Abstract
Background
Anti-programmed cell death protein 1 (PD1)/programmed death-ligand 1 (PD-L1) agents have only moderate antitumor activity in some advanced solid tumors (AST), including breast cancer (BC), prostate cancer (PC), cervical cancer (CC), and head and neck cancer (HNC). Combining anti-PD-L1 with anti-cytotoxic T-lymphocyte-associated protein (CTLA) and chemotherapy may significantly improve efficacy.
Patients and methods
MOVIE is a multicohort phase I/II study examining the combination of anti-PD-L1 durvalumab (Durv; 1500 mg IV Q4W) plus anti-CTLA tremelimumab (Trem; 75 mg IV Q4W) with metronomic vinorelbine (MVino; 20-40 mg orally daily) in various AST resistant to conventional therapies. The primary objective of the phase I part was to determine the maximum tolerated dose (MTD) and recommended dose for phase II (RP2D).
Results
Among the 14 patients enrolled during phase I, including 13 women and 1 man, 9 had BC, 1 PC, 2 CC, and 2 miscellaneous cancers with high mutational loads. Median age was 53 years. A total of 12 patients were assessable for the dose-escalation part in which only one dose-limiting toxicity (DLT) was observed [one neutropenia without fever, grade (G) 4]. Two (14.3%), four (28.6%), and four (28.6%) patients had G ≥3 adverse events (AEs) related to MVino, Durv, and Trem, respectively. Treatment-related events included mostly clinical AEs with asthenia (eight G2; three G3), colitis (one G2, one G3), diarrhea (one G3), nausea (two G2), dry skin (two G2), maculopapular rash (one G3), and hyperthyroidism (three G2). No toxic death was reported. Preliminary data showed one patient (CC) who presented a complete response and four patients with stable disease (SD).
Conclusions
MTD was not reached and dose level 2 (MVino 40 mg, Durv 1500 mg, Trem 75 mg) was selected as RP2D. The safety profile of the combination was manageable and consistent with previous reports of Trem + Durv or MVino. Phase II is currently ongoing in BC, PC, CC, HNC, and miscellaneous cohorts.
Key words: advanced solid tumors (AST), metronomic vinorelbine (MVino), immunotherapy, durvalumab (Durv), tremelimumab (Trem)
Highlights
-
•
The toxicity of MVino combined with Trem + Durv combination was manageable during MOVIE phase I.
-
•
The safety profile was consistent with previous reports of Trem + Durv or MVino.
-
•
Preliminary results showed one CC and four SD responses.
Introduction
Monoclonal antibody-based immune checkpoint blockade therapies have become a powerful clinical strategy for treating many types of cancer.1 The most well-studied checkpoint inhibitors aim programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). The binding of PD-L1 to PD-1 or to the cluster of differentiation 80 (CD80) on activated T cells delivers an inhibitory signal to the T cells, preventing them from killing target tumor cells, and protecting the tumor from immune elimination.2, 3, 4 Therefore, an anti-PD-L1 antibody enhances antitumor immune responses. As to CTLA-4, it delivers a negative regulatory signal to T cells upon binding of CD80 or CD86 ligands on antigen-presenting cells.5 Thus, blockade of CTLA-4 binding to CD80/86 results in markedly enhanced T-cell activation and leads to an increased activation of the immune system in patients with cancer. Therapeutic targeting of PD-1/PD-L1 and CTLA-4 was primarily validated in patients with advanced-stage melanoma,6 non-small-cell lung cancer (NSCLC),7 and renal cell carcinomas,8,9 and is now either part of the standard of care or under investigation in a wide range of cancers.10, 11, 12, 13
If the use of immune checkpoint regulators is a promising approach, the majority of advanced solid tumors (AST) will not respond to it or ultimately relapse after treatment. There is, therefore, an unmet medical need for patients suffering from AST to increase their sensitivity to immunotherapy and improve outcome using innovative methods. Interestingly, the efficacy of immunotherapy may be strongly enhanced when different strategies are combined and distinct targets, modulated.14,15 Indeed, if CTLA-4 and PD-1 pathways have similar negative effects on T-cell activity, they are not redundant, suggesting that dual targeting of these pathways may have additive or synergistic activity.16 When the MOVIE study was designed, the combination of anti-PD-L1 durvalumab (Durv) and anti-CTLA-4 tremelimumab (Trem) was thought to improve efficacy of therapy in difficult-to-treat AST populations, at the expense of an additional but manageable toxicity.
