Abstract
Background
The Meet-URO score allowed a more accurate prognostication than the International Metastatic RCC Database Consortium (IMDC) for patients with pre-treated metastatic renal cell carcinoma (mRCC) by adding the pre-treatment neutrophil-to-lymphocyte ratio and presence of bone metastases.
Materials and methods
A post hoc analysis was carried out to validate the Meet-URO score on the overall survival (OS) of patients with IMDC intermediate-poor-risk mRCC treated with first-line nivolumab plus ipilimumab within the prospective Italian Expanded Access Programme (EAP). We additionally considered progression-free survival (PFS) and disease response rates. Harrell’s c-index was calculated to compare the accuracy of survival prediction.
Results
Overall the EAP included 306 patients, with a median follow-up of 12.2 months, median OS was not reached, 1-year OS was 66.8% and median PFS was 7.9 months. By univariable analysis, both the IMDC score and the two additional variables of the Meet-URO score were associated with either OS or PFS (P < 0.001 for all comparisons). The four Meet-URO risk groups (G) had 1-year OS of 92%, 72%, 50% and 21% for G2 (29.1% of patients), G3 (28.8%), G4 (33.0%) and G5 (9.1%), respectively. OS was significantly shorter in each consecutive G (P = 0.001 for G3, P < 0.001 for both G4 and G5 compared to G2). Similarly, Meet-URO Gs 2-5 showed decreasing median PFS and response rates. The Meet-URO score showed the highest c-index for both OS (0.73) and PFS (0.67). Limitations include the post hoc nature of this analysis and the lack of a comparative arm to assess predictive value.
Conclusion
The Meet-URO score appeared to show better prognostic classification than the IMDC alone in patients with mRCC at IMDC intermediate-poor risk treated with first-line nivolumab and ipilimumab.
Key words: IMDC, immunotherapy, prognostic, bone metastases, NLR, Meet-URO, renal cell carcinoma
Highlights
-
•
The prognostic accuracy of the IMDC classification system in untreated mRCC patients still needs improvements.
-
•
By an analysis of the prospective Italian EAP with first-line nivolumab plus ipilimumab.
-
•
The Meet-URO score confirmed a more accurate prognostic stratification than IMDC alone in this setting.
-
•
The Meet-URO score adds to IMDC the pre-treatment neutrophil-to-lymphocyte ratio and presence of bone metastases.
Introduction
In recent years, immune checkpoint inhibitors (ICIs) have re-shaped the treatment landscape of metastatic renal cell carcinoma (mRCC).1 The ICI era in mRCC started in 2015 with nivolumab, an anti-programmed death-1 inhibitor, showing survival benefits in patients progressing to anti-angiogenic therapy.2 More recently, ICI-based combinations have become standard as first-line therapy, with the combination of nivolumab and ipilimumab, a cytotoxic T-lymphocyte-associated antigen 4 inhibitor, as the first approved for patients with intermediate- and poor-risk International Metastatic RCC Database Consortium (IMDC) mRCC.3
However, not all patients with mRCC achieve a long-term benefit from ICIs, either as single-agent or combination therapies.1,4 Therefore, identifying novel biomarkers and more accurate predictive models for immunotherapy represents a clinically unmet need and topic for clinical research.5, 6, 7
The IMDC score is the most widely used prognostic classification for mRCC and consists of clinical and laboratory parameters.8,9 It was developed in 2009, at a time when vascular endothelial growth factor (VEGF) inhibitors dominated mRCC treatment and then carried over clinical trials with new-generation tyrosine kinase inhibitors (TKIs) and ICI-based combos.10 However, patients with favourable IMDC prognosis might not benefit in terms of OS from the combination of ICIs with VEGF receptor TKIs,11 and the benefit of first-line nivolumab and ipilimumab was only confirmed by a post hoc analysis of the CheckMate 214 study for all the IMDC intermediate- and poor-risk patients regardless of the number of the IMDC risk factors they had before starting treatment.12
The Meet-URO score is a clinically useful prognostic score tested on 571 patients with mRCC treated with nivolumab in ≥2nd line.13 It combines the IMDC prognostic classification with two additional prognostic factors, the pre-treatment presence of bone metastases and peripheral blood neutrophil-to-lymphocyte ratio (NLR).13 For patients with mRCC taking ≥2nd-line nivolumab or the TKI cabozantinib, the Meet-URO score has shown more accuracy than the IMDC.13,14
We aimed to evaluate how accurately the Meet-URO score could predict the OS in patients with IMDC intermediate and poor prognosis treated with a first-line immunotherapy combination of nivolumab and ipilimumab as part of the Italian Expanded Access Programme (EAP).15
Materials and methods
The Meet-URO score was calculated using data from the Italian EAP, which included first-line nivolumab plus ipilimumab for patients with mRCC. The EAP involved 86 centres in Italy between April and October 2019. The study was carried out under the Declaration of Helsinki, Good Clinical Practice and local ethical and legal regulations. The Regional Ethical Committee approved the analysis (number 2020/139 of 16 November 2020).
Study population
The EAP included adults with metastatic clear-cell or non-clear-cell RCC and IMDC intermediate/poor risk, according to the current indication and approval based on the CheckMate 214 study results,16 who had received at least one cycle of nivolumab plus ipilimumab. Therefore, patients with IMDC favourable disease were not included in the EAP. Clinical data and laboratory parameters were obtained from patients’ electronic medical records and paper charts and included in the EAP dataset, which served as the source for this analysis.
