Abstract
Background
People living with cystic fibrosis have an increased risk of lung infection with nontuberculous mycobacteria (NTM), the prevalence of which is reportedly increasing. We conducted a systematic review of the literature to estimate the burden (prevalence and incidence) of NTM in the cystic fibrosis population.
Methods
Electronic databases, registries and grey literature sources were searched for cohort and cross-sectional studies reporting epidemiological measures (incidence and prevalence) of NTM infection or NTM pulmonary disease in cystic fibrosis. The last search was conducted in September 2021; we included reports published since database creation and registry reports published since 2010. The methodological quality of studies was appraised with the Joanna Briggs Institute tool. A random effects meta-analysis was conducted to summarise the prevalence of NTM infection, and the remaining results are presented in a narrative synthesis.
Results
This review included 95 studies. All 95 studies reported on NTM infection, and 14 of these also reported on NTM pulmonary disease. The pooled estimate for the point prevalence of NTM infection was 7.9% (95% CI 5.1–12.0%). In meta-regression, sample size and geographical location of the study modified the estimate. Longitudinal analysis of registry reports showed an increasing trend in NTM infection prevalence between 2010 and 2019.
Conclusions
The overall prevalence of NTM infection in cystic fibrosis is 7.9% and is increasing over time based on international registry reports. Future studies should report screening frequency, microbial identification methods and incidence rates of progression from NTM infection to pulmonary disease.
Short abstract
This systematic review and meta-analysis of the burden of nontuberculous mycobacteria in individuals with cystic fibrosis found that the worldwide prevalence of infection is ∼8% https://bit.ly/3dhmGvu
Introduction
Cystic fibrosis (CF) is an autosomal recessive condition caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The incidence is approximately one in 3000–4000 neonates in Caucasian populations, with variable estimates of incidence in other ethnicities [1, 2]. CF is characterised by chronic pulmonary symptoms and a progressive decline in lung function leading to respiratory failure or lung transplantation [1, 3]. The underlying pathology of CF favours microbial colonisation and confers additional susceptibility to infections with fungi, viruses and bacteria, which in turn accelerate the respiratory compromise [4].
Nontuberculous mycobacteria (NTM) are free-living organisms with pathogenic potential. Individuals with underlying immunosuppression or structural lung damage are at increased risk of infection with these bacteria [5, 6]. In the CF population, patients can have transient or chronic infection with NTM. The latter can be indolent or contribute to radiographic changes and concurrent symptoms referred to as NTM pulmonary disease (NTM-PD), which often warrants antimicrobial therapy [7]. Both chronic infection and NTM-PD are associated with poorer respiratory outcomes and are relative contraindications for lung transplant [8, 9]. Recent reports show that the overall detection rate for NTM has been increasing in the general population. For instance, in the United States of America (USA), the prevalence increased from 8.2 per 100 000 to 20 per 100 000 persons between 1997 and 2007 [10–12]. A similar trend has been described in CF populations worldwide [7, 13–15]. The recent increase in awareness about the impact of NTM on CF lung disease could account for a rise in detection rates through improved screening practices.
Despite the availability of data in CF registries, the global burden of NTM remains poorly defined. The burden of NTM infection and NTM-PD can vary according to age, environmental exposure, geographical region and microbial identification methods used [7–9]. Particularly, estimates from geographical regions without established registries are typically underrepresented. Furthermore, divergent screening and laboratory practices (internationally and nationally) make it difficult to compare or generalise estimates from different locations. To estimate the burden of NTM-related conditions in the CF population, we conducted a systematic review of the incidence and prevalence of NTM infection and NTM-PD among people living with CF and explored factors that contribute to heterogeneity in these estimates.
Methods
Review question
We designed our review question based on population, condition, outcome (epidemiological measure) and study design, as recommended by current guidelines [16, 17]. Briefly, we screened for cross-sectional or cohort studies reported in English including people with CF (population) and evaluating NTM infection or NTM-PD (condition). NTM infection was defined as isolation of any NTM on at least one occasion per patient; the criteria for NTM-PD were specified by each study. Reporting of at least one epidemiological measure among incidence rate, incidence proportion, point prevalence or period prevalence was required for inclusion. The full criteria are described in supplementary table S1. The review protocol was registered in the International Prospective Register of Systematic Reviews, PROSPERO (CRD42020200418), in July 2020. In October 2020, before the abstract screening, we updated the grey literature sources and screening procedures.
Literature search
Embase and Medline were searched in September 2020 using the criteria specified in supplementary methods 1; an updated search was conducted in September 2021. We manually reviewed the conference proceedings from relevant research meetings between 2010 and 2020 (North American Cystic Fibrosis, European Cystic Fibrosis Society, American Thoracic Society and the Infectious Diseases Society of America conferences). We performed forwards and backwards searches for highly cited references using Google Scholar and Web of Science (supplementary table S2). Finally, the US (Cystic Fibrosis Foundation), Canadian (Cystic Fibrosis Canada), European (European Cystic Fibrosis Society), Australian and Brazilian registry reports published between 2010 and 2021 were included.
Screening and data extraction
All records were retrieved and exported in Research Information Systems format. Initial manual deduplication evaluated author, title and year of publication. Then we performed automated deduplication using the Systematic Review Accelerator Deduplicator software (https://sr-accelerator.com/#/deduplicator) [18] and Covidence (Veritas Health Innovation, Melboune, Australia). Screening of reports and full-text manuscripts, data extraction and risk of bias assessment were conducted independently by two reviewers (MDP and MEA); discrepancies were solved by consensus or by a third reviewer (BSQ). Epidemiological measures of interest reported in each study were included for analysis. Abstract screening evaluated language, study type, the inclusion of a CF population and reporting of any measures of interest. Full-text screening evaluated all eligibility criteria defined in supplementary table S1. For reports that could not be retrieved, we requested access to unpublished full manuscripts from authors via email on at least two separate occasions. The Joana Briggs Institute tool was used to assess methodological and reporting quality [16, 17, 19–21]. Overall low risk of bias was defined as low risk in the assessments of the sampling frame, sample size, population description and statistical methods. High risk was determined by a high-risk assessment in any of the following: sampling frame, sampling scheme, sampling size, population description, identification methods or statistical calculation. Data extraction was based on a pre-specified data dictionary piloted with 10 studies (supplementary table S3). For period prevalence, point prevalence and incidence proportion, we extracted proportions, the number of cases and the sample size. We did not impute any missing data. In studies with unclear years of data collection, we assumed that data was obtained from the year before publication. The body of evidence was not evaluated for certainty given the lack of adapted tools for single proportion measures.
