Abstract
Background
COGNITION (Comprehensive assessment of clinical features, genomics and further molecular markers to identify patients with early breast cancer for enrolment on marker driven trials) is a diagnostic registry trial that employs genomic and transcriptomic profiling to identify biomarkers in patients with early breast cancer with a high risk for relapse after standard neoadjuvant chemotherapy (NACT) to guide genomics-driven targeted post-neoadjuvant therapy.
Patients and methods
At National Center for Tumor Diseases Heidelberg patients were biopsied before starting NACT, and for patients with residual tumors after NACT additional biopsy material was collected. Whole-genome/exome and transcriptome sequencing were applied on tumor and corresponding blood samples.
Results
In the pilot phase 255 patients were enrolled, among which 213 were assessable: thereof 48.8% were identified to be at a high risk for relapse following NACT; 86.4% of 81 patients discussed in the molecular tumor board were eligible for a targeted therapy within the interventional multiarm phase II trial COGNITION-GUIDE (Genomics-guided targeted post neoadjuvant therapy in patients with early breast cancer) starting enrolment in Q4/2022. An in-depth longitudinal analysis at baseline and in residual tumor tissue of 16 patients revealed some cases with clonal evolution but largely stable genetic alterations, suggesting restricted selective pressure of broad-acting cytotoxic neoadjuvant chemotherapies.
Conclusions
While most precision oncology initiatives focus on metastatic disease, the presented concept offers the opportunity to empower novel therapy options for patients with high-risk early breast cancer in the post-neoadjuvant setting within a biomarker-driven trial and provides the basis to test the value of precision oncology in a curative setting with the overarching goal to increase cure rates.
Keywords: early breast cancer, prospective precision oncology trial, curative precision oncology, molecularly targeted therapy, tumor evolution
Highlights
-
•
The COGNITION molecular profiling diagnostic study has been recruiting in Heidelberg since April 2019 (pilot phase).
-
•
Evidence-based alterations in early breast cancer potentially increase cure rates by targeted post-neoadjuvant treatment.
-
•
Mutational profiles/signatures remain mostly stable, suggesting restricted selective pressure during neoadjuvant treatment.
-
•
COGNITION as the basis to develop the multiarm interventional phase II precision oncology therapy trial COGNITION-GUIDE.
-
•
COGNITION is one of the first initiatives in the early setting to potentially impact cure rates by molecular-guided therapies.
Introduction
Breast cancer (BC) is the most common cancer in women, with 2.3 million newly diagnosed cases worldwide per year.1 Despite significantly higher cure rates due to improved earlier diagnosis and interdisciplinary standard-of-care (SoC) treatment, BC remains the leading cause of tumor-related deaths in women worldwide, with reports estimating 685 000 deaths annually. SoC of high-risk early BC includes cytotoxic chemotherapy, which is preferentially administered as a preoperative neoadjuvant chemotherapy (NACT).2 Given the close correlation between residual cancer burden after NACT and outcome, NACT outperforms adjuvant therapy regimens due to the prospect for early in vivo efficacy assessment of the administered therapy. This strategy facilitates the identification of high-risk patients at the timepoint of surgery, who have a poor prognosis, allowing to dynamically adapt and escalate therapeutic interventions before overt metastatic disease develops.3, 4, 5
Two standard classifiers are routinely used to identify patients with high-risk eBC after NACT: In triple-negative BC (TNBC) and human epidermal growth factor receptor 2 (HER2)-positive BC, nonpathological complete response (non-pCR) after NACT is associated with a high risk of relapse. In hormone receptor (HR)-positive, HER2-negative BC the more complex CPS-EG score (CS: pretreatment clinical stage; PS: post-treatment pathologic stage; E: pretreatment estrogen receptor status; G: pretreatment grade) is used to identify high-risk patients in the clinical routine.4,6
While a large proportion of patients present with a pathological complete response (pCR) following NACT, depending on the subtype in ∼40%-80% of patients highly chemotherapy-resistant residual tumors remain (non-pCR),7 which is presumably indicative of the overall distant tumor load giving rise to metastasis. Accordingly, a large proportion (30%-50%) of non-pCR patients relapse within the first 5 years.4 Consequently, the major advantage of NACT resides in its prognostic power to adapt therapy algorithms after surgery to lower this substantial risk of relapse by the administration of a further line of post-neoadjuvant therapy (PNACT). This concept has been corroborated in largely molecular unstratified cohorts: in patients with HER2-negative BC and a high risk of recurrence, additional post-neoadjuvant treatment with capecitabine improved overall survival within the CREATEX trial.8 By contrast, in KATHERINE improved invasive disease-free survival was shown for molecularly identified HER2-positive BC after a switch to PNACT with trastuzumab emtansine (T-DM1).9 Within the OlympiA trial, PNACT with olaparib also improved overall survival in high-risk patients with HER2-negative BC and molecularly identified pathogenic or likely pathogenic BRCA1/2 germline mutations.10 These data provide evidence for the concept that addition of PNACT in a risk-adapted manner can increase outcome in early BC. In this regard, the use of patient-individual molecular tumor profiles to personalize post-neoadjuvant therapies holds the promise to further improve cure rates.
Precision oncology trials offer treatment options by application of drugs targeting molecular alterations detected in tumor cells via genomic and/or transcriptomic profiling approaches. For example, the precision oncology trial CATCH (Comprehensive assessment of clinical features and biomarker to identify patients with advanced or metastatic breast cancer for marker driven trials in humans) for patients with advanced/metastatic BC revealed a clinical benefit for about one-third of treated patients.11 So far, however, this approach has been mainly confined to the palliative setting. Recently, application of molecular profiling is being introduced in neoadjuvant regimens to search for improved biomarkers predicting pCR or high risk (reviewed in12), with the prospect to apply and validate targeted molecular therapies also within post-neoadjuvant settings.13,14
Here we describe the results of the pilot phase of COGNITION (Comprehensive assessment of clinical features, genomics and further molecular markers to identify patients with early breast cancer for enrolment on marker driven trials) as a monocenter experience to implement genomics-guided targeted PNACT in early high-risk BC and report in-depth clonal evolution patterns in longitudinal analysis of the genomes of 16 paired pre- and post-NACT tumor tissue. The pattern of identified targets in this cohort has triggered the design of the interventional multiarm phase II trial COGNITION-GUIDE (Genomics-guided targeted post neoadjuvant therapy in patients with early breast cancer), with each biomarker-driven arm testing a single targeted drug, which will start recruitment in Q4 2022.