Treatment
Nivolumab plus ipilimumab was administered intravenously at 3 mg/kg and 1 mg/kg, respectively, every 3 weeks for four doses, followed by maintenance nivolumab at a flat dose of 240 mg every 2 weeks or 480 mg every 4 weeks. The treatment was administered until clinical or radiological disease progression, unacceptable toxicity, death or patient’s choice. Follow-up consisted of periodic physical examination, laboratory analysis and imaging assessment. Radiological assessments consisted of computed tomography scan of chest abdomen-pelvis and head (when clinically indicated), carried out at baseline and then every 2-4 months of treatment, according to local clinical practice.
Prognostic factors
The following clinical and laboratory data were examined to calculate the pre-treatment Meet-URO score: IMDC prognostic risk category (intermediate or poor risk), metastatic sites (indicating the existence of bone metastases) and full blood count values (for NLR calculation) (web calculator: https://proviso.shinyapps.io/Meet-URO15_score/).
The definition of the five prognostic groups identified by the Meet-URO score is available in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100634. As IMDC favourable-risk patients were not included in the Italian EAP, the Meet-URO prognosis group 1 could not be calculated.
Study endpoints
The primary endpoint for this analysis was validating the prognostic value of the Meet-URO score on OS. The secondary endpoint was comparing the Meet-URO score with the IMDC in terms of OS stratification and prognostic discrimination by Harrell’s c-index. The OS was calculated from the first nivolumab plus ipilimumab administration until death, censored at last follow-up for alive patients. The median follow-up was calculated as the median time from the first administration until death or last follow-up for censored patients. The prediction of the following outcome parameters was also described: progression-free survival (PFS), defined as the time from the treatment start to progression or death whichever occurred first; OS rate at 1 year (1y-OS); overall response rate (ORR); disease control rate (DCR), defined as the sum of partial response (PR), complete response (CR) and stable disease; and duration of response (DOR), defined as the time from the occurrence of PR and CR to the occurrence of progressive disease (PD). The disease response assessment was clinician-led following the Response Evaluation Criteria in Solid Tumours (RECIST 1.1) guidelines. The first evaluation was planned after four cycles of nivolumab and ipilimumab and then as per local clinical practice, approximately every 12 weeks.
Statistical analysis
Patients’ characteristics were reported using absolute frequency and percentage for categorical variables and median and range for quantitative variables.
The analysis included only patients who had information for all pre-treatment characteristics that compose the Meet-URO score, using the weights obtained for each prognostic factor during the development of the Meet-URO score.13 Missing values in other clinical characteristics were not replaced, and a complete-case analysis was carried out. We assigned patients to each Meet-URO risk class based on the three clinical and laboratory variables composing the Meet-URO score (i.e. the IMDC score, the NLR and the presence of bone metastases).13
The Kaplan–Meier method was used to estimate the OS and PFS survival curves for each of the three prognostic variables of the Meet-URO score and their combination. Univariable Cox regression model was carried out to estimate the hazard ratios (HRs), and multivariable Cox regression analysis for OS and PFS, which included the Meet-URO score and the characteristics showing a significant difference (P < 0.05) at the univariable analysis across the Meet-URO risk classes.
A logistic regression model was used to estimate odds ratios (ORs) for ORR and DCR. All HR and OR were reported alongside the 95% confidence intervals (CIs).
The Harrell’s c-index was calculated for the Meet-URO score and compared to that calculated for the IMDC to examine discriminative abilities on OS and PFS. Furthermore, the two scores were compared also using the Bayes information criterion (BIC; lower is better) and the generalized R2 (higher is better). The Meet-URO score was calibrated using a calibration plot that compared estimated and observed OS probabilities at 1 year. All statistical analyses were carried out using Stata v.16 (StataCorp Stata Statistical Software: Release 16, StataCorp LLC, College Station, TX).
Results
Patients’ characteristics
Three hundred and six patients with mRCC out of the 324 enrolled in the Italian EAP (94.4%) had available data to calculate the Meet-URO score. Patients’ characteristics are summarized in Table 1.
Table 1.