Data analysis
Data were analysed with the meta and metafor packages in RStudio (www.rstudio.com) and R version 4.1.1 (www.r-project.org) [22]. Risk of bias plots were produced with the robvis [23] and ggplot2 (https://ggplot2.tidyverse.org) packages, and tables with the flextable package (https://davidgohel.github.io/flextable/). We pre-specified the use of random effects models based on expected heterogeneity by study region and dates. To model proportion data, we used generalised linear models with LOGIT transformation [24–26]. Point and annual prevalence of NTM infection were summarised together in the meta-analysis because they contain comparable time frames of evaluation (a year or less). The remaining epidemiological measures, including period prevalence of NTM infection, incidence of NTM infection, prevalence (point or period) of NTM-PD and incidence of NTM-PD, are reported in supplementary tables and text only. Period prevalence of NTM infection and NTM-PD were not pooled owing to varying time intervals among studies, while the rest of the epidemiological measures had a small number of studies. To avoid the overrepresentation of registry reports in the meta-analysis, we included only the most recent report per registry with raw data available (numerator and denominator to calculate prevalence). Secondary data analyses of registry data were also excluded from the meta-analysis to reduce redundancy with the registry reports. Heterogeneity was assessed with the I2 index and 95% confidence intervals, with a significance level established at p<0.10. Publication bias was explored graphically using sample size as a predictor of bias in a funnel plot [27].
We pre-specified subgroup analyses by study design, age category (paediatric versus adult), year of data collection (before 2000, 2001–2009 and 2010–2019), geographical region (grouped as North America, Europe, and others) and the most common individual NTM species reported in CF (Mycobacterium abscessus complex (MABs) and Mycobacterium avium complex (MAC)). The pre-specified meta-regression model was optimised by maximum likelihood and used the same transformation as the meta-analysis (LOGIT). We evaluated the goodness of fit in the model using Akaike's information criteria by stepwise inclusion of pre-specified coefficients. Exploratory (unspecified) analyses included a longitudinal trend of prevalence in registries and subgroup analyses by region for MAC and MABs. Sensitivity analyses included three meta-analyses of NTM infection point (and annual) prevalence. The first excluded a study that screened patients only in the presence of increased symptoms, the second included only registry data and the third excluded studies that did not use standardised culture media for identification of NTM. Reporting is based on the recommendations of the Joanna Briggs Institute and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [16, 28]. The final dataset, the code for analysis, the data collection form and other study forms are available at https://github.com/azmigueldario/SR_prevalence_NTM and as a Dryad repository (https://datadryad.org/stash/share/19vSo2cbAw6I-g2f0a9DmtjdWJsmtUtX2KBB5b29xbE).
Results
Description of studies
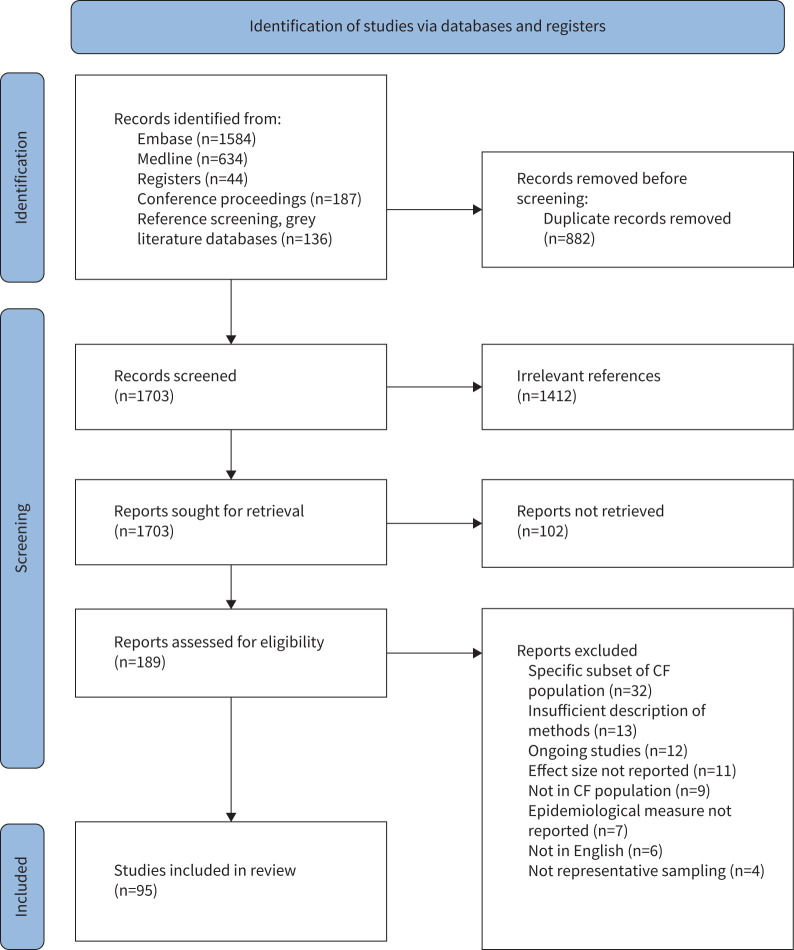
After removing duplicates, 1703 references were included for abstract screening, 291 were reviewed as full-text and 95 were included in the systematic review. The PRISMA flowchart in figure 1 summarises the screening process. The abstract and full-text screening processes had a Cohen's κ of 0.899 and 0.698, respectively, and all disagreements were resolved by consensus. The majority of the publications originated from Europe (42%) or North America (33%). The most common study design was cross-sectional registry (n=44, 46%), followed by cross-sectional non-registry (n=35, 37%) and cohort (n=16, 17%). A majority of studies (n=75, 79%) included a mixture of paediatric and adult patients. The most represented period of data capture was 2010–2019 (n=65, 68%), concordant with the availability of registry data. As expected, registry reports and studies using registry data had a larger median sample size (n=4278, IQR 2230–15 048) compared to the median of non-registry studies (n=155, IQR 92–382). Supplementary tables S4–S8 summarise the characteristics of included studies by the epidemiological measure of interest. Annual or point prevalence of NTM infection was reported in 67 studies, and period prevalence of NTM infection in 43 studies. The incidence proportion of NTM infection was reported in five studies. NTM-PD point prevalence was reported in two studies and period prevalence in 13 studies, but no studies reporting incidence of NTM-PD were found.
