Abstract
Background
The PEOPLE trial aimed to identify new immune biomarkers in negative and low programmed death-ligand 1 (PD-L1) (0%-49%) advanced non-small-cell lung cancer (aNSCLC) patients treated with first-line pembrolizumab. Here we report the main outcomes and the circulating immune biomarkers analysis.
Patients and methods
The primary endpoint of this phase II trial was the identification of immune biomarkers associated with progression-free survival (PFS). Overall survival (OS), objective response rate (ORR), disease control rate (DCR), duration of response (DoR) and safety were secondary endpoints. Absolute cell counts for 36 subsets belonging to innate and adaptive immunity were determined by multiparametric flow cytometry in peripheral blood at baseline and at first radiologic evaluation. An orthoblique principal components–based clustering approach and multivariable Cox regression model adjusted for clinical variables were used to analyze immune variables and their correlation with clinical endpoints.
Results
From May 2018 to October 2020, 65 patients were enrolled. After a median follow-up of 26.4 months, the median PFS was 2.9 months [95% confidence interval (CI) 1.8-5.6 months] and median OS was 12.1 months (95% CI 8.7-17.1 months). The ORR was 21.5%, DCR was 47.7% and median DoR was 14.5 months (95% CI 6.4-24.9 months). Drug-related grade 3-4 adverse events were 9.2%. Higher T cell and natural killer (NK) cell count at baseline and at the first radiologic evaluation were associated with improved PFS, DCR and OS. On the contrary, higher myeloid cell count at baseline or at the first radiologic evaluation was significantly associated with worse OS and DCR.
Conclusions
Circulating immune biomarkers can contribute to predict outcomes in negative and low PD-L1 aNSCLC patients treated with first-line single-agent pembrolizumab.
Key words: advanced NSCLC, circulating immune biomarkers, pembrolizumab, first line, negative and low PD-L1
Highlights
-
•
In low PD-L1 aNSCLC treated with first-line pembrolizumab, higher T and NK cell counts were associated with better outcomes.
-
•
In the same setting higher myeloid cell count was associated with worse OS and DCR.
-
•
Characterization for peripheral immune subsets may help in the patient selection process.
Introduction
During the past decade, immune checkpoint inhibitors (ICIs) have revolutionized the management of metastatic and locally advanced non-small-cell lung cancer (aNSCLC) without targetable oncogenic driver.1,2 For aNSCLC patients characterized by a programmed death-ligand 1 (PD-L1) tumor proportion score (TPS) ≥ 50%, single-agent immunotherapy is associated with improved objective response rate (ORR), longer overall survival (OS) and low toxicity rate compared to chemotherapy (KEYNOTE-024).3 Based on these data, pembrolizumab has replaced platinum-based chemotherapy as the standard first line in aNSCLC expressing high PD-L1 levels.2,3 The superiority of single-agent immunotherapy compared to chemotherapy as the first-line treatment in aNSCLC patients with high PD-L1 has been further confirmed by the results of IMpower-110 (atezolizumab) and EMPOWER-Lung 1 (cemiplimab) trials.4,5 However, patients with PD-L1 TPS ≥ 50% represent a minority (∼23% to 28%) of all patients with aNSCLC diagnosis.6 Patients with negative and low PD-L1 (TPS 0%-49%) derive greater benefit from the combination of anti-programmed cell death protein 1 (PD-1)/PD-L1 with standard platinum doublets.7,8 In particular, based on the results of KEYNOTE-021, -189 and -407 studies, pembrolizumab in combination with platinum-based chemotherapy can be considered the standard first line for both squamous and epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase wild-type (WT) non-squamous aNSCLC in all comers (any PD-L1 level).7, 8, 9 In the same setting, also the combination of chemotherapy plus atezolizumab with or without bevacizumab and chemotherapy plus nivolumab and ipilimumab showed superiority over chemotherapy alone.10, 11, 12
In the KEYNOTE-042 study, pembrolizumab improved OS in aNSCLC patients with PD-L1 TPS ≥1% compared to standard chemotherapy.13 Although in this study the overall improvement in clinical outcomes with pembrolizumab was largely driven by patients with high PD-L1 expression, in clinical practice the combination of chemotherapy and ICIs may lead to higher incidence of adverse events (AEs) compared with single-agent ICI treatment; then pembrolizumab could be considered a valid option also in low PD-L1 patients, especially in those who are unfit for chemotherapy.3, 4, 5,7, 8, 9, 10, 11, 12
No other biomarkers have been approved beyond PD-L1 for aNSCLC treated with ICIs. Among the most investigated alternative biomarkers, circulating immune profiling has shown potential prediction of ICI efficacy in aNSCLC. Circulating biomarkers are easy to use since they are less invasive and less time-consuming compared to tissue biomarkers.14,15
The phase II PEOPLE trial aims to identify circulating immune, tissue and metagenomic biomarkers associated with treatment efficacy in aNSCLC patients with negative and low PD-L1 levels treated with first-line single-agent pembrolizumab. Here we report the association of circulating immune biomarkers and clinical outcomes.