Patients’ characteristics
| Meet-URO score prognostic group |
||||||
|---|---|---|---|---|---|---|
| Characteristics | All cohort (n = 306) | 2 (n = 89, 29.1%) | 3 (n = 88, 28.8%) | 4 (n = 101, 33.0%) | 5 (n = 28, 9.2%) | P value |
| n (%) | ||||||
| Sex | 0.41 | |||||
| Male | 228 (74.5) | 61 (68.5) | 68 (77.3) | 76 (75.3) | 23 (82.1) | |
| Female | 78 (25.5) | 28 (31.5) | 20 (22.7) | 25 (24.8) | 5 (17.9) | |
| Median age, years (range) | 62.2 (24-87) | 62 (24-87) | 62.8 (37-87) | 62.4 (37-83) | 61.7 (37-82) | 0.99 |
| <70 | 229 (74.8) | 70 (78.7) | 66 (75.0) | 73 (72.3) | 20 (71.4) | |
| ≥70 | 77 (25.2) | 19 (21.3) | 22 (25.0) | 28 (27.7) | 8 (28.6) | |
| Histologic subtype | 0.040 | |||||
| Clear cell | 255 (83.3) | 78 (87.6) | 75 (85.2) | 84 (83.2) | 18 (64.3) | |
| Non-clear cell | 48 (15.7) | 9 (10.1) | 13 (14.8) | 17 (16.8) | 9 (32.1) | |
| Missing | 3 (1.0) | 2 (2.3) | 0 | 0 | 1 (3.6) | |
| Nephrectomy | <0.001 | |||||
| Yes | 200 (65.4) | 75 (84.3) | 66 (75.0) | 49 (48.5) | 10 (35.7) | |
| No | 106 (34.6) | 14 (15.7) | 22 (25.0) | 52 (51.5) | 18 (64.3) | |
| Metastatic at diagnosis | <0.001 | |||||
| Yes | 189/279 (67.7) | 44 (54.3) | 47 (57.3) | 72 (81.8) | 26 (92.9) | |
| No | 90/279 (32.3) | 37 (45.7) | 35 (42.7) | 16 (18.2) | 2 (7.1) | |
| IMDC score at start of treatment | <0.001 | |||||
| Intermediate | 206 (67.3) | 89 (100) | 88 (100) | 29 (28.7) | 0 | |
| Poor | 100 (32.7) | 0 | 0 | 72 (71.3) | 28 (100) | |
| Bone metastases | <0.001 | |||||
| Yes | 96 (31.4) | 0 | 28 (31.8) | 40 (39.6) | 28 (100) | |
| No | 210 (68.6) | 89 (100) | 60 (68.2) | 61 (60.4) | 0 | |
| NLR | <0.001 | |||||
| ≥3.2 | 160 (52.3) | 0 | 60 (68.2) | 72 (71.3) | 28 (100) | |
| <3.2 | 146 (47.7) | 89 (100) | 28 (31.8) | 29 (28.7) | 0 | |
| Liver metastases | 0.001 | |||||
| No | 250 (81.7) | 79 (88.8) | 75 (85.2) | 80 (79.2) | 16 (57.1) | |
| Yes | 56 (18.3) | 10 (11.2) | 13 (14.8) | 21 (20.8) | 12 (42.9) | |
| Sarcomatoid component | 0.70 | |||||
| No | 202 (79.2) | 57 (78.1) | 60 (80) | 71 (81.6) | 14 (70) | |
| Yes | 53 (20.8) | 16 (21.9) | 15 (20) | 16 (18.4) | 6 (30) | |
IMDC, International Metastatic RCC Database Consortium; n, number of patients; NLR, neutrophil-to-lymphocyte ratio.
Most patients were male (74%), and the median age was 62 years (range 24-87 years). Most patients had clear-cell RCC histology (86%) and previous nephrectomy (68%).
The IMDC score was intermediate and poor in 67% and 33% of patients, respectively. Baseline NLR was ≥3.2 in 45%, and bone metastases were present in 31% of patients.
Meet-URO score
Due to the lack of IMDC favourable-risk patients, the Meet-URO score identified four prognosis groups described in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100634: group 2 (29.1% of patients), group 3 (28.8%), group 4 (33.0%) and group 5 (9.1%). The distribution of patients by the Meet-URO score points is shown in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100634. According to risk classes, the characteristics of patients are reported in Table 1. Histology subtype (P = 0.04), previous nephrectomy (P < 0.001) and the presence of metastases at disease onset (P < 0.001) were significantly different across Meet-URO risk classes, alongside the IMDC class (P < 0.001), presence of bone metastases (P < 0.001), liver metastases (P = 0.001) and pre-treatment NLR value (P < 0.001) (Table 1).
Survival and disease response in the overall population
At the time of data cut-off (April 2021), with a median follow-up of 12.2 months (interquartile range 4.7-17.3 months), 31.2% of patients experienced PD, and 36.9% died. The median OS (mOS) was not reached (NR), 1y-OS was 66.8% and the median PFS (mPFS) was 7.9 months. ORR and DCR were 38.8% and 68.8%, respectively, while the median DOR (mDOR) was 14.0 months (95% CI not estimable due to the low number of responders).
Univariable analysis of OS and PFS by the Meet-URO score prognostic factors
The univariable Cox regression analysis results on the survival outcomes for the three prognostic factors included in the Meet-URO score are reported in Table 2, Figure 1A and C and Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100634.
Table 2.