At the time, combination therapy strategies with chemotherapy were also being explored to possibly ameliorate the efficacy of immunotherapies.17 Due to its excellent tolerance and favorable toxicity profile, metronomic chemotherapy (MC), corresponding to the minimum effective doses of chemotherapy given on a continuous schedule, was believed to be a more attractive partner to anti-CTLA-4 plus anti-PD-1/PD-L1 combination immunotherapy than conventionally dosed chemotherapy. Moreover, MC was also estimated to be capable of enhancing the efficacy of immunotherapies as previous data outlined its immunostimulatory potential.18 Several chemotherapeutic agents have been investigated using metronomic schedules in solid tumors,18 among which vinorelbine (Vino), a vinca alkaloid inhibits microtubule polymerization during mitosis and thus prevents cell proliferation.19,20 Vino is approved in Europe for treating NSCLC and metastatic breast cancer (BC), but is commonly used out of its marketed authorization in several solid tumors such as prostate cancer (PC), as well as other AST, with demonstrated safety and activity in relapsed and/or refractory disease.18,21 Incidentally, vinorelbine is also reported to have pro-immune properties at low dose.20
With therapeutic options being limited for patients with AST resistant to conventional treatments, investigators of the MOVIE study hypothesized that MVino, the toxicity of which is relatively low, would potentiate the efficacy of Durv + Trem combination immunotherapy in patients with AST including BC, PC, cervical cancer (CC), and head and neck cancer (HNC), in which immune checkpoint inhibitors have only moderate or no single-agent antitumor activity. In addition, this combination could also boost the already documented efficacy of single-agent immunotherapy in miscellaneous malignancies with high mutational load and/or microsatellite instability (MSI)-high.
Patients and methods
Study design
The MOVIE trial was a national, multicenter, multicohort, open-label, non-randomized and non-comparative phase I/II study that aimed to assess the antitumor activity and safety of metronomic vinorelbine (MVino) plus anti-PDL-1 (Durv)/anti-CTLA-4 (Trem) immunotherapy in patients with AST resistant to conventional therapies. The study protocol was approved by the French Ethical Committee CPP Sud-Méditerranée I and by the French regulatory authorities. Patients had to confirm their consent in writing before starting the study and before any study-related procedures. The study was sponsored by Unicancer and conducted in the rigorous standards set out in the Good Clinical Practice guidelines and in accordance with the principles in the Declaration of Helsinki. It is registered at ClinicalTrials.gov (identifier: NCT03518606). MOVIE phase I was conducted from 18 July 2018 to 15 May 2019.
The MOVIE study uses a 3 + 3 dose-escalation design for the phase I part to determine the maximum tolerated dose (MTD) and the recommended dose for phase II (RP2D) of MVino associated with Durv + Trem combination immunotherapy (Figure 1). Determination of the MTD was based on the occurrence of dose-limiting toxicity (DLT). A DLT was defined as any grade (G) ≥3 toxicity, related to MVino and/or Durv and/or Trem, occurring during treatment cycle 1 (first 28 days of treatment). Adverse events (AEs) clearly and directly related to the primary disease or to another etiology were excluded from this definition. MTD was the lowest dose level at which ≥33% of patients experienced DLT. RP2D was the dose level just below the MTD or, in case MTD was not reached, the Data Safety Managing Board set up to evaluate phase I was to review the data collected and decide whether the highest dose level used in the study was RP2D. A fixed dosing approach of immunotherapy was preferred by the prescribing community due to ease of use and reduced dosing errors.
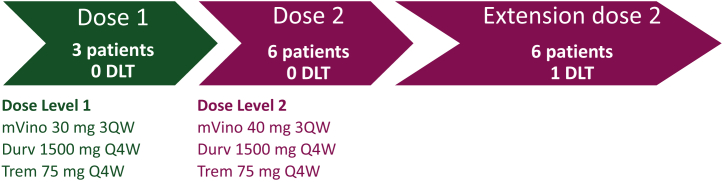
Figure 1.
Summary of the dose-escalation part. Representation of the dose-escalation part of the MOVIE study. Starting dose of MVino was 30 mg 3QW (dose level 1). The next dose level was 40 mg 3QW (dose level 2). Three patients were initially included at each dose level. In the absence of DLTs, dose level 2 was opened for inclusion and then extended to include a total of nine assessable patients.
DLT, dose-limiting toxicity.
Treatment procedures and dose escalation
During the MOVIE study, patients received 1500 mg Durv plus 75 mg Trem at day 1 of a 4-week cycle (28 days) via intravenous infusion (Q4W). Trem was administered for the first four cycles. MVino was administered orally three times a week (on days 1, 3, and 5) of every week (3QW). As the use of MVino in combination at its standard dose of 50 mg 3QW was associated with significant toxicity concerns following the CHEOPS trial results, the starting dose of MVino in the MOVIE study was set at 30 mg 3QW (dose level 1).22,23 The next dose level was 40 mg 3QW (dose level 2). Decrease to dose level 1 (MVino 20 mg 3QW) was planned if needed. MVino was administered just before starting immunotherapy infusion. Trem was given in a 1-h infusion, and then 1 h after the end of Trem infusion, Durv was administered in a 1-h infusion. Patients were treated until progression of their disease (PD), unacceptable toxicity, intercurrent conditions that preclude continuation of treatment, or patient refusal. Three patients were initially included at each dose level. In the absence of DLTs, the next level was open for inclusion. In case of one DLT, three additional patients were included for a maximum of six patients in any dose level. If no other DLT was described, the next level was open for inclusion.