Material and methods
Patients and biomaterial
Patients with histologically confirmed early BC and indication for NACT irrespective of molecular subtype were screened for eligibility and consented upon presentation at the Division of Gynecologic Oncology at the National Center for Tumor Diseases (NCT) Heidelberg. Written informed consent was retrieved according to the study approval protocol by the local IRB of Heidelberg University (S-790/2018). To retrieve suitable material(s) for molecular profiling, snap-frozen tumor samples were collected by standard bioptic procedures (14G diameter) at baseline (T1 timepoint: treatment-naïve pre-neoadjuvant) and after standard NACT in case of residual bulk tumors; T2 timepoint). Clinical remission status at timepoint T2 was assessed after NACT via ultrasound and/or breast magnetic resonance imaging. The high-risk state was assessed according to pathological state and CPS-EG score as outlined in the Supplementary Information S1, available at https://doi.org/10.1016/j.esmoop.2022.100637. If fresh tumor tissue with sufficient tumor cell content was not available at timepoint T2 from high-risk patients, formalin-fixed, paraffin-embedded (FFPE) material from the tumor specimen resected at surgery was used. Blood control samples taken at timepoint 1 were used to account for germline alterations. Pseudonymization was conducted according to standard in-house procedures.
DNA and RNA analysis
Following histological evaluation (cut-off tumor cell content ≥20%), isolation of DNA and RNA from tumor samples and preparation of the respective libraries for sequencing analyses were carried out (see Supplementary Information S1, available at https://doi.org/10.1016/j.esmoop.2022.100637). Sequencing data were processed and analyzed using in-house computational pipelines as previously described11,15,16 and outlined in the Supplementary Information S1, available at https://doi.org/10.1016/j.esmoop.2022.100637.
Predictive biomarkers and intervention arm allocation
The relationship between alterations identified by sequencing and specific targeted substances was established using molecular evidence levels of clinical actionability as defined in the classification scheme used in the end-to-end workflows of the molecular tumor boards of the NCT Heidelberg.17,18 For this purpose, the three main BC subtypes (HR-positive/HER2-negative, HER2-positive, and TNBC) were considered as different entities. Given the curative intent, only stringent molecular evidence levels based on prospective, meta-analysis, or retrospective cohorts irrespective of histology were considered sufficiently strong to qualify biomarkers as predictive for a particular intervention (see Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100637). Individual case reports and preclinical or biological data for an association between a biomarker and treatment were not considered as a sufficiently strong rationale for an intervention in this patient population treated with a curative intent. Additional predictive factors were also considered, such as the most recent immune histology of the sequenced sample [i.e. immunohistochemistry (IHC)-based HR, HER2, PD-L1, and TROP2 status], as well as whole-genome sequencing (WGS)- or whole-exome sequencing (WES)-derived tumor mutational burden defined as the number of somatic single-nucleotide variants (SNVs) per megabase of the human genome or targeted genome region in case of WES.
Results
Cohort characteristics
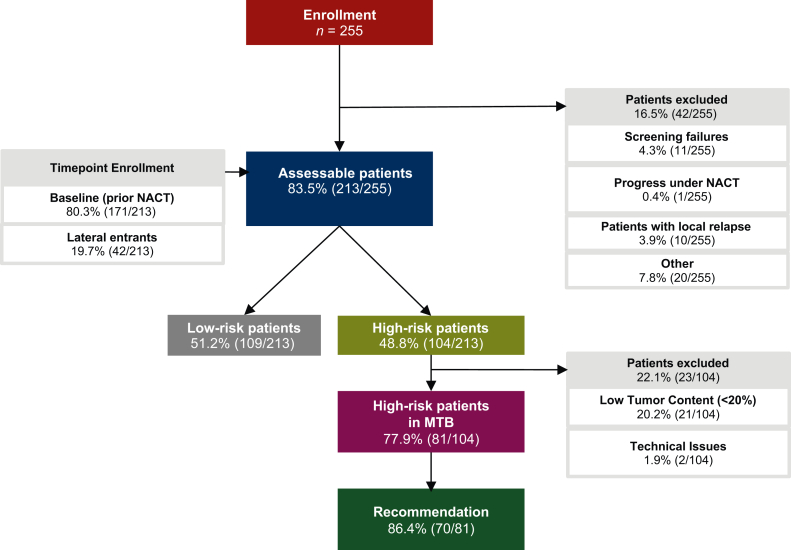
In the pilot phase (April 2019 to September 2020), 255 patients were enrolled (average age 52 years; Figure 1). A total of 42 patients were excluded from the initial analysis due to various reasons such as progress and/or local relapse under NACT, revealing 213 assessable patients. Of these, six patients had BC on both sides and seven patients had two different tumors with distinct molecular subtypes on one side. Consequently, 226 lesions were detected in 213 assessable patients.
Figure 1.
Cohort characteristics. Flow diagram displaying the number of patients enrolled, assessable, and excluded from analysis. Patients were conventionally enrolled before start of neoadjuvant chemotherapy (NACT; baseline patients), but also after the start of the course of NACT (lateral entrants). Risk status for relapse after NACT based on the pathological evaluation was assessed in a standard-of-care post-operative tumor board. In high-risk patients molecular profiling (whole-genome or whole-exome as well as transcriptome sequencing) was carried out and patients were presented in an interdisciplinary molecular tumor board (MTB) to prioritize molecular-guided therapies. A total of 213 assessable patients were distributed among immunohistochemistry-based subtypes as follows: 75 HR+HER2–, 39 HR+HER2+, 19 HER2+HR–, and 80 TNBC cases. HER2, human epidermal growth factor receptor 2; HR, hormone receptor; TNBC, triple-negative breast cancer.
Evaluation of standard pathological IHC-based parameters [estrogen receptor (ER), progesterone receptor (PR), HER2, Ki-67, Grade (G)] revealed that the majority of the 226 evaluable lesions of baseline pre-NACT tumors had a luminal subtype [55.31%, luminal-HR+/HER2– or luminal-HER2+like (HR+/HER2+); classification outlined in Supplementary Information S1, available at https://doi.org/10.1016/j.esmoop.2022.100637], followed by TNBC (35.40%, HR–/HER2–) and HER2-positive subtype (8.85%, HR–HER2+). Further, one ductal carcinoma in situ, which according to clinical convention cannot be classified according the common subtype scheme, was included because the patient had a further prognostically leading tumor (0.44% Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100637). Based on the assessment of the overall risk status, 104 patients possessed a high risk.
