Abstract
Background
Patients with microsatellite stable (MSS) colorectal carcinoma (CRC) do not respond to immune checkpoint inhibitors. Preclinical models suggested synergistic anti-tumour activity combining CXD101 and anti-programmed cell death protein 1 treatment; therefore, we assessed the clinical combination of CXD101 and nivolumab in heavily pre-treated patients with MSS metastatic CRC (mCRC).
Patients and methods
This single-arm, open-label study enrolled patients aged 18 years or older with biopsy-confirmed MSS CRC; at least two lines of systemic anticancer therapies (including oxaliplatin and irinotecan); at least one measurable lesion; Eastern Cooperative Oncology Group performance status of 0, 1 or 2; predicted life expectancy above 3 months; and adequate organ and bone marrow function. Nine patients were enrolled in a safety run-in study to define a tolerable combination schedule of CXD101 and nivolumab, followed by 46 patients in the efficacy assessment phase. Patients in the efficacy assessment cohort were treated orally with 20 mg CXD101 twice daily for 5 consecutive days every 3 weeks, and intravenously with 240 mg nivolumab every 2 weeks. The primary endpoint was immune disease control rate (iDCR).
Results
Between 2018 and 2020, 55 patients were treated with CXD101 and nivolumab. The combination therapy was well tolerated with the most frequent grade 3 or 4 adverse events being neutropenia (18%) and anaemia (7%). Immune-related adverse reactions commonly ascribed to checkpoint inhibitors were surprisingly rare although we did see single cases of pneumonitis, hypothyroidism and hypopituitarism. There were no treatment-related deaths. Of 46 patients assessable for efficacy, 4 (9%) achieved partial response and 18 (39%) achieved stable disease, translating to an immune disease control rate of 48%. The median overall survival (OS) was 7.0 months (95% confidence interval 5.13-10.22 months).
Conclusions
The primary endpoint was met in this phase II study, which showed that the combination of CXD101 and nivolumab, at full individual doses in the treatment of advanced or metastatic MSS CRC, was both well tolerated and efficacious.
Key words: CXD101, nivolumab, MSS, colorectal cancer
Highlights
-
•
Patients with refractory MSS MCRC have a poor outcome and do not respond to checkpoint inhibitors.
-
•
The histone deacetylase inhibitor CXD101 was combined with nivolumab to try to increase the response to immunotherapy.
-
•
The combination was well tolerated with surprisingly minimal immune-related toxicity.
-
•
CXD101/nivolumab is efficacious in refractory MSS MCRC with a response rate of 9%, DCR of 48% and median OS of 7.0 months.
Introduction
Colorectal carcinoma (CRC) is a malignant neoplasm with high global incidence. Based on the World Health Organization estimates, there were 1.1 million new cases of CRC diagnosed in 2020, and the incidence is expected to rise to 1.8 million by 2040.1 About one-fifth of diagnosed CRC cases are metastatic and the 5-year survival rate upon diagnosis is estimated to be 14.3%.2 While surgery may be indicated for localised disease, chemotherapy has remained the standard management for metastatic CRC (mCRC). Two agents have been approved by the US Food and Drug Administration (FDA) for the treatment of third-line and above metastatic CRC, namely trifluridine-tipiracil hydrochloride (Lonsurf®), a thymidine-based nucleoside analogue3 and regorafenib (Stivarga®), a multikinase inhibitor.4
The DNA mismatch repair system plays an essential role in maintaining fidelity of DNA replication and suppression of mutagenesis. DNA mismatch repair deficiency (dMMR) causes slippage at DNA microsatellites—referred to as microsatellite instability (MSI)—and accumulation of mutations, resulting in increased neoantigen load and tumour recognition by infiltrating lymphocytes.5
The categorisation of CRC into MSS and MSI is highly relevant to the use of immune checkpoint inhibitors as patients with MSI CRC were found to respond favourably to programmed cell death protein 1 (PD-1) inhibitors, such as nivolumab and pembrolizumab.6,7 Unfortunately, 90%-95% of mCRC patients are of the MSS subtype,8 which do not respond to immune checkpoint inhibitors.9
Nivolumab is a human immunoglobulin monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2. The drug removes inhibitory signals on T-cells and stimulates the immune system to mount an effective response against the tumour. Based on results of the phase II CheckMate-142 study, the US FDA granted accelerated approval for nivolumab in patients with MSI CRC who have progressed following treatment with a fluoropyrimidine, oxaliplatin and irinotecan.6
Histone deacetylase inhibitors (HDACi) belong to a broad class of epigenetic drugs that bind with different affinities to the 18 mammalian HDACs identified to date.10 They maintain lysine acetylation on core histones, leading to chromatin uncoiling and gene transcription, which impact on cell proliferation, angiogenesis, differentiation, motility and induction of apoptosis.11,12 Four HDACi have so far been approved by the FDA: romidepsin (Isodax®) for cutaneous T-cell lymphoma (CTCL) and peripheral T-cell lymphoma (PTCL), vorinostat (Zolinza®) for CTCL, belinostat (Beleodaq®) for PTCL and panobinostat (Farydax®) for multiple myeloma. None of these have shown clinical activity in CRC.