FIGURE 1.
PRISMA flowchart summarising identification of reports, retrieval of manuscripts and screening steps. CF: cystic fibrosis.
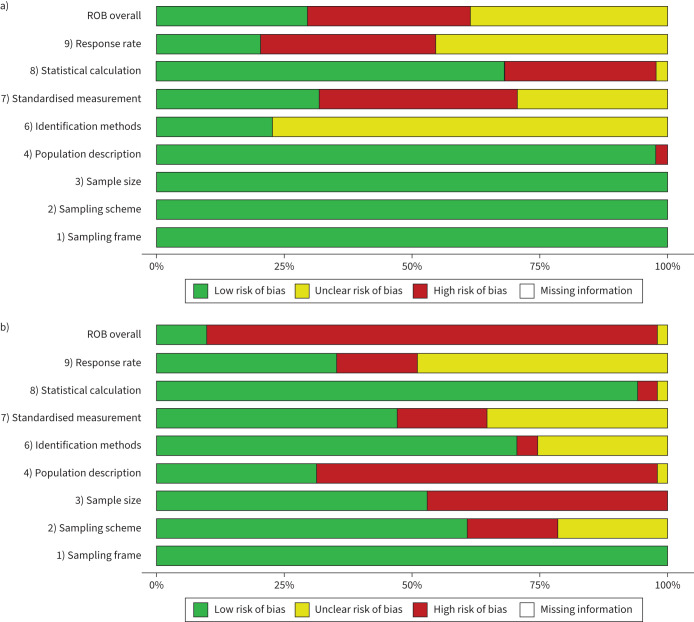
The results of the quality assessment are summarised in figure 2. Registry reports had mostly low-risk scores on the domains of sampling frame, sampling approach, sample size and population description. In contrast, registry reports had mostly unclear risk in identification methods (77%), and high or unclear risk in standardised measurement and response rate. The latter is expected because registries do not typically collect identification methods. Non-registry studies had a higher risk of bias scores in terms of sample size and population description. Also, non-registry studies showed higher quality assessment in reporting of identification methods (Fisher's test p<0.001) compared to registry reports. By epidemiological measure, studies that reported the incidence of NTM infection had, in general, a low risk of bias for all questions except sample size and response rate. Studies reporting NTM-PD also had a high risk of bias for sample size and population description, and mostly low/unclear risk for the remaining domains (supplementary figure S1).
FIGURE 2.
Summary plots of quality appraisal of studies included in the systematic review using the Joanna Briggs Institute tool for systematic reviews of prevalence. Domain 5 applies to survey studies and was not evaluated. a) Summary of all included registry reports (n=44). b) Summary of all included non-registry reports (n=51). Overall risk of bias (ROB) determined as specified in methods.
NTM infection point (annual) prevalence
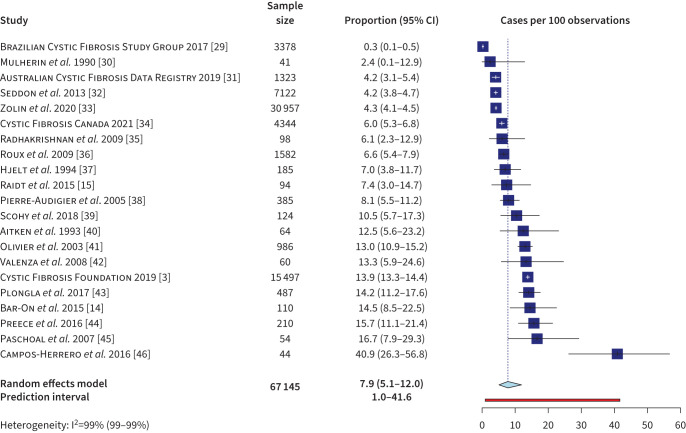
Point prevalence and annual prevalence of NTM infection were summarised together in a meta-analysis of 21 studies. For registry data, we used the most recent report that included both the number of cases and sample size. Four studies that used registry data between 2010 and 2019 were excluded to avoid duplication of data. The primary random effects model (figure 3) produced an NTM infection prevalence estimate of 7.9% (95% CI 5.1–12.0%), with a 95% prediction interval (PI), which is the interval in which a future observation is most likely to fall, of 1.0–41.6% and substantial heterogeneity in the estimate (I2=99%). The characteristics of studies reporting point and annual prevalence of NTM infection are summarised in supplementary table S4.
FIGURE 3.
Random effects meta-analysis of LOGIT transformed nontuberculous mycobacterium infection prevalence (annual and point) in the cystic fibrosis population (n=21). Includes last registry year that reported raw numbers for cases and evaluated patients. White crosses in forest plot represent the whiskers of narrow confidence intervals.