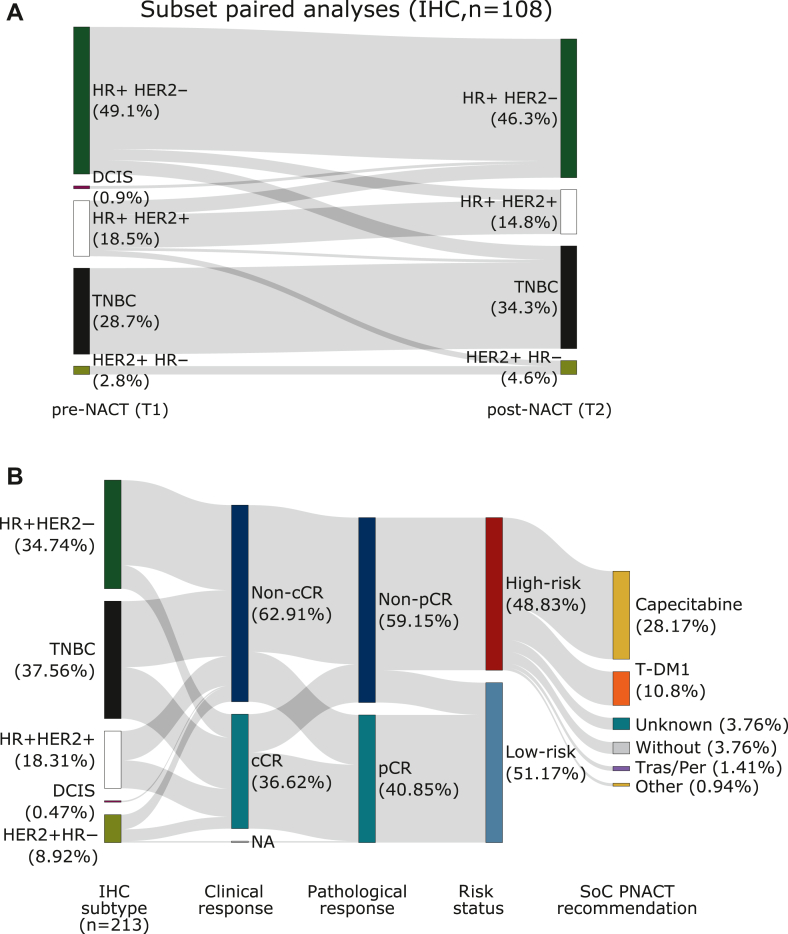
The ER, PR, and HER2 IHC-based expression dynamics between pre- and post-NACT were assessed in 108 available paired lesions, where it was possible to retrieve both a biopsy from the baseline and the residual bulk tumor following NACT (Figure 2A). A subtype switch was seen in 17.59% (19/108) of the lesions (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100637). This was predominantly attributed to a switch from luminal-B-like (HR+/HER2–/high proliferation) to luminal-A-like (HR+/HER2–/low proliferation) in 22.2% (24/108) of the lesions, which was associated with a substantial decrease in the average Ki-67 expression (% positive cells) from 42.0% before NACT to 14.6% after NACT in this subcohort.
Figure 2.
Pre- and postdynamics of subtype and prognostic risk status. (A) Sankey plot showing the distribution of paired subtypes before and after neoadjuvant chemotherapy (NACT; n = 108) determined by immunohistochemistry (IHC) for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). One patient displayed a ductal carcinoma in situ, which is conventionally not classified according to the standard IHC-based subtyping. Based on the assessment of the overall risk status, 104 patients possessed a high risk, yet 108 patients were eligible for the IHC-based paired subtyping. (B) Analysis of image-based (ultrasound, mammography, and/or magnetic resonance imaging) clinical response, classified as complete clinical response (cCR) or noncomplete clinical response (non-cCR), and its relation to the immunohistochemistry (IHC)-based subtyping, the pathology-based pathological response, classified as complete pathological response (pCR) or non-pCR, and the respective prognostic risk status. In one patient clinical response was not evaluable. Post-neoadjuvant treatment recommendations in the standard of care (SoC) include capecitabine, T-DM1, trastuzumab/pertuzumab (Tras/Per), and trastuzumab/pertuzumab/capecitabine. For 7.7% of the patients, the therapy protocol was unknown and 7.7% of the patients did not receive any post-neoadjuvant therapy (PNACT) because of intolerability of NACT and comorbidities. DCIS, ductal carcinoma in situ; HR, hormone receptor; NA, missing data; TNBC, triple-negative breast cancer.
Overall, a complete loss/gain of either ER PR or HER2 was detected in 17.59% (19/108) of the lesions.
Risk status and post-neoadjuvant standard-of-care therapy
From the 213 assessable patients, 171 were enrolled before the start of NACT (baseline patients) and 42 during NACT (lateral entrants). As there were no differences observed between baseline patients and lateral entrants with respect to the distribution of clinical complete response (cCR), pCR, and high-risk status (Figure 2B, Supplementary Tables S4 and S5, available at https://doi.org/10.1016/j.esmoop.2022.100637), both groups were pooled for further analysis. To identity high-risk patients, who require PNACT following surgery, pathological response was determined by pathological assessment of all tissue specimens resected at surgery.
The imaging-based cCR rate prior to surgery was 36.62% (78/213) and residual tumor was still evident in 62.91% (non-cCR, 134/213; Supplementary Table S4A, available at https://doi.org/10.1016/j.esmoop.2022.100637). Pathological evaluation after surgery revealed a pCR rate of 40.85% (87/213), whereas 59.15% (126/213) of patients showed non-pCR (Supplementary Table S4B, available at https://doi.org/10.1016/j.esmoop.2022.100637). The proportion of tumors, which had a discordance between cCR and pCR status, was 28.17% (60/213, 12.21% cCR/non-pCR; 15.96% non-cCR/pCR; Supplementary Table S4C, available at https://doi.org/10.1016/j.esmoop.2022.100637). Following surgery according to risk classification using the pCR status and CPS-EG score, respectively, 104/213 (48.83%) patients were classified as having a high risk. Interestingly a higher fraction of patients with HR+HER2+ (8/18, 44.44%) and TNBC (35/80, 43.75%) than patients with HR+HER2– tumor (16/74, 21.62%) showed an imaging-based cCR (see Figure 2B, Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2022.100637).