Study objectives
The primary objective of the phase I part of the study was to determine MTD and RP2D. Secondary objectives included safety profile; clinical benefit rate (CBR), defined as the rate of patients who had a complete response (CR), partial response (PR), or stable disease (SD) for at least 24 weeks as best response according to Response Evaluation Criteria In Solid Tumors (RECIST) v1.1; objective response rate (ORR), defined as the percentage of patients in each cohort with a CR or PR as best response measured at the disease assessment after initiation of treatment; and duration of overall response (DoR), defined as the time periods from documented tumor response (CR/PR) to PD.
Participants
The study population consisted of men and women ≥ 18 years old with histologically confirmed locally AST, resistant to conventional therapies and candidate to experimental therapy, from the following primary tumors: BC, PC, CC, HNC, and miscellaneous malignancies with high mutational load (defined by a local molecular tumor board after next-generation sequencing or whole-genome sequencing analyses), and/or MSI-high or mismatch repair deficient (as determined by locally carried out PCR and immunohistochemistry test). Eligible patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status ≤ 1 and a measurable disease according to RECIST v1.1. Inclusion/exclusion criteria are detailed in the Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100646.
Safety assessments
The incidence of AEs and DLTs was reported and graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI CTCAE) v.5.0. Assessments of all AEs, drug-related and non-drug-related, were monitored during treatment for 30 days after discontinuation of treatment or until an ongoing AE was resolved or deemed to continue indefinitely.
Vinorelbine pharmacokinetics
MVino plasma concentrations were monitored in a longitudinal manner. Blood samples for pharmacokinetic (PK) evaluation were collected for their trough (T0) and peak (Cmax; T2H) serum concentration levels on day 15 of cycle 1, and day 1 of cycles 2 and 3. The samples were next centrifuged and the plasma stored at −80°C until analysis. Plasma concentration of vinorelbine were quantified after simple precipitation using a fully validated liquid chromatography-tandem mass spectroscopy method (Xevo TQD, Waters, France) with a limit of quantitation of 0.1 ng/ml and a 15% precision. Because no pop-PK models was available with plasma MVino, it was not possible to derive individual parameters from the PK samples.
Statistical analysis
The global intent-to-treat (ITT) population included all enrolled patients. Patients and disease baseline characteristics were described on the global ITT population. The date of inclusion served as the reference for calculation of durations unless otherwise indicated. The full analysis set population consisted of all patients of the global population having received at least one cycle of study treatment or discontinued the treatment before the end of the first cycle for progression or toxicity (treatment failures) and with no major protocol violation that could have biased primary endpoint evaluation (e.g. wrong pathology). The safety population consisted of all patients who received at least one dose of study drug and was used for safety analyses. The DLT population included all patients of the safety population who had been observed for a full phase I duration period (4 weeks from the study treatment initiation), or having experienced a DLT. Patients who did not receive at least 80% of prescribed doses [unless treatment(s) was stopped for a DLT], did not undergo a DLT assessment at the end of phase I duration period, or definitely discontinued during this period for reasons other than DLT were not assessable for DLT and were therefore replaced. CBR and ORR were presented with the associated 95% confidence intervals (CIs). DoR was estimated using the Kaplan–Meier method and described in terms of median with the associated 95% CI.
Results
Patients’ characteristics
Between July 2018 and May 2019, 14 patients were screened, included, and started on treatment in the dose-escalation part of the MOVIE study. Repartition per cohort and main patient characteristics are described in Table 1: nine patients (64.3%) had BC, one patient (7.1%) had PC, two patients (14.3%) had CC, and two patients (14.3%) had miscellaneous cancers with high mutational loads. Median age at study entry was 53 years (range 32-79 years). Out of 14, 13 patients were women. ECOG-PS was 0 for seven patients and 1 for six patients (one missing data). At inclusion, 13 patients had advanced metastatic disease and 1 patient with BC had a locally advanced disease. All had received prior anticancer therapy and BC patients were treated with a maximum of two lines of chemotherapy in metastatic settings.
Table 1.
