whole patient population
A total of 759 consecutive patients were available for the analysis. Five hundred and sixty-nine patients were treated with lenvatinib, and 190 patients were treated with atezolizumab plus bevacizumab.
The median follow-up was 8.9 months (95% CI 7.9-10.4 months) for atezolizumab plus bevacizumab patients and 13.7 months (95% CI 12.8-15.1 months) for lenvatinib patients.
Baseline patient characteristics are shown in Table 1. The two groups differed in previous surgery, Child–Pugh class, Eastern Cooperative Oncology Group (ECOG) performance status (PS), and albumin-bilirubin (ALBI) grade.
Table 1.
Lenvatinib and atezolizumab plus bevacizumab patients’ cohort characteristics at baseline
| Whole patient population |
NASH/NAFLD population |
Non-viral/NASH/NAFLD population |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Atezolizumab plus bevacizumab, n (%) n = 190 patients | Lenvatinib, n (%) n = 569 patients | P value | Atezolizumab plus bevacizumab, n (%) n = 82 patients | Lenvatinib, n (%) n = 254 patients | P value | Atezolizumab plus bevacizumab, n (%) n = 108 patients | Lenvatinib, n (%) n = 314 patients | P value | |
| Gender | |||||||||
| Male | 149 (78.4) | 457 (80.3) | 62 (75.6) | 190 (74.8) | 87 (80.5) | 267 (85) | |||
| Female | 41 (21.6) | 112 (19.7) | 0.60 | 20 (24.4) | 64 (25.2) | 1.00 | 21 (19.5) | 47 (15) | 0.28 |
| Age, years | |||||||||
| <75 | 111 (58.4) | 319 (56) | 39 (47.5) | 124 (48.8) | 72 (66.7) | 195 (62) | |||
| ≥75 | 79 (41.6) | 250 (44) | 0.61 | 43 (52.5) | 130 (51.2) | 0.89 | 36 (33.3) | 119 (38) | 0.41 |
| Previous surgery | |||||||||
| Yes | 91 (48) | 153 (27) | 36 (44) | 82 (32) | 55 (51) | 71 (22.6) | |||
| No | 99 (52) | 416 (73) | <0.000001 | 46 (56) | 172 (68) | 0.06 | 53 (49) | 243 (77.4) | <0.000001 |
| Previous radiofrequency ablation | |||||||||
| Yes | 43 (22.6) | 98 (17.2) | 14 (17) | 47 (18.5) | 29 (27) | 51 (16.2) | |||
| No | 147 (77.4) | 471 (82.8) | 0.10 | 68 (83) | 207 (71.5) | 0.86 | 89 (73) | 263 (83.8) | 0.052 |
| Previous TACE | |||||||||
| Yes | 74 (39) | 276 (48.5) | 31 (37.8) | 126 (49.6) | 43 (39.8) | 150 (47.8) | |||
| No | 116 (61) | 293 (51.5) | 0.02 | 51 (62.2) | 128 (50.4) | 0.07 | 65 (60.2) | 164 (52.2) | 0.17 |
| Child–Pugh class | |||||||||
| A | 179 (94.2) | 488 (85.8) | 77 (94) | 223 (87.8) | 102 (94.4) | 265 (84.4) | |||
| B | 11 (5.8) | 81 (14.2) | 0.001 | 5 (6) | 31 (12.2) | 0.18 | 6 (5.6) | 49 (15.6) | 0.007 |
| BCLC stage | |||||||||
| B | 85 (44.7) | 235 (41.4) | 39 (47.5) | 108 (42.5) | 46 (42.6) | 127 (40.4) | |||
| C | 105 (55.3) | 334 (58.6) | 0.44 | 43 (52.5) | 146 (57.5) | 0.15 | 62 (57.4) | 187 (59.6) | 0.73 |
| ECOG PS | |||||||||
| 0 | 142 (74.7) | 466 (82) | 61 (74.4) | 210 (82.7) | 81 (75) | 256 (81.5) | |||
| >0 | 48 (25.3) | 103 (18) | 0.03 | 21 (25.6) | 44 (17.3) | 0.22 | 27 (25) | 58 (18.5) | 0.16 |
| Macrovascular invasion | |||||||||
| Yes | 46 (24.2) | 462 (81.2) | 17 (20.7) | 40 (15.7) | 29 (26.9) | 67 (21.3) | |||
| No | 144 (75.8) | 107 (18.8) | <0.000001 | 65 (79.3) | 214 (84.3) | 0.10 | 79 (73.1) | 247 (88.7) | 0.16 |
| AFP, ng/ml | |||||||||
| <400 | 139 (73.5) | 417 (73.4) | 63 (76.8) | 198 (78) | 76 (71) | 219 (69.7) | |||
| ≥400 | 50 (26.5) | 151 (26.6) | 1.00 | 19 (23.2) | 56 (22) | 0.87 | 31 (29) | 95 (30.3) | 0.90 |
| NLR | |||||||||
| <3 | 104 (56) | 255 (59) | 48 (58.5) | 133 (67.5) | 56 (53.3) | 122 (52) | |||
| ≥3 | 82 (44) | 176 (41) | 0.47 | 34 (41.5) | 64 (32.5) | 0.16 | 49 (46.7) | 112 (48) | 0.90 |
| ALBI grade | |||||||||
| 1 | 182 (95.8) | 489 (86) | 78 (95) | 227 (89.4) | 104 (96.3) | 262 (83.4) | |||
| 2 | 8 (4.2) | 69 (14) | 0.0008 | 4 (5) | 23 (10.6) | 0.25 | 4 (3.7) | 46 (16.6) | 0.001 |
| Aspartate aminotransferase, U/l | 41 (12-284) | 38 (8-830) | 0.2684 | 40 (18-217) | 39 (8-267) | 0.20 | 41 (12-284) | 38 (11-830) | 0.7044 |
| Alanine aminotransferase, U/l | 29 (4-361) | 30 (3-677) | 0.7127 | 31 (8-361) | 28 (3-199) | 0.50 | 28 (4-289) | 31 (8-677) | 0.2970 |
AFP, α-Fetoprotein; ALBI, albumin-bilirubin; BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NLR, neutrophil-to-lymphocyte ratio; TACE, transarterial chemoembolization.
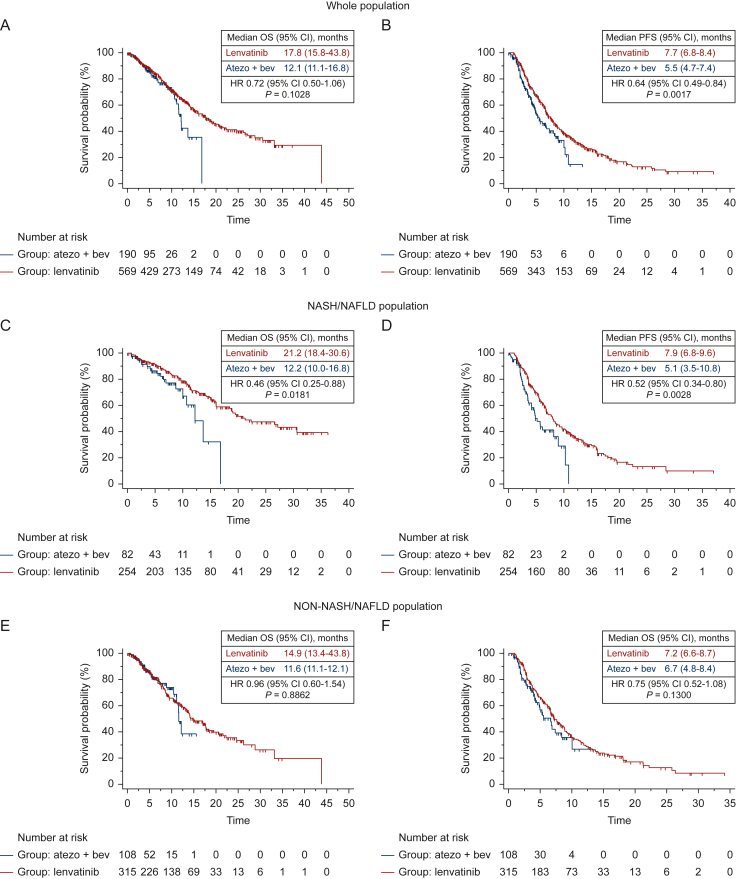
Using univariate analysis, median OS was 17.8 months (95% CI 15.8-43.8 months) for patients receiving lenvatinib, and 12.1 months (95% CI 11.1-16.8 months) for patients treated with atezolizumab plus bevacizumab (Figure 1A), with a 28% reduction in the risk of death for patients on lenvatinib (HR 0.71; 95% CI 0.50-1.06; P = 0.1028), compared with patients on atezolizumab plus bevacizumab (Table 2). The complete list of factors associated with a longer OS is reported in Table 2.