Patients and methods
Study design and patients
PEOPLE is a prospective, monocentric, open-label, phase II trial conducted at Fondazione IRCCS Istituto Nazionale dei Tumori in Milan. Eligible patients were aged ≥18 years, had confirmed diagnosis of EGFR/anaplastic lymphoma kinase WT and PD-L1 TPS 0%-49% aNSCLC (stage IIIB/IV), were treatment-naïve, had measurable disease according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2. Patients with asymptomatic central nervous system metastases were eligible for the study. Palliative radiation therapy was allowed within 2 weeks before the start or during treatment, at the investigator’s discretion. Tumor tissue availability was mandatory for inclusion in the trial.
The trial protocol and all amendments were approved by the local ethical committee. The trial was conducted in accordance with the International Conference on Harmonization Guidelines on Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent before enrollment. This study was registered at ClinicalTrials.gov (NCT03447678).
Treatment and procedures
Pembrolizumab at a flat dose of 200 mg was i.v. administered every 3 weeks (21 ± 3 days) and continued up to 2 years or 35 cycles (whichever occurred later) or until progressive disease (PD), unacceptable toxicity or patient’s consent withdrawal. Tumor response was assessed every 9 weeks according to RECIST 1.1. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) Version 4.0. PD-L1 expression was re-assessed locally by an independent pathologist during the screening phase by immunohistochemistry with DAKO 22C3 PD-L1 antibody on archival or newly conducted biopsy. Results on PD-L1 were evaluated both as dichotomous (0% or 1%-49%) and continuous variables (from 0% to 49%). Tissue samples were collected at baseline to perform gene expression analysis (data not shown). Blood samples were collected at baseline, at each radiological evaluation, at PD and at the end of treatment for circulating immune profiling. Stool samples were collected at baseline, after one treatment cycle and at PD for microbiome analysis (data not shown).
Quantitative determination of peripheral blood immune subsets by flow cytometry
Determination of absolute cell counts (cells/μl) of 36 innate and adaptive immunity subsets in peripheral blood was obtained by flow cytometry. Peripheral blood at the first radiological evaluation was collected at day 63 (±3 days). One hundred microliters of freshly collected blood was stained with fluorescently labeled monoclonal antibodies (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100645) in Trucount™ Absolute Counting Tubes (Becton Dickinson, Franklin Lakes, NJ 663028). After staining, red blood cells were lysed using 2 ml of NH4Cl and samples were incubated for 10 min at room temperature. The Trucount tubes were then analyzed using a 10-color Gallios (Beckman Coulter, Brea, CA) cytometer. Instrument optical alignment and fluidics were routinely checked by Flow-Check Fluorospheres (Beckman Coulter, Brea A63493), while Flow-Set Fluorospheres (Beckman Coulter, Brea A63492) were used for control of light scatter and fluorescence intensity. Data were analyzed using the FlowJo software (v.10.8.0, FlowJo, Franklin Lakes, LLC). Fluorescent beads emit fluorescence in different wavelengths; therefore, they can be identified in different fluorescence channels (Supplementary Figure S1A and B, available at https://doi.org/10.1016/j.esmoop.2022.100645, for representative dotplots) and then gated out for subsequent analysis. After gating out beads, and including single cells in the analysis, the peripheral blood mononuclear cells were further gated using side scatter (SSC) versus cluster differentiation (CD) 45 dotplots (Supplementary Figure S1A, available at https://doi.org/10.1016/j.esmoop.2022.100645). To calculate the absolute count (A) we used the following formula: A = X/Y × N/V, where X = number of positive cells events, Y = number of bead events, N = number of beads in the test tube and V = test volume. Validation of quantitative data generated by the Trucount method was obtained by carrying out correlation analysis of lymphocyte, granulocyte and monocyte counts with data generated on the same samples by an automated hematology cell counter (Supplementary Figure S1C, available at https://doi.org/10.1016/j.esmoop.2022.100645). The flow cytometry experimental set-up allowed to identify and count 36 distinct immune subsets (Supplementary Figure S2A-G for gating strategy for all these subsets and Supplementary Figure S2H, available at https://doi.org/10.1016/j.esmoop.2022.100645, for the related definition).
Statistical analysis
The primary objective was to identify immune biomarkers associated with progression-free survival (PFS), defined as the time from the first treatment administration to PD or death due to any cause, whichever occurs first. Patients who had not progressed or died at the time of analysis were censored at their last disease evaluation date. Secondary objectives were: to detect differences in immune biomarker distribution between pre- and post-study treatment, to estimate the activity of the study treatment in terms of ORR, disease control rate (DCR), duration of response (DoR) and OS. The DoR was measured from the date of evaluation when the measurement criteria were met for complete response (CR) or partial response (PR) (whichever was first recorded) until the first date of recurrence or PD or death. The OS was defined as the time from the first treatment administration to death due to any cause. Alive patients at the time of analysis were censored at the last follow-up.
The sample size estimation was based on pragmatic considerations that included the planned study duration and a worthwhile clinically relevant effect. Considering an enrollment period of 1 year and assuming a proportion of events at analysis time ≥75%, at least 65 patients enrolled and 48 PFS events were expected. With this number of events, setting the statistical power at 80%, we were able to detect a relative hazard per standard deviation unit equal to 1.50 using a two-sided test at the 0.05 level.