Patients’ demographic and clinical characteristics
| Total patients included in the phase I part of the study | N = 14 (100%) |
|---|---|
| Disease type Dose level 1 |
|
| Breast cancer | 2 (14.3%) |
| Triple-negative breast cancer | 2 (100.0%) |
| Hormone receptor | 0 (0.0%) |
| Cervical cancer | 1 (7.1%) |
| Miscellaneous cancers with high mutational loads | 1 (7.1%) |
| Dose level 2 | |
| Breast cancer | 7 (50.0%) |
| Triple-negative breast cancer | 5 (71.4%) |
| Hormone receptor | 2 (28.6%) |
| Cervical cancer | 1 (7.1%) |
| Prostate cancer | 1 (7.1%) |
| Miscellaneous cancers with high mutational loads | 1 (7.1%) |
| Age at study enrollment (years) | |
| Median (min; max) | 53 (32; 79) |
| Sex | |
| Male | 1 (7.1%) |
| Female | 13 (92.9%) |
| ECOG-PS at baseline | |
| Missing data | 1 (7.1%) |
| 0 | 7 (53.8%) |
| 1 | 6 (46.2%) |
| Previous cancer therapies | |
| Autograft | 1 (7.1%) |
| Chemotherapy | 14 (100.0%) |
| Hormonotherapy | 3 (21.4%) |
| NSAIDs | 1 (7.1%) |
| Radiotherapy | 10 (71.4%) |
| Surgery | 10 (71.4%) |
| Targeted therapy | 7 (50.0%) |
| Disease status at inclusion | |
| Metastatic | 13 (92.9%) |
| Locally advanced | 1 (7.1%) |
| Number of metastatic sites | |
| ≤ 3 | 10 (76.9%) |
| > 3 | 3 (23.1%) |
| Type of metastasis | |
| Liver | 3 (23.1%) |
| Bone | 6 (46.2%) |
| Pleural | 1 (7.7%) |
| Lung | 2 (15.4%) |
| Breast | 2 (15.4%) |
| Lymphatic system | 9 (69.2%) |
| Skin | 5 (38.5%) |
| Uterus | 1 (7.7%) |
| Other | 1 (7.7%) |
| Breast cancer | 1 (100.0%) |
ECOG-PS, Eastern Cooperative Oncology Group-performance status; NSAIDs, nonsteroidal anti-inflammatory drugs.
Dose escalation and RP2D
Out of the 14 patients enrolled, 12 were assessable for the dose-escalation part of the study: three patients at dose level 1 and nine at dose level 2 (Figure 1). Two patients were not assessable because they did not receive at least 80% of the intended dose of MVino on the first 28 days of treatment (compliance to treatment during DLT evaluation period is detailed in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100646). Among the three assessable patients included at dose level 1 (MVino 30 mg 3QW, Durv 1500 mg Q4W, Trem 75 mg Q4W), none experienced a DLT. Dose escalation thus proceeded to dose level 2 (MVino 40 mg 3QW, Durv 1500 mg Q4W, Trem 75 mg Q4W) at which none of the initial three assessable patients experienced DLT. The cohort was therefore expanded and six additional patients were enrolled at dose level 2 in order to confirm the RP2D on nine assessable patients. Among this extended cohort, only one patient experienced a DLT: a 70-year-old female with a history of metastatic BC developed a G4 neutropenia without fever related to vinorelbine. MTD was not reached and dose level 2 was selected as the recommended regimen used during phase II (RP2D) of the MOVIE study.
Safety and tolerability
The 14 included patients were all assessable for the safety analyses. As shown in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100646, they all (100.0%) experienced at least one AE and nine patients (64.3%) presented at least one serious AE (SAE). One patient (7.1%) presented at least one SAE related to vinorelbine, three (21.4%) to Durv, and three (21.4%) to Trem. Two (14.3%), four (28.6%), and four (28.6%) patients treated at dose level 2 had G≥3 AEs related to MVino, Durv, and Trem, respectively. Treatment-related events included clinical AEs (Table 2): asthenia (eight G2; three G3), colitis (one G2, one G3), diarrhea (one G3), nausea (two G2), dry skin (two G2), maculopapular rash (one G3), and hyperthyroidism (three G2) (Table 2). Biological AEs were also observed with neutropenia (one G4), anemia (three G2), gamma glutamyl transferase (GGT) increase (three G3), and alkaline phosphatase (ALP) increase (three G2). A total of 22 AEs led to treatment modifications during MOVIE phase I, of which 16 were treatment-related AEs. No toxic death was reported. The safety profile of the combination was consistent with previous reports of study immunotherapy combination or vinorelbine administered on a metronomic schedule.
Table 2.
Treatment-related AEs during MOVIE phase I
| Preferred term | Dose level 1 (N = 4) |
Dose level 2 (N = 10) |
Overall (N = 14) |
||||
|---|---|---|---|---|---|---|---|
| Grade 2 | Grade 2 | Grade 3 | Grade 4 | Grade 2 | Grade 3 | Grade 4 | |
| Anemia | 3 (30.0%) | 3 (21.4%) | |||||
| Asthenia | 8 (80.0%) | 3 (30.0%) | 8 (57.1%) | 3 (21.4%) | |||
| ALP increase | 3 (30.0%) | 3 (21.4%) | |||||
| Colitis | 1 (10.0%) | 1 (10.0%) | 1 (7.1%) | 1 (7.1%) | |||
| Constipation | 1 (25.0%) | 1 (7.1%) | |||||
| Decreased appetite | 1 (10.0%) | 1 (7.1%) | |||||
| Dermatitis exfoliative | 1 (10.0%) | 1 (7.1%) | |||||
| Diarrhea | 1 (10.0%) | 1 (7.1%) | |||||
| Dry skin | 2 (20.0%) | 2 (14.3%) | |||||
| GGT increase | 3 (30.0%) | 3 (21.4%) | |||||
| Hyperthyroidism | 3 (30.0%) | 3 (21.4%) | |||||
| Hypothyroidism | 1 (25.0%) | 1 (1.7%) | |||||
| Lymphocyte count increase | 1 (10.0%) | 1 (7.1%) | |||||
| Musculoskeletal pain | 1 (10.0%) | 1 (7.1%) | |||||
| Nausea | 2 (20.0%) | 2 (14.3%) | |||||
| Neutropenia | 1 (10.0%) | 1 (7.1%) | |||||
| Pruritus | 2 (20.0%) | 2 (14%) | |||||
| Rash maculopapular | 1 (10.0%) | 1 (7.1%) | |||||
AE, adverse event; ALP, alkaline phosphatase; GGT, γ-glutamyltransferase.