In addition to endocrine therapy in case of HR-positive disease, 88 out of the 104 high-risk patients received either capecitabine 57.69% (60/104), trastuzumab emtansine (T-DM1) 22.12% (23/104), trastuzumab/pertuzumab 2.88% (3/104), or trastuzumab/pertuzumab combined with capecitabine 1.92% (2/104) as post-neoadjuvant standard treatment. About 7.69% (8/104) of high-risk patients received no additional post-neoadjuvant systemic therapy due to poor tolerance of NACT or comorbidities; for 7.69% (8/104) of the patients further details were not available (Figure 2B).
Tissue sampling for molecular profiling
For 78.87% (168/213) of patients a tissue biopsy was taken prior to NACT at baseline (T1). Of these, 91.67% (154/168) had sufficient tumor cell content for subsequent WGS. The predominant site of the biopsy was the breast (165 breast, 3 lymph node biopsies). In case patients displayed a poor cCR in the preoperative imaging procedure following NACT (41.31%, 88/213), further fresh-frozen tissue was retrieved (T2), as these lesions presumably display an outgrowth of cancer cells and the respective molecular landscape driving NACT resistance. The predominant T2 biopsy site was again breast (82 breast, 6 lymph node biopsies).
One hundred four patients were classified as having high risk based on the pCR state and the CPS-EG score and from 60 patients post-NACT fresh-frozen biopsies were available. To avoid overtreatment in low-risk patients, administration of an additional molecular-guided PNACT is directed specifically toward high-risk patients only. As chemotherapy alters the tissue architecture, only in a subset of these high-risk patients (32/104, 30.77%) was the tumor cell content of the T2 biopsy sufficient for WGS. In the remaining high-risk patients (72/104, 69.23%), we used FFPE tissue from surgery specimen for WES, of which 51 had sufficient material for sequence analysis.
In total, in 77.88% (81/104) of the high-risk patients sequencing analysis was successfully conducted by either WGS using fresh tumor tissue from T2 biopsy (32/104, 30.77%) or WES using FFPE material from surgery (49/104, 47.12%, high-risk patients).
Molecular profiles
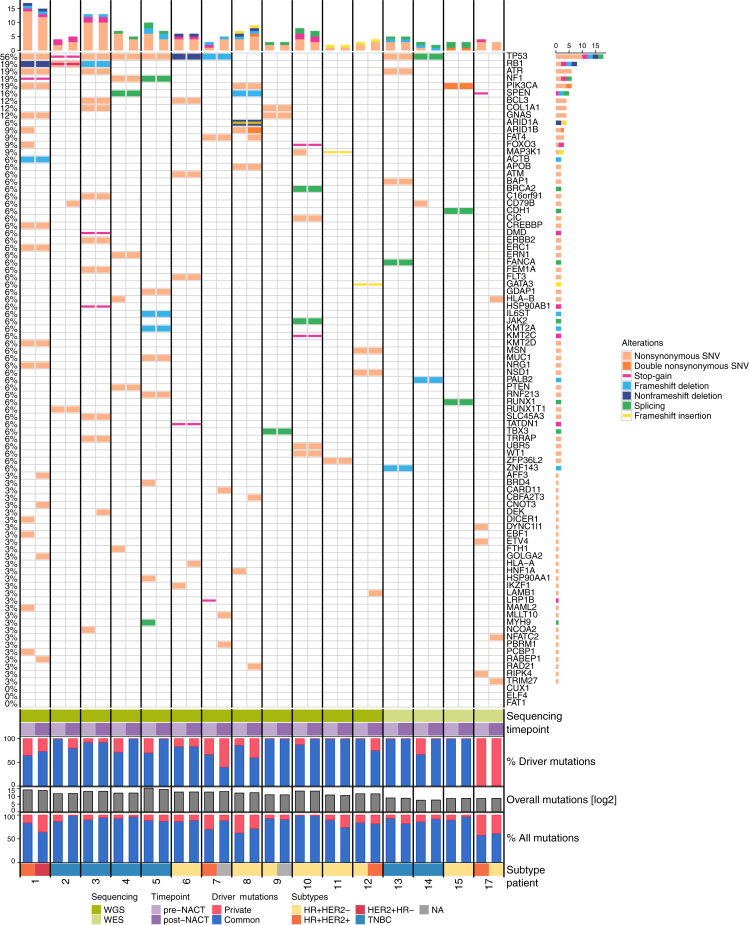
To retrieve an in-depth molecular picture of the evolutionary differences driven by NACT administration, we analyzed mutation spectra in 16 patients at baseline and after NACT (12 samples with WGS and 4 samples with WES from 15 patients with baseline and residual tumor tissues and 1 patient with material from contralateral sides before and after NACT).
One patient initially presented with a bilateral BC and the baseline tumor was sampled from the right side while the tumor biopsy after NACT was from the left side. The total number of variants [SNVs and short insertions–deletions (InDels)] was on average 10 487 (median 7964.5) in WGS (range 2177-47 727) and 477 (median 499.5) in WES (range 219-689). Across all patients each tumor harbored a median of 5 mutations in putative BC-driver genes (average 6.0), most frequently TP53 (56%), PIK3CA (19%), RB1 (19%), NF1 (19%), and ATR (19%; Figure 3). The majority (mean 86.4%, standard deviation 16.2%) of the driver mutations in a patient with unilateral disease (n = 15) were detected in both samples, suggesting that they were early events (trunk associated in the evolutionary tree) and hence not negatively selected by treatment. In particular, this was always the case for mutations in the five most frequently mutated genes, namely, TP53, PIK3CA, RB1, NF1, and ATR. Conversely, 13.6% of putative drivers were lost or gained upon treatment in patients with unilateral disease, possibly reflecting ongoing clonal evolution. Interestingly, the one patient with tumor samples from both sides of the breast showed the lowest fraction of overlapping variants between genes [overall, 689 distinct mutations; overlap before NACT 57.5% (396/689), after NACT 61.2% (422/689) versus 86.7% overlap in patients 1-15] and no overlapping driver mutations, indicating an at least partly independent tumor evolution.
Figure 3.