The association of each immune biomarker with the expression of PD-L1 was investigated by means of the Spearman correlation index and of correlation at two time points were compared applying standard Fisher’s z-transformation.
To compare the distribution of the immune biomarkers at different time points, the paired t-test or the Wilcoxon signed rank test were used, as appropriate. In order to reduce the number of immune biomarkers, a variable clustering procedure was carried out. An orthoblique principal components–based clustering approach was applied, in order to define a subset of clusters that explains at least a 60% of the total variance. Each cluster score, as well as the most representative biomarker of each cluster, selected according to the 1-R2 index, was tested for association with PFS and OS by means of univariable and multivariable Cox proportional hazard models and for association with DCR by means of univariable and multivariable logistic regression models. The multivariable models were adjusted for the impact of age, sex, smoke, ECOG, histology, number of metastatic sites and continued expression of PD-L1, and administration of steroids, antibiotics or proton-pump inhibitors (PPIs) during the treatment. The results were provided as hazard ratios (HRs) or odds ratios (ORs) for survival or response results, respectively, and their confidence interval at 95% (95% CI). The survival curves were estimated by the Kaplan–Meier (KM) method. The median follow-up was estimated using the inverse KM method. The PFS, OS, DCR and the safety profile were analyzed in the per-protocol population that included patients enrolled in the study who received at least one dose of study treatment.
For patients experiencing the same AE more than once, the extreme grade experienced by each subject was reported. All analyses were done with SAS software, version 9.4 (SAS Institute).
Results
Patient’s characteristics and compliance
From 31 May 2018 to 7 October 2020, 87 patients were screened and 65 patients were enrolled in this study (Figure 1). The immune biomarker analysis was not carried out for 8 patients at baseline (due to scanty quality of blood samples) and for 19 patients at first radiological disease evaluation (especially due to deterioration of clinical condition or death). Therefore, the biomarker analysis was carried out at baseline and at first radiological disease evaluation on 57 and 46 patients, respectively (Figure 1).
Figure 1.
Enrollment flowchart process.
ICI, immune checkpoint inhibitor; PD, progressive disease; PD-L1, programmed death-ligand 1; pts, patients; RT, radiotherapy.
aData not shown.
Demographic and clinical baseline patient characteristics are reported in the Table 1. Overall, the median age was 70.9 years (Q1-Q3 63.7-77.1 years), 12 patients (18.5%) had ECOG 2, PD-L1 expression was 0 in 19 patients (29.2%) and 54 patients (83.1%) had a non-squamous tumor histology. Brain metastases were present in 10 patients (15.4%). Steroids (>10 mg prednisone), antibiotics and PPIs were administered during treatment in 29 (44.6%), 28 (43.1%) and 41 (63.1%) patients, respectively. The most common reasons for discontinuation were radiological or clinical PD (48 patients, 77.4%) and death (12 patients, 19.4%). Three patients were under treatment at the time of the data snapshot for the final analysis (21 July 2021).
Table 1.
Demographic and clinical characteristics at baseline
| All patients |
Patients with biomarker profile at baseline |
Patients with biomarker profile at baseline and at first disease evaluation |
|
|---|---|---|---|
| N = 65 | N = 57 | N = 46 | |
| Age (years) | |||
| Median (Q1-Q3) | 70.9 (63.7-77.1) | 70.9 (63.7-75.6) | 69.3 (63.7-75.0) |
| Female sex, n (%) | 21 (32.3) | 20 (35.1) | 17 (37.0) |
| Race, n (%) | |||
| Asian | 1 (1.5) | 0 (0.0) | 0 (0.0) |
| Black or African American | 1 (1.5) | 0 (0.0) | 0 (0.0) |
| White | 63 (96.9) | 57 (100.0) | 46 (100.0) |
| Smoking habits, n (%)a | |||
| Current | 15 (23.1) | 13 (22.8) | 12 (26.1) |
| Former | 42 (64.6) | 38 (66.7) | 28 (60.9) |
| Never | 8 (12.3) | 6 (10.5) | 6 (13.0) |
| ECOG performance status, n (%) | |||
| 0 | 23 (35.4) | 22 (38.6) | 20 (43.5) |
| 1 | 30 (46.2) | 25 (43.9) | 20 (43.5) |
| 2 | 12 (18.5) | 10 (17.5) | 6 (13.0) |
| Histology, n (%) | |||
| Non-squamous | 54 (83.1) | 49 (86.0) | 42 (91.3) |
| Squamous | 11 (16.9) | 8 (14.0) | 4 (8.7) |
| Number of metastatic sites, n (%) | |||
| 0-2 | 47 (72.3) | 44 (77.2) | 38 (82.6) |
| >2 | 18 (27.7) | 13 (22.8) | 8 (17.4) |
| Liver metastases, n (%) | 5 (7.7) | 3 (5.3) | 3 (6.5) |
| Brain metastases, n (%) | 10 (15.4) | 8 (14.0) | 8 (17.4) |
| Bone metastases, n (%) | 10 (15.4) | 8 (14.0) | 8 (17.4) |
| Clinical staging at study entry (TNM seventh edition), n (%) | |||
| IIIB | 3 (4.6) | 3 (5.3) | 2 (4.3) |
| IV | 62 (95.4) | 54 (94.7) | 44 (95.7) |
| Categorical PD-L1 status, n (%) | |||
| 0 | 19 (29.2) | 17 (29.8) | 14 (30.4) |
| 1-49 | 46 (70.8) | 40 (70.2) | 32 (69.6) |
| Continuous PD-L1 status | |||
| Mean (SD) | 12.1 (16.7) | 11.2 (15.6) | 9.6 (13.9) |
| Median (Q1-Q3) | 2.3 (0.0-20.0) | 2.3 (0.0-20.0) | 2.0 (0.0-10.0) |
| Missing | 3 | 3 | 1 |
| Neo/adjuvant chemotherapy, n (%) | |||
| No | 62 (95.4) | 55 (96.5) | 45 (97.8) |
| Yes | 3 (4.6) | 2 (3.5) | 1 (2.2) |
ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death-ligand 1; Q1-Q3, first and third quartile; SD, standard deviation; TNM, tumor–node–metastasis.