CXD101 is an orally administered drug with selective inhibitory activities against class I HDACs: HDAC1 (IC50: 63 nM), HDAC2 (IC50: 570 nM) and HDAC3 (IC50: 550 nM). A phase I study conducted previously determined the maximum tolerated dose (MTD) of CXD101 to be 20 mg twice daily (b.i.d), which resulted in maximal plasma concentrations of 231–342 ± 126 nM, well within the biologically active range. The most frequent adverse events (AEs) were fatigue, nausea and reversible cytopenia. Key grade 3-4 AEs included thrombocytopenia (11%), neutropenia (17%) and neutropenic fever (2%). While the toxicity profile was similar to approved HDAC inhibitors, the frequency and severity of AEs appeared to be fewer and milder, suggesting improved safety. Anti-tumour activity was reported predominantly in lymphoma patients. Of 17 lymphoma patients evaluated, there was one complete response, three partial responses (PR) and nine stable diseases (SD), with duration of responses ranging from 161to 441 days.13
A recently published study on the mechanism of action of CXD101 describes the upregulation of differentially expressed genes involved in antigen processing and natural killer cell–mediated cytotoxicity in human cell lines (SW620, A549, HCT116), murine MSS CRC cell line (CT26) and MSS CRC tumours growing in vivo (colon26 syngeneic mouse model).14 These include increased expression of major histocompatibility complex class I and II genes which drove infiltration of CD4- and CD8-positive T lymphocytes into the tumour microenvironment. The MSS CRC mouse model also showed that CXD101 displayed minor anti-tumour effects, and murine PD-1 and Cytotoxic T-lymphocyte-associated antigen-4 inhibitors were completely inactive, whereas the combination of both agents demonstrated a profound anti-tumour effect. These results suggest synergy between CXD101 and immune checkpoint inhibitors, providing a scientific rationale to explore the combination of CXD101 and nivolumab in the treatment of patients with MSS mCRC.
Patients and methods
Study design and participants
We carried out a phase Ib limited dose-finding study followed by a phase II single-arm, open-label trial (EudraCT NUMBER 2017-004509-42) to determine the efficacy and tolerability of CXD101 given orally b.i.d. for 5 consecutive days every 3 weeks in combination with nivolumab 240 mg by intravenous (i.v.) infusion every 2 weeks in patients with metastatic, previously treated (third or later line), MSS CRC.
This multi-institution phase II trial was conducted in five cancer centres in the UK. All patients of 18 years or more had biopsy-confirmed proficient mismatch repair/MSS CRC and gave written informed consent. All patients had previous first- and second-line treatment including oxaliplatin and irinotecan unless contraindicated. Patients were required to have adequate organ and bone marrow function (haemoglobin > 100 g/dl, neutrophils > 1.5 × 109/l and platelets > 100 × 109/l; serum creatinine ≤ 1.5 × ULN, AST ≤ 3.0 × ULN, total bilirubin ≤ 1.5 × ULN).
This study was approved by the Oxford Research Ethics Committee (REC number: 18/SC/0108) and Medicines and Healthcare products Regulatory Agency, and was conducted in accordance with ICH E6 (R2) Good Clinical Practice Guidelines, and the Declaration of Helsinki.