Somatic single-nucleotide polymorphisms (SNVs) and insertions–deletions (indels) in paired biopsies.Top: Mutational spectrum in candidate driver genes. Coding genes are shown if targeted by at least one single-nucleotide variant (more than one SNV in the same gene is highlighted), indel mutations, or a variant in the splicing region. Different colors mark different types of mutations. Middle: Common (blue) and private (red) proportions of driver genes in each sample. Bottom: Variant count for each of the tumors on a log scale and the percentage of common (blue) and private (red) mutations. Shown are data of tumor samples from 16 patients, among which whole genomes were sequenced for 12 cases and whole exomes for the remaining 4. For each case, mutations identified in the baseline T1 and in residual T2 tissue after neoadjuvant chemotherapy (NACT) are shown next to each other. Individual patients are separated by lines and IHC-based subtype is displayed. HER2, human epidermal growth factor receptor 2; HR, hormone receptor; TNBC, triple-negative breast cancer; WES, whole-exome sequencing; WGS, whole-genome sequencing.
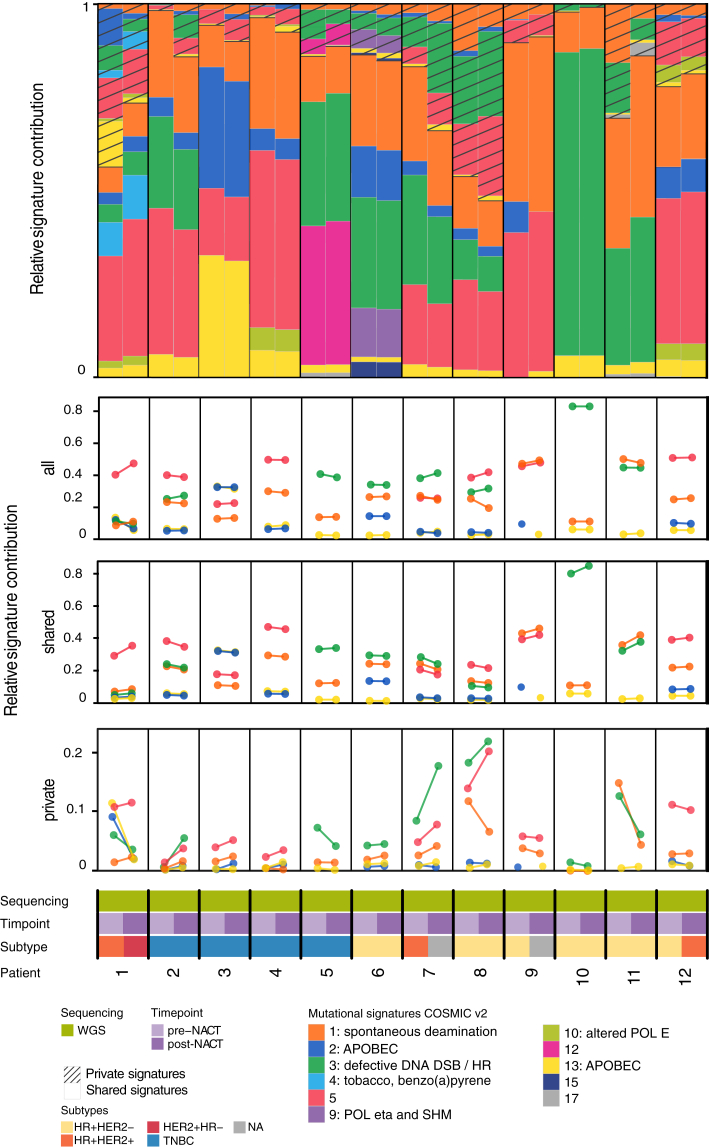
We then asked whether NACT alters the mutational processes active in BC by analyzing mutational signatures (Cosmic version 219) in the 12 cases, for which longitudinal WGS data with high tumor content were available. The predominant SNV-derived mutation signatures were AC1, associated with spontaneous deamination of 5-methylcytosine and aging; AC2 and AC13, both attributed to APOBEC enzyme activity, which in physiological situations may protect cells from viral infections; AC3, associated with defective DNA double-strand break repair; and AC5, for which etiology is unknown (Figure 4). Most signatures remained stable after NACT. However, in one case signature AC13 arose after NACT, while AC2 was lost. Although both are explained by related mechanisms by virtue of APOBEC activity, this may either indicate a possibility of differential clonal development under NACT or may be due to algorithmic limitations, as these two signatures are rather similar. Taken together, the mutational profiles and signatures remained largely stable during NACT in this subcohort, suggesting limited selective pressure on the genomes, albeit clonal evolution was observed in individual cases.
Figure 4.
Mutational signatures in paired biopsies.Top: Relative mutational signature contribution for each tumor case using YAPSA.19 Each signature is subdivided into two fractions, depending on whether the mutations contributing to it were found in both tumor tissues of the same patient or in only one. Bottom: Comparison between the contribution of the five most frequent signatures (AC1, AC2, AC3, AC5, and AC13), before and after neoadjuvant chemotherapy (NACT): the upper one displays the change in relative frequency of all signatures, whereas the middle and lower ones show the relative frequency of the shared and private signatures. For each case the type and timepoint of sequencing and the immunohistochemistry-based subtype are displayed. DSB, double-strand break; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; TNBC, triple-negative breast cancer; WES, whole-exome sequencing; WGS, whole-genome sequencing.