Univariable Cox regression analyses for survival outcomes in the overall population and according to the parameters of the Meet-URO score
| Values | No. of patients n (%) | mOS (months) | 1y-OS (%) | HR (95% CI) | P value | c-Index | mPFS (months) | HR (95% CI) | P value | c-Index | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall population | 306 | NR | 66.8 | — | — | 7.9 | — | — | |||
| IMDC score | Intermediate | 206 (67.3) | NR | 77.3 | 1.00 (ref) | 0.65 | 10.2 | 1.00 (ref) | 0.59 | ||
| Poor | 100 (32.7) | 7.8 | 41.8 | 3.45 (2.40-4.96) | <0.001 | 2.9 | 1.90 (1.43-2.52) | <0.001 | |||
| NLR | <3.2 | 146 (47.7) | NR | 81.2 | 1.00 (ref) | 0.71 | 12.5 | 1.00 (ref) | 0.65 | ||
| ≥3.2 | 160 (52.3) | 14.2 | 53.1 | 2.95 (1.97-4.43) | <0.001 | 4.2 | 2.20 (1.65-2.93) | <0.001 | |||
| Bone | No | 210 (68.6) | NR | 73.5 | 1.00 (ref) | 0.59 | 10.6 | 1.00 (ref) | 0.58 | ||
| Yes | 96 (31.4) | 12.6 | 51.9 | 2.07 (1.42-2.99) | <0.001 | 4.6 | 1.91 (1.43-2.54) | <0.001 | |||
| Meet-URO score | Group 2 | 89 (29.1) | NR | 91.6 | 1.00 (ref) | — | 0.73 | 16.6 | 1.00 (ref) | — | 0.67 |
| Group 3 | 88 (28.8) | NR | 71.8 | 3.09 (1.54-6.19) | 0.001 | 6.8 | 2.05 (1.37-3.06) | <0.001 | |||
| Group 4 | 101 (33.0) | 12.0 | 50.0 | 6.07 (3.16-11.67) | <0.001 | 4.8 | 2.77 (1.88-4.08) | <0.001 | |||
| Group 5 | 28 (9.1) | 3.2 | 20.4 | 16.03 (7.74-33.21) | <0.001 | 1.4 | 6.56 (3.97-10.82) | <0.001 |
1y-OS, overall survival at 1 year; CI, confidence interval; HR, hazard ratio; IMDC, International Metastatic RCC Database Consortium; mOS, median overall survival; mPFS, median progression-free survival; n, number of patients; NLR, neutrophil-to-lymphocyte ratio; NR, not reached; ref, reference.
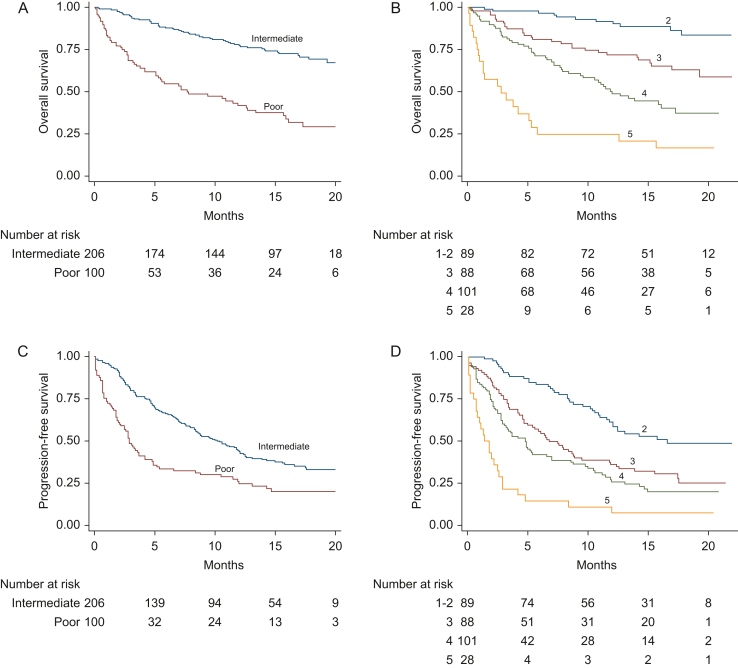
Figure 1.
Survival outcomes of 306 patients treated with first-line nivolumab plus ipilimumab within the prospective Italian Expanded Access Programme according to the International Metastatic RCC Database Consortium (IMDC) and the Meet-URO score systems. (A) Overall survival according to the IMDC intermediate- and poor-risk classes. (B) Overall survival according to the five Meet-URO risk classes. (C) Progression-free survival according to the IMDC intermediate- and poor-risk classes. (D) Progression-free survival according to the five Meet-URO risk classes.
By univariable analysis, either the IMDC score or the two additional variables of the Meet-URO score (NLR and the presence of bone metastases) resulted as significantly associated with either OS or PFS (P < 0.001 for all comparisons). The highest c-index for those three variables was observed with the NLR for both the OS (0.71) and PFS (0.65) (Table 2).
Univariable analysis of disease response by the Meet-URO score prognostic factors
Two hundred and fifty patients out of the 306 (81.7%) had available data on disease response. The univariable logistic (for the ORR, DCR) or Cox (for the DOR) regression analysis on response outcomes for the three prognostic factors included in the Meet-URO score is reported in Table 3.
Table 3.
