Abstract
Background
Sleep disturbances occur frequently in people with dementia with a reported prevalence of up to 40%. Common problems are increased number and duration of awakenings and increased percentage of light sleep. Sleep disturbances are associated with a number of problems for people with dementia, their relatives, and carers. In people with dementia, they may lead to worsening of cognitive symptoms, challenging behaviours such as restlessness or wandering, and further harms, such as accidental falls. Sleep disturbances are also associated with significant carer distress and have been reported as a factor contributing to institutionalisation of people with dementia. As pharmacological approaches have shown unsatisfactory results, there is a need to synthesise the research evidence on non‐pharmacological strategies to improve sleep in people with dementia. As interventions are often complex, consisting of more than one active component, and implemented in complex contexts, it may not be easy to identify effective intervention components.
Objectives
To evaluate the benefits and harms of non‐pharmacological interventions on sleep disturbances in people with dementia compared to usual care, no treatment, any other non‐pharmacological intervention, or any drug treatment intended to improve sleep, and to describe the components and processes of any complex intervention included.
Search methods
We used standard, extensive Cochrane search methods. The latest search was 13 January 2022.
Selection criteria
We included individually or cluster‐randomised controlled trials in people with dementia comparing non‐pharmacological interventions to improve sleep compared to usual care or to other interventions of any type. Eligible studies had to have a sleep‐related primary outcome. We included people with a diagnosis of dementia and sleep problems at baseline irrespective of age, type of dementia, severity of cognitive impairment, or setting. Studies reporting results on a mixed sample (e.g. in a nursing home) were only considered for inclusion if at least 80% of participants had dementia.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were 1. objective sleep‐related outcomes (e.g. total nocturnal sleep time, consolidated sleep time at night, sleep efficiency, total wake time at night (or time spent awake after sleep onset), number of nocturnal awakenings, sleep onset latency, daytime/night‐time sleep ratio, night‐time/total sleep ratio over 24 hours) and 2. adverse events. Our secondary outcomes were 3. subjective sleep‐related outcomes, 4. behavioural and psychological symptoms of dementia, 5. quality of life, 6. functional status, 7. institutionalisation, 8. compliance with the intervention, and 9. attrition rates. We used GRADE to assess the certainty of evidence and chose key outcomes to be included in summary of findings tables.
Main results
We included 19 randomised controlled trials with 1335 participants allocated to treatment or control groups. Fourteen studies were conducted in nursing homes, three included community residents, one included 'inpatients', one included people from a mental health centre, and one included people from district community centres for older people. Fourteen studies were conducted in the US. We also identified nine ongoing studies.
All studies applied one or more non‐pharmacological intervention aiming to improve physiological sleep in people with dementia and sleep problems. The most frequently examined single intervention was some form of light therapy (six studies), five studies included physical or social activities, three carer interventions, one daytime sleep restriction, one slow‐stroke back massage, and one transcranial electrostimulation. Seven studies examined multimodal complex interventions.
Risk of bias of included studies was frequently unclear due to incomplete reporting. Therefore, we rated no study at low risk of bias.
We are uncertain whether light therapy has any effect on sleep‐related outcomes (very low‐certainty evidence). Physical activities may slightly increase the total nocturnal sleep time and sleep efficiency, and may reduce the total time awake at night and slightly reduce the number of awakenings at night (low‐certainty evidence). Social activities may slightly increase total nocturnal sleep time and sleep efficiency (low‐certainty evidence). Carer interventions may modestly increase total nocturnal sleep time, may slightly increase sleep efficiency, and may modestly decrease the total awake time during the night (low‐certainty evidence from one study). Multimodal interventions may modestly increase total nocturnal sleep time and may modestly reduce the total wake time at night, but may result in little to no difference in number of awakenings (low‐certainty evidence). We are uncertain about the effects of multimodal interventions on sleep efficiency (very low‐certainty evidence). We found low‐certainty evidence that daytime sleep restrictions, slow‐stroke back massage, and transcranial electrostimulation may result in little to no difference in sleep‐related outcomes.
Only two studies reported information about adverse events, detecting only few such events in the intervention groups.
Authors' conclusions
Despite the inclusion of 19 randomised controlled trials, there is a lack of conclusive evidence concerning non‐pharmacological interventions for sleep problems in people with dementia. Although neither single nor multimodal interventions consistently improved sleep with sufficient certainty, we found some positive effects on physical and social activities as well as carer interventions. Future studies should use rigorous methods to develop and evaluate the effectiveness of multimodal interventions using current guidelines on the development and evaluation of complex interventions. At present, no single or multimodal intervention can be clearly identified as suitable for widespread implementation.
Keywords: Aged, Humans, Caregivers, Caregivers/psychology, Dementia, Dementia/complications, Quality of Life, Randomized Controlled Trials as Topic, Sleep Wake Disorders, Sleep Wake Disorders/epidemiology, Sleep Wake Disorders/therapy
Plain language summary
Non‐medicine interventions for sleep problems in dementia
What are sleep problems in people with dementia?
People with dementia frequently have sleep problems including an increase in the length and number of awakenings and an increased amount of light sleep. These cause a number of problems for the affected person, their relatives, and carers, possibly leading to carer distress and the admission of people with dementia to nursing homes or long‐term care homes.
Can non‐medicine interventions help?
As we do not know if medicines can help improve sleep in people with dementia, non‐medicine interventions are frequently recommended. These include light therapy, social and physical activities, changes of the environment (such as reducing noise and light at night), or avoiding daytime sleep. Also, intervention programmes consisting of more than one of these components are available (so‐called 'multimodal interventions'; e.g. combining light therapy and activities for people with dementia).
What did we want to find out?
We searched for clinical trials that tested the effects of non‐medicine interventions for people with dementia and sleep problems. We wanted to find out if these interventions or programmes can promote sleep and avoid side effects for people with dementia and their carers.
What did we do?
We searched for randomised controlled trials (a design of study that usually gives the most reliable evidence about the effects of a treatment) evaluating any non‐medicine intervention to improve sleep in people with dementia. We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and numbers of participants.
What did we find?
We identified 19 studies, including 1335 participants. The studies included 13 to 193 participants with sleep problems and dementia. All studies applied one or more non‐medicine intervention (i.e. light therapy, physical and social activities, carer interventions, daytime sleep restriction, slow‐stroke back massage, or transcranial electrostimulation (a method that delivers a low electric current to the scalp that changes brain function)). Seven studies assessed multimodal interventions. Studies assessed sleep in different ways, but most used actigraphy, which is a wristband to measure night‐time sleep.
Main results
– Physical activity interventions, social activities, carer interventions, and multimodal interventions may slightly or modestly improve night‐time sleep in people with dementia.
– We found no evidence that light therapy, slow‐stroke back massage, or transcranial electrostimulation reduce sleep problems in people with dementia.
What are the limitations of the evidence?
Although we were able to include 19 studies with 1335 participants evaluating non‐medicine interventions to avoid sleep disturbances in people with dementia, we were unable to draw firm conclusions mostly due to important differences between interventions and lack of methodological quality. Therefore, the results of this review must be interpreted with caution and high‐quality studies are urgently needed.
How up to date is this evidence?
The evidence is up to date to 13 January 2022.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Light therapy compared to usual care for sleep disturbances in people with dementia.