The heterogeneity of results was explored through subgroup analyses (supplementary figure S2). We did not examine age because 81% of included studies (17 of 21) had a mix of paediatric and adult populations without individual estimates reported for each group. No significant difference was found between subgroups of registry studies (n=5) and non-registry studies (n=16); heterogeneity was large for all subgroups (I2>90). The pre-specified subgroup meta-analyses by first year of data collection and geographical region showed no significant differences among subgroups (p>0.05). Studies conducted in other regions (Latin America and the Caribbean, Middle East, Africa and Australia) had less precise estimates (4%, 95% CI 0.2–40.1%) than those conducted in Europe or North America.
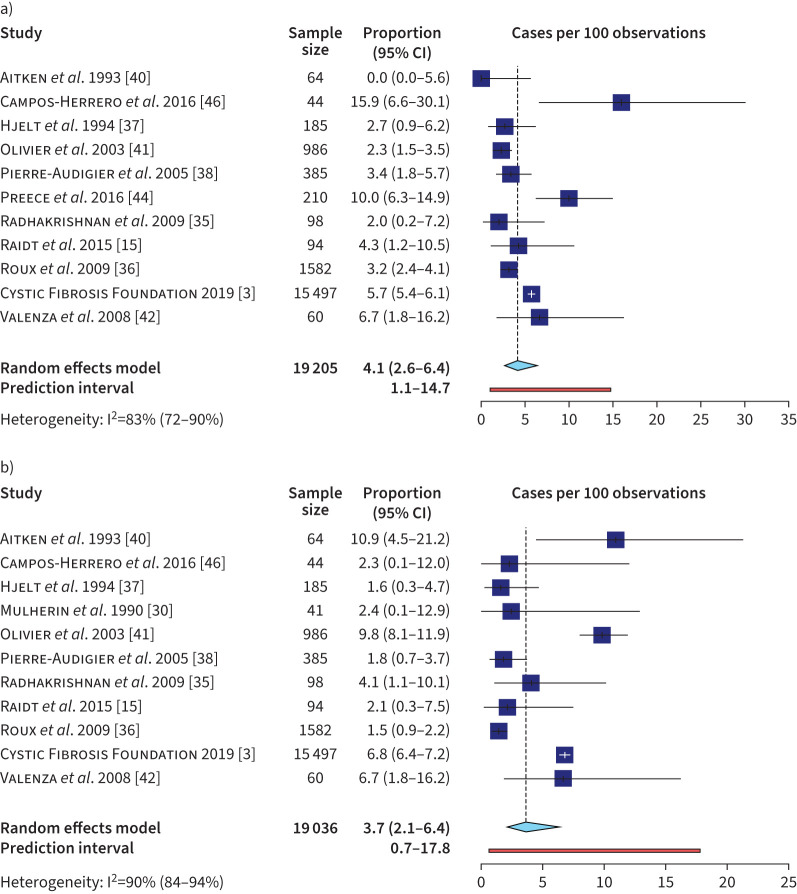
The prevalence (point and annual) of NTM infection was analysed separately for MAC and MABs (n=11 for both) (figure 4). The variability was lower for these two estimates than in the analysis including all NTM species, although heterogeneity remained >80%. The MAC estimate was 3.7% (95% PI 0.7–17.8%) and the MABs estimate was 4.1% (95% PI 1.1–14.7%). In an exploratory subgroup analysis examining prevalence by geographical region, a significantly lower prevalence (annual and point) of MAC infection was seen in Europe (1.7%, 95% CI 1.2–2.5%, I2=27%) compared to North America (7.8%, 95% CI 5.3–11.3, I2=80.7%). No differences were found in MABs infection prevalence by geographical region (supplementary figure S3). The funnel plot examining the relationship between sample size and NTM infection prevalence showed no graphical asymmetry or statistically significant difference (Peter's test, p=0.4) to suggest publication bias (supplementary figure S4) [47].
FIGURE 4.
Meta-analyses of a) Mycobacterium abscessus complex and b) Mycobacterium avium complex infection in the cystic fibrosis population including studies that reported point and annual prevalence. White crosses in forest plot represent the whiskers of narrow confidence intervals.
We evaluated which factors significantly affected the NTM infection prevalence while controlling for other covariates using meta-regression. The final model included the pre-specified variables study region, sample size category, year of data collection and study design. Age category was excluded because of the small number of studies reporting paediatric and adult estimates separately. As shown in table 1, ‘other’ geographical region and sample size <1000 had a significant effect on the estimated LOGIT prevalence (p<0.05). Proportions were obtained using the formula ecoef/(1+ecoef), where e is the mathematical constant and coef the respective coefficient. The calculated estimate for the intercept (5.3%) provides the NTM infection prevalence (point and annual) for studies with all reference categories, i.e. cross-sectional registry studies with sample sizes >3000 conducted in North America between 2010 and 2019. Each coefficient shows the magnitude of change in the associated category while holding all other covariates constant. On average, studies conducted in regions other than Europe and North America had a reduced estimate of NTM infection prevalence of 1.5% compared to those conducted in North America while all other factors were held constant. Also, studies with sample sizes below 1000 had a larger estimate on average (22.9%) compared to those with sample sizes above 3000 while holding all other covariates constant.
TABLE 1.
Results of meta-regression for nontuberculous mycobacteria infection point prevalence
| Coefficients | LOGIT-estimate | se | 95% CI | p-value |
| Intercept | −2.884 | 0.373 | −3.615– −2.153 | <0.01 |
| Design (Ref.: Cross-sectional: registry) | ||||
| Cross-sectional: non-registry | −0.290 | 0.893 | −2.040–1.461 | 0.746 |
| Cohort | −0.268 | 0.726 | −1.691–1.155 | 0.712 |
| Sample size (Ref.: >3000) | ||||
| 1000–3000 | 0.978 | 0.652 | −0.299–2.256 | 0.133 |
| <1000 | 1.671 | 0.682 | 0.335–3.007 | 0.014 |
| Region (Ref.: North American) | ||||
| European region | −0.424 | 0.396 | −1.201–0.353 | 0.285 |
| Other regions# | −1.302 | 0.501 | −2.284– −0.320 | 0.009 |
| Reporting year (Ref.: 2010–2019) | ||||
| 2000–2009 | 0.412 | 0.542 | −0.651–1.474 | 0.447 |
| Before year 2000 | −0.652 | 0.490 | −1.612–0.308 | 0.183 |
LOGIT-estimates are back-transformed to proportions through the formula ecoef/(1+ecoef). #: included Africa, Latin America and the Caribbean, and the Middle East.