Response outcomes in the overall population and according to the parameters of the Meet-URO score
| Values | No. of patients n (%) | ORR (%) | OR (95% CI) | P value | DCR (%) | OR (95% CI) | P value | DOR (months) | HR (95% CI) | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall population | 250 | 38.8 | — | — | 68.8 | — | — | 14.0 | — | — | |
| IMDC score | Intermediate | 178 (71.2) | 43.3 | 1.00 (ref) | — | 76.9 | 1.00 (ref) | — | 14.0 | 1.00 (ref) | — |
| Poor | 72 (28.8) | 27.8 | 0.50 (0.28-0.91) | 0.024 | 48.6 | 0.28 (0.16-0.51) | <0.001 | NR | 0.53 (0.19-1.53) | 0.24 | |
| NLR | <3.2 | 126 (50.4) | 45.2 | 1.00 (ref) | 82.5 | 1.00 (ref) | NR | 1.00 (ref) | |||
| ≥3.2 | 124 (49.6) | 32.3 | 0.58 (0.34-0.96) | 0.036 | 54.8 | 0.26 (0.14-0.46) | <0.001 | 12.7 | 1.31 (0.64-2.69) | 0.47 | |
| Bone | No | 170 (68.0) | 45.3 | 1.00 (ref) | 75.9 | 1.00 (ref) | NR | 1.00 (ref) | |||
| Yes | 80 (32.0) | 25.0 | 0.40 (0.22-0.73) | 0.002 | 53.8 | 0.37 (0.21-0.65) | 0.001 | 10.0 | 2.27 (1.06-4.86) | 0.035 | |
| Meet-URO score | Group 2 | 78 (31.2) | 51.3 | 1.00 (ref) | — | 89.7 | 1.00 (ref) | — | NR | 1.00 (ref) | — |
| Group 3 | 75 (30.0) | 41.3 | 0.67 (0.35-1.27) | 0.22 | 74.7 | 0.34 (0.14-0.83) | 0.018 | NR | 1.08 (0.45-2.56) | 0.86 | |
| Group 4 | 78 (31.2) | 30.8 | 0.42 (0.22-0.81) | 0.010 | 51.3 | 0.12 (0.05-0.28) | <0.001 | 12.7 | 1.20 (0.49-2.94) | 0.69 | |
| Group 5 | 19 (7.6) | 10.5 | 0.11 (0.02-0.52) | 0.005 | 31.6 | 0.05 (0.02-0.18) | <0.001 | 9.2 | 1.20 (0.16-9.29) | 0.86 |
CI, confidence interval; HR, hazard ratio; IMDC, International Metastatic RCC Database Consortium; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio.
Both the ORR and DCR were significantly different by either the IMDC (P = 0.024 and P < 0.001, respectively), NLR (P = 0.036 and P < 0.001) or presence of bone metastases (P = 0.002 and P = 0.001), whereas the DOR was significantly different only by the presence of bone metastases (P = 0.035) (Table 3).
Survival outcomes by the Meet-URO score
The four risk groups defined by the Meet-URO score showed different survival results (Table 2, Figure 1B and D).
The mOS was NR in groups 2 and 3, of 12.0 and 3.2 months in groups 4 and 5, respectively. The 1y-OS was 92%, 72%, 50% and 21% for groups 2, 3, 4 and 5, respectively, and significantly shorter in each consecutive group (P = 0.001 for group 3, and P < 0.001 for both groups 4 and 5 as compared to reference group 2; P = 0.004 and P < 0.001 for groups 4 and 5 compared to group 3 and P < 0.001 for group 5 compared to group 4) (Figure 1B). Similarly, the mPFS was 16.6, 6.8, 4.8 and 1.4 months for groups 2, 3, 4 and 5, respectively (P < 0.001 for all comparisons of groups 3, 4 and 5 to group 2) (Figure 1D). The Meet-URO score showed the highest c-index for both the OS (0.73) and PFS (0.67) compared to each of the three included variables (Table 2). Furthermore, the Meet-URO score showed a lower BIC value both for OS (1151.6) and PFS (1950.8) compared to IMDC (1168.1 and 1974.1, respectively) and a higher generalized R2 (0.059 versus 0.036 for OS and 0.026 versus 0.0082 for PFS).
Results for OS were also confirmed after adjusting by multivariable analysis for histological subtype, nephrectomy, metastatic status at diagnosis and liver metastases (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100634).
Disease response by the Meet-URO score
The four Meet-URO groups had a significantly decreasing likelihood of ORR and DCR, which did not reach a statistical difference only for ORR of group 3 compared to group 2 (P = 0.22). The mDOR was not significantly different across the four Meet-URO groups (Table 3).
Meet-URO and IMDC classification
The classification of patients according to the Meet-URO and IMDC scores is shown in Table 4 and Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2022.100634. The prevalence of IMDC factors is summarized in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100634.
Table 4.
Classification of patients by Meet-URO and IMDC scoring systems
| IMDC risk group n (%) |
||||||
|---|---|---|---|---|---|---|
| Meet-URO prognostic group n (%) | Intermediate | Poor | mOS (months) | 1y-OS (%) | Total (n) | P value |
| 2 | 89 (100) | — | NR | 92 | 89 | <0.001a |
| 3 | 88 (100) | — | NR | 72 | 88 | |
| 4 | 29 (28.7) | 72 (71.3) | 12 | 50 | 101 | |
| 5 | — | 28 (100) | 3.2 | 50 | 28 | |
| mOS (months) | NR | 7.8 | NR | 20 | ||
| Total (n) | 206 | 100 | 306 | |||
1y-OS, overall survival rate at 1 year; IMDC, International Metastatic RCC Database Consortium; mOS, median overall survival; n, number of patients; NR, not reached.
P value refers to the significant difference in OS observed across the five Meet-URO risk classes.
All patients classified as Meet-URO groups 2 and 3 were at intermediate risk by the IMDC; group 4 included 28.7% of patients at IMDC intermediate risk and 71.3% poor risk; all group 5 patients were IMDC poor risk.
Conversely, 43.2% of patients at intermediate risk by the IMDC were classified as Meet-URO group 2, 42.7% group 3 and 14.1% group 4, while 72.0% and 28.0% of IMDC poor-risk patients as groups 4 and 5, respectively.