| Light therapy compared to usual care for sleep disturbances in people with dementia | ||||||
| Patient or population: sleep disturbances in people with dementia Setting: nursing home Intervention: light therapy Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with light therapy | |||||
| Total nocturnal sleep time (minutes) | The mean total nocturnal sleep time in the control group was 512 minutes (Dowling 2005), 496 minutes (Figueiro 2019), 430 minutes (Fontana Gasio 2003), and 248 minutes (Sloane 2014). Total nocturnal sleep time between groups was 33 minutes lower (103.54 lower to 37.54 higher) in Dowling 2005, 20.40 minutes lower (63.29 lower to 22.49 higher) in Figueiro 2019, 110.00 minutes higher (19.36 higher to 200.64 higher) in Fontana Gasio 2003, and there was no clear difference in Sloane 2014 (0.23 minutes higher, 12.75 lower to 12.28 higher). | 82 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | 2 studies found a difference in favour of the control group (Dowling 2005; Figueiro 2019). Fontana Gasio 2003 found differences in favour of the intervention. Sloane 2015 found no clear difference between groups. | ||
| Consolidated sleep ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sleep efficiency | The mean sleep efficiency in the control group was 71.14% (Dowling 2005), 85.43% (Figueiro 2019), 59.9% (Fontana Gasio 2003), 78.1% (McCurry 2011), 90.84% (Nowak 2008), and 68.9% (Sloane 2014). 1 study reported no data. Sleep efficiency between groups was 4.50% lower (14.34 lower to 5.34 higher) in Dowling 2005, 2.21% lower (5.17 lower to 0.75 higher) in Figueiro 2019, 16.60% higher (6.49 higher to 26.71 higher) in Fontana Gasio 2003, 6.20% higher (0.04 lower to 12.44 higher) in McCurry 2011, and 5.60% higher (0.47 higher to 10.73 higher) in Nowak 2008. 2 studies found no difference between groups, 0% (3.45 lower to 3.45 higher) in Sloane 2014, Ancoli‐Israel 2003 reported no data. | 133 (7 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Dowling 2005 reported differences between groups in favour of the control group using actigraphy after 11 weeks. 3 studies found small improvements in the intervention group after 10 weeks, and 2 months and 2 weeks (Fontana Gasio 2003; McCurry 2011; Nowak 2008). 2 studies found no difference between groups after 15 days and 6 weeks (Ancoli‐Israel 2003; Sloane 2014) | ||
| Total wake time at night (minutes) | The mean night‐time total wake time in the control group was 207 minutes (Dowling 2005) and 123 minutes (McCurry 2011), 1 study did not offer this information. Night‐time total wake time between groups was 32.00 minutes higher (38.54 lower to 102.54 higher) in Dowling 2005 and 39.00 minutes lower (74.40 lower to 3.60 lower) in McCurry 2011. 1 study found no difference between groups, but no further information was reported. | 205 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Dowling 2005 reported a small improvement in the control group and McCurry 2011 found a small improvement in the intervention group using actigraphy after 11 weeks and 2 months. Ancoli‐Israel 2002 found no differences between groups after 15 days. | ||
| Number of nocturnal awakenings | The mean night‐time number of awakenings in the control group was 37.99 (Dowling 2005), 14.9 (Sloane 2014), 17.6 (McCurry 2011), and 4.6 (Nowak 2008). Night‐time number of awakenings for light therapy was 4.89 higher (3.31 lower to 13.09 higher) in Dowling 2005, 2.90 lower (7.09 lower to 1.29 higher) in McCurry 2011, and 2.31 lower (4.17 lower to 0.45 lower) in Nowak 2008. Sloane 2014 found no clear difference between groups (0.81 lower, 2.64 lower to 1.03 higher). | 136 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Dowling 2005 reported small improvements in the control group after 11 weeks and 2 studies reported a small improvement in the intervention group after 2 weeks and 2 months (McCurry 2011; Nowak 2008). Sloane 2015 found no difference between groups. | ||
| Sleep onset latency | The mean sleep onset latency in the control group was 12.32 minutes (Figueiro 2019), 1 minute (Fontana Gasio 2003), and 24 minutes (Sloane 2015). Sleep onset latency between groups was 6.05 minutes higher (0.60 lower to 12.70 higher) in Figueiro 2019, 1.02 minutes lower (3.34 lower to 1.30 higher) in Fontana Gasio 2003, and 3.72 minutes lower (9.54 lower to 2.10 higher) in Sloane 2015. | (3 RCTs) | ⊕⊕⊝⊝ Lowc,d | 3 studies reported no clear differences between groups after 3, 4, and 6 weeks (Figueiro 2019; Fontana Gasio 2003; Sloane 2015). | ||
| Adverse events | None of the studies reported any unexpected or serious adverse events | 318 (7 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_424365942749483426. | ||||||
a Downgraded one level for risk of bias: unclear risk of bias in several studies; high risk of bias in blinding participants and personnel in one study (McCurry 2011). b Downgraded one level for inconsistency: inconsistent results between studies. c Downgraded one level for imprecision: wide confidence intervals in individual studies. d Downgraded one level for risk of bias: unclear risk of selection bias in all studies, high risk of reporting bias in one study (Figueiro 2019).
Summary of findings 2. Summary of findings table ‐ Physical activities compared to usual care for sleep disturbances in people with dementia.
| Physical activities compared to usual care for sleep disturbances in people with dementia | ||||||
| Patient or population: sleep disturbances in people with dementia Setting: nursing home Intervention: physical activities Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with physical activities | |||||
| Total nocturnal sleep time (minutes) | The mean total nocturnal sleep time in the control group was 438.3 minutes (McCurry 2011) and 328.9 minutes (Richards 2011). Total nocturnal sleep time with physical activity was 11.80 minutes higher (28.63 lower to 52.23 higher) in McCurry 2011 and 11.8 minutes higher (16.14 lower to 39.74 higher) in Richards 2011. | 167 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | Both studies reported differences between groups for total nocturnal sleep time in favour of the intervention group after 7 weeks and 2 months (McCurry 2011; Richards 2011). | ||
| Consolidated sleep ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sleep efficiency | The mean sleep efficiency in the control group was 78.1% (McCurry 2011) and 68.5% (Richards 2011). Sleep efficiency for physical activity was 4.90% higher (0.43 lower to 10.23 higher) in McCurry 2011 and 2.60% higher (1.29 lower to 6.49 higher) in Richards 2011. | 167 (2 RCTs) | ⊕⊕⊝⊝ Lowa,c | Richards 2011 reported differences between groups in favour of the intervention group using actigraphy after 7 weeks and McCurry found little to no difference between groups after 6 months. | ||
| Total wake time at night (minutes) | The mean total wake time at night (minutes) was 122 minutes | 33.2 minutes lower (65.11 lower to 1.29 lower) | ‐ | 65 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | McCurry 2011 reported improvements in the intervention group using actigraphy after 2 months. |
| Number of nocturnal awakenings | The mean number of nocturnal awakenings was 18.4 | 3.3 lower (6.77 lower to 0.17 higher) | ‐ | 65 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | McCurry 2011 found differences between groups in favour of the intervention using actigraphy after 2 months. |
| Sleep onset latency | 1 study reported no changes after 6 months, but reported no data. | (1 RCT) | ⊕⊕⊕⊝ Moderated | Richards 2011 reported no differences between groups using actigraphy after 7 weeks. | ||
| Adverse events | 1 study reported unexpected and serious adverse events (Richards 2011). 1 participant had substernal chest pain 15 hours after exercising, but was negative for myocardial infarction; 1 had back, hip, and leg pain; and 1 had multifocal premature ventricular contractions or non‐specific t‐wave changes in their electrocardiogram. | 167 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_424365659920486101. | ||||||
a Downgraded one level for risk of bias: high risk of a performance bias in one study. b Downgraded one level for imprecision: only one study with a small number of participants. c Downgraded one level for imprecision: wide confidence intervals. d Downgraded one level for imprecision: no data reported.
Summary of findings 3. Summary of findings table ‐ Social activities compared to usual care for sleep disturbances in people with dementia.
| Social activities compared to usual care for sleep disturbances in people with dementia | ||||||
| Patient or population: sleep disturbances in people with dementia Setting: nursing home Intervention: social activities Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with social activities | |||||
| Total nocturnal sleep time (minutes) | The mean total nocturnal sleep time (minutes) was 328.9 minutes | MD 16.78 minutes higher (7.78 lower to 41.34 higher) | ‐ | 236 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | Both studies reported differences between groups in favour of the interventions using actigraphy after 21 days and 7 weeks (Richards 2005; Richards 2011). |
| Consolidated sleep ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sleep efficiency | The mean sleep efficiency was 52.69 % | MD 2.65 % higher (1.79 lower to 7.09 higher) | ‐ | 236 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | Both studies reported differences between groups in favour of the interventions using actigraphy after 21 days and 7 weeks (Richards 2005; Richards 2011). |
| Total wake time at night (minutes) ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Number of nocturnal awakenings ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Sleep onset latency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events | None of the studies reported any unexpected or serious adverse event | 236 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_424365232111962546. | ||||||
a Downgraded one level for risk of bias: unclear risk of selection, performance, and detection bias in at least one study. b Downgraded one level for imprecision: wide confidence interval.
Summary of findings 4. Summary of findings table ‐ Carer interventions compared to usual care for sleep disturbances in people with dementia.
| Carer interventions compared to usual care for sleep disturbances in people with dementia | ||||||
| Patient or population: sleep disturbances in people with dementia Setting: nursing home Intervention: carer interventions Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with carer interventions | |||||
| Total nocturnal sleep time (minutes) | The mean total nocturnal sleep time (minutes) was 468 minutes | 108 minutes higher (10.6 higher to 205.4 higher) | ‐ | 33 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | McCurry 2012 reported small differences between groups in favour of the intervention using actigraphy after 6 months. |
| Consolidated sleep ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sleep efficiency | The mean sleep efficiency was 75.8 % | 8.4 % higher (1.55 lower to 18.35 higher) | ‐ | 33 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | McCurry 2012 reported small differences between groups in favour of the intervention using actigraphy after 6 months. |
| Total wake time at night (minutes) | The mean total wake time at night (minutes) was 138 minutes | 24 minutes lower (79.01 lower to 31.01 higher) | ‐ | 33 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | McCurry 2012 reported small differences between groups in favour of the intervention using actigraphyafter 6 months. |
| Number of nocturnal awakenings ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Sleep onset latency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events | None of the studies reported any serious unexpected events. | (2 RCTs) | ⊕⊕⊕⊝ Moderatea | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_424366201603052337. | ||||||
a Downgraded one level for risk of bias: unclear risk of selection and performance bias. b Downgraded one level for imprecision: only one study with a small number of participants.