Current smokers: more than 100 cigarettes smoked in life, smokers in the last six months; former smokers: at least 100 cigarettes smoked in life, non smokers for at least 6 months; never smokers: less than 100 cigarettes smoked in life.
Efficacy analysis
The median follow-up was 26.4 months (95% CI 22.9-31.8 months). Overall, 60 PFS events (92.3%) were observed and 46 patients (70.8%) died. The median PFS was 2.9 months (95% CI 1.8-5.8 months) and the median OS was 12.1 months (95% CI 9.00-20.2 months). Supplementary Figure S3A and B, available at https://doi.org/10.1016/j.esmoop.2022.100645, shows the KM curves for PFS and OS.
Two patients (3.1%) experienced CR, 12 (18.5%) PR, 17 (26.2%) stable disease (SD) and 27 (41.5%) PD as best response, whereas 7 patients (10.8%) died before the first radiological evaluation. The ORR and DCR were 21.5% (95% CI 12.3% to 33.5%) and 47.7% (95% CI 35.2% to 60.5%), respectively. The median DoR in 14 responder patients was 14.5 months (95% CI 6.4-24.9 months).
Safety analysis
Toxicity was reported as the reason for treatment discontinuation for two patients (3.2%). Grade (G)3/G4 AEs were reported for 26 patients (40.0%) (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100645). Six G3/G4 AEs (9.2%) were judged related to the treatment. In detail, two increase of lipase, two hyponatremia, one increase in gamma-glutamyltransferase and one infection (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100645). Two G3/G4 pneumonitis were recorded as not-drug-related. Two drug-related serious AEs were reported for two patients: one hyponatremia and one infection.
Immune biomarkers: cluster analysis and association with PFS, OS and DCR
Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100645, provides the distribution of circulating immune subset counts at baseline, at first radiological evaluation and the differences between the two time points for 57 patients and for 46 patients, respectively.
Statistically significant differences in terms of correlation between the expression of PD-L1 at baseline and the expression of immune biomarkers at baseline and at the first radiological evaluation were found for CD14+, CD14+/human leukocyte antigen - DR isotype (HLA-DR)+, classical CD14+CD16− and classical CD14+CD16−/HLA-DR+ at baseline, and for CD3+, granulocyte-/HLA-DRdimCD14−, lymphocytes (CD3+ or CD19+ or CD56+), CD19+ and CD19+/HLA-DR+ at the first radiological evaluation (Supplementary Tables S5 and S6, available at https://doi.org/10.1016/j.esmoop.2022.100645).
At univariable analysis, both worse PFS and OS were detected in patients with an ECOG 2 [PFS: HR 3.03 (95% CI 1.43-6.43), P = 0.004; OS:HR 9.57 (95% CI 3.89-23.6), P < 0.001] and in patients with more than two metastatic sites at baseline [PFS: HR 3.54 (95% CI 1.79-6.99), P < 0.001; OS: HR 6.82 (95% CI 3.32-14.0), P < 0.001]. No statistically significant correlation was found with the other variables analyzed (Supplementary Tables S7 and S8, available at https://doi.org/10.1016/j.esmoop.2022.100645).