The phase Ib single-arm dose-escalation trial determined the safety, tolerability and dose-limiting toxicities and, therefore, the MTD of multiple oral doses of CXD101 and nivolumab. The incidence and severity of AEs (evaluated according to Common Terminology Criteria for Adverse Events version 4.03), vital signs, electrocardiogram parameters, biochemistry, haematology and urinalysis were recorded to determine tolerability. Three patients were treated starting at CXD101 30 mg (days 1-5) q 3 weekly and nivolumab 240 mg iv 2 weekly and a further six patients at CXD101 20 mg b.i.d on days 1-5 q 3 weekly and nivolumab 240 mg iv 2 weekly, which was the recommended dose for the phase II trial.
Efficacy was measured using Immune Response Evaluation Criteria in Solid Tumours (iRECIST) imaging studies, typically computed tomography scan of the chest, abdomen and pelvis, supplemented by magnetic resonance imaging of the liver when required, carried out at baseline and after every 6 weeks, with objective confirmation of response 6 weeks (±1 week) after observation. A total of 46 subjects were enrolled in the phase II study. Tumour response data collected in phase II were pooled with n = 6 subjects from phase Ib, where possible.
Outcomes
The primary endpoint was the anti-tumour activity of CXD101 + nivolumab in terms of investigator-assessed disease control [complete and PR and SD (iCR + iPR + iSD, at 8 weeks)] according to immune RECIST 1.1 criteria. The secondary endpoints evaluated by the study investigators were progression-free survival (PFS, time from the date of first infusion to disease progression or death from any cause); overall survival (OS, time from the date of first infusion to death or loss to follow-up); and type, incidence, severity, seriousness and relationship to study medications of AEs and any laboratory abnormalities.
Statistical design
The trial design for the phase II part of the study followed a two-stage design. Subjects who signed informed consent and received at least one dose (which may only have been a partial course) of study treatment, and who had at least one follow-up tumour assessment were included in a full analysis set.
Subjects who received any amount of study treatment and had at least one safety assessment (including ‘death’) were included in a safety set. Tumour response data collected in phase II were pooled with dose-matched subjects from phase Ib.
Sixteen subjects were assessed in the first phase II stage. If there were seven or fewer cases of disease control in these subjects, the study would have been stopped. Otherwise, 30 additional subjects would be recruited into the second stage (i.e. a total of 46).
Consented subjects who did not receive any study medication (i.e. withdrawn from the study during the screening phase) were replaced, and did not contribute to the assessment of numbers of responders.
Role of funding source
The study was conducted by Syneos Health, and was sponsored by Celleron Therapeutics Ltd.
Results
Between July 2018 and June 2020, 55 patients were treated with CXD101 and nivolumab. Nine patients were enrolled in a safety run-in study (phase Ib) to define a tolerable combination dose schedule of CXD101 and nivolumab, which was determined to be 20 mg CXD101 b.i.d for 5 consecutive days every 3 weeks, and intravenously with 240 mg nivolumab (i.e. full monotherapy doses for both drugs) every 2 weeks, with both drugs commencing on the same day in the initial cycle. This was followed by a two-stage efficacy evaluation (phase II). As 8 out of 16 patients in stage I exhibited disease control, the efficacy assessment progressed into stage II, which enrolled an additional 30 patients.
The data cut-off was Jun 2020, although survival follow-up continues for a small group of subjects at the time of publication. The median follow-up was 17.1 months (interquartile range 6.5-25.3 months). The median age of the study cohort was 58 years, ranging from 18 to 81. Of the 55 patients who received combination treatment, 17 (31%) were female and 38 (69%) were male. Twenty-one (38%) patients had an Eastern Cooperative Oncology Group (ECOG) performance score of 0, and 34 (62%) had a score of 1. The median number of prior treatments received was three, ranging from two to seven. The mean baseline lymphocyte count was 1.25 × 109/l, ranging from 0.4 × 109/l to 2.49 × 109/l. The mean alkaline phosphatase count was 201 U/l, ranging from 43 U/l to 1242 U/l. We had the RAS status in 52 patients and 21 (40%) were wild-type and 31 (60%) mutated. There was no significant association between RAS status and treatment efficacy. Out of 47 patients with known BRAF status, all were wild-type. A summary of the baseline characteristics is presented in Table 1.
