Abstract
Background
Preclinical studies showed a synergistic effect for 5-fluorouracil and lurbinectedin against solid tumors. This phase I trial evaluated a combination of capecitabine plus lurbinectedin in patients with selected advanced solid tumors. Results in patients with relapsed metastatic breast cancer (MBC) are described.
Patients and methods
Patients received capecitabine daily on day (D)1-D14 combined with lurbinectedin on D1, D8 or D1 every 3 weeks (q3w) intravenously, following a standard 3 + 3 escalation design and expansion at the recommended dose (RD).
Results
Of the 81 enrolled patients, 28 had relapsed MBC: 20 with hormone receptor (HR)-positive tumors and 8 with triple-negative tumors; 3 treated in the D1,D8 schedule and 25 in the D1 schedule. The RD was capecitabine 1650 mg/m2 daily on D1-D14 plus lurbinectedin 2.2 mg/m2 on D1 q3w. Sixteen confirmed responses and two prolonged disease stabilizations (≥6 months) were observed [overall response rate (ORR)/clinical benefit rate (CBR) = 57%/64% at all dose levels; 47%/60% at the RD]. Twelve responses and both prolonged stabilizations occurred in HR-positive tumors (ORR/CBR = 60%/70% at all dose levels, 56%/78% at the RD). Four responses were found in triple-negative tumors (ORR and CBR = 50% at all dose levels; 33% at the RD). Myelotoxicity was reversible and manageable at the RD; most non-hematological toxicities were mild/moderate. No episodes of febrile neutropenia or severe palmar-plantar erythrodysesthesia syndrome occurred. No major pharmacokinetic drug–drug interaction was found between lurbinectedin, capecitabine or capecitabine metabolites.
Conclusions
The capecitabine/lurbinectedin combination showed encouraging clinical activity in relapsed MBC, especially in HR-positive tumors. Toxicity was manageable at the RD. Further development is warranted in relapsed MBC.
Key words: lurbinectedin, capecitabine, phase I study, breast cancer
Highlights
-
•
Capecitabine daily on D1-D14 plus lurbinectedin on D1 q3w showed encouraging activity in relapsed MBC.
-
•
At the RD (capecitabine 1650 mg/m2 plus lurbinectedin 2.2 mg/m2), ORR was 47% and CBR after 6 months was 60%.
-
•
Antitumor activity at the RD was higher in patients with HR-positive tumors (ORR = 56%; CBR = 78% after 6 months).
-
•
Myelotoxicity was reversible and manageable at the RD, and most non-hematological toxicities were mild/moderate.
-
•
Further development of the capecitabine/lurbinectedin combination is warranted in relapsed MBC.
Introduction
Lurbinectedin is a synthetic tetrahydroisoquinoline alkaloid structurally related to trabectedin that inhibits oncogenic transcription primarily through binding to the exocyclic amino group of guanine-rich DNA sequences around promoters of protein-coding genes. This binding alters the three-dimensional DNA structure, evicting oncogenic transcription factors from their binding sites, and thus halting their aberrant transcription programs.1, 2, 3 Lurbinectedin adducts can also stop transcribing (phosphorylated) RNA polymerase II, decreasing messenger RNA synthesis and inducing the ubiquitination and degradation of RNA polymerase II.4 Lurbinectedin adducts may also favor the production of DNA double-strand breaks and trigger apoptotic cell death.5
Previous phase I studies defined recommended doses (RDs) for two schedules of single-agent lurbinectedin given as 1-h intravenous (i.v.) infusions every 3 weeks (q3w): 7.0 mg flat dose (FD) for a day (D)1 q3w schedule, and 5.0 mg FD for a D1,D8 q3w schedule. Transient grade 3/4 neutropenia and mild gastrointestinal disorders and fatigue were common at these RDs.6,7
Preclinical studies showed a synergistic effect for the combination of lurbinectedin and 5-fluorouracil (5-FU) in vitro in gastric and colon tumor cells, and in vivo in mice bearing xenografted gastric, colon or pancreatic tumors.8 The toxicity profile of lurbinectedin does not completely overlap that of capecitabine, an oral pro-drug of 5-FU used as monotherapy for the treatment of metastatic colorectal cancer (mCRC) and metastatic breast cancer (MBC).
The aim of this phase I study was to determine the RD, safety profile, antitumor activity and pharmacokinetics (PK) of lurbinectedin in combination with capecitabine in patients with selected advanced solid tumors. Due to the promising antitumor activity observed during escalation, the results shown here are focused on MBC patients.
Patients and methods
This clinical trial was conducted in Spain and Belgium, in compliance with International Conference on Harmonisation (ICH) Good Clinical Practice guidelines. The protocol was approved by the centers’ research ethics committees. Signed written informed consent was obtained for each patient before study-specific procedures. The trial is registered at https://www.clinicaltrials.gov as NCT02210364.
Eligibility criteria
Eligible patients were aged 18-75 years; with unresectable mCRC, MBC or pancreatic cancer (PC); life expectancy ≥3 months; Eastern Cooperative Oncology Group performance status score ≤1; adequate bone marrow, hepatic, renal and metabolic function; and normal left ventricular ejection fraction who had recovered from any previous toxicities. Patients in the expansion cohort at the RD also had measurable disease according to RECIST v.1.1 and documented disease progression.
Patients were excluded if they had been pretreated with lurbinectedin or capecitabine-containing therapy for advanced disease; had received ≥3 prior chemotherapy lines for advanced disease, or bone marrow, stem cell transplantation or extensive radiotherapy; were lactating women or were not using effective contraceptives; or had symptomatic brain metastases or leptomeningeal disease, ongoing chronic hepatopathy, active infection, relevant cardiac disease, external drainages, dyspnea requiring oxygen support, known dihydropyrimidine dehydrogenase deficit or any disease interfering with study outcome.
Study treatment
Initially, treatment consisted of escalating doses of oral capecitabine administered twice daily on D1-D14, followed by lurbinectedin as a 1-h i.v. infusion on D1 and D8, both q3w. If half or more assessable patients at any given dose level were unable to receive their corresponding lurbinectedin D8 infusion in cycles 1 and/or 2, or if the starting dose of this combination proved to be unfeasible, the lurbinectedin schedule could be switched to D1 q3w without modifying the original capecitabine schedule.