Efficacy
At MOVIE phase I data cut-off (DCO) date (15 May 2019), the four patients included at dose level 1 had disease progression at their first evaluation. Among the patients included at dose level 2, five patients with BC had PD, four patients (n = 2 BC, n = 1 PC, and n = 1 miscellaneous) had SD, and one patient with CC had a CR. This led to an overall ORR of 7.0% as one patient achieved a CR as the best response at disease assessment after initiation. Duration of response at DCO for this patient was 78 days. At the time of the analysis, among the four patients who had a best response of SD, three had <16 weeks of follow-up and were thus not assessable for the CBR, and one had progressed within 24 weeks after inclusion (Table 3). Figure 2 shows each patient’s response to treatment over time and Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100646, indicates tumor response from baseline in each patient.
Table 3.
Treatment antitumor activity
| Best response overall | Total patients included |
||
|---|---|---|---|
| Dose level 1 | Dose level 2 | Overall | |
| Complete response | 1 | 1 | |
| Stable disease | 4 | 4 | |
| Progressive disease | 4 | 5 | 9 |
| Objective response rate (%)a | 0.0 | 10.0 | 7.0 |
Objective response rate (%): percentage of patients with complete or partial response as the best response at disease assessment after initiation of treatment according to RECIST v1.1.
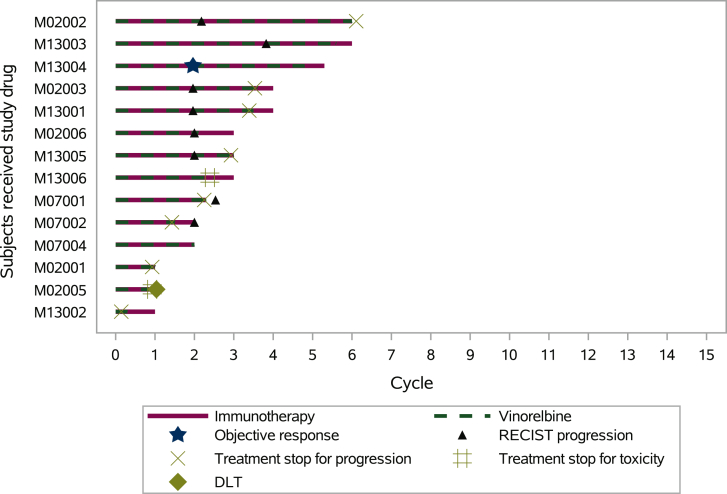
Figure 2.
Swimmer plot. Each bar represents one subject from their inclusion until phase I data cut-off (15 May 2019). In total, 14 patients were included in the phase I MOVIE study. Patients M02002, M13001, M02001, and M13002 received dose level 1 (MVino 30 mg 3QW, Durv 1500 mg Q4W, Trem 75 mg Q4W). The other 10 patients received dose level 2 (MVino 40 mg 3QW, Durv 1500 mg Q4W, Trem 75 mg Q4W). Patients M07001 and M13002 were not assessable for DLT due to temporary treatment withdrawal for AE, and definite treatment stop for progression (cycle 1). One DLT was recorded (M02005).
AE, adverse event; DLT, dose-limiting toxicity.
Pharmacokinetics
PK investigation was carried out in the patients enrolled during the MOVIE study. Eight patients were fully monitored over cycles 1-3, and the trough (Cmin) and maximum concentrations (Cmax) of vinorelbine were assessed in 60 samples. PK results are reported in Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100646. A huge inter-patient variability was observed on both Cmin and Cmax values, with a coefficient of variation ranging from 45% to 127%. At dose level 1 (MVino 30 mg), concentrations ranged from <0.1 (below the limit of quantification) to 1.12 ng/ml and from 1.8 to 10.8 ng/ml for Cmin and Cmax, respectively. At dose level 2 (MVino 40 mg; RP2D), Cmin ranged from 0.1 to 0.86 ng/ml, and Cmax from 2.7 to 54.7 ng/ml.
Discussion
The MOVIE study established the feasibility of combining oral MVino at 40 mg thrice a week with monthly i.v. Durv at 1500 mg and Trem at 75 mg, each immunotherapy dose being the single agent recommended dose. Tolerance profile included AEs expected to occur with PD-L1 and CTLA-4 inhibitors, such as colitis, diarrhea, maculopapular rash, and hyperthyroidism. All these AEs had been previously associated with combination immunotherapies.24 Hematological toxicities were more likely vinorelbine-related and only one G4 neutropenia without fever was reported. Overall, the combination was well tolerated, with a lower rate of colitis and diarrhea than in other trials.25 Half of the patients included at dose level 2 achieved disease control (one CR and four SDs). In view of the preliminary efficacy results and reassuring toxicity profile, dose level 2 was adopted for the phase II part of the study.