Table 1.
Baseline characteristics
| Patients (n = 55) | |
|---|---|
| Age in years, median (range) | 58 (18-81) |
| Sex | |
| Female | 17 (31%) |
| Male | 38 (69%) |
| ECOG status | |
| Score 0 | 21 (38%) |
| Score 1 | 34 (62%) |
| Previous therapies, median (range) | 3 (2-7) |
| Baseline lymphocytes, mean (range) | 1.25 × 109/l (0.4-2.49) |
| Baseline alkaline phosphatase, mean (range) | 201 U/l (43-1242) |
Data are in n (%) unless specified otherwise.
ECOG, Eastern Cooperative Oncology Group.
Out of a total of 84 patients consented, 55 patients received at least one dose of CXD101 and nivolumab and were evaluated in the safety set (n = 29 ineligible for treatment). The combination was generally well tolerated and was consistent with the recognised side-effect profile of the individual agents. The most frequent AEs regardless of grade were fatigue (58%), nausea (45%) and decreased appetite (29%). Grade 3-4 AEs occurring in >5% of the treated patients include neutropenia (18%) and anaemia (7%). Other grade 3-4 AEs occurring only once or twice include thrombocytopenia, diarrhoea, nausea, fatigue, amylase increase, aspartate aminotransferase increase and lethargy. Ten (18%) patients had treatment-related serious AEs (SAEs), including one with anaemia, two with neutropenia, one with diarrhoea, two with nausea, one with fatigue, one with lower respiratory tract infection, one with decreased appetite and one with headache (associated with cerebral metastases). No single SAE occurred more than twice (5%). All AEs reported were manageable and no toxicity-related deaths occurred. Immune-related adverse reactions commonly ascribed to checkpoint inhibitors were surprisingly rare, although we did see single cases of pneumonitis, hypothyroidism and hypopituitarism.15 A summary of the safety profile is presented in Table 2.
Table 2.
Treatment-related (possibly, probably or definitely) AEs and SAEs with a frequency of 5% or more
| Adverse event | All grades (%) | G1-2 (%) | G3-4 (%) | SAEs (%) |
|---|---|---|---|---|
| Blood and lymphatic system disorders | ||||
| Anaemia | 15 (27%) | 11 (20%) | 4 (7%) | 1 (2%) |
| Neutropenia | 14 (25%) | 4 (7%) | 10 (18%) | 2 (4%) |
| Thrombocytopenia | 10 (18%) | 9 (16%) | 1 (2%) | |
| Gastrointestinal disorders | ||||
| Abdominal pain upper | 3 (5%) | 3 (5%) | ||
| Constipation | 3 (5%) | 3 (5%) | ||
| Diarrhoea | 9 (16%) | 8 (15%) | 1 (2%) | 1 (2%) |
| Dry mouth | 3 (5%) | 3 (5%) | ||
| Dyspepsia | 5 (9%) | 5 (9%) | ||
| Nausea | 25 (45%) | 24 (44%) | 1 (2%) | 2 (4%) |
| Vomiting | 12 (22%) | 12 (22%) | ||
| General disorders and administrative site conditions | ||||
| Chills | 3 (5%) | 3 (5%) | ||
| Fatigue | 32 (58%) | 30 (55%) | 2 (4%) | 1 (2%) |
| Infections and infestations | ||||
| Lower respiratory tract infection | 3(5%) | 3 (5%) | 1 (2%) | |
| Oral candidiasis | 4 (7%) | 4 (7%) | ||
| Investigations | ||||
| Alanine aminotransferase increased | 3 (5%) | 3 (5%) | ||
| Amylase increased | 5 (9%) | 3 (5%) | 2 (4%) | |
| Aspartate aminotransferase increased | 4 (7%) | 3 (5%) | 1 (2%) | |
| Weight decreased | 3 (5%) | 3 (5%) | ||
| Metabolism and nutrition disorders | ||||
| Decreased appetite | 16 (29%) | 16 (29%) | 1 (2%) | |
| Musculoskeletal and connective-tissue disorders | ||||
| Arthralgia | 3 (5%) | 3 (5%) | ||
| Nervous system disorders | ||||
| Headache | 6 (11%) | 6 (11%) | 1 (2%) | |
| Lethargy | 11 (20%) | 9 (16%) | 2 (4%) | |
| Respiratory, thoracic and mediastinal disorders | ||||
| Dyspnoea | 5 (9%) | 5 (9%) | ||
| Skin and subcutaneous tissue disorders | ||||
| Dry skin | 3 (5%) | 3 (5%) | ||
| Pruritus | 7 (13%) | 7 (13%) | ||
| Rash | 3 (5%) | 3 (5%) |
Safety population, n = 55.