Additional potential sources of variability included differences in the study populations, NTM testing frequency, microbial identification methods and NTM species distribution. Among the patient characteristics of the included studies, the distribution of female sex was homogeneous (median 47.9%, range 43.3–56.2%, n=15). Most studies included mixed paediatric and adult populations. Because of missing data, we could not determine whether differences in ethnicity or lung disease severity (i.e. forced expiratory volume in 1 s) could have affected the estimates. The frequency of testing was also difficult to assess as a source of variability because it was only reported in 28.6% of studies (six of 21) in the meta-analysis. Furthermore, a single study screened for NTM only in the presence of symptoms but a sensitivity analysis removing this study had no impact on the primary meta-analysis results (supplementary figure S3c). Out of 67 studies reporting NTM infection point prevalence or annual prevalence, only 24 described the specimen analysed (all used sputum alone or with other samples), and 14 the culturing method. In the meta-analysis, five studies did not report the specimen and seven failed to report the culturing method. Mycobacterial growth indicator tubes (MGIT) and Lowenstein–Jensen (LJ) medium were the most frequently used methods in 12 of 14 studies for point or annual prevalence of NTM infection [48]. However, the length of incubation, method of speciation and decontamination procedures varied significantly among studies.
Because most registries effectively capture the CF population in a region, we conducted a sensitivity analysis with only registry data (supplementary figure S5a). The results with only registry reports differed from those in the main meta-analysis; the estimate was 3.4% (95% CI 0.7–16.1%) with significant heterogeneity (I2=100%, n=5). Also, to evaluate the impact of using culture media other than those recommended by clinical practice guidelines (MGIT and LJ medium), we conducted a sensitivity analysis excluding four studies (supplementary figure S5b). The estimate was close to the one in the main meta-analysis at 7.1% (95% CI 4.2–12%), suggesting that the use of non-standardised culture media does not affect the overall results.
NTM infection period prevalence
Supplementary table S5 summarises the characteristics of studies that reported period prevalence of NTM infection in an interval longer than 1 year (n=32). The majority were cross-sectional non-registry studies (n=22, 69%) conducted in Europe (n=20, 62%) with mixed paediatric and adult populations (n=17, 53%). Typically, studies collected data spanning ≥5 years (n=8, 56%), while the longest study period was 14 years [49]. The variability in prevalence estimates was larger in studies with longer study periods (supplementary figure S6). In summary, most estimates of NTM infection period prevalence were between 6.6% and 19% (IQR). No meta-analysis was conducted due to diverging study periods. The median sample size was 192 (IQR 104–444), and only 12 studies had sample sizes larger than 300 participants.
NTM infection incidence
Incidence was reported as incidence proportion in five studies, with no reports of incidence rate [14, 46, 50–52]. Supplementary table S6 summarises the characteristics and estimates of these studies. Besides secondary registry analyses [51, 52], studies had small sample sizes (n≤110). The annual estimates of incidence proportion per year were typically <10%. The highest estimate (14.3% in 2002) was reported by the study with the smallest sample size (n=44) [46]. In contrast, the estimates of the study with the largest sample size ranged from 1.3% to 1.8% between 2011 and 2016 [51].
NTM-PD
Point prevalence of NTM-PD was only reported in two studies, and both had small sample sizes. Radhakrishnan et al. [35] reported a prevalence of one out of 98 (1.0%) using data collected in 2004 and based on the American Thoracic Society (ATS) 2007 criteria [53]. Bar-On et al. [14] evaluated annual prevalence in Israel between 2002 and 2011 using the ATS 2007 criteria and reported a prevalence between 2.5% and 11.3% (supplementary table S7).
NTM-PD period prevalence was reported in 13 studies, with estimates ranging between 0.8% (3-year period) and 22.7% (10-year period) (supplementary table S8) [46, 54]. Most studies were conducted in Europe (n=7), with the remaining ones in Israel, Brazil and a French territory in Africa. Most of them applied the ATS 2007 criteria (n=8), two used ATS 1997 criteria and three studies failed to report the criteria used to define NTM-PD. Only three studies had sample sizes above 300 participants. No reports of NTM-PD incidence were identified.
Discussion
This is the most comprehensive systematic review on the prevalence and incidence of NTM infection and NTM-PD in the CF population. The estimated prevalence (annual and point) of NTM infection in CF was 7.9% based on a meta-analysis of all non-registry and registry studies. With regards the most common mycobacteria in CF, the prevalence of infection with MABs was estimated at 4.1% and with MAC at 3.7%. NTM-PD had only two reports of point prevalence, and estimates of period prevalence were usually <10%, despite variable interval lengths per study (n=13). In general, all included studies had good quality in the appraisal of sampling and statistical methods, but poorer scores in reporting of microbiological methods and screening approaches.
We employed meta-regression to elucidate the contributors to heterogeneity in the meta-analysis of NTM infection prevalence (point and annual) and showed that a smaller sample size and a geographical region outside of North America and Europe produced significantly different estimates. However, only four studies were represented in this ‘other’ geographic region group, and it included a mixture of minimally represented populations in Asia, Latin America and the Middle East, likely with variable screening practices. In an exploratory analysis, we observed a lower prevalence of infection with MAC in European studies. Interestingly, some studies from Western Europe have reported a predominance of MABs infection in contrast to the dominance of MAC often seen in North America [36, 55–57].