When this study was designed, a phase Ib study (102 patients) enrolling advanced squamous or non-squamous NSCLCs had demonstrated a manageable tolerability profile with antitumor activity of Durv + Trem immunotherapies in both PD-L1-positive and PD-L1-negative patients.26 More recently, the MYSTIC trial evidenced that first-line treatment with Durv was associated with greater overall survival (OS) compared to patients treated with chemotherapy alone, whereas Durv + Trem did not significantly improve OS or progression-free survival compared with chemotherapy in NSCLCs.27 Durv and a Durv + Trem combination also failed to show an OS improvement over standard of care in treatment-naïve bladder cancer patients with high PD-L1 expression (DANUBE trial).28
On another hand, a phase I/II study evaluated the efficacy and safety of Durv monotherapy and different Durv + Trem combinations and demonstrated that all tested regimen were tolerable and clinically active for patients with unresectable hepatocellular carcinoma (HCC). In this study, the combination regimen, featuring Single Tremelimumab Regular Interval Durvalumab (STRIDE) and comprising a single priming dose of Trem 300 mg added to Durv 1500 mg followed by Durv 1500 mg Q4W, displayed the best benefit-risk profile.29 Following these findings, the phase III HIMALAYA trial’s (NCT03298451) early results further showed a statistically significant and clinically meaningful OS benefit versus sorafenib in patients with no prior systemic therapy for HCC when treated with the STRIDE regimen.30 Given the expectation of similar PK exposure and variability, it was deemed feasible to use fixed dosing regimens of immunotherapy in the MOVIE study, and this approach was preferred by the prescribing community, due to ease of use and reduced dosing errors. However, despite these recent promising results combining Durv + Trem, most benefits in terms of survival regarding the use of dual immune check-point inhibitors were obtained with ipilimumab plus nivolumab.9,16,31, 32, 33, 34, 35 Therefore, we cannot exclude that another combination of immunotherapies could improve the outcome and further research is required to evaluate new doses and regimen to develop the optimal therapeutic combination.
To guide therapeutic adjustments, PK parameters were assessed. Unlike previous works, vinorelbine concentration was described in the plasma of MOVIE patients, eight of whom were monitored during three cycles, rather than in whole-blood. To be able to compare our data, we divided reported whole-blood values by 1.9 in order to take into account the usual blood-to-plasma ratio.36 Our results were similar to the corrected values described by Briasoulis et al.23 Also in line with previous observations, the monitoring of vinorelbine concentrations in the MOVIE cohort was characterized by high inter- and intra-patient variability.36 However, this variability was extremely important in our study with a 50%-100% range depending on time points, and outlier patients detected with extremely high and low concentrations extending from 0.1 to 1.12 ng/ml and 1.8 to 10.8 at MVino 30 mg and MVino 40 mg, respectively. Among the 60 analyzed samples, 7 exhibited values below the limit of quantification (<0.1 ng/ml) suggesting that exposure levels were too low to exert any anti-proliferative efficacy. The wide distribution of MVino plasma concentrations could be explained by several reasons such as co-morbidities, polypharmacy, or poor adherence. The possibility that it may be related to the methods used in drug pharmaceutical formulation needs to be further investigated as such variability has been particularly noted when vinorelbine was administered orally.37 From a clinical standpoint, the high variability of MVino represents an important challenge since it could affect patients’ outcome and safety. These results highlight the importance to implement therapeutic drug monitoring when administrating vinorelbine as a metronomic regimen.
At the time of this analysis, out of 14 patients, 9 presented BC, and there was 1 success (one CR), 6 failures (five PD and one SD < 24 weeks), and 3 not yet assessable for CBR (<16 weeks of follow-up) leading to an ORR of only 7%. In a population mostly made of heavily pre-treated BC patients who received no less than two lines of chemotherapy in the metastatic settings, the dual immunotherapy agents associated with MC seem to display a modest activity. This lack of efficacy is comparable to previous reports showing limited benefits in heavily pre-treated BC patients.38,39 However, results are still very early and the efficacy of the treatment will further be confirmed in the currently ongoing phase II. As we anticipated differences in antitumor activity and prolonged survival according to cancer types, the phase II of the study was divided into distinct cohorts thought to benefit from the treatment combination proposed in MOVIE. Namely, BC and PC, not genuinely sensitive to single-agent monotherapies; CC and HNC, which displayed significant but partial efficacy to checkpoint inhibitors; and miscellaneous tumors with high mutational load and/or MSI-high, sensitive to immunotherapies but in which efficacy could be potentially increased by a combination regimen. Despite the breakthrough in clinical treatment, the success of immune checkpoint blockade monotherapies is limited to clinical responses and modest better survival depending on patients and cancers. Combining immune checkpoint inhibitors with anticancer drugs could generate durable effects in patients who do not respond or respond weakly to single-agent immunotherapies and improve the benefits observed in those who respond.