Lurbinectedin was supplied as a lyophilized powder concentrate, reconstituted and diluted with glucose 5% or sodium chloride 0.9% solution. Commercially available capecitabine was provided. Antiemetic prophylaxis with i.v. dexamethasone and ondansetron was given before each lurbinectedin infusion; oral metoclopramide and other antiemetics could be added if required (except for aprepitant, which was not allowed). Treatment was administered until disease progression, unacceptable toxicity, intercurrent illness precluding study continuation, patient refusal and/or non-compliance with study requirements, treatment delay >15 days (except if clear clinical benefit) and requirement of >2 dose reductions.
Dose escalation and dose-limiting toxicities
Dose escalation followed a standard 3 + 3 design. The starting capecitabine dose (1650 mg/m2 daily, split into two doses at least 12 h apart) was ∼66% of the single-agent RD of 2510 mg/m2 daily using this intermittent schedule,9 and the starting lurbinectedin dose (2.0 mg FD) was 40% of the RD of 5.0 mg FD defined for single-agent lurbinectedin as 1-h i.v. infusions on D1,D8 q3w.7
The following dose-limiting toxicities (DLTs) were defined: grade 4 neutropenia >3 days; febrile neutropenia or neutropenic sepsis; grade 4 thrombocytopenia (or grade 3 requiring transfusion); grade 4 transaminase increase (or grade 3 for >7 days); grade ≥2 transaminase increase with total bilirubin increase ≥2× upper limit of normal and normal alkaline phosphatase (AP); any clinically relevant grade ≥3 toxicity; and toxicity resulting in cycle delay >15 days, interruption of capecitabine administration for >5 consecutive days or for >7 days in a cycle or omission of lurbinectedin D8 infusion and subsequent cycle delay.
Determination of the recommended dose
The primary endpoint of the study was the RD of lurbinectedin in combination with capecitabine, defined as the highest dose level explored at which less than one-third of assessable patients experienced a DLT in cycle 1 during dose escalation. This dose level was then expanded, and confirmed as the RD if less than one-third of the first nine assessable patients at this dose level had DLTs during cycle 1.
Safety assessments
Hematology and biochemistry tests were done at baseline, weekly during cycle 1 (and cycle 2 for hematology tests) and on D1 and D8 during subsequent cycles. Electrocardiograms were done at baseline and repeated if clinically indicated.
Adverse events (AEs) and laboratory abnormalities were graded with the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v.4,10 and coded using the Medical Dictionary for Regulatory Activities (MedDRA) v.16.0.
Efficacy assessments
Antitumor activity was evaluated according to the RECIST v.1.111 every 6 weeks until cycle 6 and every 9 weeks afterward. Overall response rate (ORR) was the percentage of patients with complete (CR) or partial response (PR). Clinical benefit rate (CBR) was the percentage of patients with CR, PR or prolonged disease stabilization. Time-to-event parameters were progression-free survival (PFS) and duration of response (DoR).
Pharmacokinetic analyses
Twelve samples were collected from each patient to quantify plasma concentrations of lurbinectedin, capecitabine and capecitabine metabolites [5′-deoxy-5-fluorouridine (5′-DFUR), 5-FU and α-fluoro-β-alanine (FBAL)] at baseline and at different times during 3 weeks after the first treatment administration. Both drugs were measured by validated liquid extraction methods followed by ultra-performance liquid chromatography tandem mass spectrometry detection (Dynakin, Derio, Spain). Calibration ranges were 0.1-50 ng/ml for lurbinectedin, 100-10 000 ng/ml for capecitabine, 500-10 000 ng/ml for 5′-DFUR, 5-1000 ng/ml for 5-FU and 200-10 000 ng/ml for FBAL. Individual PK parameters of lurbinectedin were calculated using non-compartmental analysis. Calculation of individual PK parameters of capecitabine and its metabolites was carried out by means of a population pharmacokinetic (PopPK) approach based on the model proposed by Urien et al.12 and pooling PK data from all patients treated in the study, regardless of tumor type. Assessments of dose linearity of PK parameters and potential PK drug–drug interactions also included all patients to obtain more conclusive results.
Statistical analysis
Continuous variables were presented with summary statistics and categorical variables in frequency tables. Time-to-event variables were calculated using the Kaplan–Meier approach. Binomial exact distribution was used to calculate 95% confidence intervals (95% CIs) for categorical variables. Blood and plasma concentration–time profiles were analyzed by standard non-compartmental methods. Individual PK parameters were tabulated and summarized.
Results
Dose escalation
A total of 81 patients participated in this study between April 2013 and October 2016: 31 with mCRC, 28 with MBC and 22 with PC (see Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100651). Seven dose levels were evaluated in this study: two with the capecitabine D1-D14 plus lurbinectedin D1,D8 q3w schedule, and five with an alternative schedule of capecitabine D1-D14 plus lurbinectedin D1 q3w (see Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100651). The latter was evaluated following the finding of a shorter duration of nadir blood cell counts with single-agent lurbinectedin using a D1 q3w schedule compared to a D1,D8 q3w schedule.6,7 In addition, lurbinectedin was converted from an FD to a body surface area (BSA)-based dose (calculated by dividing the FD value by a BSA of 1.8 m2), owing to the finding of a greater probability of grade 3/4 thrombocytopenia with single-agent lurbinectedin among patients with the lowest BSA values in an exploratory analysis of pooled phase II data. Most DLTs in both schedules were related to myelosuppression and occurred above the RDs. The RDs were defined at capecitabine 1650 mg/m2 D1-D14 plus lurbinectedin 2.0 mg FD D1,D8 q3w, and capecitabine 1650 mg/m2 D1-D14 plus lurbinectedin 2.2 mg/m2 D1 q3w; the latter was chosen as the RD for phase II studies with the combination.