Potential options of individualized post-neoadjuvant systemic treatment
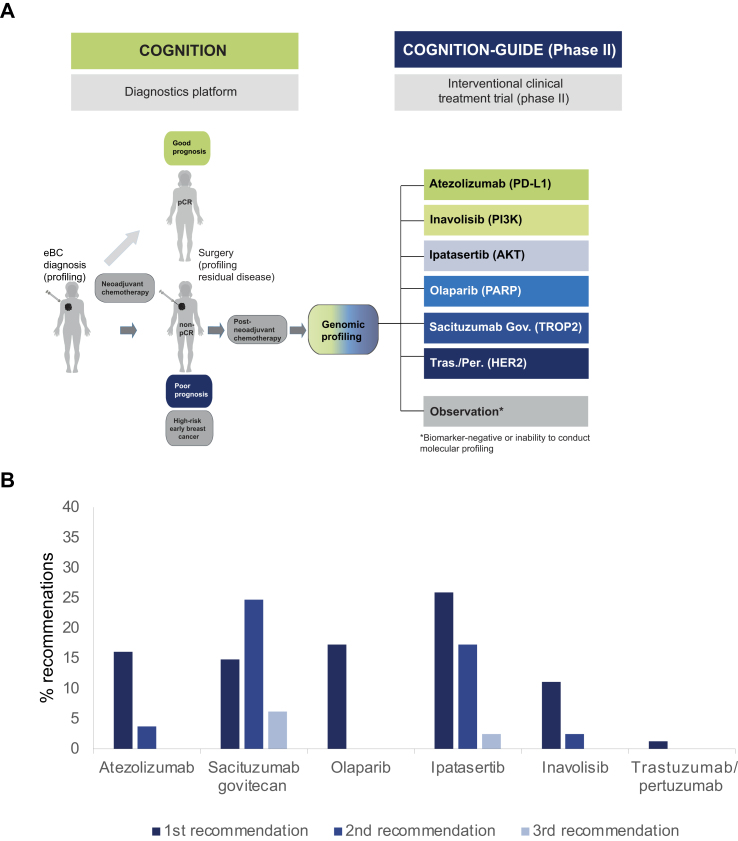
The major objective of COGNITION is to characterize the residual tumor of high-risk patients to tailor post-neoadjuvant treatment according to genomic alterations detected in NACT-resistant cell clones to reduce the substantial risk of a relapse. Given the curative treatment intent, administration of off-label therapies is not feasible, and so the patients will be assigned to one of the treatment arms in a newly designed phase II treatment trial (COGNITION-GUIDE; NCT05332561). Within this study, patients who are still at high risk for relapse following NACT and surgery will subsequent to post-neoadjuvant SoC treatment being offered a personalized therapy. According to the tumors biomarker profile they will be assigned to one of the six treatment arms (atezolizumab, inavolisib, ipatasertib, olaparib, sacituzumab govitecan, trastuzumab/pertuzumab) (Figure 5A, Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100637, Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100637). For the successfully sequenced patients (n = 81) an interdisciplinary molecular tumor board at NCT Heidelberg prioritized one to three treatment options. At least one therapy was recommended in 86.42% (70/81) of all high-risk patients with successfully conducted sequencing analysis, a second recommendation in 48.15% (39/81), and a third recommendation in 8.64% (7/81; Figure 5B).
Figure 5.
Trial design of COGNITION and COGNITION-GUIDE and preliminary arm assignment of analyzed patients. (A) Concept of COGNITION and COGNITION GUIDE. Tumor residues from high-risk patients identified after neoadjuvant chemotherapy (NACT) are molecularly profiled to identify biomarkers, which are used to enroll patients in one of the six arms of the biomarker-driven phase II study COGNITION-GUIDE. (B) Hypothetical arm assignment of high-risk patients within COGNITION-GUIDE according to molecular tumor board (MTB) biomarker assignment (n = 81). AKT, protein kinase B; COGNITION, Comprehensive assessment of clinical features, genomics and further molecular markers to identify patients with early breast cancer for enrolment on marker driven trials; COGNITION-GUIDE, Genomics-guided targeted post neoadjuvant therapy in patients with early breast cancer; eBC, early breast cancer; HER2, human epidermal growth factor receptor 2; PARP, poly (ADP-ribose) polymerase; pCR, pathological complete response; PD-L1, programmed death-ligand 1; PI3K, phosphoinositide 3-kinase; Sacituzumab Gov., sacituzumab govitecan; Tras./Per., trastuzumab/pertuzumab; TROP2, Tumor-associated calcium signal transducer 2.
Beyond identification of somatic actionable alterations, broad genomic profiling possesses the advantage to uncover precious information on germline alterations. Genomic profiling of 81 high-risk patients unraveled (likely) pathogenic cancer-relevant germline variants in 17.28% (14/81) of the patients (in 8/14 a variant in BRCA1 or BRCA2, 3/14 in the FANC gene family, 2/14 in CHEK2, and 1/14 in ATM); 50% (7/14) of the patients with a pathogenic or likely pathogenic variant had a novel indication for genetic counseling based on the broad genomic profiling approach, either because the patients did not meet the family criteria for genetic counseling (n = 5) or the variant was not detected during genetic counseling before (n = 2).
Discussion
This is the first report of the feasibility of comprehensive molecular profiling and its clinical applicability within the molecular precision registry trial COGNITION for patients with early BC with NACT indication. We carried out WGS/WES and RNA sequencing to identify molecular targets for individualized post-neoadjuvant treatment of patients who are still at high risk for relapse following SoC NACT and surgery. Thus COGNITON is one of the first precision oncology trials addressing potentially curative situations, intended to reduce the risk of relapse and to increase cure rates.
Of the first 213 assessable patients enrolled in this program, 40.85% presented with pCR defined as ypT0/Tis ypN0. This rate is in line with previously described reports (18%-40%3,7) and steadily increasing pCR rates due to better patient selection, improved treatment regimens, and supportive measures. On the post-operative tumor board 48.83% of patients were identified as having a high risk for relapse according to non-pCR status (patients with TNBC or HER2-positive BC) and non-pCR plus high CPS-EG score (patients with HR-positive, HER2-negative tumors), respectively. To obtain fresh tumor tissue resistant to NACT, patients with clinically residual tumor were biopsied before surgery. The overall low rate of post-NACT biopsies with a tumor content sufficient for WGS (30.8%) has to be attributed to the effects of the NACT on the tissue composition. Considering pre-NACT samples 91.7% possessed a tumor content sufficient for WGS.
In line with published results, clinical and pathological response were discordant in 28.2% of patients.20,21 Thus, in high-risk patients without sufficient fresh tumor tissue biopsied before surgery, we used FFPE material from surgical specimens for WES. In summary, in 77.9% of high-risk patients sequencing analysis could be successfully carried out by either WGS (n = 32) or WES (n = 49).