Figure 1.
Kaplan-Meier curves for OS and PFS in the whole population, in NASH/NAFLD population and in no-NASH/NAFLD population, according to the first line treatment received (atezolizumab plus bevacizumab Vs lenvatinib). Kaplan–Meier curves for OS (A) and PFS (B) in the whole population, OS (C) and PFS (D) in the NASH/NAFLD population, and OS (E) and PFS (F) in the non-NASH/NAFLD population, in patients treated with lenvatinib versus atezolizumab plus bevacizumab.
CI, confidence interval; HR, hazard ratio; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; OS, overall survival; PFS, progression-free survival.
Table 2.
Univariate and multivariate analysis of lenvatinib and atezolizumab plus bevacizumab patients’ cohort
| Whole patient population |
NASH/NAFLD population |
Non-viral/NASH/NAFLD population |
||||
|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
Univariate |
Multivariate |
|
| HR (95% CI); P value | HR (95% CI); P value | HR (95% CI); P Value | HR (95% CI); P value | HR (95% CI); P value | HR (95% CI); P value | |
| Treatment arm | ||||||
| Atezolizumab plus bevacizumab | 1 | 1 | 1 | |||
| Lenvatinib | 0.65 (0.44-0.95) | 0.46 (0.26-0.84) | 0.71 (0.42-1.18) | |||
| P value | 0.0268 | 0.0110 | 0.1852 | |||
| Gender | ||||||
| Male | 1 | 1 | 1 | 1 | 1 | |
| Female | 0.73 (0.54-0.99) | 0.74 (0.50-1.09) | 0.91 (0.59-1.42) | 0.64 (0.42-0.98) | 0.55 (0.30-1.02) | |
| P value | 0.0430 | 0.1240 | 0.6900 | 0.0429 | 0.0588 | |
| Age, years | ||||||
| <75 | 1 | 1 | 1 | 1 | 1 | |
| ≥75 | 0.94 (0.73-1.20) | 1.54 (1.05-2.27) | 2.60 (1.61-4.21) | 0.70 (0.51-0.97) | 0.77 (0.52-1.14) | |
| P value | 0.6035 | 0.0280 | 0.0110 | 0.0314 | 0.1995 | |
| Previous surgery | ||||||
| Yes | 1 | 1 | 1 | 1 | 1 | |
| No | 1.50 (1.16-1.94) | 1.78 (1.26-2.14) | 1.36 (0.92-2.02) | 1.55 (1.10-2.19) | 1.94 (1.28-2.32) | |
| P value | 0.0021 | 0.0072 | 0.1259 | 0.0129 | 0.0114 | |
| Previous radiofrequency ablation | ||||||
| Yes | 1 | 1 | 1 | |||
| No | 0.87 (0.63-1.21) | 1.08 (0.66-1.76) | 0.71 (0.46-1.11) | |||
| P value | 0.6932 | 0.7561 | 0.2435 | |||
| Previous TACE | ||||||
| Yes | 1 | 1 | 1 | 1 | 1 | |
| No | 1.44 (1.12-1.84) | 1.70 (0.89-1.98) | 1.99 (1.34-2.94) | 1.58 (0.95-2.64) | 1.12 (0.82-1.54) | |
| P value | 0.0038 | 0.6591 | 0.0006 | 0.1637 | 0.4730 | |
| Child–Pugh class | ||||||
| A | 1 | 1 | 1 | 1 | 1 | |
| B | 2.19 (1.45-3.33) | 1.36 (0.83-2.01) | 1.82 (0.89-3.69) | 2.27 (1.36-3.76) | 1.52 (0.82-1.72) | |
| P value | 0.0002 | 0.4270 | 0.0988 | 0.0016 | 0.3412 | |
| BCLC stage | ||||||
| B | 1 | 1 | 1 | 1 | 1 | 1 |
| C | 1.60 (1.25-2.05) | 1.47 (1.07-2.01) | 1.66 (1.13-2.45) | 1.63 (1.01-2.63) | 1.56 (1.13-2.14) | 1.38 (0.92-2.06) |
| P value | 0.0002 | 0.0167 | 0.0103 | 0.0448 | 0.0064 | 0.1171 |
| ECOG PS | ||||||
| 0 | 1 | 1 | 1 | |||
| >0 | 0.92 (0.67-1.26) | 1.25 (0.74-2.11) | 1.85 (1.16-2.95) | |||
| P value | 0.5913 | 0.4021 | 0.0094 | |||
| Macrovascular invasion | ||||||
| Yes | 1 | 1 | 1 | |||
| No | 0.63 (0.46-0.87) | 0.67 (0.39-1.17) | 1.52 (1.03-2.24) | |||
| P value | 0.0048 | 0.1576 | 0.0357 | |||
| AFP, ng/ml | ||||||
| <400 | 1 | 1 | 1 | 1 | 1 | |
| ≥400 | 1.69 (1.26-2.28) | 1.08 (0.77-1.51) | 1.25 (0.77-2.02) | 1.99 (1.37-2.90) | 1.60 (1.08-2.37) | |
| P value | 0.0005 | 0.6487 | 0.3617 | 0.0003 | 0.0196 | |
| NLR | ||||||
| <3 | 1 | 1 | 1 | 1 | 1 | 1 |
| ≥3 | 2.02 (1.51-2.70) | 1.66 (1.24-2.22) | 1.67 (1.04-2.68) | 1.54 (0.96-2.47) | 2.12 (1.48-3.06) | 1.75 (1.21-2.55) |
| P value | <0.0001 | 0.0005 | 0.0334 | 0.0757 | 0.0001 | 0.0032 |
| ALBI grade | ||||||
| 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 5.20 (3.19-8.47) | 1.94 (1.22-3.08) | 2.86 (1.25-6.52) | 1.68 (0.83-3.36) | 6.48 (3.57-11.7) | 2.47 (1.40-4.36) |
| P value | <0.0001 | 0.0045 | 0.0125 | 0.1440 | <0.0001 | 0.0019 |
| Aspartate aminotransferase, | ||||||
| U/l | 1.00 (1.00-1.00) | 1.01 (1.00-1.01) | 1.01 (1.00-1.01) | 1.01 (1.00-1.01) | 1.00 (0.99-1.00) | |
| P value | 0.0033 | 0.0001 | 0.0004 | 0.0046 | 0.1524 | |
| Alanine aminotransferase, U/l | 1.00 (0.99-1.00) | 1.00 (0.99-1.00) | 1.00 (1.00-1.00) | |||
| P value | 0.0202 | 0.6483 | 0.7226 | |||
Values given in bold are the ones which resulted to be statistically significant (P < 0.05) at uni-and multi-variate analysis.