Nowadays, combination of therapy strategies with chemotherapy is also being explored to possibly improve the efficacy of immunotherapies.17 At a time, conventional chemotherapy was administered in high doses followed by a treatment-free period allowing patients to recover. This MTD approach has proven effective. Nevertheless, treatment-free periods may allow the development of resistance and ultimately disease progression. MC relies on frequent administration of low doses of chemotherapy, and coincidentally, although its administration is supposed to primarily target tumor angiogenesis, immunomodulation also occurs, shifting the immunological balance from immunosuppression to immunostimulation through various mechanisms.18 Thus, MC presents a high potential for synergism with immunotherapies, including anti-CTLA-4 and anti-PD-1/PD-L1 monoclonal antibody-based immune checkpoint blockade strategies.40 In addition, MVino has recently proven to reduce tumor vascularization and reduce interleukin-2 levels in mice model, suggesting that MVino could have a variety of immunomodulating features.41 Nonetheless, considering the erratic vinorelbine concentrations measured in MOVIE patients, the actual impact of MVino on tumor microenvironment and tumor immunity and its capacity to boost the efficacy of immune checkpoint inhibitors are currently unpredictable and may probably vary from one patient to another depending on exposure levels. Additionally, if combining immune checkpoint regulators with chemotherapy is likely to increase antitumor activity compared to either single agent alone, the tolerance may also be altered with an increased rate of side effects.42 In this instance, MC has proven less toxic and could therefore be an advantageous treatment option combined to immunotherapy, especially dual immunotherapy regimen. In the MOVIE study phase I, if the toxicities were clearly manageable, more solid evidence, from phase II and other trials, is required to conclude on the tolerance of MVino combined with Durv + Trem.
AST are a heterogeneous group of diseases with a high unmet medical requirement. Newer approaches are needed in order to enhance cure rate and ameliorate toxicity profile for these difficult-to-treat tumors. Combining MC and checkpoint blockade immunotherapies such as MVino with Durv + Trem could prove to be an efficient form of cancer therapy for such tumors. However, more studies focusing on dose and schedule regimens are necessary to tailor combinations depending on cancer types and translate into clinical benefits. Individualized patients’ settings might also be a way to select those who will benefit the most from MVino combined with Durv + Trem. In this context, identifying biomarkers to pinpoint the most appropriate candidates for immunotherapies will be critical in the future.
Acknowledgements
The authors thank the patients and their families as well as all of the investigators and their staff involved in the MOVIE trial. We also thank Dr Emilie Dassé who provided medical writing services on behalf of Unicancer and the members of the independent data and safety monitoring committee (IDMC), Dr Luis Teixeira, Dr Carlos Gomez-Roca, and Dr Jocelyn Gal.
Funding
This work was supported by the French Ministry of Health [grant number PHRC-K_2016-078], La Ligue Nationale Contre le Cancer (no grant number), AstraZeneca [grant number #2018-00706/ESR-16-12287], and Pierre Fabre [grant number #2018-00603].
Disclosure
The authors have declared no conflicts of interest.
Supplmentary data
References
- 1.Kohrt H.E., Tumeh P.C., Benson D., et al. Immunodynamics: a cancer immunotherapy trials network review of immune monitoring in immuno-oncology clinical trials. J Immunother Cancer. 2016;4:15. doi: 10.1186/s40425-016-0118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zou W., Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 3.Butte M.J., Keir M.E., Phamduy T.B., Sharpe A.H., Freeman G.J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterson A.M., Brown K.E., Keir M.E., et al. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol. 2011;187(3):1097–1105. doi: 10.4049/jimmunol.1003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fife B.T., Bluestone J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 6.Hodi F.S., O'Day S.J., McDermott D.F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer J., Reckamp K.L., Baas P., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer R.J., McHenry M.B., Chen A.C. Immune checkpoint blockade in advanced renal-cell carcinoma. N Engl J Med. 2018;379(1):92–93. doi: 10.1056/NEJMc1805988. [DOI] [PubMed] [Google Scholar]
- 9.Motzer R.J., Tannir N.M., McDermott D.F., et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian S.L., Hodi F.S., Brahmer J.R., et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patnaik M.M., Hanson C.A., Hodnefield J.M., et al. Differential prognostic effect of IDH1 versus IDH2 mutations in myelodysplastic syndromes: a Mayo Clinic study of 277 patients. Leukemia. 2012;26(1):101–105. doi: 10.1038/leu.2011.298. [DOI] [PubMed] [Google Scholar]
- 12.Patnaik A., Kang S.P., Rasco D., et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21(19):4286–4293. doi: 10.1158/1078-0432.CCR-14-2607. [DOI] [PubMed] [Google Scholar]