AEs, adverse events; SAEs, serious adverse events.
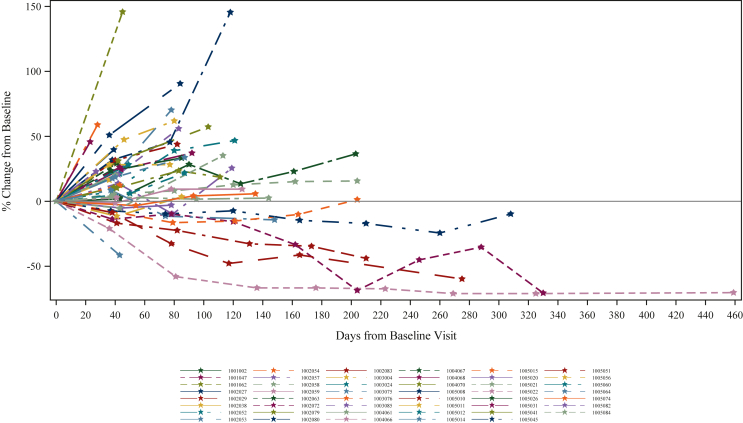
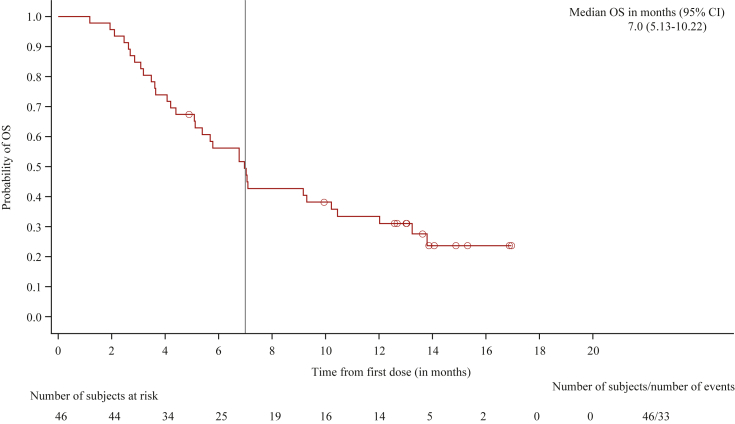
Of 46 patients assessable for efficacy in the full analysis set, 4 (9%) achieved PR and 18 (39%) achieved SD, translating to an immune disease control rate (iDCR) of 48% and an immune objective response rate (iORR) of 9%. Tumour sizes measured as percentage change in sum of largest diameter (SLD) of target lesions from baseline are presented in Figure 1, with each line representing an individual patient. The median PFS was 2.1 months [95% confidence interval (CI) 1.4-3.9 months] and the median OS was 7.0 months (95% CI 5.13-10.22 months) (Table 3). At the time of publication, survival follow-up continues in a small group of subjects, but the median was imputed once the 50% population endpoint had been achieved. At 2 years from the start of treatment, eight patients (14.5%) were in survival, and at 3 years four subjects were still alive (7.3%). A Kaplan–Meier curve describing the OS is presented in Figure 2.
Figure 1.
Activity of CXD101 plus nivolumab in patients with microsatellite-stable colorectal cancer. Tumour sizes measured as percentage change in SLD of target lesions from baseline. SLD, sum of largest diameter.
Table 3.