AFP, α-Fetoprotein; ALBI, albumin-bilirubin; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NLR, neutrophil-to-lymphocyte ratio; TACE, transarterial chemoembolization.
Using univariate analysis, median PFS was 7.7 months (95% CI 6.8-8.4 months) for patients receiving lenvatinib, and 5.5 months (95% CI 4.7-7.4 months) for patients treated with atezolizumab plus bevacizumab (Figure 1B), with a 36% reduction in the risk of progression for patients on lenvatinib (HR 0.64; 95% CI 0.49-0.84; P = 0.0017), compared with patients on atezolizumab plus bevacizumab.
Following adjustment for clinical covariates associated with a longer OS at univariate analysis and for imbalances in baseline patient characteristics, multivariate analysis confirmed treatment with lenvatinib, compared to atezolizumab plus bevacizumab, as an independent prognostic factor for OS (HR 0.65; 95% CI 0.44-0.95; P = 0.0268) and PFS (HR 0.67; 95% CI 0.51-0.86; P = 0.002) (Table 2).
In addition, lenvatinib was associated with a longer OS compared to atezolizumab plus bevacizumab in patients with ALBI grade 1, age >75 years, and neutrophil-to-lymphocyte ratio (NLR) >3 (Supplementary Figures S1 and S2, available at https://doi.org/10.1016/j.esmoop.2022.100591). However, the interaction test was statistically negative for all these factors.
Patients treated with lenvatinib had a higher overall response rate (ORR) compared to patients treated with atezolizumab plus bevacizumab (39.9% versus 28.4%; P = 0.006), while no difference was observed in the disease control rate (DCR) (88.1% versus 91.9%; P = 0.33) (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2022.100591).
Safety results are summarized in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100591.
Clinical outcomes in the NAFLD/NASH population
A total of 336 consecutive patients were available for the analysis. Two hundred and fifty-four patients were treated with lenvatinib, and 82 patients were treated with atezolizumab plus bevacizumab.
The median follow-up was 9.7 months (95% CI 7.8-10.6 months) for atezolizumab plus bevacizumab patients and 14.5 months (95% CI 13.0-37.2 months) for lenvatinib patients.
Baseline patient characteristics were well balanced between the two treatment groups (Table 1).
Using univariate analysis, median OS was 21.2 months (95% CI 18.4-30.6 months) for patients treated with lenvatinib, and 12.2 months (95% CI 10.0-16.8 months) for patients treated with atezolizumab plus bevacizumab (Figure 1C), with a 54% reduction in the risk of death for patients on lenvatinib (HR 0.46; 95% CI 0.25-0.88; P = 0.0181), compared with patients on atezolizumab plus bevacizumab. The complete list of factors associated with a longer OS is reported in Table 2.
Using univariate analysis, median PFS was 7.9 months (95% CI 6.8-9.6 months) for patients receiving lenvatinib, and 5.1 months (95% CI 3.5-10.8 months) for patients treated with atezolizumab plus bevacizumab (Figure 1D), with a 48% reduction in the risk of progression for patients on lenvatinib (HR 0.52; 95% CI 0.34-0.80; P = 0.0028), compared with patients on atezolizumab plus bevacizumab.
Following adjustment for clinical covariates associated with a longer OS at univariate analysis, multivariate analysis confirmed treatment with lenvatinib compared to atezolizumab plus bevacizumab as an independent prognostic factor for OS (HR 0.46; 95% CI 0.26-0.84; P = 0.0110) and PFS (HR 0.55; 95% CI 0.38-0.82; P = 0.031) (Table 2).
Also, lenvatinib was associated with a longer OS compared to atezolizumab plus bevacizumab in male patients, with Child–Pugh class A, BCLC-C, α-fetoprotein <400 ng/ml, ALBI grade 1, age >75 years, and NLR >3 (Supplementary Figures S1 and S3, available at https://doi.org/10.1016/j.esmoop.2022.100591). The interaction test showed the positive predictive role of NLR for lenvatinib (P = 0.04).
Patients treated with lenvatinib had a higher ORR compared to patients treated with atezolizumab plus bevacizumab (38.5% versus 24.3%; P = 0.02) with no difference in DCR (79.8% versus 82.4%; P = 0.73) (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2022.100591).
Safety results are summarized in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100591.
Clinical outcomes in the non-NAFLD/NASH population
A total of 423 consecutive patients were available for the analysis. Three hundred and fifteen patients were treated with lenvatinib, and 108 patients were treated with atezolizumab plus bevacizumab.
The median follow-up was 8.3 months (95% CI 6.0-15.5 months) for atezolizumab plus bevacizumab patients and 13.5 months (95% CI 12.0-15.4 months) for lenvatinib patients.
Baseline patient characteristics are shown in Table 1. The two groups differed in previous surgery, Child–Pugh class, and ALBI grade.
Using univariate analysis, median OS was 14.9 months (95% CI 13.4-43.8 months) for patients receiving lenvatinib, and 11.6 months (95% CI 11.1-12.1 months) for patients treated with atezolizumab plus bevacizumab (Figure 1E), with a 4% reduction in the risk of death for patients on lenvatinib (HR 0.96; 95% CI 0.60-1.54; P = 0.8862), compared with patients on atezolizumab plus bevacizumab. The complete list of factors associated with a longer OS is reported in Table 2.
Using univariate analysis, median PFS was 7.2 months (95% CI 6.6-8.7 months) for patients receiving lenvatinib, and 6.7 months (95% CI 4.8-8.4 months) for patients treated with atezolizumab plus bevacizumab (Figure 1F), with a 25% reduction in the risk of progression for patients on lenvatinib compared with patients on atezolizumab plus bevacizumab (HR 0.75; 95% CI 0.52-1.08; P = 0.1300).
Following adjustment for clinical covariates associated with a longer OS at univariate analysis and for imbalances in baseline patient characteristics, multivariate analysis did not confirm treatment of lenvatinib compared to atezolizumab plus bevacizumab as an independent prognostic factor for OS (HR 0.71; 95% CI 0.42-1.18; P = 0.1852) or PFS (HR 0.74; 95% CI 0.51-1.07; P = 0.1137) (Table 2).
Also, no differences were found between lenvatinib and atezolizumab plus bevacizumab in all baseline patient characteristics (Supplementary Figures S1 and S5, available at https://doi.org/10.1016/j.esmoop.2022.100591).
Patients treated with lenvatinib tended to have a higher ORR compared to patients treated with atezolizumab plus bevacizumab (41.2% versus 32.0%; P = 0.09) with no difference in DCR (76.2% versus 82.0%; P = 0.40) (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2022.100591).
Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100591, reports the observed adverse events.
Propensity score matching analysis
Clinical outcomes in the whole patient population
Propensity score matching analysis was carried out on the whole patient population. Once the population derived from propensity score matching analysis was obtained, we carried out the survival analysis on the whole population, and then on the NASH/NAFLD population and the non-NASH/NAFLD population.
After propensity score matching, 187 patients treated with atezolizumab plus bevacizumab and 187 patients treated with lenvatinib were available for the analysis (Supplementary Figure S6, available at https://doi.org/10.1016/j.esmoop.2022.100591).
The median follow-up was 9.1 months (95% CI 8.0-10.7 months) for atezolizumab plus bevacizumab patients and 13.5 months (95% CI 11.9-15.4 months) for lenvatinib patients.
Baseline patient characteristics were well balanced between the two treatment groups (Table 3).
Table 3.