Characteristics of patients with metastatic breast cancer and treatment
Baseline characteristics of the 28 patients with MBC treated with capecitabine plus lurbinectedin in the present study are summarized in Table 1. All patients were female, with a median age of 51.5 years (range, 29-71 years). Nineteen patients (68%) had hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative MBC, one patient (4%) had HR-positive, HER2-positive MBC, and eight patients (29%) had triple-negative MBC. BRCA status was evaluated in 11 patients; of these, 5 had known BRCA1 or BRCA2 mutations. At baseline, 27 (96%) had visceral disease and 22 (79%) had liver metastases. Median number of lines of prior therapy for advanced disease was 1 (range, 0-3 lines). Most patients had received prior therapy with taxanes (89%), anthracyclines (86%) and nitrogen mustard analogues (cyclophosphamide) (82%). Nineteen patients (68%) received prior hormone therapy, including tamoxifen (n = 16 patients; 57%), letrozole (n = 11; 39%), fulvestrant (n = 8; 29%), exemestane and goserelin (n = 5; 18% each) and anastrozole (n = 2; 7%). Two patients (7%) received prior therapy with cyclin-dependent kinase (CDK4/6) inhibitors (abemaciclib and ribociclib, n = 1 each, both experimental treatments at the time the study was conducted). In addition, the patient with HR-positive, HER2-positive MBC was treated with trastuzumab for ∼12 months before participating in the present study.
Table 1.
Baseline characteristics of patients with metastatic breast cancer
| Capecitabine plus lurbinectedin |
||
|---|---|---|
| RD (capecitabine 1650 mg/m2 D1-D14/lurbinectedin 2.2 mg/m2 D1) (n = 15) |
All dose levels (n = 28) | |
| Age, years | ||
| Median (range) | 46.0 (29-71) | 51.5 (29-71) |
| ≤40 | 2 (13) | 3 (11) |
| 41-60 | 8 (53) | 17 (61) |
| >60 | 5 (33) | 8 (29) |
| ECOG performance status | ||
| 0 | 11 (73) | 18 (64) |
| 1 | 4 (27) | 10 (36) |
| Median BSA, m2 (range) | 1.7 (1.5-2.1) | 1.7 (1.3-2.2) |
| HR and HER2/neu expression | ||
| HR positive | 9 (60) | 20 (71) |
| HR positive/HER2/neu negative | 8 (53) | 19 (68) |
| HR positive/HER2/neu positive | 1 (7) | 1 (4) |
| Triple negative | 6 (40) | 8 (29) |
| BRCA mutation | ||
| BRCA1+ | 4 (27) | 4 (14) |
| BRCA2+ | 1 (4) | |
| BRCA1/2 wild type | 4 (27) | 6 (21) |
| Unknown | 7 (47) | 17 (61) |
| Bulky disease (any target lesion ≥50 mm) | 5 (33) | 7 (25) |
| No. of metastatic sites | ||
| Median (range) | 3.0 (2-6) | 3.0 (1-6) |
| 1 | 1 (4) | |
| 2 | 6 (40) | 7 (25) |
| ≥3 | 9 (60) | 20 (71) |
| Sites of disease | ||
| Viscerala | 15 (100) | 27 (96) |
| Liver | 12 (80) | 22 (79) |
| Bone | 10 (67) | 18 (64) |
| CNS | 1 (4) | |
| No. of lines of prior therapy for advanced disease | ||
| Median (range) | 1.0 (0-3) | 1.0 (0-3) |
| 0b | 3 (20) | 8 (29) |
| 1 | 5 (33) | 9 (32) |
| 2 | 6 (40) | 10 (36) |
| 3 | 1 (7)c | 1 (4)c |
| Prior anticancer agents | ||
| Taxanes | 14 (93) | 25 (89) |
| Anthracyclines and related substances | 12 (80) | 24 (86) |
| Nitrogen mustard analogues | 13 (87) | 23 (82) |
| Pyrimidine analogues | 5 (33) | 11 (39) |
| Platinum compounds | 4 (27) | 4 (14) |
| Prior hormone therapy | 8 (53) | 19 (68) |
| Prior CDK4/6 inhibitor therapy | — | 2 (7) |
Data shown are n (percentage) of patients, except for median (range).
BEV, bevacizumab; BSA, body surface area; CDK4/6, cyclin-dependent kinase 4/6; CNS, central nervous system; D, day; ECOG, Eastern Cooperative Oncology Group; FOLFIRI, folinic acid, 5-fluorouracil and irinotecan; HR, hormone receptor; MBC, metastatic breast cancer; RD, recommended dose.
Includes lung, liver and adrenal glands.
These patients received prior therapy in the neoadjuvant and/or the adjuvant settings.
This patient received FOLFIRI for 2 months, followed by locoregional administration of a single cycle of mitomycin and then by re-challenge with FOLFIRI plus BEV.
Fifteen of the 28 patients with MBC were treated at the RD (capecitabine 1650 mg/m2 D1-D14 plus lurbinectedin 2.2 mg/m2 D1 q3w). Median age of these patients was 46.0 years (range, 29-71 years). Nine patients (60%) had HR-positive MBC and six patients (40%) had triple-negative MBC. BRCA status was evaluated in eight patients, of whom four had known BRCA1 mutations. At baseline, all 15 patients had visceral disease and 12 (80%) had liver metastases. Median number of lines of prior therapy for advanced disease was 1 (range, 0-3 lines). Most patients had received prior therapy with taxanes (93%), nitrogen mustard analogues (cyclophosphamide) (87%) and anthracyclines (80%) (Table 1). Eight patients (53%) received prior hormone therapy with tamoxifen (n = 6; 40%), letrozole (n = 5; 33%), fulvestrant and goserelin (n = 3; 20% each), exemestane (n = 2; 13%) and anastrozole (n = 1; 7%). No patients at the RD received prior therapy with CDK4/6 inhibitors.
A total of 248 cycles of capecitabine and lurbinectedin (median, 7.5 cycles per patient) were administered at all dose levels, including 108 cycles (median, 7 cycles per patient) at the RD. Median dose intensities (DIs) at the RD were 5810 mg/m2/week for capecitabine and 0.7 mg/m2/week for lurbinectedin, and median relative DIs compared to planned dose were 75.4% and 91.1%, respectively.