Survival data
| Total (n = 46) | |
|---|---|
| Progression-free survival | |
| iRECIST confirmation of progression, n (%) | 42 (91.3) |
| Death in the absence of progression, n (%) | 2 (4.3) |
| Censored subjects, n (%) | 2 (4.3) |
| 25th percentile progression-free survival (months)a | 1.3 |
| Median progression-free survival (months)a | 2.1 |
| 95% CI for median progression-free survival (months)a | 1.41-3.91 |
| 75th percentile progression-free survival (months)a | 4.7 |
| Overall survival | |
| Death, n (%) | 33 (71.7) |
| Censored subjects, n (%) | 13 (28.3) |
| 25th percentile overall survival (months)a | 3.6 |
| Median overall survival (months)a | 7.0 |
| 95% CI for median overall survival (months)a | 5.13-10.22 |
| 75th percentile overall survival (months)a | 13.8 |
CI, confidence interval.
Calculated using the Kaplan–Meier technique.
Figure 2.
Kaplan–Meier survival analysis. OS, overall survival.
Discussion
This phase II trial met its primary endpoint and showed that the combination of CXD101 and nivolumab was active as a third-line and above treatment for patients with advanced, late-stage MSS CRC. Disease control assessed by central adjudication was 48% and met the per-protocol statistical boundaries to show anti-tumour activity. These results are supported by the durability of responses (14.5% alive at 2 years), and the median OS of 7.0 months, which is noteworthy in the refractory third-/fourth-/fifth-line CRC setting (median number of previous therapies three with a range of two to seven). The combination was well tolerated with mild fatigue and neutropenia and had a low SAE rate. Given that the broad class of HDACi and immune checkpoint inhibitors have been shown to be clinically inactive as single agents in MSS CRC, it is plausible that the degree of disease control demonstrated in this study reflects or recapitulates the syngeneic mouse model in which the drug combination was synergistic, based on tumoural immune reactivation.
The main strengths of our study are the size of the treated cohort in the context of a multicentre study; the enrolment of a representative population of MSS patients with resistant disease; confirmation of responses at least 4 weeks following the documentation of response; and the use of an independent review committee to confirm investigator assessments and to minimise data interpretation bias. The limitations of our study are the single-arm design with no control group and the absence of assessment of tissue-associated immune parameters and other exploratory biomarkers. We have designed a window of opportunity study to collect biopsies before and after treatment and a randomised phase III trial to provide these definitive data. Acknowledging the inherent limitations of cross-trial comparisons, an indirect comparison using the data from randomised trials of regorafenib4 and trifluridine/tipiracil3 carried out with similar assessment criteria (RECIST assessed by the investigator) suggests a higher overall response rate for CXD101 and nivolumab, with similar median OS and PFS. It also suggests a more favourable safety profile for CXD101 and nivolumab than regorafenib and trifluridine/tipiracil, with respective SAE rates of 18%, 44% and 30%. Our study had a partial response rate of 9%, a disease control rate of 48% and a median OS of 7.0 months. In separate randomised trials of patients with advanced CRC in the third-line setting versus best supportive care, the median OS for TAS 102 was 7.1 months, with a disease control rate of 44% and response rate of 2%. The most frequently observed clinically significant AEs associated with trifluridine/tipiracil were neutropenia, which occurred in 38% of those treated, and leukopenia, which occurred in 21%. Median OS was 6·4 months for regorafenib-treated patients, with a disease control rate of 41% and response rate of 1%. The most common AEs of grade 3 or higher related to regorafenib were hand–foot–skin reaction (17%), fatigue (10%), diarrhoea (7%), hypertension (7%) and rash or desquamation (6%). In the international PRECONNECT phase IIIb study of 161 patients in Italy, the median disease control rate was only 28.6% but PFS was reached at 3.0 months.16
Deficient mismatch repair (dMMR; MSI) is one of the key genetic mechanisms driving the occurrence and progression of CRC. One consequence of dMMR is that these tumour cells carry a very high neoantigen load driven by their tumour mutational burden (TMB), increasing the likelihood of immune recognition. Perhaps unsurprisingly, MSI colon tumours have a strong lymphocyte infiltration and have a significantly better prognosis than their MSS counterparts, and when diagnosed with stage II disease, PD-1 inhibitors in metastatic colorectal carcinoma with MSI have demonstrated a high disease control rate and favourable PFS; however, reported response rates to pembrolizumab and nivolumab are variable and often < 50%, which may be related to heterogeneity of TMB.