Baseline characteristics of lenvatinib and atezolizumab plus bevacizumab patients’ cohorts after propensity score matching analysis
| Whole patient population |
NASH/NAFLD population |
Non-NASH/NAFLD population |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Atezolizumab plus bevacizumab, n (%) n = 187 patients | Lenvatinib, n (%) n = 187 patients | P value | Atezolizumab plus bevacizumab, n (%) n = 81 patients | Lenvatinib, n (%) n = 102 patients | P value | Atezolizumab plus bevacizumab, n (%) n = 106 patients | Lenvatinib, n (%) n = 85 patients |
P value | |
| Gender | |||||||||
| Male | 146 (78) | 148 (79) | 61 (75) | 77 (75.5) | 85 (80) | 71 (83.5) | |||
| Female | 41 (22) | 39 (21) | 0.80 | 20 (25) | 25 (24.5) | 1.00 | 21 (20) | 14 (16.5) | 0.57 |
| Age, years | |||||||||
| <75 | 110 (59) | 102 (54.5) | 39 (48) | 50 (49) | 71 (67) | 52 (61) | |||
| ≥75 | 77 (41) | 85 (45.5) | 0.40 | 42 (52) | 52 (51) | 1.00 | 35 (33) | 33 (39) | 0.44 |
| Previous surgery | |||||||||
| Yes | 88 (47) | 87 (46.5) | 35 (43) | 55 (54) | 53 (50) | 32 (38) | |||
| No | 99 (53) | 100 (53.5) | 0.91 | 46 (57) | 47 (46) | 0.18 | 53 (50) | 53 (62) | 0.11 |
| Previous radiofrequency ablation | |||||||||
| Yes | 42 (22.5) | 33 (18) | 14 (17) | 18 (17.5) | 28 (26) | 15 (17.5) | |||
| No | 145 (77.5) | 154 (82) | 0.30 | 67 (83) | 84 (82.5) | 1.00 | 78 (74) | 70 (82.5) | 0.16 |
| Previous TACE | |||||||||
| Yes | 83 (44) | 91 (49) | 31 (38) | 52 (51) | 42 (39.5) | 39 (46) | |||
| No | 104 (56) | 96 (51) | 0.46 | 40 (62) | 50 (49) | 0.35 | 64 (60.5) | 46 (54) | 0.46 |
| Child–Pugh class | |||||||||
| A | 176 (94) | 176 (94) | 76 (94) | 98 (96) | 100 (94) | 78 (92) | |||
| B | 11 (6) | 11 (6) | 1.00 | 5 (6) | 4 (4) | 0.51 | 6 (6) | 7 (8) | 0.58 |
| BCLC stage | |||||||||
| B | 85 (45.5) | 74 (40) | 39 (48) | 46 (45) | 46 (43) | 28 (33) | |||
| C | 102 (54.5) | 113 (60) | 0.29 | 42 (52) | 56 (55) | 0.76 | 60 (57) | 57 (67) | 0.18 |
| ECOG PS | |||||||||
| 0 | 139 (74) | 153 (82) | 70 (86.5) | 90 (88) | 69 (65) | 63 (74) | |||
| >0 | 48 (26) | 34 (18) | 0.10 | 11 (13.5) | 12 (12) | 0.82 | 37 (35) | 22 (26) | 0.21 |
| Macrovascular invasion | |||||||||
| Yes | 43 (23) | 43 (23) | 16 (20) | 16 (15.5) | 27 (25) | 27 (32) | |||
| No | 144 (77) | 144 (77) | 1.00 | 65 (80) | 86 (84.5) | 0.55 | 89 (75) | 67 (68) | 0.42 |
| AFP, ng/ml | |||||||||
| <400 | 137 (73) | 145 (77.5) | 62 (76.5) | 72 (70.5) | 75 (71) | 65 (76.5) | |||
| ≥400 | 50 (27) | 42 (22.5) | 0.34 | 19 (23.5) | 22 (29.5) | 1.00 | 31 (29) | 20 (23.5) | 0.41 |
| AFP, ng/ml CVa | 19.1 (8-43) | 11.6 (7.7-19.4) | 0.32 | 15.1 (10.5-47.4) | 10.4 (5.8-24.8) | 0.1660 | 33 (15.2-75.76) | 12.3 (8.8-39.1) | 0.37 |
| NLR | |||||||||
| <3 | 124 (66) | 102 (54.5) | 60 (74) | 60 (59) | 64 (60) | 42 (49.5) | |||
| ≥3 | 69 (34) | 47 (45.5) | 0.42 | 20 (26) | 24 (41) | 0.72 | 49 (40) | 23 (50.5) | 0.34 |
| ALBI grade | |||||||||
| 1 | 179 (96) | 174 (93) | 77 (95) | 98 (96) | 102 (96) | 76 (89.5) | |||
| 2 | 8 (4) | 10 (7) | 0.63 | 4 (5) | 3 (4) | 0.70 | 4 (4) | 7 (10.5) | 0.21 |
| ALBI CVa | −3.24 (−3.33 to −3.17) | −3.30 (−3.38 to −3.20) | 0.86 | −3.27 (−3.34 to −3.21) | −3.28 (−3.38 to −3.24) | 0.37 | −3.26 (−3.33 to −3.22) | −3.27(−3.33 to −3.25) | 0.65 |
| Albumin CVa | 3.6 (3.5-3.7) | 3.7 (3.6-3.7) | 0.42 | 3.8 (3.7-3.9) | 3.7 (3.6-3.8) | 0.42 | 3.6 (3.5-3.7) | 3.7 (3.6-3.8) | 0.39 |
| Bilirubin CVa | 0.8 (0.7-0.9) | 0.8 (0.7-0.8) | 0.56 | 0.8 (0.7-0.8) | 0.9 (0.8-0.9) | 0.49 | 0.7 (0.7-0.8) | 0.8 (0.7-0.8) | 0.49 |
| Aspartate aminotransferase, U/l CVa | 39 (15-276) | 36 (8-471) | 0.67 | 40 (18-276) | 39 (8-267) | 0.53 | 46 (15-284) | 45 (11-471) | 0.78 |
| Alanine aminotransferase, U/l CVa | 31 (8-243) | 33 (3-477) | 0.62 | 35 (8-285) | 31 (3-202) | 0.56 | 33 (8-243) | 29 (14-477) | 0.39 |
AFP, α-Fetoprotein; ALBI, albumin-bilirubin; BCLC, Barcelona Clinic Liver Cancer; CV, continuous variables; ECOG PS, Eastern Cooperative Oncology Group performance status; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NLR, neutrophil-to-lymphocyte ratio; TACE, transarterial chemoembolization.
CV were expressed as median (95% confidence interval).