Summary of findings 5. Summary of findings table ‐ Multimodal interventions compared to usual care for sleep disturbances in dementia.
| Multimodal interventions compared to usual care for sleep disturbances in dementia | ||||||
| Patient or population: sleep disturbances in dementia Setting: nursing home Intervention: multimodal interventions Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with multimodal interventions | |||||
| Total nocturnal sleep time (minutes) | The mean total nocturnal sleep time in the control group was 384 minutes (Alessi 2005), 438.3 minutes (McCurry 2011), and 328.9 minutes (Richards 2011). Total nocturnal sleep time for multimodal interventions was 24.00 minutes higher (3.51 lower to 51.51 higher) in Alessi 2005, 29.4 minutes higher (25.90 lower to 84.70 higher) in McCurry 2011, and 35.3 minutes higher (7.99 higher to 62.61 higher) in Richards 2011. | 272 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | All studies reported differences between groups in favour of the interventions using actigraphy after 32 days (Alessi 2005), 7 weeks (Richards 2011), and 6 months (McCurry 2011). | ||
| Consolidated sleep ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sleep efficiency | The mean sleep efficiency in the control group was 66.3% (Alessi 1999), 80% (Alessi 2005), 78.1% (McCurry 2011), 68.5% (Richards 2011), and 60.8% (Schnelle 1999). Sleep efficiency for multimodal interventions was 3.80% lower (17.96% lower to 10.36% higher) in Alessi 1999, 4% higher (1.42% lower to 9.42% higher) in Alessi 2005, 2.3% higher (5.08% lower to 9.68% higher) in McCurry 2011, 4.80% higher (0.47% higher to 9.13% higher) in Richards 2011, and without a difference in Schnelle 1999 (MD 0%, 4.61% lower to 4.61% higher). | 485 (5 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | 3 studies found improvements in favour of the interventions after 32 days (Alessi 2005), 7 weeks (Richards 2011), and 6 months (McCurry 2011). 1 study found small differences in favour of the control group after 14 weeks (Alessi 1999). 1 study found no differences between groups (Schnelle 1999). | ||
| Total wake time at night (minutes) | The mean night‐time total wake time in the control group was 108 minutes (McCurry 2005) and 122 minutes (McCurry 2011).Night‐time total wake time for multimodal interventions was 36.00 minutes lower (89.66 lower to 17.66 higher) in McCurry 2005 and 7.00 minutes lower (52.90 lower to 38.90 higher) in McCurry 2011. | 102 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | Both studies reported differences between groups in favour of the interventions using actigraphy after 6 months (McCurry 2005; McCurry 2011). | ||
| Number of nocturnal awakenings | The mean number of awakenings in the control group was 22.4 (Alessi 2005), 12.2 (McCurry 2005), 18.4 (McCurry 2011), and 4.5 (Schnelle 1999). Number of awakenings for multimodal interventions was 0.1 higher (5.25 lower to 5.45 higher) in Alessi 2005, 4 lower (10.10 lower to 2.10 higher) in McCurry 2005, 4.7 lower (9.29 lower to 0.11 lower) in McCurry 2011, and 0.3 lower (0.76 lower to 0.16 higher) in Schnelle 1999. | 404 (4 RCTs) | ⊕⊕⊝⊝ Lowa,c | 2 studies found improvements in favour of the intervention using actigraphy after 5 nights and 6 months (McCurry 2005; McCurry 2011). 2 studies found no differences between study groups (Alessi 2005; Schnelle 1999). | ||
| Sleep onset latency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events | 1 study reported unexpected and serious adverse events (Richards 2011). 1 participant had substernal chest pain 15 hours after exercising, but was negative for myocardial infarction; 1 had back, hip, and leg pain; and 1 had multifocal premature ventricular contractions or non‐specific t‐wave changes in their electrocardiogram. | 589 (7 RCTs) | ⊕⊕⊝⊝ Lowa,b | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_424366361397646497. | ||||||
a Downgraded one level for risk of bias: high or unclear risk of performance and detection bias in all studies b Downgraded one level for imprecision: wide confidence intervals c Downgraded one level for inconsistency: inconsistent results
Background
Description of the condition
Dementia is a clinical syndrome characterised by cognitive, neuropsychiatric, and functional symptoms. It involves difficulties in memory, disturbances in language, psychological and psychiatric changes, as well as impairments in activities of daily living (Burns 2009).
Dementia can be due to various underlying pathologies. Approximately 60% to 70% of dementia cases are due to Alzheimer's disease. Other major forms are vascular dementia, dementia with Lewy bodies, or dementia by other causes (e.g. stroke, infections, alcohol). Mixed forms with more than one pathology are also very common (WHO 2020).
Worldwide about 24 million people are affected by dementia. Because of the age profile of the population, numbers are especially high in Western European and Northern American countries where approximately 6% of people over 60 years old are affected. Worldwide, the prevalence rate in people over 60 years old has been estimated between 5% and 8% (Prince 2015). Predictions that the number of people with dementia will increase steadily, doubling every 20 years (Burns 2009; Ferri 2005), have been challenged by the results of more‐recent studies indicating increasing age of dementia onset, possibly leading to a less marked increase in incidence than previously predicted (Larson 2013).
Sleep disturbance and insomnia occur frequently in people with dementia. Common problems are increases in the duration and number of awakenings and an increased percentage of time spent in stage 1 sleep (Colton 2006). Prevalences of sleep disturbances of up to 40% have been reported in different settings (Dauvilliers 2007; McCurry 1999; Ritchie 1996; Wilfling 2019). Effects of progressive dementia (e.g. AD) on sleep can be distinguished from normal ageing, and are particularly evident in fragmentation of the sleep–wake cycle and disruption of the circadian regulation of sleep (Song 2010). These changes in sleep regulation and architecture have been related to the deterioration of brain structures and the supply of neurotransmitters relevant for sleep, as well as psychosocial and behavioural changes occurring in people with dementia (Ancoli‐Israel 2006; Cipriani 2015; Saeed 2017). AD is frequently associated with lesions of multiple brain systems leading to insufficient regulation of the sleep–wake cycles (Li 2019). Also, a bidirectional relationship between sleep and AD has been suggested with disrupted sleep promoting the development of AD pathology (van Egroo 2019).
Sleep disturbances are associated with several problems for the affected people, their relatives, and carers. In people with dementia it may lead to worsening of cognitive symptoms, challenging behaviours (such as restlessness and wandering), and further harms (such as accidental falls). In addition, sleep disturbances can be associated with significant carer distress and have been reported as an important factor contributing to decisions to admit people with dementia to institutional care (Ancoli‐Israel 2006; Gibson 2014; Lee 2011).
Increases in costs attributable to dementia have been shown for impairment in activities of daily living and cognitive deficits (Hurd 2013; Leicht 2013), but there are insufficient data for reliable estimates of the costs associated with sleep disturbances in people with dementia.
Description of the intervention
Non‐pharmacological interventions include all treatment options that are not medication or drug therapies (Capezuti 2018; Livingston 2014; O'Caoimh 2019). A number of classifications of non‐pharmacological interventions for behavioural and psychological symptoms of dementia have been used in earlier systematic reviews (Livingston 2014; O'Neil 2011). However, a preplanned categorisation of interventions seems inappropriate in this context as non‐pharmacological interventions that have been proposed to improve sleep in people with dementia are frequently multifaceted. Components may include environmental modification (e.g. increased exposure to natural light, decreased night‐time noise and light), changes to care routines (e.g. decreased time in bed during the day, structured bedtime routines), behavioural interventions (e.g. increased daytime physical activity and exercise), or other sleep hygiene measures (e.g. avoidance of caffeinated drinks). Other interventions may include sensory stimulation (e.g. aromatherapy, touch and massage, transcutaneous electrical nerve stimulation (O'Neil 2011)), individual relaxation therapies, and complementary therapies (e.g. acupuncture). Also, bright light therapy has been suggested as an intervention of specific benefit to sleep, which may be implemented in combination with other interventions or as stand‐alone intervention (Forbes 2014; Hjetland 2020).
How the intervention might work
Non‐pharmacological interventions apply different mechanisms to improve the management of sleep disorders in people with dementia. For example, environmental interventions aim to improve sleep by providing conditions that allow for physiological sleep, while sensory stimulation targets the lack of sensory input in people with dementia that could cause disruptions in internal circadian rhythms. There are several advantages of non‐pharmacological over pharmacological interventions. Depending on the intervention, compliance tends to be good and there are usually few adverse events. Furthermore, treatment efficacy may last longer compared to pharmacological treatments for sleep, where positive effects tend to stop with treatment cessation, while behavioural interventions may lead to sustained effects. Because of the risk of adverse effects of drug treatments for sleep in older people with dementia, non‐pharmacological management of sleep problems has been proposed as a first‐line treatment option (David 2010).