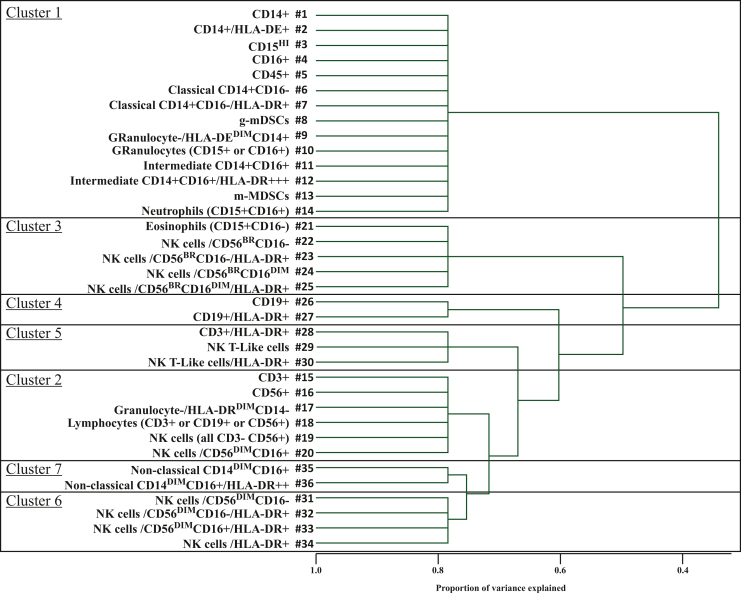
A variable clustering procedure was carried out in order to reduce the number of immune biomarkers. Seven clusters were identified, each including 2-14 immune subsets (Figure 2). According to cluster scores analysis, at multivariable analysis, cluster 2 containing lymphocytes, T cells and natural killer (NK) cells (P = 0.002) and cluster 6, containing specific NK cell subsets (P = 0.010), were statistically associated with PFS (Supplementary Table S9, available at https://doi.org/10.1016/j.esmoop.2022.100645). Moreover, a statistically significant better PFS was observed for both the most representative subsets of clusters 2 and 6. In detail, a positive association was found for higher levels of lymphocytes defined as ‘granulo-/HLA-DR CD14’ [cluster 2, subset #17; HR 0.92 (95% CI 0.86-0.97), P = 0.005] (Figure 3A, Supplementary Table S9, available at https://doi.org/10.1016/j.esmoop.2022.100645) and of NK cells/HLA-DR+ [cluster 6, subset #34; HR 0.98 (95% CI 0.96-1.00), P = 0.016] (Figure 3B, Supplementary Table S9, available at https://doi.org/10.1016/j.esmoop.2022.100645). A statistically significant association was found between cluster 2 (in terms of cluster score) and both OS (P = 0.037) (Supplementary Table S10, available at https://doi.org/10.1016/j.esmoop.2022.100645) and DCR (P = 0.013) (Supplementary Table S11, available at https://doi.org/10.1016/j.esmoop.2022.100645). A trend toward significant association of cluster #17 (granulocyte-/HLA-DRdimCD14−) with OS was found (Supplementary Table S10, available at https://doi.org/10.1016/j.esmoop.2022.100645 and Figure 3C), while the same subset significantly correlated with a higher DCR [OR 1.18 (95% CI 1.02-1.36), P = 0.022] (Supplementary Table S11, available at https://doi.org/10.1016/j.esmoop.2022.100645).
Figure 2.
Cluster representation of the 36 subsets of circulating immune biomarkers. CD, cluster of differentiation; HLA-DR, human leukocyte antigen - DR isotype; MDSC, myeloid derived suppressor cell; NK, natural killer.
Figure 3.
Kaplan–Meier curves related to the most representative biomarker according to multivariable analysis for PFS and cluster 2 (A), PFS and cluster 6 (B), OS and cluster 2 (C) and OS and cluster 1 (D).
CD, cluster differentiation; CI, confidence interval; HLA-DR, human leukocyte antigen - DR isotype; NE, not evaluable; NK, natural killer; OS, overall survival; PFS, progression-free survival.
Exploratory analysis on immune biomarkers at baseline
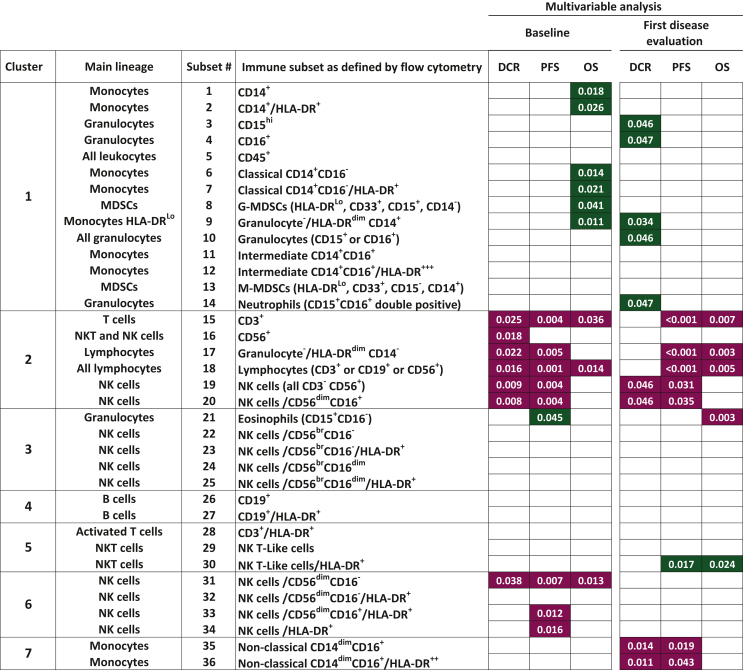
Results of univariable and multivariable analyses on PFS, OS and DCR for all 36 biomarkers were summarized in Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2022.100645, and in Figure 4, respectively (details are provided in Supplementary Tables S12-S17, available at https://doi.org/10.1016/j.esmoop.2022.100645). Both univariable and multivariable analyses indicated that, at baseline, several immune subsets in cluster 2 (six different lymphocyte subsets) (Figure 4) and in cluster 1 (14 subsets belonging to the myeloid compartment) had opposite associations with PFS, OS and DCR. At multivariable analyses, higher counts of five out of six biomarkers of cluster 2 (subset #15: CD3+ T cells, #17, #18: all lymphocytes, #19, #20: all NK cells and the most frequent NK subset, i.e. the CD56dim CD16+ cells) and three out of four NK subsets within cluster 6 (#31: CD56dimCD16−, #33, #34: HLA-DR+ fractions of NK cells) were significantly associated with a better PFS. A statistically significant negative association with PFS was found for eosinophil counts (cluster 3, subset #21: CD15+ CD16− granulocytes). The multivariable analyses for OS detected a statistically significant positive correlation for higher level of CD3+ T cells (cluster 2, subset #15), of all lymphocytes (cluster 2, subset#18) and for CD56dimCD16− NK cells (cluster 6, subset #31) and a negative correlation with several myeloid subsets in cluster 1 [#1, #2: CD14+ monocytes and HLA-DR+ monocytes, #6, #7: CD14+CD16− classical monocytes, #9: HLA-DRLo monocytes, #8: granulocytic myeloid derived suppressor cells (MDSCs)]. In particular, higher count of CD14+ cells (cluster 1, subset #1) were significantly associated with worse OS [HR 1.24 (95% CI 1.04-1.49), P = 0.018, Figure 3D, Supplementary Table S10, available at https://doi.org/10.1016/j.esmoop.2022.100645].