Although not statistically compared, the c-index of the Meet-URO was found to be higher than the IMDC score for both OS (0.73 versus 0.65) and PFS (0.67 versus 0.59) (Table 2). The Meet-URO score also showed a good calibration when expected, and observed mean OS probability at 1 year was compared (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2022.100634).
Discussion
Several immunotherapy-based combinations provided survival benefits compared to the TKI sunitinib as first-line treatment of patients with mRCC, with different related toxicity and efficacy profiles.1,7,17 While a survival benefit was reported in the IMDC intermediate- and poor-risk groups across all clinical studies with first-line immunotherapy-based combos, it was not substantial in patients at favourable risk.7,11,18 Therefore, more accurate prognostic scores or predictive biomarkers based on the host immune response and tumour biology are critical to improving patients’ selection and treatment decision making.
Programmed death-ligand 1 expression, whole-exome sequencing and gene-expression profiling have not yet been translated into therapeutic practice in untreated mRCC patients,19,20 while the IMDC score still needs improvements. Notably, the IMDC intermediate-risk group is highly heterogeneous, and there have been attempts to improve the prognostic stratification of these patients by considering the number of IMDC prognostic factors.21, 22, 23, 24 A post hoc analysis of the CheckMate 214 study confirmed the prognostic value of the IMDC and the survival benefit of nivolumab and ipilimumab in both the IMDC intermediate- and poor-risk groups regardless of the number of IMDC risk factors.12
Here, we tested the Meet-URO score on the Italian EAP population of patients with mRCC treated with first-line nivolumab and ipilimumab, confirming its prognostic value in this treatment setting, alongside a higher accuracy than the IMDC alone.13,25
The prognostic value of pre-treatment NLR in patients with mRCC treated with nivolumab and ipilimumab has been investigated by three retrospective analyses involving 35-110 patients,26, 27, 28 and was confirmed as an independent prognostic factor by a post hoc analysis of the CheckMate 214 trial at 3 years of follow-up.29 An absolute neutrophil count (ANC) higher than the upper limit of normal is one of six parameters considered by the IMDC classification.8,9 However, ANC is different from the ratio between neutrophils and lymphocytes (i.e. the NLR); for instance, a high NLR could also be found in normal ANCs. For this reason, we did not adjust the Meet-URO score model for the neutrophil count level recorded by the IMDC.13 Bone metastases are associated with worse survival and response outcomes in mRCC patients.30,31
The Meet-URO score allowed a better risk stratification within both the IMDC intermediate- and poor-risk groups. In particular, the IMDC intermediate-risk group was classified into three prognostically different subgroups by the Meet-URO score and the IMDC poor-risk group into two separate ones. The Harrell’s c-index confirmed the discriminatory superiority of the Meet-URO score, although a statistical comparison of predictive power was not carried out due to the high risk of inflating type I errors by using a test statistic to compare two Harrell’s C-indexes.32 The Meet-URO score also provided a more precise prediction of disease response than the IMDC score, NLR and presence of bone metastases separately.
Among the limitations of this study, we acknowledge the post hoc nature of this analysis and the lack of a comparative arm which do not allow any conclusion about the predictive value of the Meet-URO score. Furthermore, the predictive role for PFS and ORR needs validation in prospective trials with an independent radiological review of the imaging for the disease reassessments. A relative limitation is that analysis was restricted to patients classified as at intermediate or poor prognosis risk by the IMDC classification, which means that results cannot be directly translated to the IMDC favourable-risk patients.
Nevertheless, the Italian EAP was a prospective and multicentric study with an adequate sample size and follow-up (given the absence of the IMDC favourable-risk disease), which included, for instance, patients with non-clear-cell RCC (19%), unlike the CheckMate 214 trial.33
Conclusions
By post hoc analysis of a prospective study, the Meet-URO score seemed to show a better prognostic classification than the IMDC alone in patients with mRCC at IMDC intermediate-poor risk treated with first-line nivolumab and ipilimumab.
The Meet-URO score can be considered an additional prognostic classification system for patients with IMDC intermediate- or poor-risk mRCC eligible for first-line treatment with ipilimumab and nivolumab. This analysis paved the way for future investigations and external validation on different immunotherapy-based combinations and comparative studies to confirm the prognostic role of the Meet-URO score for other current first-line treatments and explore the potential predictive value.
Acknowledgements
PR’s work is funded by Prostate Cancer Foundation through a PCF YI award, and by the PTC RC SEE PROS ONCOLOGIA-FPRC 5 PER MILLE-MS 2017. GLB’s work is supported by FPRC 5xmille Ministero Salute 2017 PTCRC-Intra 2020 ‘CTU-Lung’. SER and GF would like to thank the Italian Ministry of Health (Ricerca Corrente 2018-2021 grants) that financially support their current research focused on identifying prognostic and predictive markers for patients with genitourinary tumours. All authors would like to thank the Italian Network for Research in Urologic-Oncology (Meet-URO).
Funding
None declared.