Safety
All treated patients with MBC were assessable for safety. Treatment-related AEs and laboratory abnormalities at all dose levels and at the RD (capecitabine 1650 mg/m2 D1-D14 plus lurbinectedin 2.2 mg/m2 D1 q3w) are shown in Table 2.
Table 2.
Treatment-related adverse events (>10% of patients or grade ≥3) and laboratory abnormalities (hematological and biochemical) in patients with metastatic breast cancer treated with capecitabine plus lurbinectedin at all dose levels and at the recommended dose
| Capecitabine plus lurbinectedin |
||||||||
|---|---|---|---|---|---|---|---|---|
| RD (capecitabine 1650 mg/m2 D1-D14/lurbinectedin 2.2 mg/m2 D1) (n = 15) |
All dose levels (n = 28) |
|||||||
| NCI-CTCAE grade | 1-2 | 3 | 4 | Total | 1-2 | 3 | 4 | Total |
| Hematological laboratory abnormalities | ||||||||
| Anemia | 80 | 13 | — | 93 | 82 | 11 | — | 93 |
| Neutropenia | 27 | 40 | 7 | 73 | 25 | 32 | 25 | 82 |
| Thrombocytopenia | 73 | — | — | 73 | 64 | 7 | — | 71 |
| Biochemical laboratory abnormalities | ||||||||
| ALT increased | 87 | 7 | — | 93 | 79 | 11 | — | 89 |
| AP increased | 47 | — | — | 47 | 54 | — | — | 54 |
| AST increased | 80 | 7 | — | 87 | 71 | 7 | — | 79 |
| Bilirubin increased | 40 | — | — | 40 | 29 | — | — | 29 |
| CPK increased | 20 | — | — | 20 | 21 | — | — | 21 |
| Creatinine increased | 93 | — | — | 93 | 96 | — | — | 96 |
| Adverse events | ||||||||
| Constipation | 13 | — | — | 13 | 18 | — | — | 18 |
| Decreased appetite | 33 | — | — | 33 | 39 | — | — | 39 |
| Diarrhea | 33 | — | — | 33 | 46 | — | — | 46 |
| Dyspepsia | 33 | — | — | 33 | 43 | — | — | 43 |
| Fatigue | 40 | 7 | — | 47 | 50 | 7 | — | 57 |
| Headache | 7 | — | — | 7 | 11 | — | — | 11 |
| Hypertriglyceridemia | — | — | — | — | — | 4 | — | 4 |
| Mucositis | 33 | — | — | 33 | 32 | — | — | 32 |
| Myalgia | 13 | — | — | 13 | 7 | — | — | 7 |
| Nausea | 60 | — | — | 60 | 71 | — | — | 71 |
| Palmar-plantar erythrodysesthesia syndrome | 33 | — | — | 33 | 43 | — | — | 43 |
| Paronychia | 20 | — | — | 20 | 14 | — | — | 14 |
| Peripheral sensory neuropathy | 13 | — | — | 13 | 21 | — | — | 21 |
| Pulmonary embolism | — | — | — | — | — | 4 | — | 4 |
| Vomiting | 20 | — | — | 20 | 29 | — | — | 29 |
| Weight decreased | 13 | — | — | 13 | 7 | — | — | 7 |
| Xerosis | — | — | — | — | 11 | — | — | 11 |
Data shown are percentage of patients. Hematological and biochemical abnormalities are shown regardless of relationship to treatment.
ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; CPK, creatine phosphokinase; D, day; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; RD, recommended dose.
All 15 patients treated at the RD had at least one AE related to treatment or with unknown relationship. Most of these AEs were grade 1/2, with the most common being nausea (60% of patients), fatigue (47%), decreased appetite, diarrhea, dyspepsia, mucositis and palmar-plantar erythrodysesthesia syndrome (33% each). The only severe treatment-related AE was grade 3 fatigue in one patient (7%). No treatment-related AEs reached grade 4. Hematological abnormalities consisted of anemia (93%; grade 3 in 13%), neutropenia (73%; grade 3/4 in 47%, with no febrile neutropenia) and thrombocytopenia (73%). The most frequent biochemical abnormalities were increases in creatinine (93%), alanine aminotransferase (93%; grade 3 in 7%), aspartate aminotransferase (87%; grade 3 in 7%), AP (47%) and bilirubin (40%). One patient (6.7%) required red blood cell transfusions. No patients required granulocyte colony-stimulating factor support. No treatment-related discontinuations or deaths were observed.
Efficacy
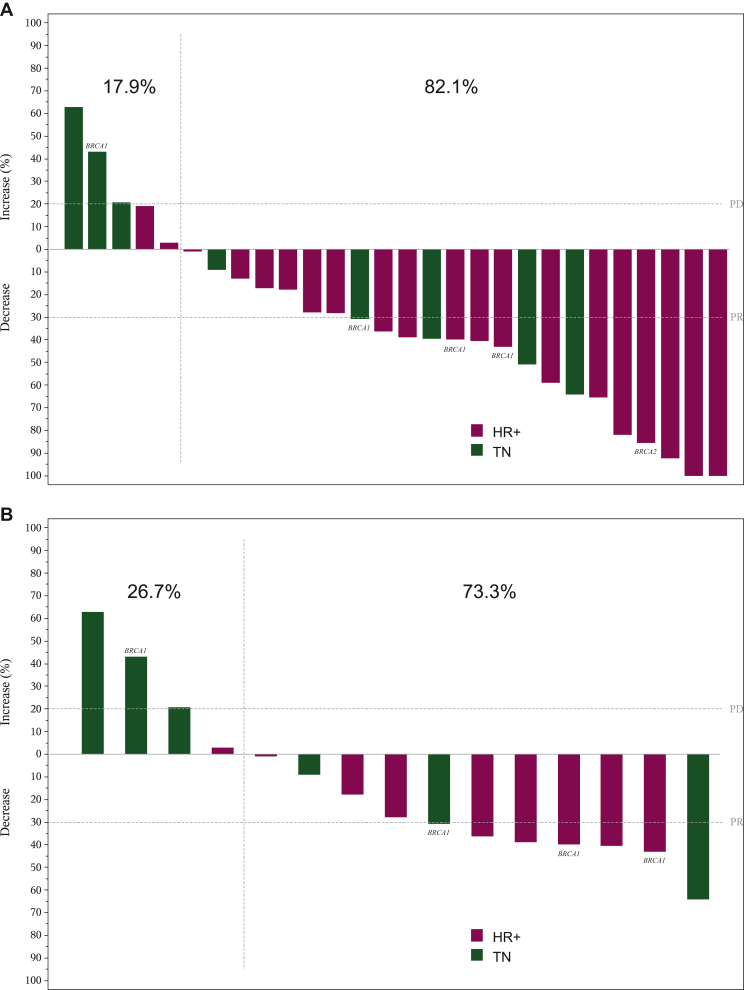
All 28 patients with MBC were assessable for efficacy. Sixteen confirmed responses, including one CR, were observed at all dose levels [ORR = 57% (95% CI 37.2% to 75.5%)]. In addition, two disease stabilizations lasted ≥6 months [CBR = 64% (95% CI 44.1% to 84.1%) after 6 months] (Table 3). Median DoR was 6.8 months (95% CI 3.4-12.5 months) and median PFS was 7.3 months (95% CI 3.9-10.2 months). Objective tumor shrinkage was found in 23 patients (82%) (Figure 1A).