- 13.Westin J.R., Chu F., Zhang M., et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15(1):69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarhini A.A. Tremelimumab: a review of development to date in solid tumors. Immunotherapy. 2013;5(3):215–229. doi: 10.2217/imt.13.9. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara R., Imbimbo M., Malouf R., et al. Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2020;12:CD013257. doi: 10.1002/14651858.CD013257.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larkin J., Chiarion-Sileni V., Gonzalez R., et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y.L., Chang M.C., Cheng W.F. Metronomic chemotherapy and immunotherapy in cancer treatment. Cancer Lett. 2017;400:282–292. doi: 10.1016/j.canlet.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Andre N., Carre M., Pasquier E. Metronomics: towards personalized chemotherapy? Nat Rev Clin Oncol. 2014;11(7):413–431. doi: 10.1038/nrclinonc.2014.89. [DOI] [PubMed] [Google Scholar]
- 19.Mukhtar E., Adhami V.M., Mukhtar H. Targeting microtubules by natural agents for cancer therapy. Mol Cancer Ther. 2014;13(2):275–284. doi: 10.1158/1535-7163.MCT-13-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasquier E., Andre N., Braguer D. Targeting microtubules to inhibit angiogenesis and disrupt tumour vasculature: implications for cancer treatment. Curr Cancer Drug Targets. 2007;7(6):566–581. doi: 10.2174/156800907781662266. [DOI] [PubMed] [Google Scholar]
- 21.Pasquier E., Kavallaris M., Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7(8):455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 22.Heudel P., Arnaud A., Frenel J.S., et al. A GINECO randomized phase II assessing addition of an aromatase inhibitor to oral vinorelbine in pretreated metastatic breast cancer patients. J Clin Oncol. 2019;37(15_suppl) 1043. [Google Scholar]
- 23.Briasoulis E., Aravantinos G., Kouvatseas G., et al. Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a hellenic cooperative oncology group clinical translational study. BMC Cancer. 2013;13:263. doi: 10.1186/1471-2407-13-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darnell E.P., Mooradian M.J., Baruch E.N., Yilmaz M., Reynolds K.L. Immune-related adverse events (irAEs): diagnosis, management, and clinical pearls. Curr Oncol Rep. 2020;22(4):39. doi: 10.1007/s11912-020-0897-9. [DOI] [PubMed] [Google Scholar]
- 25.Wolchok J.D., Kluger H., Callahan M.K., et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Planchard D., Yokoi T., McCleod M.J., et al. A phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: rationale and protocol design of the ARCTIC study. Clin Lung Cancer. 2016;17(3):232–236.e1. doi: 10.1016/j.cllc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Rizvi N.A., Cho B.C., Reinmuth N., et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020;6(5):661–674. doi: 10.1001/jamaoncol.2020.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powles T., van der Heijden M.S., Castellano D., et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1574–1588. doi: 10.1016/S1470-2045(20)30541-6. [DOI] [PubMed] [Google Scholar]
- 29.Kelley R.K., Sangro B., Harris W., et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol. 2021;39(27):2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abou-Alfa G.K., Chan S.L., Kudo M., et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(4_suppl):379. [Google Scholar]
- 31.Baas P., Scherpereel A., Nowak A.K., et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397(10272):375–386. doi: 10.1016/S0140-6736(20)32714-8. [DOI] [PubMed] [Google Scholar]
- 32.Hellmann M.D., Paz-Ares L., Bernabe Caro R., et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 33.Overman M.J., Lonardi S., Wong K.Y.M., et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 34.Paz-Ares L., Ciuleanu T.E., Cobo M., et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 35.Yau T., Kang Y.K., Kim T.Y., et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11) doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gauvin A., Pinguet F., Culine S., Astre C., Cupissol D., Bressolle F. Blood and plasma pharmacokinetics of vinorelbine in elderly patients with advanced metastatic cancer. Cancer Chemother Pharmacol. 2002;49(1):48–56. doi: 10.1007/s00280-001-0378-2. [DOI] [PubMed] [Google Scholar]
- 37.Rowinsky E.K., Noe D.A., Trump D.L., et al. Pharmacokinetic, bioavailability, and feasibility study of oral vinorelbine in patients with solid tumors. J Clin Oncol. 1994;12(9):1754–1763. doi: 10.1200/JCO.1994.12.9.1754. [DOI] [PubMed] [Google Scholar]
- 38.Mezni E., Behi K., Goncalves A. Immunotherapy and breast cancer: an overview. Curr Opin Oncol. 2022;34(5):587–594. doi: 10.1097/CCO.0000000000000878. [DOI] [PubMed] [Google Scholar]
- 39.Winer E.P., Lipatov O., Im S.A., et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(4):499–511. doi: 10.1016/S1470-2045(20)30754-3. [DOI] [PubMed] [Google Scholar]
- 40.Kareva I., Waxman D.J., Lakka Klement G. Metronomic chemotherapy: an attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Cancer Lett. 2015;358(2):100–106. doi: 10.1016/j.canlet.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orlandi P., Banchi M., Alì G., et al. Active metronomic vinorelbine schedules decrease plasma interleukin-2 levels in mice with Lewis lung carcinoma. J Chemother. 2021;33(3):198–202. doi: 10.1080/1120009X.2020.1819069. [DOI] [PubMed] [Google Scholar]
- 42.Boutros C., Tarhini A., Routier E., et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.