The differential estimate of NTM infection prevalence according to sample size is likely driven by differences in study design. In a subgroup analysis (supplementary figure S2a), registry studies, which tended to be larger with unclear microbial identification methods and screening practices of participating registry sites, had a lower estimate of NTM infection prevalence (point/annual) than non-registry studies. This difference was not statistically significant. In contrast, non-registry studies had good concordance in microbiological identification methods (culture and specimens) and reported screening frequency more often but the studies were generally small with the potential for selection bias. Heterogeneity due to included population characteristics could not be evaluated owing to differences in primary data reporting of summary (mean, median) and distribution (median, mean, IQR, range) measures. Information related to the age range of first NTM detection could help with screening efforts because culturing frequency can be intensified during this higher risk period. Yet, the primary data reporting for age did not allow further exploration of its effect on NTM infection prevalence. To obtain comparable estimates from different countries and regions, harmonisation of screening practices and identification methods is necessary [7]. We encourage adherence to published reporting guidelines for observational studies (i.e. Strengthening the Reporting of Observational studies in Epidemiology (STROBE)) and standardisation of registry reporting to facilitate longitudinal and global comparisons [58, 59].
In recent years there has been an increase in the global burden of NTM in all populations [8, 14, 60–62]. Our analyses did not show significant differences in prevalence in the subgroup by years of data collection. Given the methodological variability between studies, we explored the longitudinal report of NTM infection annual prevalence within individual registries with comparable methods over the years. An increasing trend of prevalence was observed in all but the Brazilian registry (supplementary table S4 and figure S7), for which an overall low prevalence of NTM infection has been consistently reported [63]. Improved screening rates, novel detection methods and increased awareness may explain this increase [7].
Only a few studies reported NTM infection incidence measures (n=5) or NTM-PD prevalence (n=13 for period prevalence and n=2 for point prevalence). From a limited set of studies with small sample sizes, the incidence proportion of NTM infection was <10% per year in European populations [46, 51], without sufficient data from North America or other regions to make meaningful conclusions. Moreover, the conversion rate to NTM-PD after initial NTM infection remains unclear because no studies have reported the incidence of NTM-PD. Hopefully, ongoing studies like the PREDICT trial (NCT02073409), which is evaluating a standardised approach to NTM-PD diagnosis in CF, will help establish an approximate risk of progression [64].
Overall, the results from this systematic review present a comprehensive view of the known burden of NTM in CF while pointing out gaps in knowledge [65]. Accurate epidemiological data about the frequency and risk of NTM infection in CF is necessary to inform clinical decision-making and health policy. Identification of high-risk groups can help facilitate prevention and surveillance strategies to improve prognosis and quality of life. There was large variability and a wide prediction interval for our NTM infection meta-analysis, which may limit its utility for decision-making. Unfortunately, a lack of reported data in primary studies did not allow for further exploration of the sources of heterogeneity beyond the ones already described. Once CFTR modulators are widely implemented, their impact on infection prevalence is likely to change and our results may serve as a baseline to measure the impact on NTM and NTM-PD. However, future prevalence and incidence estimates might also be difficult to interpret and compare against historical estimates given that obtaining sputum samples on demand for surveillance is becoming more challenging for patients on highly effective CFTR modulators. Finally, moving forwards, we advocate for a stronger emphasis on reporting standards of microbiological identification methods and screening procedures for registry and non-registry studies [59]. A significant and relatively low-cost way to build upon this work is to create a living systematic review of the NTM burden in CF, which could be updated annually with new registry and observational data to enhance surveillance of trends [66].
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods, figures and tables 00336-2022.supplement (1.7MB, pdf)
Acknowledgments
We would like to thank Dean Giustini from the University of British Columbia Biomedical branch library for his support and guidance in the design and improvement of systematic searches of the Medline and Embase databases.
Provenance: Submitted article, peer reviewed
Support statement: A.N. Franciosi is supported by a Michael Smith Foundation for Health Research Trainee Award (#RT-2020-0493). B.S. Quon is supported by a Michael Smith Foundation for Health Research Scholar Award. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: None declared.
References
- 1.O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 2009; 373: 1891–1904. doi: 10.1016/S0140-6736(09)60327-5 [DOI] [PubMed] [Google Scholar]
- 2.Spoonhower KA, Davis PB. Epidemiology of cystic fibrosis. Clin Chest Med 2016; 37: 1–8. doi: 10.1016/j.ccm.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 3.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2019 Annual Data Report. Bethesda, MD Cystic Fibrosis Foundation, 2020. [Google Scholar]
- 4.Blanchard AC, Waters VJ. Microbiology of cystic fibrosis airway disease. Semin Respir Crit Care Med 2019; 40: 727–736. doi: 10.1055/s-0039-1698464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falkinham JO. Environmental sources of nontuberculous mycobacteria. Clin Chest Med 2015; 36: 35–41. doi: 10.1016/j.ccm.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 6.Koh W-J. Nontuberculous mycobacteria-overview. Microbiol Spectr 2017; 5 : 5.1.11. [DOI] [PubMed] [Google Scholar]