Table 3.
Best response according to RECIST in patients with metastatic breast cancer treated with capecitabine plus lurbinectedin
| Schedule |
||||||||
|---|---|---|---|---|---|---|---|---|
| Capecitabine D1-D14/lurbinectedin D1,D8 |
Capecitabine D1-D14/lurbinectedin D1 |
|||||||
| All dose levels (n = 3) |
RD (1650 mg/m2/2.2 mg/m2) (n = 15) |
All dose levels (n = 25) |
All dose levels (n = 28) |
|||||
| n | % | n | % | n | % | n | % | |
| CR | — | — | — | — | 1 | 4 | 1 | 4 |
| PR | 1 | 33 | 7 | 47 | 14 | 56 | 15 | 54 |
| SD ≥4 months | — | — | 3 | 20 | 3 | 12 | 3 | 11 |
| SD <4 months | 1 | 33 | 2 | 13 | 4 | 16 | 5 | 18 |
| PD | 1 | 33 | 3 | 20 | 3 | 12 | 4 | 14 |
| ORR (%) (95% CI) |
33% (0.8% to 90.6%) | 47% (21.3% to 73.4%) | 60% (38.7% to 78.9%) | 57% (37.2% to 75.5%) | ||||
| CBR (%) after 4 monthsa (95% CI) |
33% (0.8% to 90.6%) | 67% (38.4% to 88.2%) | 72% (50.6% to 87.9%) | 68% (47.7% to 84.1%) | ||||
| CBR (%) after 6 monthsb (95% CI) |
33% (0.8% to 90.6%) | 60% (32.3% to 83.7%) | 68% (46.5% to 85.1%) | 64% (44.1% to 84.1%) | ||||
| Median DoR (months) (95% CI) |
12.5 (—) | 6.8 (1.3-NR) | 6.8 (2.4-12.5) | 6.8 (3.4-12.5) | ||||
| Median PFS (months) (95% CI) |
16.9 (1.1-16.9) | 5.5 (1.1-10.2) | 7.3 (3.9-10.2) | 7.3 (3.9-10.2) | ||||
CBR, clinical benefit rate; CI, confidence interval; CR, complete response; D, day; DoR, duration of response; NR, not reached; ORR, overall response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; RD, recommended dose; SD, stable disease.
Patients with CR + PR + SD ≥4 months.
Patients with CR + PR + SD ≥6 months.
Figure 1.
Maximum variation of target lesions in patients with metastatic breast cancer, measurable disease and at least one radiological tumor assessment who were treated with capecitabine plus lurbinectedin. (A) At all dose levels (n = 28). (B) At the RD of capecitabine 1650 mg/m2 D1-D14 plus lurbinectedin 2.2 mg/m2 D1) (n = 15). Patients with known BRCA-mutated tumors are noted.
D, day; HR, hormone receptor; PD, progressive disease; PR, partial response; RD, recommended dose; TN, triple negative.
Seven confirmed responses and both disease stabilizations ≥6 months were observed among the 15 patients treated at the RD (capecitabine 1650 mg/m2 D1-D14 plus lurbinectedin 2.2 mg/m2 D1 q3w) [ORR = 47% (95% CI 21.3% to 73.4%); CBR = 60% (95% CI 32.3% to 83.7%) after 6 months] (Table 3). Median DoR was 6.8 months (95% CI 1.3 months-not reached) and median PFS was 5.5 months (95% CI 1.1-10.2 months). Eleven patients (73%) showed objective tumor shrinkage (Figure 1B).
Most confirmed responses (n = 12, including the CR) and all prolonged disease stabilizations were found in patients with HR-positive tumors [ORR = 60% (95% CI 36.1% to 80.9%), CBR = 70% (95% CI 45.7% to 88.1%) after 6 months at all dose levels; ORR = 56% (95% CI 21.2% to 86.3%), CBR = 78% (95% CI 40.0% to 97.2%) after 6 months at the RD] (Table 4). Responses were durable [median DoR, 10.9 months (95% CI 2.4 months-not reached) and 7.0 months (95% CI 1.3 months-not reached), respectively] and median PFS was 10.2 months at all dose levels (95% CI 3.9-16.9 months) and at the RD (95% CI 2.3-10.2 months). Most patients with HR-positive tumors showed objective tumor shrinkage: 90% at all dose levels and 89% at the RD (Figure 1A and B).
Table 4.