Part of the reason why MSS tumours are unresponsive to single-agent immune checkpoint inhibitors may be due to the low TMB which is ∼10-fold less than MSI tumours. One may hypothesise that by adding an HDACi may increase immune recognition and cytolysis. This may lead to an increase in TMB due to upregulating the machinery of antigen presentation, increasing tumoural infiltration by cytotoxic T lymphocytes and reducing T reg cell numbers in MSS CRC. We believe that this may be the mechanism underpinning the clinical activity demonstrated in this clinical trial, which showed that the combination of CXD101 and nivolumab, at full individual doses in the treatment of advanced or MSS mCRC, was both well tolerated and efficacious.
Acknowledgements
The team would like to thank all the patients involved in the study and their families. The views expressed are those of the authors and not necessarily those of the funding body. The research was funded/supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Funding
This trial was supported by Celleron Therapeutics. This work was supported by The Oxford NIHR Comprehensive Biomedical Research Centre (BRC), and a Cancer Research UK Advanced Clinician Scientist Fellowship [grant number C26642/A27963] to DNC.
Disclosure
MS: Merck, Amgen, Servier. JG: Pierre Fabre, BMS, Bayer, Servier, Nucana, Amgen, MSD. DCu: OVIBIO and to To Royal Marsden Hospital: MedImmune/AZ, Clovis, Eli Lilly, 4SC, Bayer, Celgene, LEAP, Roche. RP: AZ, Novartis, Bayer, Tesaro, BMS, MSD, Pierre Fabre, Biosceptre, Cybrexa, Ellipses, Astex, CV6, Medivir, GammaDelta, Sanofi. DCh: MSD. RK: -. SC: Employee of Celleron and share options. SZ: Celleron share options. N. LaT: Celleron shares. DK: Personal Holdings and payments from Celleron + share options.
Supplementary Data
References
- 1.Ferlay J., Colombet M., Soerjomataram I., et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Cancer of the Colon and Rectum - Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/colorect.html Available at.
- 3.Mayer R.J., Van Cutsem E., Falcone A., et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 4.Grothey A., Van Cutsem E., Sobrero A., et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 5.Smyrk T.C., Watson P., Kaul K., Lynch H.T. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91:2417–2422. [PubMed] [Google Scholar]
- 6.Overman M.J., McDermott R., Leach J.L., et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le D.T., Uram J.N., Wang H., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hewish M., Lord C.J., Martin S.A., Cunningham D., Ashworth A. Mismatch repair deficient colorectal cancer in the era of personalized treatment. Nat Rev Clin Oncol. 2010;7:197–208. doi: 10.1038/nrclinonc.2010.18. [DOI] [PubMed] [Google Scholar]
- 9.Topalian S.L., Hodi F.S., Brahmer J.R., et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P., Wang Z., Liu J. Role of HDACs in normal and malignant hematopoiesis. Mol Cancer. 2020;19:5. doi: 10.1186/s12943-019-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ropero S., Esteller M. The role of histone deacetylases (HDACs) in human cancer. Mol Oncol. 2007;1:19–25. doi: 10.1016/j.molonc.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barneda-Zahonero B., Parra M. Histone deacetylases and cancer. Mol Oncol. 2012;6:579–589. doi: 10.1016/j.molonc.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eyre T.A., Collins G.P., Gupta A., et al. A phase 1 study to assess the safety, tolerability, and pharmacokinetics of CXD101 in patients with advanced cancer. Cancer. 2019;125:99–108. doi: 10.1002/cncr.31791. [DOI] [PubMed] [Google Scholar]
- 14.Blaszczak W., Liu G., Zhu H., et al. Immune modulation underpins the anti-cancer activity of HDAC inhibitors. Mol Oncol. 2021;15:3280–3298. doi: 10.1002/1878-0261.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bristol-Myers Squibb OPDIVO (nivolumab) SmPC. https://www.medicines.org.uk/emc/product/6888/smpc#gref Available at.
- 16.Zaniboni A., Antonio Barone C., Chiara Banzi M., et al. Italian results of the PRECONNECT study: safety and efficacy of trifluridine/tipiracil in metastatic colorectal cancer. Future Oncol. 2021;17(18):2315–2324. doi: 10.2217/fon-2020-1278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.