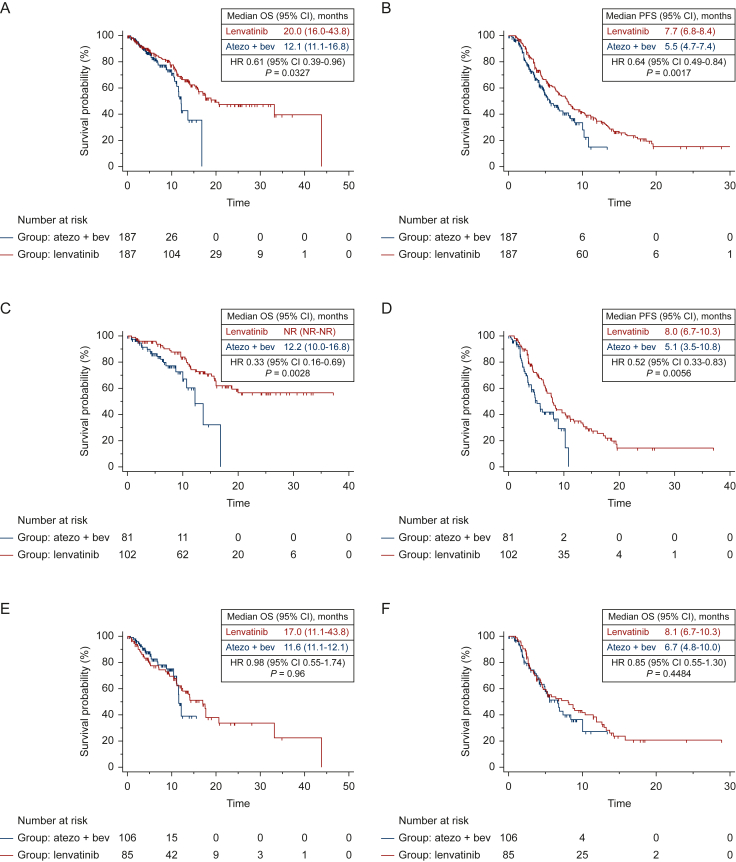
Median OS was 20.0 months (95% CI 16.0-43.8 months) for patients receiving lenvatinib, and 12.1 months (95% CI 11.1-16.8 months) for patients treated with atezolizumab plus bevacizumab (Figure 2A), with a 39% reduction in the risk of death for patients on lenvatinib (HR 0.61; 95% CI 0.39-0.96; P = 0.0327), compared with patients on atezolizumab plus bevacizumab.
Figure 2.
Kaplan-Meier curves for OS and PFS after propensity score matching in the whole population, in NASH/NAFLD population and in no-NASH/NAFLD population, according to the first line treatment received (atezolizumab plus bevacizumab Vs lenvatinib).
Kaplan–Meier curves for OS (A) and PFS (B) in the whole population, OS (C) and PFS (D) in the NASH/NAFLD population, and OS (E) and PFS (F) in the non-NASH/NAFLD population, in patients treated with lenvatinib versus atezolizumab plus bevacizumab after propensity score matching analysis.
CI, confidence interval; HR, hazard ratio; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NR, not reached; OS, overall survival; PFS, progression-free survival.
Median PFS was 8.0 months (95% CI 4.7-8.1 months) for patients receiving lenvatinib, and 5.7 months (95% CI 4.7-8.1 months) for patients treated with atezolizumab plus bevacizumab (Figure 2B) (HR 0.67; 95% CI 0.49-0.92; P = 0.0124), compared with patients on atezolizumab plus bevacizumab.
Patients treated with lenvatinib had a higher ORR compared to patients treated with atezolizumab plus bevacizumab (41.3% versus 28.8%; P = 0.01), while no difference was observed in DCR (81.1% versus 81.8%; P = 0.87).
Clinical outcomes in the NAFLD/NASH population
After propensity score matching in the whole patient population, 81 patients treated with atezolizumab plus bevacizumab and 102 patients treated with lenvatinib were available for the analysis. Baseline patient characteristics were well balanced between the two treatment groups (Table 3).
Median OS was not reached (NR) (95% CI NR-NR) for patients receiving lenvatinib, and 12.2 months (95% CI 10.0-16.8 months) for patients treated with atezolizumab plus bevacizumab (Figure 2C), with a 67% reduction in the risk of death for patients on lenvatinib (HR 0.33; 95% CI 0.16-0.69; P = 0.0028), compared with patients on atezolizumab plus bevacizumab.
Median PFS was 8.0 months (95% CI 6.7-10.3 months) for patients receiving lenvatinib, and 5.1 months (95% CI 3.5-10.8 months) for patients treated with atezolizumab plus bevacizumab (Figure 2D), with a 48% reduction in the risk of progression for patients on lenvatinib (HR 0.52; 95% CI 0.33-0.83; P = 0.0056), compared with patients on atezolizumab plus bevacizumab.
Patients treated with lenvatinib had a higher ORR compared to patients treated with atezolizumab plus bevacizumab (41.6% versus 24.6%; P = 0.03), while no difference was observed in DCR (83.4% versus 81.2%; P = 0.65).
Clinical outcomes in the non-NAFLD/NASH population
After propensity score matching in the whole patient population, 106 patients treated with atezolizumab plus bevacizumab and 85 patients treated with lenvatinib were available for the analysis. Baseline patient characteristics were well balanced between the two treatment groups (Table 3).
Median OS was 17.0 months (95% CI 11.1-43.8 months) for patients receiving lenvatinib, and 11.6 months (95% CI 11.1-12.1 months) for patients treated with atezolizumab plus bevacizumab (Figure 2E), with a 2% reduction in the risk of death for patients on atezolizumab plus bevacizumab (HR 0.98; 95% CI 0.55-1.74; P = 0.96), compared with patients on lenvatinib.
Median PFS was 8.1 months (95% CI 6.7-10.3 months) for patients receiving lenvatinib, and 6.7 months (95% CI 4.8-10.0 months) for patients treated with atezolizumab plus bevacizumab (Figure 2F), with a 15% reduction in the risk of progression for patients on lenvatinib (HR 0.85; 95% CI 0.55-1.30; P = 0.4484), compared with patients on atezolizumab plus bevacizumab.
Patients treated with lenvatinib tended to have a higher ORR compared to patients treated with atezolizumab plus bevacizumab (43.3% versus 36.3%; P = 0.23) with no difference in DCR (78.3% versus 81.6%; P = 0.53).
Atezolizumab plus bevacizumab versus sorafenib
Clinical outcomes in the whole patient population
Four hundred consecutive patients were available for the analysis. Two hundred and ten patients were treated with sorafenib and 190 patients were treated with atezolizumab plus bevacizumab.
Baseline patient characteristics are shown in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100591. The two groups differed in gender, age, previous surgery, BCLC stage, ECOG PS, and presence of macrovascular invasion.
Median OS was 11.4 months (95% CI 9.6-13.0 months) for patients receiving sorafenib, and 12.1 months (95% CI 11.1-16.8 months) for patients treated with atezolizumab plus bevacizumab (Supplementary Figure S7A, available at https://doi.org/10.1016/j.esmoop.2022.100591), with a 31% reduction in the risk of death for patients on atezolizumab plus bevacizumab compared with patients on sorafenib (HR 0.69; 95% CI 0.49-0.98; P = 0.0360).
Median PFS was 5.6 months (95% CI 4.1-8.0 months) for patients receiving sorafenib, and 5.5 months (95% CI 4.7-7.4 months) for patients treated with atezolizumab plus bevacizumab (Supplementary Figure S7B, available at https://doi.org/10.1016/j.esmoop.2022.100591), with a 5% reduction in the risk of progression for patients on atezolizumab plus bevacizumab compared with patients on sorafenib (HR 0.95; 95% CI 0.72-1.27; P = 0.7524).
Following adjustment for clinical covariates associated with a longer OS at univariate analysis and for imbalances in baseline patient characteristics, multivariate analysis did not confirm treatment with atezolizumab plus bevacizumab versus sorafenib as an independent prognostic factor for OS (HR 0.85; 95% CI 0.66-1.26; P = 0.1918) or PFS (HR 0.98; 95% CI 0.53-1.92; P = 0.8274).
Clinical outcomes in the NAFLD/NASH population
One hundred and fifty consecutive patients were available for the analysis. Sixty-eight patients were treated with sorafenib and 82 patients were treated with atezolizumab plus bevacizumab.
Baseline patient characteristics are shown in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100591. The two groups differed in gender, age, previous surgery, BCLC stage, ECOG PS, and NLR.