Why it is important to do this review
Sleep disturbances are associated with a number of problems for people with dementia as well as carers. Therefore, there is a need to rigorously synthesise the research evidence on strategies to improve sleep in people with dementia.
One recent Cochrane Review showed a distinct lack of evidence regarding successful pharmacological interventions to manage sleep problems in people with dementia, while a number of non‐pharmacological interventions have been proposed (McCleery 2020). Most published systematic reviews have analysed specific interventions or specific settings (or both), but did not always focus on people with dementia. There are two older systematic reviews of non‐pharmacological interventions for sleep disturbances in people with dementia including 13 (Brown 2013) and nine (Salami 2011) randomised controlled trials (RCTs). These reviews also included a range of other study designs and did not use optimal tools to assess risk of bias. There are two more‐recent, general systematic reviews on non‐pharmacological interventions for people with dementia that do not specifically focus on sleep disturbances (Livingston 2014; O'Neil 2011). Four more reviews focused on nursing home residents (Capezuti 2018; Shang 2019), mild dementia (O'Caoimh 2019), or light therapy (Hjetland 2020).
The results of this review may overlap with two Cochrane Reviews on light therapy for people with dementia (Forbes 2014), and for adults aged 60+ (Montgomery 2003), but we decided to still include studies on bright light therapy in this review as we aim to be comprehensive in reviewing the evidence on non‐pharmacological interventions for sleep problems in people with dementia.
This review gives an overview of any type of non‐pharmacological interventions, irrespective of setting and type of dementia. Importantly, none of the available reviews has adequately considered the challenges of synthesising complex interventions (Anderson 2013). Recently, we (Möhler 2015) and others (Datta 2013; Higgins 2019; Noyes 2019) have highlighted the need to adequately describe and summarise important factors concerning the development, evaluation, and implementation of interventions used in systematic reviews of complex interventions. This seems warranted in order to identify effective intervention approaches and also to inform the development of new interventions on the basis of the current best evidence.
Therefore, the aim of this review is to systematically review the evidence from RCTs of non‐pharmacological interventions for sleep disturbances in people with dementia in order to inform clinical practice and identify research needs. The review is needed because of the importance of sleep disturbances for people with dementia and their relatives and carers as well as the widespread use of drug treatments of questionable effectiveness which may cause significant harm.
Objectives
To evaluate the benefits and harms of non‐pharmacological interventions on sleep disturbances in people with dementia compared to usual care, no treatment, any other non‐pharmacological intervention, or any drug treatment intended to improve sleep, and to describe the components and processes of any complex intervention included.
Methods
Criteria for considering studies for this review
Types of studies
As planned in the review protocol (Wilfling 2015), we included all individually or cluster‐RCTs investigating the effects of interventions to improve physiological sleep in people with dementia. First period data from trials with a cross‐over design were also eligible. To be included, studies had to have a primary sleep focus and a sleep‐related outcome measure as a primary outcome. We included studies published in any language.
Types of participants
We included people of any age and in any setting with a diagnosis of dementia, of any subtype or severity, or a Mini‐Mental State Examination (MMSE) score of less than 24. Diagnoses of dementia could have been made using any established diagnostic criteria. In studies also including people without dementia, we aimed to use results for the subgroup of people with dementia. If these data were not available, we included studies only if at least 80% of participants had dementia. If necessary, we contacted study authors to determine rates of people with dementia. Participants needed to have a sleep problem at baseline, diagnosed on the basis of any subjective or objective measure. We excluded studies of people with dementia and sleep apnoea, as this is primarily a respiratory problem requiring different treatment strategies.
Types of interventions
We included all non‐pharmacological interventions aiming to improve physiological sleep in people with dementia. We excluded studies where participants received medication (e.g. hypnotic drugs) to improve sleep but no other type of intervention.
As expected, several interventions were designed as complex interventions (Craig 2008), making it difficult to extract single effective components of the interventions (Higgins 2019; Noyes 2019). Therefore, we described components of included programmes in detail using the TIDieR guideline (template for intervention description and replication; Hoffmann 2014), as well as the CReDECI 2 criteria (criteria for reporting the development and evaluation of complex interventions in healthcare: revised guideline; Möhler 2015).
We included studies with any type of comparator intervention, including usual care (which could be described as 'no treatment') and optimised usual care, any other non‐pharmacological intervention, or any drug treatment intended to improve sleep.
Types of outcome measures
We used objective sleep measures and adverse events as primary outcomes, while secondary outcomes also included subjective measures of sleep quality.
Primary outcomes
-
Objective sleep‐related outcomes. We considered any of the following outcome measures:
Total nocturnal sleep time
Consolidated sleep time at night (i.e. the longest period of uninterrupted sleep between nocturnal sleep onset and final awakening)
Sleep efficiency (i.e. % of time in bed at night spent asleep)
Total wake time at night (or time spent awake after sleep onset)
Number of nocturnal awakenings
Sleep onset latency (i.e. time taken to fall asleep after going to bed)
Ratio of daytime sleep to night‐time sleep
Ratio of night‐time sleep to total sleep over 24 hours.
Outcomes had to be assessed by objective measurement. This could be done via technology (i.e. (wrist) actigraphy or polysomnography), or via repeated, direct observation during the night (e.g. using the Observational Sleep Assessment Instrument (OSAI) or other observation‐based, sleep‐related rating scales).
Adverse events as reported in the primary studies. This could include use of physical restraints or prescription of psychotropic medication.
Secondary outcomes
Subjective sleep‐related outcomes (e.g. quality of sleep, patient‐ or carer‐reported sleep satisfaction assessed using sleep‐related rating scales (e.g. Pittsburgh Sleep Quality Index (PSQI)), sleep logs, diaries, surveys, or sleep charts)
Behavioural and psychological symptoms of dementia, including agitation and 'sundowning'
Quality of life
Functional status
Institutionalisation
Compliance with the intervention
Attrition rates (as indicator for intervention acceptability)
Carer outcomes (e.g. distress and quality of life).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Dementia and Cognitive Improvement Group's (CDCIG) Specialised Register on 13 January 2022. The Information Specialists of the CDCIG maintain the register, which contains studies in the areas of dementia (prevention and treatment), mild cognitive impairment, and cognitive improvement. The studies are identified from:
monthly searches of several major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and LILACS;
monthly searches of the trial registers: the World Health Organization (WHO) International Clinical Trials Registry Platform (which covers ClinicalTrials.gov, ISRCTN, the Chinese Clinical Trials Register, the German Clinical Trials Register, the Iranian Registry of Clinical Trials, and the Netherlands National Trials Register, plus others) and ClinicalTrials.gov;
quarterly searches of the Cochrane Library's Central Register of Controlled Trials (CENTRAL);
six‐monthly searches of a number of grey literature sources from Web of Science Core Collection.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the 'Methods used in reviews' section within the editorial information about the Dementia and Cognitive Improvement Group (dementia.cochrane.org/our-trials-register). We performed additional searches in many of the sources listed above, to cover the timeframe from the last searches performed for the Register to ensure that the search for the review was as up‐to‐date and as comprehensive as possible.
The search strategies used are described in Appendix 1. The most recent search was carried out on 13 January 2022.
Searching other resources
We reviewed reference lists of included studies and relevant reviews as well as other potentially relevant trials identified through the search. We contacted study authors and experts in the field for unpublished and ongoing studies.
Data collection and analysis
Selection of studies
We obtained lists of references from different sources and merged these to check for duplicates. Independently, two review authors (from DW, SC, MD, SK) assessed titles and abstracts from all search results to identify eligible studies. After selection of potentially relevant articles, we obtained full reports and assessed them for inclusion and exclusion criteria. When necessary, we resolved any disagreement on the eligibility of studies through discussion to reach consensus or, if required, by involving a third experienced review author (from DW, SC, MD, SK).
We accessed full texts that were not published in English or German, using a language translation service.
We collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram.
Data extraction and management
Two review authors (from DW, SC, RM, SK) independently read and extracted the data from each included study. In case of disagreement or discrepancies, we involved a third review author (from DW, SC, RM, SK) to reach consensus. We used a standardised data extraction form, including source, study characteristics, methods, participants, interventions, comparators, outcomes, results, and adverse events according to the Cochrane Handbook for Systematic Reviews of Interventions (Li 2020).
Assessment of risk of bias in included studies
Assessment of risk of bias of included studies followed the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). Two review authors (from DW, SC, SK) independently assessed and scored included studies' methodological quality in order to identify any potential sources of systematic bias. Criteria for appraisal of studies were internal validity and low risk of bias through selection bias, performance bias, attrition bias, detection bias and additional design‐related criteria for cluster‐RCTs. Study validity was determined by categorising individual studies into following categories.
Low risk of bias: plausible bias that is unlikely to seriously alter the results (categorised as 'Yes' in the risk of bias table).