Disclosure
SER received honoraria as a speaker at scientific events and travel accommodation from Amgen, GSK, BMS, MSD. SB received honoraria as a speaker at scientific events and advisory role by BMS, Pfizer, MSD, Ipsen, Roche, Eli Lilly, AstraZeneca, Pierre-Fabre, Novartis. GLB reported personal fees from Astellas and AstraZeneca. MM reports personal fees from BMS, Pfizer, Merck Serono, Astellas, Janssen, MSD. UDG served as an advisory/board member of Astellas, Bayer, BMS, IPSEN, Janssen, Merck, Pfizer, Sanofi, received research grant/funding to the institution from AstraZeneca, Roche, Sanofi and travel/accommodations/expenses from BMS, IPSEN, Janssen, Pfizer. GP served as a consultant and/or a speaker for Bayer, BMS, Novartis, Amgen, Pfizer, Janssen, Ipsen, Boehringer. FC has received consulting fees from Merck Sharp & Dohme Oncology and Pfizer. GC works as responsible for research and development at Kerubin Digital Therapeutic. CP served as a consultant and/or a speaker for Ipsen, BMS, MSD, Astra Zeneca, Pfizer, Eisai, EUSA and General Electrics, as an expert testimony for both Pfizer and EUSA and is a protocol steering committee member for BMS, EUSA and Eisai. PR served as an advisory board for MSD and Astra Zeneca Italy. GF served as an advisory board member for Astellas, Janssen, Pfizer, Bayer, MSD, Merck and received travel accommodation from Astellas, Janssen, Bayer. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Quhal F., Mori K., Bruchbacher A., et al. First-line immunotherapy-based combinations for metastatic renal cell carcinoma: a systematic review and network meta-analysis. Eur Urol Oncol. 2021;4(5):755–765. doi: 10.1016/j.euo.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Motzer R.J., Escudier B., McDermott D.F., et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer R.J., Tannir N.M., McDermott D.F., et al. Nivolumab plus Ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedke J., Albiges L., Capitanio U., et al. The 2021 updated European Association of Urology guidelines on renal cell carcinoma: immune checkpoint inhibitor–based combination therapies for treatment-naive metastatic clear-cell renal cell carcinoma are standard of care. Eur Urol. 2021;80(4):393–397. doi: 10.1016/j.eururo.2021.04.042. [DOI] [PubMed] [Google Scholar]
- 5.Rebuzzi S.E., Perrone F., Bersanelli M., et al. Prognostic and predictive molecular biomarkers in metastatic renal cell carcinoma patients treated with immune checkpoint inhibitors: a systematic review. Expert Rev Mol Diagn. 2019;20(2):169–185. doi: 10.1080/14737159.2019.1680286. [DOI] [PubMed] [Google Scholar]
- 6.Pourmir I., Noel J., Simonaggio A., et al. Update on the most promising biomarkers of response to immune checkpoint inhibitors in clear cell renal cell carcinoma. World J Urol. 2021;39(5):1377–1385. doi: 10.1007/s00345-020-03528-x. [DOI] [PubMed] [Google Scholar]
- 7.Cattrini C., Messina C., Airoldi C., et al. Is there a preferred first-line therapy for metastatic renal cell carcinoma? A network meta-analysis. Ther Adv Urol. 2021;13 doi: 10.1177/17562872211053189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heng D.Y., Xie W., Regan M.M., et al. Prognostic factors for overall survival (OS) in patients with metastatic renal cell carcinoma (RCC) treated with vascular endothelial growth factor (VEGF)-targeted agents: results from a large multicenter study. J Clin Oncol. 2009;27(15_suppl) doi: 10.1200/JCO.2008.21.4809. 5041. [DOI] [PubMed] [Google Scholar]
- 9.Heng D.Y.C., Xie W., Regan M.M., et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzo A., Mollica V., Santoni M., et al. Comparative effectiveness of first-line immune checkpoint inhibitors plus tyrosine kinase inhibitors according to IMDC risk groups in metastatic renal cell carcinoma: a meta-analysis. Immunotherapy. 2021;13(9):783–793. doi: 10.2217/imt-2021-0005. [DOI] [PubMed] [Google Scholar]
- 11.Ciccarese C., Iacovelli R., Porta C., et al. Efficacy of VEGFR-TKIs plus immune checkpoint inhibitors in metastatic renal cell carcinoma patients with favorable IMDC prognosis. Cancer Treat Rev. 2021;100 doi: 10.1016/j.ctrv.2021.102295. [DOI] [PubMed] [Google Scholar]
- 12.Escudier B., Motzer R.J., Tannir N.M., et al. Efficacy of nivolumab plus ipilimumab according to number of IMDC risk factors in CheckMate 214. Eur Urol. 2020;77(4):449–453. doi: 10.1016/j.eururo.2019.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebuzzi S.E., Signori A., Banna G.L., et al. Inflammatory indices and clinical factors in metastatic renal cell carcinoma patients treated with nivolumab: the development of a novel prognostic score (Meet-URO 15 study) Ther Adv Med Oncol. 2021;13 doi: 10.1177/17588359211019642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebuzzi S.E., Cerbone L., Signori A., et al. Application of the Meet-URO score to metastatic renal cell carcinoma patients treated with second- and third-line cabozantinib. Ther Adv Med Oncol. 2022;14 doi: 10.1177/17588359221079580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basso U., Paolieri F., Rizzo M., et al. Compassionate use program of ipilimumab and nivolumab in intermediate or poor risk metastatic renal cell carcinoma: a large multicenter Italian study. Cancers. 2022;14(9):2293. doi: 10.3390/cancers14092293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer R.J., Tannir N.M., McDermott D.F., et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori K., Pradere B., Quhal F., et al. Differences in oncological and toxicity outcomes between programmed cell death-1 and programmed cell death ligand-1 inhibitors in metastatic renal cell carcinoma: a systematic review and meta-analysis. Cancer Treat Rev. 2021;99 doi: 10.1016/j.ctrv.2021.102242. [DOI] [PubMed] [Google Scholar]