- 7.Floto RA, Olivier KN, Saiman L, et al. . US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 2016; 71: i1–i22. doi: 10.1136/thoraxjnl-2015-207360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esther CR, Jr., Esserman DA, Gilligan P, et al. . Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros 2010; 9: 117–123. doi: 10.1016/j.jcf.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martiniano SL, Sontag MK, Daley CL, et al. . Clinical significance of a first positive nontuberculous mycobacteria culture in cystic fibrosis. Ann Am Thorac Soc 2014; 11: 36–44. doi: 10.1513/AnnalsATS.201309-310OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adjemian J, Olivier KN, Seitz AE, et al. . Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 2012; 185: 881–886. doi: 10.1164/rccm.201111-2016OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marras TK, Mendelson D, Marchand-Austin A, et al. . Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998–2010. Emerg Infect Dis 2013; 19: 1889–1891. doi: 10.3201/eid1911.130737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park YS, Lee CH, Lee SM, et al. . Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int J Tuberc Lung Dis 2010; 14: 1069–1071. [PubMed] [Google Scholar]
- 13.Salsgiver EL, Fink AK, Knapp EA, et al. . Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis. Chest 2016; 149: 390–400. doi: 10.1378/chest.15-0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bar-On O, Mussaffi H, Mei-Zahav M, et al. . Increasing nontuberculous mycobacteria infection in cystic fibrosis. J Cyst Fibros 2015; 14: 53–62. doi: 10.1016/j.jcf.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 15.Raidt L, Idelevich EA, Dübbers A, et al. . Increased prevalence and resistance of important pathogens recovered from respiratory specimens of cystic fibrosis patients during a decade. Pediatr Infect Dis J 2015; 34: 700–705. doi: 10.1097/INF.0000000000000714 [DOI] [PubMed] [Google Scholar]
- 16.Aromataris E, Munn Z. JBI Reviewer's Manual. Adelaide, Joanna Briggs Institute, 2019. [Google Scholar]
- 17.Munn Z, MClinSc SM, Lisy K, et al. . Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 2015; 13: 147–153. doi: 10.1097/XEB.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 18.Rathbone J, Carter M, Hoffmann T, et al. . Better duplicate detection for systematic reviewers: evaluation of systematic review assistant-deduplication module. Syst Rev 2015; 4: 6. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Migliavaca CB, Stein C, Colpani V, et al. . Quality assessment of prevalence studies: a systematic review. J Clin Epidemiol 2020; 127: 59–68. doi: 10.1016/j.jclinepi.2020.06.039 [DOI] [PubMed] [Google Scholar]
- 20.Borges Migliavaca C, Stein C, Colpani V, et al. . How are systematic reviews of prevalence conducted? A methodological study. BMC Med Res Methodol 2020; 20: 1–9. doi: 10.1186/s12874-020-00975-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munn Z, Moola S, Riitano D, et al. . The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag 2014; 3: 123–128. doi: 10.15171/ijhpm.2014.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 23.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and web application for visualising risk-of-bias assessments. Res Synth Methods 2020; 12: 55–61. doi: 10.1002/jrsm.1411 [DOI] [PubMed] [Google Scholar]
- 24.Schwarzer G, Chemaitelly H, Abu-Raddad LJ, et al. . Seriously misleading results using inverse of Freeman–Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods 2019; 10: 476–483. doi: 10.1002/jrsm.1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin L, Xu C. Arcsine-based transformations for meta-analysis of proportions: pros, cons, and alternatives. Health Sci Rep 2020; 3: e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin L, Chu H. Meta-analysis of proportions using generalized linear mixed models. Epidemiology 2020; 31: 713–717. doi: 10.1097/EDE.0000000000001232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter JP, Saratzis A, Sutton AJ, et al. . In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol 2014; 67: 897–903. doi: 10.1016/j.jclinepi.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 28.Page MJ, McKenzie JE, Bossuyt PM, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Brazilian Cystic Fibrosis Study Group . The Brazilian Cystic Fibrosis Patient Registry 2017 Annual Report. http://www.gbefc.org.br/ckfinder/userfiles/files/REBRAFC_2017_EN.pdf Date last accessed: 10 February 2022.
- 30.Mulherin D, Coffey MJ, Halloran DO, et al. . Skin reactivity to atypical mycobacteria in cystic fibrosis. Respir Med 1990; 84: 273–276. [DOI] [PubMed] [Google Scholar]
- 31.Ruseckaite R, Ahern S, Ranger T, et al. . The Australian Cystic Fibrosis Data Registry Annual Report, 2017. Melbourne, Dept of Epidemiology and Preventive Medicine Monash University, 2019. [Google Scholar]
- 32.Seddon P, Fidler K, Raman S, et al. . Prevalence of nontuberculous mycobacteria in cystic fibrosis clinics, United Kingdom, 2009. Emerg Infect Dis 2013; 19: 1128–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zolin A, Orenti A, Naehrlich L, et al. . 2018 European Cystic Fibrosis Society Patient Registry Annual Data Report. 2020. Available from: https://www.ecfs.eu/projects/ecfs-patient-registry/annual-reports. [Google Scholar]
- 34.Cystic Fibrosis Canada . The Canadian Cystic Fibrosis Registry 2019 Annual Data Report. 2021. Available from: https://www.cysticfibrosis.ca/about-us/publications-and-financials [Google Scholar]
- 35.Radhakrishnan DK, Yau Y, Corey M, et al. . Non-tuberculous mycobacteria in children with cystic fibrosis: isolation, prevalence, and predictors. Pediatr Pulmonol 2009; 44: 1100–1106. doi: 10.1002/ppul.21106 [DOI] [PubMed] [Google Scholar]
- 36.Roux AL, Catherinot E, Ripoll F, et al. . Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in France. J Clin Microbiol 2009; 47: 4124–4128. doi: 10.1128/JCM.01257-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hjelt K, Hojlyng N, Howitz P, et al. . The role of mycobacteria other than tuberculosis (MOTT) in patients with cystic fibrosis. Scand J Infect Dis 1994; 26: 569–576. [DOI] [PubMed] [Google Scholar]