High risk of bias: plausible bias that seriously weakens confidence in the results (categorised as 'No' in the risk of bias table).
Unclear risk of bias: plausible bias that raises some doubts about the results (categorised as 'Unclear' in the risk of bias table).
As recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020), we used a tool to assess the risk of bias of included studies. We used the Cochrane RoB 1 tool to assess risk of bias in included studies, addressing the domains of sequence generation, allocation concealment (avoidance of selection bias), and selective outcome reporting (avoidance of reporting bias) by a single entry for each study and considered blinding of participants, staff, and outcome assessors (avoidance of performance bias and detection bias) separately for objective and subjective outcomes.
Measures of treatment effect
For continuous outcome data, we calculated mean differences (MD) with 95% confidence intervals (CIs). If studies used different instruments, we planned to calculate the standardised mean difference (SMD). For the analysis of dichotomous outcome data, we calculated risk ratios (RR) with 95% CIs. We performed all statistical analyses using Review Manager Web (RevMan Web 2022).
For all interventions that were not expected to have a prolonged intervention effect, we used outcome data directly after the intervention period for primary analyses (i.e. light therapy, physical and social activities, daytime sleep restriction, slow‐stroke back massage, and transcranial electrostimulation). We used the last follow‐up data presented in the studies for interventions including education or other components (such as case conferences), or both education and other components, which we expected to need a longer follow‐up period to be fully implemented in clinical practice.
Unit of analysis issues
Cluster‐randomised controlled trials
We investigated whether individuals or groups of people were randomised. For cluster‐RCTs, we extracted information about the intracluster correlation coefficient (ICC) if available.
Studies with multiple treatment groups
If a study compared two or more eligible intervention groups, we checked if each intervention group met our inclusion criteria. As all intervention groups in studies evaluating different interventions met our inclusion criteria, we included all groups in the analysis.
Cross‐over studies
For cross‐over studies investigating interventions including education or other components (or both), which we expected to need a longer follow‐up period to be fully implemented in clinical practice, we only used first period data up to the first point of cross‐over to rule out carry‐over effects. For all other interventions that are not expected to have a prolonged intervention effect (e.g. light therapy), we included data from the complete study period, since carry‐over effect are unlikely.
Dealing with missing data
We used intention‐to‐treat (ITT) data if available, reporting on any imputation methods used in the primary studies. Where necessary, we contacted the study authors for additional information about missing data. We did not undertake any imputation method or other statistical methods to account for missing data, but used completer‐only data if no other data were available.
Assessment of heterogeneity
For the assessment of clinical heterogeneity, we examined extracted data for between‐study variability with respect to participants, interventions, and outcomes. As there was only one meta‐analysis, we did not further check for statistical heterogeneity.
Assessment of reporting biases
In order to minimise the risk of publication bias, we performed comprehensive searches in multiple databases, including searching for unpublished studies. We included all studies in any language. Due to the small number of studies for each intervention category, we did not prepare funnel plots.
Data synthesis
Two review authors (DW, SK) grouped studies according to the interventions in the following not predefined groups: light therapy, physical activities, social activities, carer interventions, daytime sleep restriction, slow‐stroke back massage, transcranial electrostimulation, and multimodal interventions.
There was only one intervention category (social activities) in which we considered studies to be sufficiently clinically and statistically homogeneous to allow meta‐analysis. We conducted the meta‐analysis using a random‐effects model.
As the studies in the other intervention categories were too heterogeneous for pooling in meta‐analyses, we described and compared the results of the studies (e.g. MD with standard deviations (SD)) at baseline and follow‐up narratively. If it was not possible to calculate the study results as described above and if the studies provided no aggregated data, as for some of the secondary outcomes, we compared the direction of the effects and the P values from the different studies (Campbell 2020).
Subgroup analysis and investigation of heterogeneity
We did not conduct subgroup analyses.
Sensitivity analysis
We did not perform sensitivity analyses.
Carer involvement
We used the results from the carer involvement activities applied in the review by McCleery 2020 on pharmacological interventions. For this updated review, the authors had sought advice of carers in order to identify the aspects of sleep most important for them, leading to the identification of one additional primary sleep outcome (duration of consolidated sleep) compared to an earlier version of their review.
Summary of findings and assessment of the certainty of the evidence
For the primary outcomes, we assessed the certainty of the evidence with reference to overall risk of bias of included studies, directness of the evidence, consistency of results, and precision of estimates. We displayed results for all primary outcomes of all groups of interventions in summary of findings tables using GRADEpro GDT software (GRADEpro GDT) according to the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Li 2020). We categorised the certainty of the evidence for each of the primary outcomes as high, moderate, low, or very low (Schünemann 2011).
Results
Description of studies
Results of the search
We last searched for eligible studies in January 2022. After deduplication and first assessment by the Trials Search team of CDCIG, two review authors screened 5104 records by title and abstract and 119 in full text. We finally included 19 studies (reported in 32 publications). We excluded 63 studies (74 articles) and found nine ongoing studies (10 articles) and three studies awaiting classification (three articles). The study selection process is summarised in Figure 1.
1.
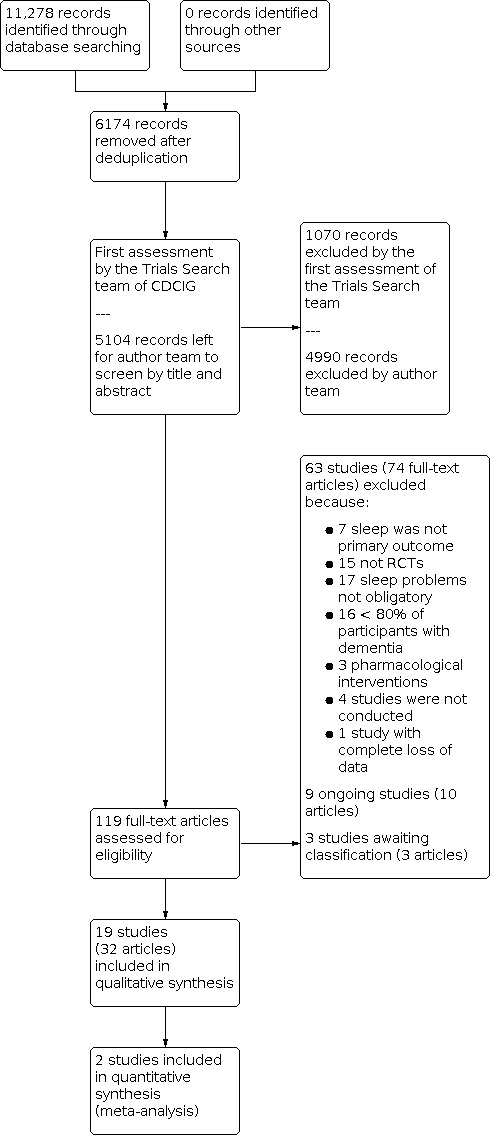
PRISMA flow diagram.
Included studies
All included studies were RCTs allocating either clusters or individuals to treatment and control groups. Two studies used a cross‐over design. We describe the study characteristics in detail in the Characteristics of included studies table.
Setting and participants
Thirteen studies were conducted in nursing homes; three studies included community dwelling people; one study described its participants as inpatients, probably on a geriatric ward in a hospital; one study included patients from a mental health centre; and one study included people from district community centres for older people. Fourteen studies were conducted in the USA, two in China, two in Switzerland, and one in Japan. Eighteen trials were published in English and one trial was in Chinese (Li 2009).
Sample sizes of included studies ranged from 13 (Fontana Gasio 2003) to 193 participants (Richards 2011). Overall, 1335 participants were included, with a mean of 70 participants per study. In all studies, at least 80% of participants had a diagnosis of dementia or an MMSE score less than 24, or both. All included participants had night‐time behaviours that could be associated with sleep disturbances.
Interventions
All studies applied one or more non‐pharmacological intervention aiming to improve physiological sleep in people with dementia (see Figure 2). The most frequent intervention was some form of light therapy, which seven studies applied as a stand‐alone intervention (Ancoli‐Israel 2003; Dowling 2005; Figueiro 2019; Fontana Gasio 2003; McCurry 2011; Nowak 2008; Sloane 2015). The next most frequent was an activity intervention, applied in four studies: physical activities in three studies (Chan 2016; McCurry 2011; Richards 2011), and social activities in two studies (Richards 2005; Richards 2011). Carer interventions were applied in two studies (Gattinger 2017; McCurry 2012), and daytime sleep restriction in one study (Ancoli‐Israel 2003). Other identified interventions were slow‐stroke back massage (Harris 2012), and transcranial electrostimulation (Hozumi 1996). Seven studies examined multimodal interventions (Alessi 1999; Alessi 2005; Li 2009; McCurry 2005; McCurry 2011; Richards 2011; Schnelle 1999).
2.