Figure 4.
Effect of all biomarkers at baseline and at first disease evaluation on DCR, PFS and OS. Multivariable Cox proportional hazard models. The 36 identified biomarkers are grouped according to the clusters they belong. The maroon or green color code of the P values indicates a positive or negative impact, respectively, of higher values of each subset on DCR, PFS or OS.
CD, cluster of differentiation; DCR, disease control rate; NK, natural killer; HLA-DR, human leukocyte antigen - DR isotype; MDSC, myeloid derived suppressor cell; OS, overall survival; PFS, progression-free survival.
Finally, higher counts of each of the six lymphocyte subsets included in cluster 2 (subsets #15 to subset #20) correlated at multivariable analysis with a statistically significant better DCR.
Exploratory analysis on immune biomarkers at first radiologic evaluation
A better PFS was detected in patients with higher counts of most lymphocyte subsets belonging to cluster 2 (subsets#15 and #17 to #20, Figure 4). A positive association of higher counts of three of these lymphocyte subsets (#15, #17 and #18) and with eosinophils (subset #21) was detected also with OS. Moreover, higher frequency of two subsets in cluster 7 (#35 and #36: nonclassical monocytes with a CD14dim CD16+ profile) was associated with better PFS. In contrast, a shorter PFS was detected in patients with higher counts of NK T-like/HLA-DR+ (cluster 5, subset#30: CD3+ CD56+ cells). A negative association for DCR was found with higher counts of several subsets in cluster 1, containing most cell types belonging to the myeloid compartment, including subset #3, #4 and #10 (granulocytes) and HLA-DRLo monocytes (subset #9). In keeping with the association detected at baseline with DCR, even at first radiologic evaluation, NK cells (cluster 2, subsets #19 and #20) were found to be positively correlated with DCR.
Discussion
Immunotherapy is a mainstay of treatment in the great part of aNSCLC patients. Biomarkers for ICI efficacy still remain a crucial unmet clinical need. PD-L1 is the only biomarker used and approved.6 Tumor mutational burden was widely assessed in prospective and retrospective clinical trials; however, its value is still debatable in predicting single-agent immunotherapy outcomes.16 Often nowadays, clinicians have to make decisions without a satisfactory clinical tool.
The results of our phase II study, focused on negative and low PD-L1 (0%-49%) aNSCLC patients, confirmed that pembrolizumab, as first-line single agent, is safe and may be also active in this setting, although in a limited number of patients. Moreover, the PEOPLE trial identified some circulating immune subsets as useful biomarkers for ICI efficacy among negative and low PD-L1 aNSCLC patients, suggesting that probably in selected cases, we can avoid the adding toxicity of chemotherapy in the first line.
The median follow-up of 26.4 months, with 60 PFS events and 46 deaths registered, make our study mature enough to draw conclusions, despite the relatively small number of patients.
Compared with the patients enrolled in the registration phase III trials in the ICI-based first-line setting, the patients of the PEOPLE trial were older. The median age was 70.9 years and ranged from 63 to 65 years in the KEYNOTE studies (KEYNOTE 189, 024 and 042).3,8,13 Moreover, patients with ECOG 2 were excluded from these trials. These two factors confer to the population of the PEOPLE trial similarity to real-life patients, partially explaining the reported low median PFS (2.9 months).