- 38.Pierre-Audigier C, Ferroni AA, Sermet-Gaudelus I, et al. . Age-related prevalence and distribution of nontuberculous mycobacterial species among patients with cystic fibrosis. J Clin Microbiol 2005; 43: 3467–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scohy A, Gohy S, Mathys V, et al. . Comparison of the RGM medium and the mycobacterial growth indicator tube automated system for isolation of non-tuberculous mycobacteria from sputum samples of cystic fibrosis patients in Belgium. J Clin Tuberc Other Mycobact Dis 2018; 13: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aitken ML, Burke W, McDonald G, et al. . Nontuberculous mycobacterial disease in adult cystic fibrosis patients. Chest 1993; 103: 1096–1099. [DOI] [PubMed] [Google Scholar]
- 41.Olivier KN, Weber DJ, Wallace RJ, et al. . Nontuberculous mycobacteria: I: Multicenter prevalence study in cystic fibrosis. Ame J Respir Crit Care Med 2003; 167: 828–834. [DOI] [PubMed] [Google Scholar]
- 42.Valenza G, Tappe D, Turnwald D, et al. . Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J Cyst Fibros 2008; 7: 123–127. [DOI] [PubMed] [Google Scholar]
- 43.Plongla R, Preece CL, Perry JD, et al. . Evaluation of RGM medium for isolation of nontuberculous mycobacteria from respiratory samples from patients with cystic fibrosis in the United States. J Clin Microbiol 2017; 55: 1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Preece CL, Perry A, Gray B, et al. . A novel culture medium for isolation of rapidly growing mycobacteria from the sputum of patients with cystic fibrosis. J Cyst Fibros 2016; 15: 186–191. [DOI] [PubMed] [Google Scholar]
- 45.Paschoal IA, De Oliveira Villalba W, Bertuzza CS, et al. . Cystic fibrosis in adults. Lung 2007; 185: 81–87. [DOI] [PubMed] [Google Scholar]
- 46.Campos-Herrero M, Chamizo FJ, Caminero JA, et al. . Nontuberculous mycobacteria in cystic fibrosis patients on the Island of Gran Canaria. A population study. J Infect Chemother 2016; 22: 526–531. doi: 10.1016/j.jiac.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 47.Peters JL, Sutton AJ, Jones DR, et al. . Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006; 295: 676–680. doi: 10.1001/jama.295.6.676 [DOI] [PubMed] [Google Scholar]
- 48.Forbes BA, Hall GS, Miller MB, et al. . Practical guidance for clinical microbiology laboratories: mycobacteria. Clin Microbiol Rev 2018; 31: e00038-17. doi: 10.1128/CMR.00038-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho D, Andre M, Gazaille V, et al. . High prevalence of nontuberculous mycobacteria in cystic fibrosis patients in tropical French reunion Island. Pediatr Infect Dis J 2021; 40: E120–E122. doi: 10.1097/INF.0000000000002999 [DOI] [PubMed] [Google Scholar]
- 50.Leitritz L, Griese M, Roggenkamp A, et al. . Prospective study on nontuberculous mycobacteria in patients with and without cystic fibrosis. Med Microbiol Immunol 2004; 193: 209–217. doi: 10.1007/s00430-003-0195-9 [DOI] [PubMed] [Google Scholar]
- 51.Hatziagorou E, Orenti A, Drevinek P, et al. . Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis-data from the European Cystic Fibrosis Society patient registry. J Cyst Fibros 2020; 19: 376–383. doi: 10.1016/j.jcf.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 52.Binder AM, Adjemian J, Olivier KN, et al. . Epidemiology of nontuberculous mycobacterial infections and associated macrolide use among persons with cystic fibrosis. Pediatr Pulmonol 2013; 48: 303–305. doi: 10.1002/ppul.22583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griffith DE, Aksamit T, Brown-Elliott BA, et al. . An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175: 367–416. doi: 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 54.Candido PHC, de Nunes L, Marques EA, et al. . Multidrug-resistant nontuberculous mycobacteria isolated from cystic fibrosis patients. J Clin Microbiol 2014; 52: 2990–2997. doi: 10.1128/JCM.00549-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Russell CD, Claxton P, Doig C, et al. . Non-tuberculous mycobacteria: a retrospective review of Scottish isolates from 2000 to 2010. Thorax 2014; 69: 593–595. doi: 10.1136/thoraxjnl-2013-204260 [DOI] [PubMed] [Google Scholar]
- 56.Catherinot E, Roux AL, Vibet MA, et al. . Mycobacterium avium and Mycobacterium abscessus complex target distinct cystic fibrosis patient subpopulations. J Cyst Fibros 2013; 12: 74–80. doi: 10.1016/j.jcf.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 57.Qvist T, Pressler T, Høiby N, et al. . Shifting paradigms of nontuberculous mycobacteria in cystic fibrosis. Respir Res 2014; 15: 41. doi: 10.1186/1465-9921-15-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fink AK, Loeffler DR, Marshall BC, et al. . Data that empower: the success and promise of CF patient registries. Pediatr Pulmonol 2017; 52: S44–S51. doi: 10.1002/ppul.23790 [DOI] [PubMed] [Google Scholar]
- 59.von Elm E, Altman DG, Egger M, et al. . The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 60.Cavalli ZZ, Reynaud Q, Bricca R, et al. . High incidence of non-tuberculous mycobacteria-positive cultures among adolescent with cystic fibrosis. J Cyst Fibros 2017; 16: 579–584. doi: 10.1016/j.jcf.2017.01.017 [DOI] [PubMed] [Google Scholar]
- 61.Winthrop KL, Marras TK, Adjemian J, et al. . Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. managed care health plan, 2008–2015. Ann Am Thorac Soc 2020; 17: 178–185. doi: 10.1513/AnnalsATS.201804-236OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H, Myung W, Koh WJ, et al. . Epidemiology of nontuberculous mycobacterial infection, South Korea, 2007–2016. Emerg Infect Dis 2019; 25: 569–572. doi: 10.3201/eid2503.181597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aiello TB, Levy CE, Zaccariotto TR, et al. . Prevalence and clinical outcomes of nontuberculous mycobacteria in a Brazilian cystic fibrosis reference center. Pathog Dis 2018; 76: fty051. doi: 10.1093/femspd/fty051 [DOI] [PubMed] [Google Scholar]
- 64.Nick JA. PREDICT trial: PRospective evaluation of NTM disease in CysTic fibrosis. ClinicalTrials.gov identifier: NCT02073409. 2021. Date last accessed: 29 November 2021.
- 65.Saiman L. Improving outcomes of infections in cystic fibrosis in the era of CFTR modulator therapy. Pediatr Pulmonol 2019; 54: S18–S26. doi: 10.1002/ppul.24522 [DOI] [PubMed] [Google Scholar]
- 66.Macdonald H, Loder E, Abbasi K. Living systematic reviews at The BMJ. BMJ 2020; 370: m2925. doi: 10.1136/bmj.m2925 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods, figures and tables 00336-2022.supplement (1.7MB, pdf)