Best response according to RECIST and hormone receptor expression in patients with metastatic breast cancer treated with capecitabine plus lurbinectedin at all dose levels and at the recommended dose
| HR positive |
Triple negative |
|||||||
|---|---|---|---|---|---|---|---|---|
| RD (capecitabine 1650 mg/m2 D1-D14/lurbinectedin 2.2 mg/m2 D1) (n = 9) |
All dose levels (n = 20) |
RD (capecitabine 1650 mg/m2 D1-D14/lurbinectedin 2.2 mg/m2 D1) (n = 6) |
All dose levels (n = 8) |
|||||
| n | % | n | % | n | % | n | % | |
| CR | — | — | 1 | 5 | — | — | — | — |
| PR | 5 | 56 | 11 | 55 | 2 | 33 | 4 | 50 |
| SD ≥4 months | 3 | 33 | 3 | 15 | — | — | — | — |
| SD <4 months | 1 | 11 | 4 | 20 | 1 | 17 | 1 | 13 |
| PD | — | — | 1 | 5 | 3 | 50 | 3 | 38 |
| ORR (%) (95% CI) |
56% (21.2% to 86.3%) | 60% (36.1% to 80.9%) | 33% (4.3% to 77.7%) | 50% (15.7% to 84.3%) | ||||
| CBR (%) after 4 monthsa (95% CI) |
89% (51.8% to 99.7%) | 75% (50.9% to 91.3%) | 33% (4.3% to 77.7%) | 50% (15.7% to 84.3%) | ||||
| CBR (%) after 6 monthsb (95% CI) |
78% (40.0% to 97.2%) | 70% (45.7% to 88.1%) | 33% (4.3% to 77.7%) | 50% (15.7% to 84.3%) | ||||
| Median DoR (months) (95% CI) |
7.0 (1.3-NR) | 10.9 (2.4-NR) | 5.6 (4.4-6.8) | 3.9 (1.6-6.8) | ||||
| Median PFS (months) (95% CI) |
10.2 (2.3-10.2) | 10.2 (3.9-16.9) | 1.5 (1.1-8.3) | 3.0 (1.1-8.0) | ||||
CBR, clinical benefit rate; CI, confidence interval; CR, complete response; D, day; DoR, duration of response; HR, hormone receptor; MBC, metastatic breast cancer; NR, not reached; ORR, overall response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; RD, recommended dose; SD, stable disease.
Patients with CR + PR + SD ≥4 months.
Patients with CR + PR + SD ≥6 months.
In contrast, four confirmed responses and no prolonged disease stabilizations were observed in patients with triple-negative tumors [ORR and CBR = 50% (95% CI 15.7% to 84.3%) at all dose levels; 33% (95% CI 4.3% to 77.7%) at the RD] (Table 4). Median time-to-event endpoints were shorter: median DoR was 3.9 months (95% CI 1.6-6.8 months) at all dose levels and 5.6 months (95% CI 4.4-6.8 months) at the RD, and median PFS was 3.0 months (95% CI 1.1-8.0 months) and 1.5 months (95% CI 1.1-8.3 months), respectively. Less patients with triple-negative tumors showed objective tumor shrinkage: 63% at all dose levels and 50% at the RD (Figure 1A and B).
Four of the five patients with known BRCA mutations showed confirmed response to capecitabine and lurbinectedin at all dose levels: three with known BRCA1 mutations and one with known BRCA2 mutations. All three patients with known BRCA1 mutations and confirmed response had been treated at the RD. Overall, confirmed PR and tumor shrinkage were observed in four of five (80%) patients with known BRCA1 or BRCA2 mutations at all dose levels, including three of four (75%) patients at the RD (Figure 1B).
Best response to capecitabine and lurbinectedin was compared with response to the last prior therapy in patients treated at the RD. Overall, 6 of 15 patients (40%) showed greater antitumor activity with capecitabine and lurbinectedin compared to the last prior therapy: 4 of 9 (44%) with HR-positive tumors and 2 of 6 (33%) with triple-negative tumors (see Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100651). Of note, all four patients with HR-positive tumors and greater antitumor activity with capecitabine and lurbinectedin had received prior chemotherapy for advanced disease and prior hormone therapy.
Pharmacokinetics
PK data were available from all 28 MBC patients. At the RD (capecitabine 1650 mg/m2 D1-D14 plus lurbinectedin 2.2 mg/m2 D1 q3w), mean half-life was 32.7 h and mean clearance (CL) was 13.5 l/h for lurbinectedin (see Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100651). Area under the concentration–time curve and maximum plasma concentration for lurbinectedin increased with dose in a linear manner. The PK profile of capecitabine and its metabolites showed great variability.
Discussion
The capecitabine and lurbinectedin combination showed encouraging antitumor activity in relapsed MBC. At the RD (capecitabine 1650 mg/m2 D1-D14 plus lurbinectedin 2.2 mg/m2 D1 q3w), ORR was 47%, and CBR was 60% after 6 months. Antitumor activity was greater in HR-positive tumors (ORR = 56%; CBR = 78% after 6 months) than in triple-negative tumors (ORR and CBR = 33%). Responses at the RD were durable: 7.0 months (HR-positive tumors) and 5.6 months (triple-negative tumors). Median PFS was 5.5 months (HR-positive tumors: 10.2 months; triple-negative tumors: 1.5 months).
Despite the many advances achieved in the treatment of breast cancer over the last two decades, MBC remains an incurable disease and prognosis after diagnosis of metastatic disease is poor: 4-5 years for HR-positive or HER2/4neu-positive tumors, and ∼1 year for triple-negative tumors.13 Currently unmet medical needs in relapsed MBC include treatment after CDK4/6 therapy in HR-positive/HER2-negative tumors, after immuno-chemotherapy combination in programmed death-ligand 1-positive tumors, and after poly-(ADP-ribose) polymerase therapy in BRCA1/2-mutated tumors. Anthracycline- and taxane-containing therapies are frequently recommended for first-line treatment of MBC, with capecitabine often being the first treatment of choice after disease progression. Studies evaluating capecitabine in relapsed MBC patients unselected for HR expression reported ORRs of 12%-29% and median PFS of 3.1-4.9 months when given as monotherapy,14, 15, 16, 17, 18, 19 and ORRs of 35%-43% and median PFS of 5.8-6.2 months when combined with other drugs (e.g. docetaxel, ixabepilone).17,18,20 Thus, the ORR observed herein for capecitabine plus lurbinectedin at the RD is at the high limit of the range of ORRs reported for other capecitabine-containing combinations in patients with relapsed MBC regardless of HR expression. Of note, 75% of patients with known BRCA mutations treated at the RD had confirmed response or tumor shrinkage. Furthermore, 40% of patients treated at the RD in the present study showed longer PFS with capecitabine/lurbinectedin than with the last prior therapy.