Median OS was 11.0 months (95% CI 6.4-12.4 months) for patients receiving sorafenib, and 12.2 months (95% CI 10.0-16.8 months) for patients treated with atezolizumab plus bevacizumab (Supplementary Figure S5C, available at https://doi.org/10.1016/j.esmoop.2022.100591), with a 15% reduction in the risk of death for patients on sorafenib compared with patients on atezolizumab plus bevacizumab (HR 0.85; 95% CI 0.85-2.53; P = 0.1661).
Median PFS was 7.6 months (95% CI 3.2-45.0 months) for patients receiving sorafenib, and 5.1 months (95% CI 3.5-10.8 months) for patients treated with atezolizumab plus bevacizumab (Supplementary Figure S5D, available at https://doi.org/10.1016/j.esmoop.2022.100591), with an 11% reduction in the risk of progression for patients on sorafenib compared with patients on atezolizumab plus bevacizumab (HR 0.89; 95% CI 0.56-1.41; P = 0.6221).
Following adjustment for clinical covariates associated with a longer OS at univariate analysis and for imbalances in baseline patient characteristics, multivariate analysis did not show treatment with sorafenib versus atezolizumab plus bevacizumab as an independent prognostic factor for OS (HR 0.93; 95% CI 0.53-2.43; P = 0.837) or PFS (HR 0.97; 95% CI 0.84-1.67; P = 0.9373).
Clinical outcomes in the non-NAFLD/NASH population
Two hundred and fifty consecutive patients were available for the analysis. One hundred and forty-two patients were treated with sorafenib and 108 patients were treated with atezolizumab plus bevacizumab.
Baseline patient characteristics are shown in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100591. The two groups differed in gender, previous surgery, and ECOG PS.
Median OS was 11.6 months (95% CI 11.1-12.1 months) for patients receiving sorafenib and 11.6 months (95% CI 11.1-12.1 months) for patients receiving atezolizumab plus bevacizumab (Supplementary Figure S5E, available at https://doi.org/10.1016/j.esmoop.2022.100591), with a 32% reduction in the risk of death for patients on atezolizumab plus bevacizumab compared with patients on sorafenib (HR 0.68; 95% CI 0.44-1.07; P = 0.0970).
Median PFS was 5.1 months (95% CI 4.0-8.1 months) for patients receiving sorafenib, and 6.7 months (95% CI 4.8-8.4 months) for patients treated with atezolizumab plus bevacizumab (Supplementary Figure S5F, available at https://doi.org/10.1016/j.esmoop.2022.100591), with a 15% reduction in the risk of progression for patients on atezolizumab plus bevacizumab compared with patients on sorafenib (HR 0.85; 95% CI 0.59-1.23; P = 0.3975).
Following adjustment for clinical covariates associated with a longer OS at univariate analysis and for imbalances in baseline patient characteristics, multivariate analysis did not confirm treatment with sorafenib versus atezolizumab plus bevacizumab as an independent prognostic factor for OS (HR 0.81; 95% CI 0.49-1.36; P = 0.4512) or PFS (HR 0.95; 95% CI 0.84-1.29; P = 0.6317).
Discussion
To the best of our knowledge, the present study is the largest study to date aimed at identifying the role of etiology, and, in particular, the role of NAFLD/NASH, in the survival outcomes of patients treated with the combination of atezolizumab plus bevacizumab and with MKIs. Our results showed that treatment with lenvatinib compared to atezolizumab plus bevacizumab was associated with a longer OS and PFS in patients with non-viral HCC, mainly NAFLD/NASH-related HCC, following multivariate and propensity score matching analyses. The observed association of lenvatinib with an improved outcome in non-viral HCC patients, and, in particular, in NAFLD/NASH-related HCC patients, may have a number of explanations.
The primary hypothesis is that NASH-associated HCC may be immunologically and metabolically different from other etiologies. In support of this hypothesis, Pfister and colleagues have recently shown an increase in the frequency of activated CD8+ T cells in mice with NASH, which are supposed to be involved in tissue damage and carcinogenesis.20 In concordance with these findings, a recent comprehensive molecular analysis conducted by Pinyol and collaborators showed that NASH-related HCCs are enriched in gene expression signatures related to bile and fatty acid metabolisms, oxidative stress, and inflammation, with the consequence of expansion and overactivation of lymphocyte population.33
On the other hand, the advent of new single-cell transcriptomic techniques allowed to demonstrate the significant alteration, with immunosuppressive consequences, of several immune-cell populations in the NASH mouse model, including B cells, innate-like T cells, such as variant natural killer (iNKT) cells, and conventional CD8+ and CD4+ T-cell subsets.34 Indeed, pro-inflammatory B-cell population expanded and became overactivated in NASH mouse models, thus producing a high level of immunoglobulin A+, which is involved in CD8+ T-cell exhaustion, secretion of pro-inflammatory cytokines, and, consequently, progression to HCC.34, 35, 36, 37 This immunosuppressive effect is potentiated by the loss of CD4+ T cells due to fatty acid-mediated cytotoxic action, thus limiting antitumor potential and contributing to carcinogenesis in NASH models.37
It is conceivable that the imbalance between an intense inflammatory microenvironment and the immune exhaustion, which characterizes NASH-related HCC patients, could act as a crucial factor in determining inferior outcome response from immunotherapy. Nevertheless, several mechanisms underlying the crosstalk between immune system, carcinogenesis, and immune checkpoint inhibition remain far to be understood and further investigations are needed.
Interestingly, we found that sorafenib, differently from lenvatinib, performed similarly to atezolizumab plus bevacizumab in the same setting of patients, which is consistent with the data reported in the subgroup analysis of the IMbrave150 trial.17 Interestingly, previous preclinical evidence demonstrated differences in terms of immunomodulatory activity between lenvatinib and sorafenib, which probably involve CD8+ T-cell and NKT cell population38,39 and which could be put in correlation with different target profiles. In fact, lenvatinib shows a more potent inhibitory activity against VEGF receptors (VEGFRs) and fibroblast growth factor receptors (FGFRs), which both play a crucial suppressive role in immune responses.40,41 As already mentioned, the chronic inflammation underlying the environment of hepatic cells with steatosis could be responsible of an early CD8+ T-cell exhaustion, thus compromising their immunocompetence. Based on our results and above-mentioned evidence, we could speculate that lenvatinib might be able to revert the role of CD8+ T cells in the NASH microenvironment, probably through its inhibition of VEGFR and FGFR pathways, thus leading to an efficient immune response. On the other hand, it is possible that sorafenib, due to its different kinase inhibition profile, may not be enough to revert the efficiency of CD8+ T cells, thus leading to scarce outcomes in the same setting of patients. Surely, further evidence is needed to confirm our hypothesis.
Interestingly, lenvatinib showed a better ORR compared to atezolizumab, but a similar DCR in the whole population and in the NASH/NAFLD population. These data seem to suggest that lenvatinib could better perform in terms of efficacy in these subgroups, thus increasing the response rate. Contrarily, immunotherapy could be less efficient compared to lenvatinib, thus achieving a significant proportion of stable diseases compared to responses. Another point that is noteworthy is that in our analysis lenvatinib showed significant differences in terms of OS in NASH/NAFLD and non-NASH/NAFLD patients, which does not correspond to a significant difference in terms of PFS.
These results do not make us less confident about our conclusion. In a real-world retrospective experience which involves a large number of institutions, the evaluation of PFS could be partially invalidated. Different time points in the disease evaluation by computed tomography scan depending on the single institution’s protocol make the evaluation of PFS less precise compared to OS, which remains the main parameter to evaluate for comparing clinical outcomes in such kind of studies.
We showed that lenvatinib was associated with better survival outcomes in all subgroups of patients with NAFLD/NASH-related HCC, but a special benefit has been shown in patients with NLR >3 and in patients >75 years old.