- 18.Buti S., Bersanelli M., Mazzaschi G., et al. Can we identify a preferred first-line strategy for sarcomatoid renal cell carcinoma? A network meta-analysis. Immunotherapy. 2022;14(2):145–153. doi: 10.2217/imt-2021-0157. [DOI] [PubMed] [Google Scholar]
- 19.Motzer R.J., Choueiri T.K., McDermott D.F., et al. Biomarker analysis from CheckMate 214: nivolumab plus ipilimumab versus sunitinib in renal cell carcinoma. J Immunother Cancer. 2022;10(3) doi: 10.1136/jitc-2021-004316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vano Y.A., Elaidi R., Bennamoun M., et al. Nivolumab, nivolumab-ipilimumab, and VEGFR-tyrosine kinase inhibitors as first-line treatment for metastatic clear-cell renal cell carcinoma (BIONIKK): a biomarker-driven, open-label, non-comparative, randomised, phase 2 trial. Lancet Oncol. 2022;23(5):612–624. doi: 10.1016/S1470-2045(22)00128-0. [DOI] [PubMed] [Google Scholar]
- 21.Iacovelli R., De Giorgi U., Galli L., et al. Is it possible to improve prognostic classification in patients affected by metastatic renal cell carcinoma with an intermediate or poor prognosis? Clin Genitourin Cancer. 2018;16(5):355–359.e1. doi: 10.1016/j.clgc.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Di Nunno V., Mollica V., Schiavina R., et al. Improving IMDC prognostic prediction through evaluation of initial site of metastasis in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer. 2020;18(2):e83–e90. doi: 10.1016/j.clgc.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Takahara K., Ando R., Kanao K., et al. Prognostic stratification of the IMDC intermediate risk group after treatment with first-line molecular-targeted therapy for metastatic renal cell carcinoma. Anticancer Res. 2020;40(8):4395–4400. doi: 10.21873/anticanres.14443. [DOI] [PubMed] [Google Scholar]
- 24.Massari F., Di Nunno V., Guida A., et al. Addition of primary metastatic site on bone, brain, and liver to IMDC criteria in patients with metastatic renal cell carcinoma: a validation study. Clin Genitourin Cancer. 2021;19(1):32–40. doi: 10.1016/j.clgc.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Rebuzzi S.E., Santoni M., Signori A., et al. The prognostic stratification of the Meet-URO score compared with the IMDC score in pretreated metastatic renal cell carcinoma (mRCC) patients (pts) receiving cabozantinib. Tumori J. 2021;107(2S) abstract C10. [Google Scholar]
- 26.Iinuma K., Enomoto T., Kawada K., et al. Utility of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune inflammation index as prognostic, predictive biomarkers in patients with metastatic renal cell carcinoma treated with nivolumab and ipilimumab. J Clin Med. 2021;10(22):5325. doi: 10.3390/jcm10225325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iinuma K., Kameyama K., Kawada K., et al. Efficacy and safety of nivolumab and ipilimumab for advanced or metastatic renal cell carcinoma: a multicenter retrospective cohort study. Curr Oncol. 2021;28(2):1402–1411. doi: 10.3390/curroncol28020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tucker M.D., Brown L.C., Chen Y.-W., et al. Association of baseline neutrophil-to-eosinophil ratio with response to nivolumab plus ipilimumab in patients with metastatic renal cell carcinoma. Biomark Res. 2021;9(1) doi: 10.1186/s40364-021-00334-4. 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motzer R.J., Choueiri T.K., McDermott D.F., et al. Biomarker analyses from the phase III CheckMate 214 trial of nivolumab plus ipilimumab (N+I) or sunitinib (S) in advanced renal cell carcinoma (aRCC) J Clin Oncol. 2020;38(15_suppl) 5009. [Google Scholar]
- 30.Gan C.L., Wells J.C., Schmidt A.L., et al. Outcomes of first-line (1L) ipilimumab and nivolumab (IPI-NIVO) and subsequent therapy in metastatic renal cell carcinoma (mRCC): results from the International mRCC Database Consortium (IMDC) J Clin Oncol. 2021;39(15_suppl) 4554. [Google Scholar]
- 31.Desai K., Brown L., Wei W., et al. A multi-institutional, retrospective analysis of patients with metastatic renal cell carcinoma to bone treated with combination ipilimumab and nivolumab. Targeted Oncol. 2021;16(5):633–642. doi: 10.1007/s11523-021-00832-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han X., Zhang Y., Shao Y. On comparing 2 correlated C indices with censored survival data. Stat Med. 2017;36(25):4041–4049. doi: 10.1002/sim.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basso U., Paolieri F., Porta C.G., et al. 721P Ipilimumab and nivolumab compassionate use program in metastatic renal cell carcinoma patients with intermediate or poor IMDC risk score: the large multicenter Italian study. Ann Oncol. 2020;31:S566. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.