No data on PFS and OS, deriving from existing prospective trials, are available in order to carry out a correct comparison with single-agent ICI therapy in patients with PD-L1 0%-49%. The PFS values reported in first-line chemo-immunotherapy studies are always clearly better than those registered in our study (median PFS of 6.2 months in the PD-L1 0% population of KEYNOTE 189).8 Moreover, in those trials using single-agent ICIs, in patients with PD-L1 ≥ 50%, the PFS is obviously higher with a median of 10.3 months (KEYNOTE 024)3 while in patients with PD-L1 ≥ 1% the reported median PFS in the KEYNOTE 042 study was 5.4 months.13
Interesting results in terms of ORR, median OS and DoR were reported in the PEOPLE trial. The ORR was 21.5%, with a DCR of 47.7%. Among the 12 PRs, 4 patients were PD-L1 negative. These results are similar to those reported with nivolumab first-line in patients with PD-L1 ≥ 5% (ORR 26%, CheckMate 026)17 and to the results reported in patients with PD-L1 1%-49% treated with pembrolizumab first-line in the KEYNOTE 042 trial (ORR 27%).13 Better results were reported with the addition of chemotherapy in the PD-L1-negative population in KEYNOTE 189 with an ORR of 33% versus 22.2% in our negative population.8
The median DoR of 14.5 months and the median OS of 12.1 months reported in the PEOPLE trial were comparable to those obtained in the first line, in similar populations, with single-agent immunotherapy (median OS 13.4 months in the PD-L1 1%-49% population of KEYNOTE 042).13 Finally, pembrolizumab was confirmed to be a safe drug, as previously reported in other trials, with low incidence of G3/G4 treatment-related AEs (9.2%).3, 4, 5,7, 8, 9, 10, 11, 12, 13
Profiling of innate and adaptive immune cell types was carried out by determination of absolute cell counts for 36 peripheral subsets. Results from the cluster analysis of these subsets have demonstrated that in the overall population, cluster 2 and 6 (lymphocyte and NK cells populations) correlated with better PFS. In accordance with these results, cluster analysis could be useful to identify those subgroups who might benefit more from ICI single agent (e.g. #34 PFS 5.8 versus 1.8, Figure 3B). Moreover, cluster 2 correlated also with better OS (e.g. #17 OS 14.6 versus 7.8, Figure 3C).
Exploratory multivariate analyses on single immune biomarker confirmed that high baseline frequency of eight subsets, including lymphocytes and several NK cells, were predictive of improved PFS. For five of these subsets, the association was retained even in the blood samples obtained at the first radiologic evaluation. These findings corroborate and extend previous evidence on the association of high frequency of T cells (PD-1+, CD4+) and NK cells14,15 with improved PFS in aNSCLC patients receiving ICIs. The association of high lymphocyte frequency (including CD3+ T cells) with improved PFS found in the PEOPLE trial is in line with the central role of T cells in immunotherapy targeting the PD-1/PD-L1 axis.18 However, the predicting significance of high NK cell frequency for PFS challenges the simple view that immune checkpoint blockade depends mostly on the activity of T cells. Indeed, several lines of evidence in pre-clinical models, as well as in clinical trials, underscore the key role of innate immune cells in the context of PD-1-targeted therapies. Barry et al.19 found that NK cell frequency (at tumor site) correlates with stimulatory dendritic cells and in melanoma patients NK cells correlate with responsiveness to anti-PD-1 agent and with increased OS. Interestingly, Dong et al.20 found that patients with PD-L1-negative tumors can respond to ICIs with anti-PD-L1, but such response depends on PD-L1-positive NK cells. In pre-clinical models, Hsu et al.21 found that PD-1 and PD-L1 blockade promotes an NK cell response that is essential for the efficacy of immunotherapy.
The multivariable analysis on innate and adaptive immune subset frequencies also revealed that the myeloid compartment of peripheral blood had opposite associations with DCR and OS compared to the lymphocyte compartment. In fact, higher baseline frequencies of six myeloid cell subsets, CD14+, classical CD14+ CD16− monocytes and G-MDSCs, were significantly associated with worse OS (e.g. #1 OS 4.0 versus 31.3, Figure 3D) while higher CD3+, lymphocytes and CD56dim CD16− NK frequencies were associated with improved OS. Higher granulocyte and M-MDSC frequencies at first disease evaluation were associated with worse DCR. These results are in keeping with the negative predictive role exerted on main outcome parameters by neutrophils in aNSCLC.22,23 In melanoma, increased monocyte counts were significantly associated with decreased OS in the context of nivolumab monotherapy and in the context of nivolumab plus ipilimumab combination therapy.24,25
In conclusion, the PEOPLE trial has some limits, especially related to the non-randomized design and the low sample size. In Italy, at the time of the study design and at the start of trial enrollment, the standard of care for patients with low PD-L1 aNSCLC was platinum-based chemotherapy. Moreover, in those years the role of PD-L1 had not yet been clarified. However, during the course of the trial enrollment there was the approval of chemo-immunotherapy and the data of the superiority of chemo-immunotherapy over single-agent immunotherapy began to be clear, especially in the negative PD-L1 population. This caused a significant selection bias in the later stages of enrollment, as PD-L1-negative patients eligible for chemo-immunotherapy were no longer enrolled.
Anyhow, to our knowledge, this is the first prospective trial using the association of peripheral immune biomarkers and clinical outcomes as primary endpoint in negative and low PD-L1 aNSCLC patients treated with first-line pembrolizumab. In this setting, our data confirm that chemo-immunotherapy must be considered the gold standard and pembrolizumab cannot be proposed without careful patient selection. However, our biomarker analysis suggests that the characterization for peripheral innate and adaptive immune subsets may help in the identification of some low PD-L1 aNSCLC patients who may benefit from first-line single-agent pembrolizumab, avoiding the added toxicity of chemotherapy.
Acknowledgements
We acknowledge Dr Vito Ladisa and our nurses for their contribution in assisting the patients, and our trial center for support in this study.