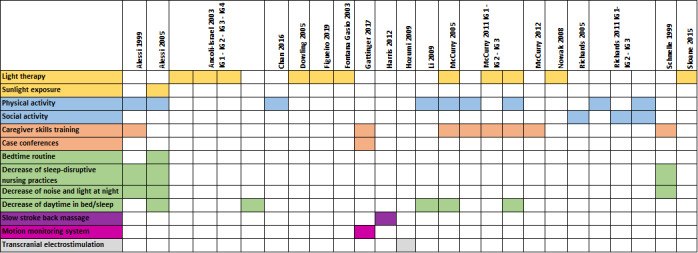
Overview: interventions and components. IG: intervention group.
Light therapy
Seven studies assessed the effects of different light therapy interventions (Ancoli‐Israel 2003; Dowling 2005; Figueiro 2019; Fontana Gasio 2003; McCurry 2011; Nowak 2008; Sloane 2015).
Residents in Ancoli‐Israel 2003 received a morning bright light, an evening bright light, or a morning dim light intervention. Morning bright light was a two‐hour light exposure at 2500 lux from 9.30 a.m. to 11.30 a.m. Participants in the evening bright light intervention group received the same light exposure (2500 lux) from 17.30 p.m. to 19.30 p.m. In comparison, residents in the morning dim light intervention were exposed to less than 300 lux of red light.
Residents in Dowling 2005 received bright light exposure (2500 lux or greater in gaze direction) from 9.30 a.m. to 10.30 a.m. (Monday to Friday) for 10 weeks.
Figueiro 2019 delivered an active lighting intervention that provided high circadian stimulus (CS). The intervention consisted of floor luminaires (550 lux or 600 lux), light boxes (350 lux), and light tables (750 lux).
Fontana Gasio 2003 investigated light therapy through a low‐intensity dawn–dusk simulation. For this purpose, an overhead halogen lamp behind a diffusing membrane was placed behind the participant's bed. A computer algorithm controlled this lamp, exposing the participant to light ranging from 0.001 lux to a maximum of 400 lux, simulating a dusk, dawn, and dark period.
Participants in McCurry 2011 sat 1 m from a sunray light box (approximately 2500 lux) for one hour a day. Light sessions were supervised by carers and supported by trainers to plan activities to perform during sessions (e.g. watching television, looking at pictures). Furthermore, carers tried to reduce light at night.
Light therapy in Nowak 2008 consisted of blue–green light exposure (12,000 lux) for 30 minutes between 6 a.m. and 7 a.m. for 14 consecutive days via cap visors.
Sloane 2015 integrated blue–white light in the participants' homes. Light bulbs (13,000 K) were placed in all lamps in areas where the participants spent most of the time. Additionally, a light box was installed at places where participants ate breakfast and lunch.
Physical activities
Three studies assessed the effects of different physical activity interventions (Chan 2016; McCurry 2011; Richards 2011).
Physical activities were Tai Chi Qigong (Chan 2016), walking (McCurry 2011), and high‐intensity resistance strength training (Richards 2011).
In Chan 2016, participants received two 60‐minute Tai Chi Qigong training sessions a week for two months. An experienced Tai Chi instructor chose 10 movements. Participants tried to replicate movements and postures. A Tai Chi Qigong expert evaluated validity and feasibility of the training for aged people with cognitive impairment.
The two‐month walking programme in McCurry 2011 consisted of three sessions. In session one, a carer conducted a 30‐minute walking programme per day. Frail residents started with less than 30 minutes to reduce the risk of injuries and aimed to increase the duration of walking. In the second and third sessions, trainers gave advice in implementing the walking plan.
Richards 2011 evaluated a high‐intensity physical resistance strength training and walking programme over seven weeks. It was hypothesised that the combination of both activities would have positive effects on total physical activity. The strength training consisted of hip extensions on a hip‐extension/leg‐press chair as well as arm extensions from a seated position in a chest‐press chair. Trained nurses supervised exercises. The high‐intensity physical resistance strength training was performed three days a week and on two further days participants walked for up to 45 minutes.
Social activities
Two studies assessed the effects of different social activity interventions (Richards 2005; Richards 2011).
In Richards 2005, two certified experienced therapeutic recreation specialists developed a catalogue including more than 100 social activities. The catalogue was divided into activities for everyone (e.g. listening music), for residents with severe dementia (MMSE less than 5) (e.g. petting a stuffed toy cat or looking in a mirror), for residents with moderate dementia (MMSE 5 to 15) (e.g. writing a letter), and for residents with mild cognitive impairment (MMSE greater than 15) (e.g. playing draughts). Participants received one to two hours of social activities in 15‐ to 30‐minute sessions, based on their interest, cognition, functional status, and napping time. The intervention was performed on 21 consecutive days.
In Richards 2011, participants received individualised social activities for one hour daily, on five days a week for a total of 7 weeks. Nursing assistants in the research project performed social activities following 40 hours of training in order to be able to plan and guide activities for residents.
Carer interventions
Two studies assessed the effects of carer interventions (Gattinger 2017; McCurry 2012). Carer interventions were skills training (Gattinger 2017; McCurry 2012), and case conferences (Gattinger 2017). Dose and content of interventions differed between studies.
In Gattinger 2017, nurses in the intervention group received two different types of in‐house training. In a 60‐minute training session, nurses were introduced to evidence‐based nursing interventions to reduce sleep disturbances in dementia and to the need‐driven dementia‐compromised behaviour (NDB) model. According to this model, behaviours of people with dementia are understood to be the consequence of their inability to express their needs. Furthermore, nurses received training on the monitoring system. In a second 60‐minute training session, nurses learned how to use the system and how to interpret the data. In each nursing home, one or two nurses were trained as key nurses with deeper knowledge of the monitoring system. Additionally, case conferences were conducted when anticipated problems, favoured outcomes, and planned interventions were documented. In Phase 1, an external registered nurse directed case conferences, and in Phase 2, internal registered nurses supervised the case conferences. Data from the monitoring system were used to assess activity and movement patterns of each resident and to promote the implementation of nursing interventions to improve sleep.
The training programme in McCurry 2012 consisted of four training sessions. The main topics were non‐pharmacological interventions to improve sleep in nursing home residents with dementia and how to implement individualised sleep plans for residents. An experienced trainer conducted the sleep education programme.
Multimodal interventions
Seven studies assessed the effects of multimodal interventions consisting of more than one element from the intervention categories mentioned above (Alessi 1999; Alessi 2005; Li 2009; McCurry 2005; McCurry 2011; Richards 2011; Schnelle 1999). We analysed these multimodal interventions in detail using the TIDieR guideline (Hoffmann 2014) and CReDECI 2 criteria (Möhler 2015). Reporting about the development and piloting of the interventions was generally incomplete. All studies reported information about the intervention components and their delivery, but none described whether and how components were intended to interact and whether contextual factors were considered during the modelling of the intervention. Also, none of the studies included a process evaluation (Figure 3).
3.
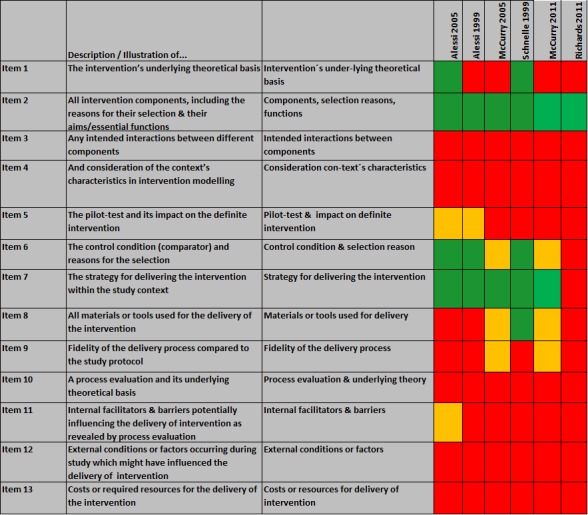
Analysis based on CReDECI 2 criteria.
Residents in Alessi 1999 participated in an intervention consisting of 1. a physical activity intervention (functional incidental training (FIT)) and 2. a night‐time programme. FIT was performed during daily nursing care routines (e.g. toileting). Training included arm and leg exercises, sit‐to‐stands, and walking or wheelchair propulsion, depending on the participants' abilities. Trained research staff conducted the training sessions every two hours between 8.00 and 16.00 (maximum five sessions a day). The intervention was performed five days a week, for 14 weeks in total. After the intervention period of 14 weeks, the additional night‐time programme was introduced for five nights, which aimed to minimise light, noise, and sleep‐disruptive nursing care interventions at night.
Alessi 2005 provided the intervention on five consecutive days and nights to five or six residents at the same time. The intervention consisted of 1. keeping residents out of bed between 8.00 a.m. and 18.00 p.m., and a minimum duration of 30 minutes of sunlight exposure a day (at 10,000 lux); 2. participating in a low‐level physical activity programme three times a day; and 3. an individualised bedtime routine (between 20.00 p.m. and 22.00 p.m.), including personal care and reduced light and noise. The study aimed to minimise night‐time noise and light for the whole night (22.00 p.m. to 6.00 a.m.). All aspects of the intervention were documented in detail.