The role of NLR as a prognostic and predictive biomarker in patients treated with immunotherapy has already been investigated in several cancer settings.42, 43, 44, 45 In advanced HCC, previous evidence revealed that a composite model of post-treatment high value of NLR and platelet-to-lymphocyte ratio was associated with an eightfold increase in the risk of death in patients treated with anti-programmed cell death protein 1.46,47 Our results are complementary, since they suggest a prognostic value of baseline NLR in HCC patients treated with lenvatinib. In cancer patients, neutrophilia is related to an increased production of neutrophil-derived cytokines, including VEGF, matrix metalloproteinases, and interleukin-18, which all contribute to cancer development and immune impairing,48 thus eventually reducing the response to immune checkpoint inhibitors.
The explanation of a greater benefit from lenvatinib in older patients is more complex.49 Treatment of older patients is challenging, since it must take into account multiple issues related to physical frailty and comorbidities, which frequently limit available therapeutic approaches. In addition, the tolerability of systemic treatments could be reduced in these patients, thus leading to unsatisfactory survival outcomes. Nevertheless, both lenvatinib and atezolizumab plus bevacizumab, as well as sorafenib, have been previously shown to be well tolerated in older patients.50, 51, 52, 53
Beginning with the sixth decade of life, the human immunity undergoes crucial aging-related changes, which lead to the state of immunosenescence.54 This is characterized by the loss of ability to protect against infections and cancer along with a more intense inflammatory response which makes elderly patients susceptible to tissue-damaging immunity and chronic inflammatory diseases.54,55 For this reason, elderly patients present even more immune alterations which could be added to those sustained by NAFLD/NASH. We could hypothesize that immune alterations which characterize elderly patients could contribute to differences in response to treatments. However, further investigations are needed to clarify this aspect.
Several limitations could be ascribed to the present study. Firstly, the retrospective nature of the work could not exclude selection biases, which should be taken into account in the interpretation of our results especially given the differences in clinical management and prescribing practice across centers. Furthermore, we must take into account the limitation of the definition of NAFLD and/or NASH, as the allocation of a patient to the NAFLD or NASH group could be complicated by the existence of different definitions in the literature. However, to reduce this bias, we have considered the definition from the European Association for the Study of the Liver (EASL) guidelines. Due to the multicenter and multinational nature of the analysis, a centralized imaging review was not possible, and the criteria for tumor assessments were based on each center internal protocol. Moreover, in the interpretation of survival outcomes of lenvatinib versus atezolizumab plus bevacizumab, a difference in terms of median follow-up has to be considered, which is correlated to the more recent introduction of the combination therapy in clinical practice. Finally, we analyzed different cohorts of patients presenting differences in baseline characteristics, mainly if considering the whole study population treated with lenvatinib versus atezolizumab and bevacizumab. Noteworthy, we carried out multivariate analysis and propensity score matching analysis including all the characteristics which differed in the two cohorts of patients, thus reducing the risk of bias and reinforcing our results.
The strengths of our analysis derive from the large number of patients who have been enrolled consecutively at each center, thus reducing the possible selection bias and limiting the confounding effect. Moreover, patients included in the analysis were both European and Asian, thus increasing the representativeness of our sample.
In conclusion, the present analysis conducted on a large number of non-viral HCC patients showed for the first time an association with a superior outcome of lenvatinib compared to atezolizumab plus bevacizumab, in particular in patients with NAFLD/NASH-related HCC. In light of the lack of level I evidence comparing lenvatinib to atezolizumab and bevacizumab, our study adds important and novel evidence highlighting the clinical impact of underlying etiology as a factor influencing outcome from treatment of advanced HCC patients. Future large prospective trials are needed to validate our results and to deepen the potential role of etiology in the clinical management of these patients.
Acknowledgments
Funding
None declared.
Disclosure
LR has received consulting fees from Amgen, ArQule, AstraZeneca, Basilea, Bayer, BMS, Celgene, Eisai, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Lilly, MSD, Nerviano Medical Sciences, Roche, Sanofi, Servier, Taiho Oncology, Zymeworks; lecture fees from AbbVie, Amgen, Bayer, Eisai, Gilead, Incyte, Ipsen, Lilly, Merck Serono, Roche, Sanofi; travel expenses from Ipsen; and institutional research funding from Agios, ARMO BioSciences, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Zymeworks. ACG has received grants and personal fees from MSD, Eisai, Bayer, and is an advisor for MSD, Eisai, Bayer, Bristol-Myers Squibb, AstraZeneca, and GSK. MK has received grants from Taiho Pharmaceuticals, Chugai Pharmaceuticals, Otsuka, Takeda, Sumitomo Dainippon-Sumitomo, Daiichi Sankyo, AbbVie, Astellas Pharma, and Bristol-Myers Squibb; has received grants and personal fees from MSD, Eisai, and Bayer; and is an adviser for MSD, Eisai, Bayer, Bristol-Myers Squibb, Eli Lilly, and ONO Pharmaceutical. FPi has received consulting or lecture fees from in the last 2 years from Astrazeneca, Bayer, Bracco, EISAI, ESAOTE, Exact Sciences, IPSEN, MSD, Roche, Samsung, and Tiziana Life Sciences. All other authors have declared no conflicts of interest.
Data sharing
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Supplementary data
References
- 1.Llovet J.M., Kelley R.K., Villanueva A., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 2.Llovet J.M., Ricci S., Mazzaferro V., et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Cheng A.L., Kang Y.K., Chen Z., et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J., Qin S., Merle P., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. Erratum in: Lancet. 2017;389(10064):36. [DOI] [PubMed] [Google Scholar]
- 5.Abou-Alfa G.K., Meyer T., Cheng A.L., et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu A.X., Kang Y.K., Yen C.J., et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M., Finn R.S., Qin S., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 8.Casadei-Gardini A., Rimini M., Kudo M., et al. Liver Cancer; 2022. REal life study of LEnVAtiNib therapy for HepAtocellular carcinoma: RELEVANT study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rimini M., Shimose S., Lonardi S., et al. Lenvatinib versus sorafenib as first-line treatment in hepatocellular carcinoma: a multi-institutional matched case-control study. Hepatol Res. 2021;51(12):1229–1241. doi: 10.1111/hepr.13718. [DOI] [PubMed] [Google Scholar]
- 10.Casadei-Gardini A., Scartozzi M., Tada T., et al. Lenvatinib versus sorafenib in first-line treatment of unresectable hepatocellular carcinoma: an inverse probability of treatment weighting analysis. Liver Int. 2021;41(6):1389–1397. doi: 10.1111/liv.14817. [DOI] [PubMed] [Google Scholar]
- 11.Burgio V., Iavarone M., Di Costanzo G.G., et al. Real-life clinical data of lenvatinib versus sorafenib for unresectable hepatocellular carcinoma in Italy. Cancer Manag Res. 2021;13:9379–9389. doi: 10.2147/CMAR.S330195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapposelli I.G., Shimose S., Kumada T., et al. Identification of lenvatinib prognostic index via recursive partitioning analysis in advanced hepatocellular carcinoma. ESMO Open. 2021;6(4) doi: 10.1016/j.esmoop.2021.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rimini M., Kang W., Burgio V., et al. Validation of the easy-to-use lenvatinib prognostic (LEP) index to predict prognosis in advanced hepatocellular carcinoma patients treated with lenvatinib. Hepatol Res. 2022 doi: 10.1111/hepr.13824. [DOI] [PubMed] [Google Scholar]
- 14.Finn R.S., Ryoo B.Y., Merle P., et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 15.Yau T., Park J.W., Finn R.S., et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 16.Merck announces KEYTRUDA® (pembrolizumab) met primary endpoint of overall survival (OS) in patients with advanced hepatocellular carcinoma previously treated with sorafenib. News release. Merck. September 27, 2021. https://www.merck.com/news/merck-announces-keytruda-pembrolizumab-met-primary-endpoint-of-overall-survival-os-in-patients-with-advanced-hepatocellular-carcinoma-previously-treated-with-sorafenib/. Accessed September 28, 2021.