Funding
This work was supported by Merck Sharp & Dohme [grant number IIS52278].
Disclosure
GLR provided consultation, attended advisory boards and/or provided lectures for the following organizations, from whom honoraria or education grants were received: Merck Sharp and Dohme, Takeda, Amgen, Eli Lilly, BMS, Roche, Italfarmaco, Novartis, Sanofi, Pfizer and AstraZeneca. AP declares personal fees from AstraZeneca, Italfarmaco, Roche and BMS. RF declares advisory role from Merck Sharp and Dohme. DS declares personal fees from Italfarmaco, AstraZeneca, MSD, Boehringer Ingelheim and BMS. CP declares personal fees from Italfarmaco, AstraZeneca, BMS and Merck Sharp and Dohme. FdB provided consultation, attended advisory boards and/or provided lectures for the following organizations, from whom honoraria or education grants were received: Amgen, AstraZeneca, Boehringer-Ingelheim, BMS, Eli Lilly, F. Hoffmann-La Roche, Ignyta, Merck Sharp and Dohme, Merck Serono, Novartis and Pfizer. MCG declares personal financial interests with the following organizations: AstraZeneca, MSD International GmbH, BMS, Boehringer Ingelheim Italia S.p.A, Celgene, Eli Lilly, Ignyta, Incyte, Inivata, MedImmune, Novartis, Pfizer, Roche and Takeda; she also declares Institutional financial interests with the following organizations: Eli Lilly, MSD, Pfizer, AstraZeneca, MSD International GmbH, BMS, Boehringer Ingelheim Italia S.p.A, Celgene, Ignyta, Incyte, Inivata, MedImmune, Novartis, Pfizer, Roche, Takeda and Foundation Medicine. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Proto C., Ferrara R., Signorelli D., et al. Choosing wisely first line immunotherapy in non-small cell lung cancer (NSCLC): what to add and what to leave out. Cancer Treat Rev. 2019;75:39–45. doi: 10.1016/j.ctrv.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Reck M., Rodrıguez-Abreu D., Robinson A.G., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non–small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4.Herbst R.S., Giaccone G., de Marinis F., et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 5.Sezer A., Kilickap S., Gümüş M., et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397:592–604. doi: 10.1016/S0140-6736(21)00228-2. [DOI] [PubMed] [Google Scholar]
- 6.Paver E.C., Cooper W.A., Colebatch A.J., et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: a guide to immunohistochemistry implementation and interpretation. Pathology. 2021;53:141–156. doi: 10.1016/j.pathol.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Paz-Ares L., Luft A., Vicente D., et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi L., Rodrıguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 9.Langer C.J., Gadgeel S.M., Borghaei H., et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Socinski M.A., Jotte R.M., Cappuzzo F., et al. Atezolizumab for first-line treatment of metastatic non squamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 11.West H., McCleod M., Hussein M., et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 12.Paz-Ares L., Ciuleanu T.E., Cobo M., et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 13.Mok T.S.K., Wu Y.L., Kudaba I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 14.Inomata M., Kado T., Okazawa S., et al. Peripheral PD1-positive CD4 T-lymphocyte count can predict progression-free survival in patients with non-small cell lung cancer receiving immune checkpoint inhibitor. Anticancer Res. 2019;39:6887–6893. doi: 10.21873/anticanres.13908. [DOI] [PubMed] [Google Scholar]
- 15.Li S., Zhang C., Pang G., Wang P. Emerging blood-based biomarkers for predicting response to checkpoint immunotherapy in non-small-cell lung cancer. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.603157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellmann M.D., Ciuleanu T.E., Pluzanski A., et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carbone D.P., Reck M., Paz-Ares L., et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barry K.C., Hsu J., Broz M.L., et al. A natural killer/dendritic cell axis defines responsive tumor microenvironments in melanoma. Nat Med. 2018;24:1178–1191. doi: 10.1038/s41591-018-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong W., Wu X., Ma S., et al. The mechanism of anti–PD-L1 antibody efficacy against PD-L1–negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov. 2019;9:1422–1437. doi: 10.1158/2159-8290.CD-18-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu J., Hodgins J.J., Marathe M., et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. 2018;128:4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kargl J., Zhu X., Zhang H., et al. Neutrophil content predicts lymphocyte depletion and anti-PD1 treatment failure in NSCLC. JCI Insight. 2019;4 doi: 10.1172/jci.insight.130850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasegawa T., Yanagitani N., Utsumi H., et al. Association of high neutrophil-to-lymphocyte ratio with poor outcomes of pembrolizumab therapy in high-PD-L1-expressing non-small cell lung cancer. Anticancer Res. 2019;39:6851–6857. doi: 10.21873/anticanres.13902. [DOI] [PubMed] [Google Scholar]
- 24.Chasseuil E., Saint-Jean M., Chasseuil H., et al. Blood predictive biomarkers for nivolumab in advanced melanoma. Acta Derm Venereol. 2018;98:406–410. doi: 10.2340/00015555-2872. [DOI] [PubMed] [Google Scholar]
- 25.Rosner S., Kwong E., Shoushtari A.N., et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med. 2018;7:690–697. doi: 10.1002/cam4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.