Li 2009 applied a sleep restriction and exercise and activity programme. Participants received 1. morning exercise from 8.00 a.m. to 9.00 a.m. outdoor activities for 60 minutes, 2. afternoon activities according to participants' interest, such as painting, games, and music; 3. no napping: participants were allowed to go to bed only when sleepy without reading or television in bed as well as limited food intake 15 to 30 minutes before going to bed, and 4. getting up at 6.30 a.m. every morning. The intervention was delivered daily for 12 weeks. We were unable to analyse the intervention components in more detail as the study was published in Chinese and after unsuccessful attempts to contact the authors, there were only limited translated data available.
The multimodal intervention in McCurry 2005 included a night‐time insomnia treatment and education programme. Before participating in the night‐time programme, the intervention group received six one‐hour educational sessions over two months. The programme consisted of 1. the development of an individual sleep hygiene programme for participants by carers; 2. participant walked daily for 30 minutes; and 3. increased daytime light exposure via a SunRay light box (2500 lux). The light intervention was performed within a three‐hour window before participants went to bed. Interventions were performed over three weekly treatment sessions by a gerontopsychologist experienced in behavioural interventions with people with dementia.
In McCurry 2011, one treatment group received a combination of 1. education, 2. light exposure, and 3. physical activity. Intervention components for exercise and light exposure are described in detail above (see under 'Physical activities' and 'Light therapy'). The carer training consisted of six training sessions. In session one, carers learned to develop an individualised sleep plan for residents, aiming to reduce daytime napping, establish bedtime routine, and identify reasons for night‐time awakenings. In session two, carers were trained about implementing the daily light exposure programme as described under 'Light therapy'. The focus of sessions three to six was on identifying reasons of night‐time awakenings as well as challenges in implementing the sleep, walking, and light exposure plans.
Richards 2011 combine 1. social activity and 2. a high‐intensity physical resistance strength training. Both interventions are described above (see under 'Social activities' and 'Physical activities').
Schnelle 1999 implemented an intervention with four major components: 1. 30‐minute in‐service education on general sleep issues and the intervention; 2. verbal and visual feedback (noise levels recorded in the nursing home were presented and verbal feedback about noise levels and sources of noise given); 3. noise abatement: implementation of procedures to reduce noise (e.g. turn off unwatched television sets); 4. individualised incontinence care: research staff provided incontinence care during hourly rounds when residents were awake. Otherwise, the frequency of waking residents up for incontinence care was based on residents' risk for skin problems. During incontinence care, staff attempted to reduce noise and light exposure.
The analysis based on CReDECI 2 criteria (Möhler 2015) showed that none of the studies investigated interactions between components or considered contextual characteristics, or internal and external facilitators or barriers to the delivery of the intervention. Furthermore, none of the studies reported costs and resources needed for the delivery of the interventions. Only two studies reported information about materials or tools used for intervention delivery (McCurry 2005; Schnelle 1999). None of the studies carried out a comprehensive process evaluation in order to be able to describe the effect of individual intervention components in more detail. We were unable to assess Li 2009 in detail, because of the limited translation of the full text (Figure 3).
Daytime sleep restriction
Ancoli‐Israel 2003 assessed the effects of a daytime sleep restriction regimen. For this purpose, one staff member had to attend each patient for six hours during the day from 9.00 a.m. to 12.00 p.m. and from 14.00 p.m. to 17.00 p.m. in order to hinder residents from falling asleep during this time.
Slow‐stroke back massage
Residents in Harris 2012 received a three‐minute slow‐stroke back massage intervention at bedtime in their bedroom for two nights from a certified geriatric advanced practice nurse trained in slow‐stroke back massage.
Transcranial electrostimulation
Hozumi 1996 used a HESS‐100 device to deliver transcranial electrostimulation via electrodes attached through a headband. This device delivered electric pulses of 6 V to 8 V at increasing frequencies from 6 Hz to 80 Hz. The electrostimulation was applied for 20 minutes at 10.00 a.m. every morning for two weeks.
Control groups
Eight studies offered usual care to the control group (Alessi 2005; Chan 2016; Harris 2012; Li 2009; McCurry 2012; Richards 2005; Richards 2011; Schnelle 1999). In Chan 2016, the control group received a weekly health talk in a community centre over the course of two months, but offered usual care. These studies did not report further details about the characteristics of usual care.
The control group of Alessi 1999 received a night‐time noise reduction programme, and two studies offered a nondirective support to the staff (McCurry 2005; McCurry 2011).
Several studies used controlled lighting; Ancoli‐Israel 2003 used dim red light of less than 300 lux for two hours; Dowling 2005 used usual indoor light (150 lux to 200 lux) for 10 weeks; Figueiro 2019 used individualised low circadian stimulus lighting (below the threshold for activation of the circadian system); Fontana Gasio 2003 used placebo dim red light (at 5 lux of white light); Nowak 2008 used dim red light at 5 lux for 30 minutes between 6 a.m. and 7 a.m. for 14 consecutive days via cap visors; and Sloane 2015 used red–yellow light.
In Gattinger 2017, the only difference between intervention and control groups was that the control group received no monitoring system.
Hozumi 1996 performed transcranial electrostimulation with a placebo device in the control group.
Outcomes
Of the 19 included studies, 16 reported objective sleep‐related outcomes as primary endpoints (Alessi 1999; Alessi 2005; Ancoli‐Israel 2003; Dowling 2005; Figueiro 2019; Fontana Gasio 2003; Harris 2012; Hozumi 1996; McCurry 2005; McCurry 2011; McCurry 2012; Nowak 2008; Richards 2005; Richards 2011; Schnelle 1999; Sloane 2015). Outcomes were night‐time total sleep in minutes, percentage of night‐time sleep (i.e. sleep efficiency), night‐time number of awakenings, night‐time total wake time in minutes, day/night sleep ratio, and sleep latency. None of the studies reported consolidated sleep time.
Six studies reported the subjective outcome 'quality of sleep'; five studies used the PSQI (Chan 2016; Figueiro 2019; Gattinger 2017; Li 2009; Sloane 2015), and one used the Sleep Disorders Inventory (McCurry 2011). Hozumi 1996 assessed the outcome sleep disorder. Nowak 2008 assessed excessive daytime sleep by the use of the Stanford Sleepiness Scale. Ancoli‐Israel 2003 assessed circadian activity rhythm parameters.
None of the studies reported data on quality of life, functional status, institutionalisation, compliance with the intervention, or attrition rates.
Excluded studies
We excluded 63 studies after full‐text screening. Main reasons for exclusion were that sleep was not a primary outcome or sleep problems were not obligatory for participants to be included; less than 80% of participants had a diagnosis of dementia; or the study design did not match our inclusion criteria. For details see Characteristics of excluded studies table.
Studies awaiting classification
Three studies are awaiting classification (see Characteristics of studies awaiting classification table).
Ongoing studies
We identified nine ongoing studies. Three are investigating the effect of therapeutic light on sleep (ChiCTR2000039991; NCT03777722/NCT03933696; NCT04073628), two the effect of sleep education (NCT03455569; NCT04533815), two multicomponent interventions (Dichter 2021; ISRCTN13072268), one Tai Chi (UMIN000042051), and one physical activities (Hodgson 2021) (see Characteristics of ongoing studies table).
Risk of bias in included studies
Overall, risk of bias in included studies was frequently unclear and there were areas of incomplete reporting (e.g. blinding and attrition). Overall and individual assessments of risk of bias are detailed in the Characteristics of included studies table; Figure 4; and Figure 5.
4.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
5.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Seven studies were at low risk of bias for methods of sequence generation as they reported detailed information (Ancoli‐Israel 2003; Chan 2016; Harris 2012; McCurry 2005; McCurry 2011; Richards 2011; Sloane 2015). The remaining studies had incomplete reporting of methods of sequence generation.
Only McCurry 2011 and Richards 2011 reported details of the methods used for allocation concealment (low risk of bias). Therefore, most studies were at unclear risk of selection bias.
Blinding
Most studies provided no information on blinding of participants or personnel (or both), and were at unclear risk of bias.
For objective sleep‐related outcomes blinding of outcome assessment was adequate in 14 studies, and we judged risk of detection bias to be low. Alessi 2005 and Gattinger 2017 were at high risk of bias, as the research staff who performed the outcome assessment was not blinded to group allocation.
The subjective sleep‐related outcomes were mainly rated by carers and most studies did not provide sufficient information about blinding. Carers were blinded in only one study and risk of bias was low (Figueiro 2019). Carers were not blinded to group allocation in one study (McCurry 2011), and in another study the unblinded principal investigator assessed subjective outcomes (Nowak 2008); risk of bias was high in both studies.