In 20.29% (17/84) of patients with non-pCR there was a switch in IHC-based subtype from before to after NACT. Remarkably, this affected predominantly HR-driven tumors, while the baseline TNBC (stable: 30/31) and HER2-positive (stable: 3/3) ones appeared mostly stable. This phenomenon is in concordance with subtype switches reported in metastases.22,23
While COGNITION relies on molecular biomarkers that are generally considered for precision oncology trials in BC, recent findings concerning these markers are carefully considered with respect to potential improvements in the future, such as modification of diagnostic cut-off scores. In this context, we refer to interesting current debates about whether anti-trop2 agents are still active despite low TROP2 expression24 and whether a tumor mutation burden score >10 might not be relevant for considering immunotherapy in BC.25
Mutational profiles and signatures of 16 paired samples taken before and after NACT revealed only occasional clonal evolution but largely stable genomes in a subset of patients selected based on maximum technical quality criteria to carry out in-depth molecular analyses. This may be attributed to the rather little selective pressure on the genome inferred by broad-acting cytotoxic chemotherapies in nonresponders and requires further elucidation if targeted agents drive a different clonal pattern. However, we here demonstrate that even in unilateral tumors, 13.6% of mutated putative drivers were found lost or gained upon treatment, possibly reflecting ongoing clonal evolution that is not visible by bulk analysis or being undetected due to variations in, for example, coverage and tumor cell content. This finding is consistent with recent studies reporting major differences after NACT in TNBC or neoadjuvant aromatase inhibitor therapy of HR-positive disease.14,26, 27, 28 To elucidate the underlying processes and mechanisms, it will be necessary to further decipher spatial and temporal heterogeneity down to the resolution of single cells in future studies.
The COGNITION framework and the results of the pilot analyses provide the basis for the design of the interventional phase II biomarker-guided multiarm umbrella trial COGNITION-GUIDE (Figure 5). At present, existing precision oncology trials predominantly address patients in a metastatic incurable situation and implement therapy recommendations in the context of biomarker-driven clinical trials or by reimbursement in the off-label use.11,15,29 On the molecular level, this strategy displays the disadvantage of an overall higher tumor burden and clonal complexity, and hence less representative genomic profiles. Consequently, moving broad genomic profiling to an earlier disease stage with only minimal residual disease circumvents these limitations and has a high potential to address critical clinical targets at an early timepoint. Patients in the curative setting are currently dependent on enrollment into biomarker-driven studies to implement therapy recommendations, as the current ethical and legal framework requires an incurable disease for off-label use. In this context, COGNITION-GUIDE provides a framework to grant drug access and to provide robust efficacy data on the value of precision oncology in early high-risk BC as patients will be stratified according to biomarkers identified within COGNITION to one of the six therapy arms encompassing atezolizumab, inavolisib, ipatasertib, olaparib, sacituzumab govitecan, and trastuzumab/pertuzumab, respectively. This personalized targeted PNACT will be administered in addition to SoC, including PNACT with capecitabine according to the CREATEX trial8 and T-DM1 according to the KATHERINE trial.9 The biomarkers mandatory for enrollment in the respective treatment arm were selected based on their previously described predictive value and classified according to Leichsenring et al.17 Indeed, 67.3% patients still at high risk for relapse following SoC NACT could be assigned to at least one of those treatment arms, highlighting the feasibility of the approach as a prerequisite to expand the platform to further sites.
In summary, the workflow of COGNITION demonstrates that WGS, WES, and transcriptome sequencing-based precision oncology are feasible for the clinical management of patients with early BC and provides a strong basis for clinical applications in interventional follow-up trials and further clinical trial sites to ease access for larger patient populations.
Acknowledgements
We highly appreciate the excellent study nurse support by Eileen Kurre, Janina Egner, Tina Bausewein, Christiane Dobeleit, Barbara Pader, and Dagmar Giesen. In addition, we are very grateful for the strong support of the breast care team. Moreover, we thank the NCT Molecular Precision Oncology Program (in particular, Ursula Weber, Viktoria Brendel, and Jana Maier), the NCT Trial Center (Luise Straßl, Haniyeh Yazdanparast, Ernst Riewe, and Marlene Diewald), and the teams of the NCT Tissue Bank and NCT Liquid Bank, the Sample Processing Lab, and the Genomics High-Throughput Sequencing facility at the DKFZ for excellent organizational and/or technical support. Further, we thank Laura Gieldon and Steffen Hirsch for their precious support regarding the evaluation of germline alterations. The authors further sincerely thank the patients and their families for the study participation.
Funding
This study was supported in part by the National Center for Tumor Diseases (NCT) Heidelberg through the Molecular Precision Oncology Program and the NCT Proof-of-Concept programs, by the Federal Ministry of Education and Research (BMBF COGNITION-/GUIDE, grant number 01EK2202A), and by the German Cancer Aid (Deutsche Krebshilfe, INTEGRATE-TN, grant number 70113450).
Disclosure
MH has received speaker’s fee from Merck Sharp & Dohme (MSD). KS has received honoraria from Pfizer, MSD, and Gilead. LM has received speaker honoraria or travel support from Roche, Eisai, Pfizer, Gilead, AstraZeneca, Lilly, MSD, Celgene, and Novartis. CF has received honoraria for advisory activity from Roche, Pfizer, MSD, and Eisai; travel grants from Celgene, Roche, and Amgen; and honoraria from Roche, Celgene/BMS, Pfizer, Astra Zeneca, GSK, and Novartis. SH reports research support, honoraria, and/or travel expenses from AstraZeneca, Clovis, Novartis, Pfizer, and GSK outside the submitted work. BS is supported by a Physician Scientist scholarship of the Medical Faculty of Heidelberg University. MW received speaker honoraria from AstraZeneca Celgene Roche, MSD, and Novartis. A. Stenzinger has received honoraria for advisory board/speaker’s bureau activities at Astra Zeneca, AGCT, Bayer, BMS, Eli Lilly, Illumina, Janssen, MSD, Novartis, Pfizer, Roche, Seattle Genetics, Takeda, and Thermo Fisher; and grants from Bayer, BMS, Chugai, and Incyte. DJ has received consulting fees for participation on a data safety monitoring board or advisory board from CureVac AG, Definiens, F. Hoffmann-La Roche Ltd, Genmab A-S, Life Science Inkubator GmbH, VAXIMM AG, OncoOne Research & Development Research GmbH, Oncolytics Biotech Inc., Zelluna, HDIT GmbH, AYOXXA, Seattle Genetics, BreakBio Corp., and Roche Pharma AG; honoraria from SKK Kliniken Heilbronn GmbH, Georg Thieme Verlag, Terrapinn, Touch Medical Media, BMS GmbH & Co KGaA, MSD, Gruppe 5 Filmproduktion GmbH, AstraZeneca GmbH, Department of Radiation Medicine University Kentucky, and The Norwegian Cancer Society Oslo; payment for expert testimony from expert opinions for courts, Wilhelm-Sander-Stiftung, Else Kröner-Fresenius-Stiftung, Schering Stiftung, and NordForsk; support for attending meeting and/or travel from Amgen Inc., Oryx GmbH, Roche Glycart AG, Parexel.com, IKTZ HD GmbH, and BMS; honoraria for a leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from BMS Stiftung Immunonkologie. RS reports consulting for or advisory board membership with Daiichi Sankyo, Pfizer, Astellas, and Novartis; research funding from PharmaMar, AstraZeneca, Pfizer, Roche, Boehringer Ingelheim, and Daiichi Sankyo; travel, accommodations, and expenses covered by Daiichi Sankyo. A. Schneeweiss has received research grants from Celgene, Roche, and AbbVie; honoraria from Roche, Celgene, Pfizer, AstraZeneca, Novartis, MSD, Tesaro, Lilly, GSK, Seagen, Gilead, Bayer, Amgen, Pierre Fabre, streamedup!, promedicis, onkowissen.de, Metaplan, and Connectmedica Sp; and travel support from Roche, Celgene, and Pfizer. All remaining authors have declared no conflicts of interest.