- 17.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 18.Abou-Alfa G, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. Paper presented at 2022 Gastrointestinal Cancers Symposium. January 20-22, 2022; San Francisco, California. Abstract 379.
- 19.Kelley R.K., Rimassa L., Cheng A.L., et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(8):995–1008. doi: 10.1016/S1470-2045(22)00326-6. [DOI] [PubMed] [Google Scholar]
- 20.Pfister D., Núñez N.G., Pinyol R., et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell E.E., Wong V.W., Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–2224. doi: 10.1016/S0140-6736(20)32511-3. [DOI] [PubMed] [Google Scholar]
- 22.Wolf M.J., Adili A., Piotrowitz K., et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26(4):549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Ma C., Kesarwala A.H., Eggert T., et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531(7593):253–257. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malehmir M., Pfister D., Gallage S., et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019;25(4):641–655. doi: 10.1038/s41591-019-0379-5. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa H., Umemura A., Taniguchi K., et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell. 2014;26(3):331–343. doi: 10.1016/j.ccr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ringelhan M., Pfister D., O’Connor T., Pikarsky E., Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19(3):222–232. doi: 10.1038/s41590-018-0044-z. [DOI] [PubMed] [Google Scholar]
- 27.Michelotti G.A., Machado M.V., Diehl A.M. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10(11):656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 28.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rimini M., Kudo M., Tada T., et al. Nonalcoholic steatohepatitis in hepatocarcinoma: new insights about its prognostic role in patients treated with lenvatinib. ESMO Open. 2021;6(6) doi: 10.1016/j.esmoop.2021.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Freites-Martinez A., Santana N., Arias-Santiago S., Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) 2021;112(1):90–92. doi: 10.1016/j.ad.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 32.D’Agostino R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 33.Pinyol R., Torrecilla S., Wang H., et al. Molecular characterisation of hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol. 2021;75(4):865–878. doi: 10.1016/j.jhep.2021.04.049. Erratum in: J Hepatol. 2021;75(6):1515. [DOI] [PubMed] [Google Scholar]
- 34.Anstee Q.M., Reeves H.L., Kotsiliti E., Govaere O., Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16(7):411–428. doi: 10.1038/s41575-019-0145-7. [DOI] [PubMed] [Google Scholar]
- 35.Ramadori P., Kam S., Heikenwalder M. T cells: friends and foes in NASH pathogenesis and hepatocarcinogenesis. Hepatology. 2022 doi: 10.1002/hep.32336. [DOI] [PubMed] [Google Scholar]
- 36.Huby T., Gautier E.L. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat Rev Immunol. 2022;22(7):429–443. doi: 10.1038/s41577-021-00639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruzzì S., Sutti S., Giudici G., et al. B2-lymphocyte responses to oxidative stress-derived antigens contribute to the evolution of nonalcoholic fatty liver disease (NAFLD) Free Radic Biol Med. 2018;124:249–259. doi: 10.1016/j.freeradbiomed.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Kimura T., Kato Y., Ozawa Y., et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109(12):3993–4002. doi: 10.1111/cas.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Q., Liu H., Wang H., et al. Lenvatinib promotes antitumor immunity by enhancing the tumor infiltration and activation of NK cells. Am J Cancer Res. 2019;9(7):1382–1395. [PMC free article] [PubMed] [Google Scholar]
- 40.Courau T., Nehar-Belaid D., Florez L., et al. TGF-β and VEGF cooperatively control the immunotolerant tumor environment and the efficacy of cancer immunotherapies. JCI Insight. 2016;1(9) doi: 10.1172/jci.insight.85974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hato T., Zhu A.X., Duda D.G. Rationally combining anti-VEGF therapy with checkpoint inhibitors in hepatocellular carcinoma. Immunotherapy. 2016;8(3):299–313. doi: 10.2217/imt.15.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardet E., Acosta L.P., Ferreira M.G., et al. Neutrophil-lymphocyte radio (NLR) as a possible predictive factor in patients with advanced NSCLC WHO received immunotherapy. J Clin Oncol. 2021;39(suppl 15) doi: 10.1200/JCO.2021.39.15_suppl.e21042. [DOI] [Google Scholar]
- 43.Capone M., Giannarelli D., Mallardo D., et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6(1):74. doi: 10.1186/s40425-018-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takenaka Y., Kitamura T., Oya R., et al. Prognostic role of neutrophil-lymphocyte ratio in nasopharyngeal carcinoma: a meta-analysis. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0181478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nunno V.D., Mollica V., Gatto L., et al. Prognostic impact of neutrophil-to-lymphocyte ratio in renal cell carcinoma: a systematic review and meta-analysis. Immunotherapy. 2019;11(7):631–643. doi: 10.2217/imt-2018-0175. [DOI] [PubMed] [Google Scholar]
- 46.Dharmapuri S., Özbek U., Lin J.Y., et al. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti-PD-1 therapy. Cancer Med. 2020;9(14):4962–4970. doi: 10.1002/cam4.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muhammed A., Fulgenzi C.A.M., Dharmapuri S., et al. The systemic inflammatory response identifies patients with adverse clinical outcome from immunotherapy in hepatocellular carcinoma. Cancers (Basel) 2021;14(1):186. doi: 10.3390/cancers14010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li M., Spakowicz D., Burkart J., et al. Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J Cancer Res Clin Oncol. 2019;145(10):2541–2546. doi: 10.1007/s00432-019-02982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hajiev S. Impact of age on sorafenib outcomes in hepatocellular carcinoma: an international cohort study. Br J Cancer. 2021;124(2):407–413. doi: 10.1038/s41416-020-01116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tada T., Kumada T., Hiraoka A., et al. Safety and efficacy of lenvatinib in elderly patients with unresectable hepatocellular carcinoma: a multicenter analysis with propensity score matching. Hepatol Res. 2020;50(1):75–83. doi: 10.1111/hepr.13427. [DOI] [PubMed] [Google Scholar]
- 51.Li D, Toh H, Merle P, et al. Atezolizumab + bevacizumab vs sorafenib for unresectable hepatocellular carcinoma: results from older adults enrolled in IMbrave150. Paper presented at ESMO World Congress on Gastrointestinal Cancer 2020. July 1-4, 2020; Virtual. Abstract O-8.
- 52.Hajiev S., Allara E., Motedayеn Aval L., et al. Impact of age on sorafenib outcomes in hepatocellular carcinoma: an international cohort study. Br J Cancer. 2021;124(2):407–413. doi: 10.1038/s41416-020-01116-9. Erratum in: Br J Cancer. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nebhan C.A., Cortellini A., Ma W., et al. Clinical outcomes and toxic effects of single-agent immune checkpoint inhibitors among patients aged 80 years or older with cancer: a multicenter international cohort study. JAMA Oncol. 2021;7(12):1856–1861. doi: 10.1001/jamaoncol.2021.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valiathan R., Ashman M., Asthana D. Effects of ageing on the immune system: infants to elderly. Scand J Immunol. 2016;83(4):255–266. doi: 10.1111/sji.12413. [DOI] [PubMed] [Google Scholar]
- 55.Rimassa L., Personeni N., Czauderna C., Foerster F., Galle P. Systemic treatment of HCC in special populations. J Hepatol. 2021;74(4):931–943. doi: 10.1016/j.jhep.2020.11.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.