The safety profile of the capecitabine/lurbinectedin combination was predictable and manageable. At the RD, myelosuppression was common but reversible. Non-hematological toxicities were mostly mild/moderate, and the most frequent were fatigue, gastrointestinal disorders (nausea, diarrhea, dyspepsia), decreased appetite and palmar-plantar erythrodysesthesia syndrome. As expected, the incidences of severe neutropenia and most of these non-hematological toxicities at the RD for the combination were higher than those reported in previous studies with each drug alone at their approved monotherapy doses, i.e. lurbinectedin 3.2 mg/m2 D1 q3w21,22 and capecitabine 2500 mg/m2 D1-14 q3w.14,23,24 In particular, the higher rates of diarrhea, mucositis, decreased appetite and palmar-plantar erythrodysesthesia syndrome observed with the combination compared to single-agent lurbinectedin may be attributed to addition of capecitabine, as these toxicities are frequently found in patients treated with single-agent capecitabine. Combination of capecitabine with other antitumor drugs has been associated with higher incidences of severe gastrointestinal disorders and severe palmar-plantar erythrodysesthesia syndrome compared to these drugs alone.25 Nevertheless, all gastrointestinal disorders and palmar-plantar erythrodysesthesia episodes reported with capecitabine/lurbinectedin at the RD in the present study were mild or moderate. Notably, some dermatological AEs commonly associated with taxane therapy, such as alopecia or nail disorders,26 were not found at this RD. In addition, no toxic deaths or discontinuations due to toxicity occurred at this RD, thereby further suggesting an acceptable safety profile.
The PK parameters of lurbinectedin described herein were very similar to those reported as a single agent.6 The concentration–time plasma profiles of capecitabine and its metabolites in the present study showed high variability. A PopPK model based on these profiles had similar absorption rate values, a lower volume of distribution for capecitabine and lower CL values for metabolites compared to a PopPK model based on data from other phase I trials.12 Exposure values for capecitabine and its metabolites were about twofold or threefold higher, and time to maximum plasma concentration for capecitabine was shorter, than that reported elsewhere.27 However, no relationship was established between lurbinectedin dose or exposure and capecitabine exposure or its metabolites, and therefore no evidence of drug–drug interactions between capecitabine and lurbinectedin or with capecitabine metabolites was found.
In conclusion, oral capecitabine daily on D1-D14 combined with i.v. lurbinectedin on D1 q3w showed a manageable safety profile and promising antitumor activity in patients with relapsed MBC, especially in those with HR-positive tumors. These results support further development of the capecitabine/lurbinectedin combination in this indication.
Acknowledgements
The authors thank all the motivated patients, the caregivers who enrolled on the trial and all the clinical trial support staff in all the enrolling sites.
Funding
This work was supported by Pharma Mar S.A., including grants from the Centro para el Desarrollo Tecnológico Industrial (CDTI) during the conduct of the study [grant number IDI-20130013].
Disclosure
VB reports employment as Director of Clinical Cancer Research, NEXT Madrid, Universitary Hospital QuirónSalud Pozuelo; institutional financial support for clinical trials from Abbvie, ACEO, Adaptaimmune, Amcure, AMGEN, Amunix, AstraZeneca, BMS Cytomx, GSK, Genentech/Roche, H3, Incyte, Janssen, Kura, Lilly, Loxo, Nektar, Macrogenics, Menarini, Merck, Merus, Nanobiotix, Novartis, Pfizer, PharmaMar, Principia, PUMA, Sanofi, Taiho, Tesaro, BeiGene, Transgene, Takeda, Incyte, Innovio, MSD, PsiOxus, Seattle Genetics, Mersana, Daiichi, Nektar, Astellas, ORCA, Boston Therapeutics, Dynavax, DebioPharm, Boehringer Ingelheim, Regeneron, Millennium, Synthon, Spectrum, Rigontec and Zenith; advisory board/consultant position with Puma Biotechnology, Ideaya Biosciences, Loxo Therapeutics, CytomX Therapeutics, Guidepoint, Oncoart and IDMC Nanobiotix NANORAY-312; honoraria (for speaking) from Eli Lilly and MSD; and travel/inscription/accommodation from Bayer (ESMO GI), all outside the submitted work. PA reports non-financial support from MSD, Roche, Pfizer and Amgen; and personal fees from Boehringer Ingelheim, Macrogenics, Amcure, Synthon, Servier, G1 Therapeutics, Roche, Novartis, Amgen, Radius, Deloitte, Menarini and Gilead, all outside the submitted work. CK reports personal fees for salary as full-time employee and stock ownership from Pharma Mar, outside the submitted work. XELE, MS and MCY report personal fees for salary as full-time employees from Pharma Mar, outside the submitted work. CFT was an employee of Pharma Mar at the time the study was carried out. JT reports scientific consultant position with Array Biopharma, AstraZeneca, Avvinity, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Ikena Oncology, Inspirna Inc, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, Neophore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Scandion Oncology, Servier, Sotio Biotech, Taiho, Tessa Therapeutics and TheraMyc; and educational collaboration with Imedex, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education and Physicians Education Resource (PER), all outside the submitted work. All other authors have declared no conflicts of interest.
Data sharing
Individual participant data are not publicly available since this requirement was not anticipated in the study protocol considering that this trial started patient enrolment in 2013. Clinical trial summary results were placed in the European Clinical Trials Database (EudraCT; https://eudract.ema.europa.eu; study 2012-005268-86) and ClinicalTrials.gov (Identifier: NCT02210364).