Supplementary data
References
- 1.Ferlay J., Colombet M., Soerjomataram I., et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021 doi: 10.1002/ijc.33588. In press. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39. doi: 10.1016/S1470-2045(17)30777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortazar P., Zhang L., Untch M., et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 4.von Minckwitz G., Untch M., Blohmer J.U., et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 5.von Minckwitz G., Blohmer J.U., Costa S.D., et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013;31(29):3623–3630. doi: 10.1200/JCO.2012.45.0940. [DOI] [PubMed] [Google Scholar]
- 6.Marme F., Lederer B., Blohmer J.U., et al. Utility of the CPS+EG staging system in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer treated with neoadjuvant chemotherapy. Eur J Cancer. 2016;53:65–74. doi: 10.1016/j.ejca.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Riedel F., Hoffmann A.S., Moderow M., et al. Time trends of neoadjuvant chemotherapy for early breast cancer. Int J Cancer. 2020;147(11):3049–3058. doi: 10.1002/ijc.33122. [DOI] [PubMed] [Google Scholar]
- 8.Masuda N., Lee S.J., Ohtani S., et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 9.von Minckwitz G., Huang C.S., Mano M.S., et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 10.Tutt A.N.J., Garber J.E., Kaufman B., et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hlevnjak M., Schulze M., Elgaafary S., et al. CATCH: a prospective precision oncology trial in metastatic breast cancer. JCO Precis Oncol. 2021;5 doi: 10.1200/PO.20.00248. PO.20.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freitas A.J.A., Causin R.L., Varuzza M.B., et al. Molecular biomarkers predict pathological complete response of neoadjuvant chemotherapy in breast cancer patients: review. Cancers. 2021;13(21):5477. doi: 10.3390/cancers13215477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balko J.M., Cook R.S., Vaught D.B., et al. Profiling of residual breast cancers after neoadjuvant chemotherapy identifies DUSP4 deficiency as a mechanism of drug resistance. Nat Med. 2012;18(7):1052–1059. doi: 10.1038/nm.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balko J.M., Giltnane J.M., Wang K., et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 2014;4(2):232–245. doi: 10.1158/2159-8290.CD-13-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horak P., Heining C., Kreutzfeldt S., et al. Comprehensive genomic and transcriptomic analysis for guiding therapeutic decisions in patients with rare cancers. Cancer Discov. 2021;11(11):2780–2795. doi: 10.1158/2159-8290.CD-21-0126. [DOI] [PubMed] [Google Scholar]
- 16.Reisinger E., Genthner L., Kerssemakers J., et al. OTP: an automatized system for managing and processing NGS data. J Biotechnol. 2017;261:53–62. doi: 10.1016/j.jbiotec.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Leichsenring J., Horak P., Kreutzfeldt S., et al. Variant classification in precision oncology. Int J Cancer. 2019;145(11):2996–3010. doi: 10.1002/ijc.32358. [DOI] [PubMed] [Google Scholar]
- 18.Horak P., Leichsenring J., Goldschmid H., et al. Assigning evidence to actionability: an introduction to variant interpretation in precision cancer medicine. Genes Chromosomes Cancer. 2022;61(6):303–313. doi: 10.1002/gcc.22987. [DOI] [PubMed] [Google Scholar]
- 19.Hubschmann D., Jopp-Saile L., Andresen C., et al. Analysis of mutational signatures with yet another package for signature analysis. Genes Chromosomes Cancer. 2021;60(5):314–331. doi: 10.1002/gcc.22918. [DOI] [PubMed] [Google Scholar]
- 20.Baumgartner A., Tausch C., Hosch S., et al. Ultrasound-based prediction of pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast. 2018;39:19–23. doi: 10.1016/j.breast.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 21.Schaefgen B., Mati M., Sinn H.P., et al. Can routine imaging after neoadjuvant chemotherapy in breast cancer predict pathologic complete response? Ann Surg Oncol. 2016;23(3):789–795. doi: 10.1245/s10434-015-4918-0. [DOI] [PubMed] [Google Scholar]
- 22.Amir E., Miller N., Geddie W., et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012;30(6):587–592. doi: 10.1200/JCO.2010.33.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botteri E., Disalvatore D., Curigliano G., et al. Biopsy of liver metastasis for women with breast cancer: impact on survival. Breast. 2012;21(3):284–288. doi: 10.1016/j.breast.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Bardia A., Tolaney S.M., Punie K., et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021;32(9):1148–1156. doi: 10.1016/j.annonc.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 25.McGrail D.J., Pilié P.G., Rashid N.U., et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32(5):661–672. doi: 10.1016/j.annonc.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim C., Gao R., Sei E., et al. Chemoresistance evolution in triple-negative breast cancer delineated by single-cell sequencing. Cell. 2018;173(4):879–893 e13. doi: 10.1016/j.cell.2018.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klintman M., Buus R., Cheang M.C., Sheri A., Smith I.E., Dowsett M. Changes in expression of genes representing key biologic processes after neoadjuvant chemotherapy in breast cancer, and prognostic implications in residual disease. Clin Cancer Res. 2016;22(10):2405–2416. doi: 10.1158/1078-0432.CCR-15-1488. [DOI] [PubMed] [Google Scholar]
- 28.Miller C.A., Gindin Y., Lu C., et al. Aromatase inhibition remodels the clonal architecture of estrogen-receptor-positive breast cancers. Nat Commun. 2016;7 doi: 10.1038/ncomms12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andre F., Bachelot T., Commo F., et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER) Lancet Oncol. 2014;15(3):267–274. doi: 10.1016/S1470-2045(13)70611-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.