Supplementary data
References
- 1.Cuevas C., Perez M., Martin M.J., et al. Synthesis of ecteinascidin ET-743 and phthalascidin Pt-650 from cyanosafracin B. Org Lett. 2000;2:2545–2548. doi: 10.1021/ol0062502. [DOI] [PubMed] [Google Scholar]
- 2.Bueren-Calabuig J.A., Giraudon C., Galmarini C.M., Egly J.M., Gago F. Temperature-induced melting of double-stranded DNA in the absence and presence of covalently bonded antitumour drugs: insight from molecular dynamics simulations. Nucleic Acids Res. 2011;39:8248–8257. doi: 10.1093/nar/gkr512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harlow M.L., Maloney N., Roland J., et al. Lurbinectedin inactivates the Ewing sarcoma oncoprotein EWS-FLI1 by redistributing it within the nucleus. Cancer Res. 2016;76:6657–6668. doi: 10.1158/0008-5472.CAN-16-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santamaria Nunez G., Robles C.M., Giraudon C., et al. Lurbinectedin specifically triggers the degradation of phosphorylated RNA polymerase II and the formation of DNA breaks in cancer cells. Mol Cancer Ther. 2016;15:1–14. doi: 10.1158/1535-7163.MCT-16-0172. [DOI] [PubMed] [Google Scholar]
- 5.Leal J.F., Martinez-Diez M., Garcia-Hernandez V., et al. PM01183, a new DNA minor groove covalent binder with potent in vitro and in vivo anti-tumour activity. Br J Pharmacol. 2010;161:1099–1110. doi: 10.1111/j.1476-5381.2010.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elez M.E., Tabernero J., Geary D., et al. First-in-human phase I study of Lurbinectedin (PM01183) in patients with advanced solid tumors. Clin Cancer Res. 2014;20:2205–2214. doi: 10.1158/1078-0432.CCR-13-1880. [DOI] [PubMed] [Google Scholar]
- 7.Jimeno A., Sharma M.R., Szyldergemajn S., et al. Phase I study of lurbinectedin, a synthetic tetrahydroisoquinoline that inhibits activated transcription, induces DNA single- and double-strand breaks, on a weekly × 2 every-3-week schedule. Invest New Drugs. 2017;35:471–477. doi: 10.1007/s10637-017-0427-2. [DOI] [PubMed] [Google Scholar]
- 8.Aviles P., Guillen M.J., Galmarini C., et al. Synergism of lurbinectedin (PM01183) combined with 5-fluorouracil (5-FU): in vitro and in vivo studies. Cancer Res. 2013;73 Abstract 5498. [Google Scholar]
- 9.Mackean M., Planting A., Twelves C., et al. Phase I and pharmacologic study of intermittent twice-daily oral therapy with capecitabine in patients with advanced and/or metastatic cancer. J Clin Oncol. 1998;16:2977–2985. doi: 10.1200/JCO.1998.16.9.2977. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute Common Terminology Criteria for Adverse Events v.4.0 (CTCAE) 2009. https://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5x7.pdf Available at.
- 11.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Urien S., Rezai K., Lokiec F. Pharmacokinetic modelling of 5-FU production from capecitabine – a population study in 40 adult patients with metastatic cancer. J Pharmacokinet Pharmacodyn. 2005;32:817–833. doi: 10.1007/s10928-005-0018-2. [DOI] [PubMed] [Google Scholar]
- 13.Waks A.G., Winer E.P. Breast cancer treatment: a review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 14.Blum J.L., Jones S.E., Buzdar A.U., et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–493. doi: 10.1200/JCO.1999.17.2.485. [DOI] [PubMed] [Google Scholar]
- 15.Fumoleau P., Largillier R., Clippe C., et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004;40:536–542. doi: 10.1016/j.ejca.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Pallis A.G., Boukovinas I., Ardavanis A., et al. A multicenter randomized phase III trial of vinorelbine/gemcitabine doublet versus capecitabine monotherapy in anthracycline- and taxane-pretreated women with metastatic breast cancer. Ann Oncol. 2012;23:1164–1169. doi: 10.1093/annonc/mdr405. [DOI] [PubMed] [Google Scholar]
- 17.Thomas E.S., Gomez H.L., Li R.K., et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–5217. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 18.Sparano J.A., Vrdoljak E., Rixe O., et al. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010;28:3256–3263. doi: 10.1200/JCO.2009.24.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman P.A., Awada A., Twelves C., et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015;33:594–601. doi: 10.1200/JCO.2013.52.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Shaughnessy J., Miles D., Vukelja S., et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol. 2002;20:2812–2823. doi: 10.1200/JCO.2002.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Trigo J., Subbiah V., Besse B., et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020;21:645–654. doi: 10.1016/S1470-2045(20)30068-1. [DOI] [PubMed] [Google Scholar]
- 22.Gaillard S., Oaknin A., Ray-Coquard I., et al. Lurbinectedin versus pegylated liposomal doxorubicin or topotecan in patients with platinum-resistant ovarian cancer: a multicenter, randomized, controlled, open-label phase 3 study (CORAIL) Gynecol Oncol. 2021;163:237–245. doi: 10.1016/j.ygyno.2021.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Van Cutsem E., Findlay M., Osterwalder B., et al. Capecitabine, an oral fluoropyrimidine carbamate with substantial activity in advanced colorectal cancer: results of a randomized phase II study. J Clin Oncol. 2000;18:1337–1345. doi: 10.1200/JCO.2000.18.6.1337. [DOI] [PubMed] [Google Scholar]
- 24.Nishijima T.F., Suzuki M., Muss H.B. A comparison of toxicity profiles between the lower and standard dose capecitabine in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2016;156:227–236. doi: 10.1007/s10549-016-3756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y., Yang H., Wei J.F., Meng L. Efficacy and toxicity of capecitabine-based chemotherapy in patients with metastatic or advanced breast cancer: results from ten randomized trials. Curr Med Res Opin. 2012;28:1911–1919. doi: 10.1185/03007995.2012.748655. [DOI] [PubMed] [Google Scholar]
- 26.Marks D.H., Qureshi A., Friedman A. Evaluation of prevention interventions for taxane-induced dermatologic adverse events: a systematic review. JAMA Dermatol. 2018;154:1465–1472. doi: 10.1001/jamadermatol.2018.3465. [DOI] [PubMed] [Google Scholar]
- 27.Reigner B., Blesch K., Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet. 2001;40:85–104. doi: 10.2165/00003088-200140020-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.