Incomplete outcome data
Most studies were at low risk of bias (Alessi 1999; Alessi 2005; Dowling 2005; Fontana Gasio 2003; Gattinger 2017; Harris 2012; Li 2009; McCurry 2005; McCurry 2011; McCurry 2012; Nowak 2008; Richards 2005; Richards 2011; Sloane 2015), with some at unclear risk (Ancoli‐Israel 2003; Chan 2016; Dowling 2008; Figueiro 2019; Schnelle 1999).
Hozumi 1996 reported results only for participants completing the study without mentioning attrition and were therefore considered to be at high risk of bias in this domain.
Selective reporting
Study protocols were only available for five studies allowing a check for selective outcome reporting (Figueiro 2019; Gattinger 2017; McCurry 2011; McCurry 2012; Richards 2011). Two studies were at high risk of bias because outcomes differed between registration and publication (Figueiro 2019; Gattinger 2017), and the other studies had a low risk of bias, because all outcomes were reported as planned.
Other potential sources of bias
We assessed three studies at high risk of bias for other reasons. Alessi 2005 used a delayed time series approach to avoid contamination and, therefore, follow‐up in the control group was twice as long as in the intervention group. The intervention was also provided at different time points. Fontana Gasio 2003 had unbalanced group sizes (intervention group nine participants; control group four participants). In Gattinger 2017, we identified a potential source of bias as intervention and control clusters were wards from the same nursing homes with a high risk of contamination between clusters.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
See Table 1; Table 2; Table 3; Table 4; and Table 5.
Light therapy
Studies assessed different sleep‐related outcomes for light therapy interventions. We presented results for all primary outcomes in Table 1.
Primary outcomes
Objective sleep‐related outcomes
Total nocturnal sleep time
Four studies (105 participants) reported total nocturnal sleep time. Two studies found a difference in favour of the control group using actigraphy for total nocturnal sleep time compared with usual care (after 10 weeks: MD −33.00 minutes, 95% CI −103.54 to 37.54; 46 participants; Dowling 2005; after 4 weeks: MD −20.40 minutes, 95% CI −63.29 to 22.49; 32 participants; Figueiro 2019; Analysis 1.1). Sloane 2015 found no clear difference between groups after six weeks (MD −0.23 minutes, 95% CI −12.75 to 12.28; 14 participants) and Fontana Gasio 2003 found a difference in favour of the intervention group after three weeks (MD 110.00 minutes, 95% CI 19.36 to 200.64, 13 participants, Analysis 1.1). We found very low‐certainty evidence (downgraded one level each for risk of bias, imprecision, and inconsistency) and we are uncertain whether light therapy has any effect on total nocturnal sleep time.
1.1. Analysis.

Comparison 1: Light therapy, Outcome 1: Total nocturnal sleep time (minutes)
Sleep efficiency
Seven studies (284 participants) reported sleep efficiency (%). Sleep efficiency slightly increased in three studies in the intervention groups (after 2 weeks: MD 5.60%, 95% CI 0.47% to 10.73%, 20 participants; Nowak 2008; after 3 weeks: MD 16.60%, 95% CI 6.49% to 26.71%; 13 participants; Fontana Gasio 2003; after 2 months: MD 6.20%, 95% CI −0.04% to 12.44%; 67 participants; McCurry 2011; Analysis 1.2). Two studies found an increase of sleep efficiency in favour of the control group (after 11 weeks: MD −4.50%, 95% CI −14.34% to 5.34%; 46 participants; Dowling 2005; after 4 weeks: −2.21%, 95% CI −5.17% to 0.75%; 32 participants; Figueiro 2019; Analysis 1.2). Two studies found no difference between groups (after 6 weeks: MD 0, 95% CI −3.45 to 3.45; 14 participants; Sloane 2015; after 15 days: MD and CIs not reported; 92 participants; Ancoli‐Israel 2003). We found very low‐certainty evidence (downgraded one level each for risk of bias, imprecision, and inconsistency) and we are uncertain whether light therapy improves sleep efficiency.
1.2. Analysis.

Comparison 1: Light therapy, Outcome 2: Sleep efficiency
Total wake time at night
Three studies (205 participants) reported total wake time at night. Dowling 2005 found an increase of the total wake time at night in the intervention group after 11 weeks (MD 32.00 minutes, −38.54 to 102.54; 46 participants; Analysis 1.3), McCurry 2011 found a decrease of the total wake time at night in the intervention group after two months (MD −39.00 minutes, 95% CI −74.40 to −3.60; 67 participants; Analysis 1.3), and Ancoli‐Israel 2003 found no difference in the total time awake at night between groups after 15 days (MD and CIs not reported; 92 participants). We found very low‐certainty evidence (downgraded one level each for risk of bias, imprecision, and inconsistency) and we are uncertain whether light therapy improves total wake time at night.
1.3. Analysis.

Comparison 1: Light therapy, Outcome 3: Total wake time at night (minutes)
Number of nocturnal awakenings
Four studies (147 participants) reported the mean number of nocturnal awakenings. In two studies, the number of nocturnal awakenings was slightly reduced in the intervention group (after 2 weeks: MD −2.31, 95% CI −4.17 to −0.45; 20 participants; Nowak 2008; after 2 months: MD −2.90, 95% CI −7.09 to 1.29; 67 participants; McCurry 2011; Analysis 1.4). In Dowling 2005, the number of nocturnal awakenings increased slightly in the intervention group after 11 weeks (MD 4.89, 95% CI −3.31 to 13.09; 46 participants; Analysis 1.4). Sloane 2015 found no difference between groups (MD −0.81, 95% CI −2.64 to 1.03; 14 participants). We found very low‐certainty evidence (downgraded one level each for risk of bias, imprecision, and inconsistency), and we are uncertain whether light therapy improves nocturnal time number of awakenings.
1.4. Analysis.

Comparison 1: Light therapy, Outcome 4: Number of nocturnal awakenings
Sleep onset latency
Three studies (59 participants) reported sleep latency and found no clear differences between groups (after 4 weeks: MD 6.05 minutes, 95 % CI −0.60 to 12.70; 32 participants; Analysis 1.5; Figueiro 2019; after 6 weeks: MD −3.72 minutes, 95% CI −9.54 to 2.10; 14 participants; Sloane 2015; after 3 weeks: −1.02 minutes, 95% CI −3.34 to 1.30; 13 participants; Analysis 1.5; Fontana Gasio 2003). We found low‐certainty evidence (downgraded one level each for risk of bias and imprecision).
1.5. Analysis.

Comparison 1: Light therapy, Outcome 5: Sleep onset latency
Other night‐time sleep‐related outcomes
No studies reported the effect of light therapy interventions on consolidated sleep time at night, ratio of daytime sleep to night‐time sleep, or ratio of night‐time sleep to total sleep over 24 hours.
For other night‐time sleep‐related outcomes (awake after sleep onset, Ancoli‐Israel 2003), and sleep‐related outcomes during daytime (e.g. total sleep (Ancoli‐Israel 2003; McCurry 2011), and percentage wake (Ancoli‐Israel 2003), there were no clear differences between groups (no further information reported; low‐certainty evidence (downgraded one level each for risk of bias and imprecision)).
Adverse events
No studies reported any adverse events or serious adverse events with light therapy interventions.
Secondary outcomes
Subjective sleep‐related outcomes
Three studies assessed sleep quality. Two studies found an improved sleep quality in the intervention group using the PSQI (MD −2.24, 95% CI −3.39 to −1.09; 41 participants; Figueiro 2019; Analysis 1.6; Fontana Gasio 2003 no further information). The third study found nearly no change in sleep quality in both study groups assessed by the Sleep Disorders Inventory (MD −0.4, 95% CI −0.95 to 0.15; 67 participants, McCurry 2011; Analysis 1.6).
1.6. Analysis.

Comparison 1: Light therapy, Outcome 6: Sleep quality
Behavioural and psychological symptoms of dementia
Dowling 2005 found a difference in favour of the control group between morning light and usual indoor light (control group) on agitation/aggression based on the Neuropsychiatric Inventory Nursing Home Version (NPI‐NH). Scores increased for participants exposed to morning light, whereas scores decreased for participants in the control group (P = 0.015).
Fontana Gasio 2003 found no significant effects in the neuropsychological evaluations (CERAD, MMS‐E, NPI, and GDS, P = 0.2).
In Ancoli‐Israel 2003 agitation was assessed using the Cohen‐Mansfield Agitation Inventory (CMAI) as well as the Agitated Behavior Rating Scale (ABRS). Nurses rated less agitation after light treatment (P = 0.007). No significant changes were found for total physical agitation or total verbal agitation.
Other secondary outcomes
No studies reported the effect of light therapy interventions on quality of life, functional status, institutionalisation, compliance with the intervention, attrition rates, or carer outcomes.
Physical activities
We presented results for all primary outcomes in Table 2.
Primary outcomes
Objective sleep‐related outcomes
Total nocturnal sleep time
Two studies (167 participants) reported total nocturnal sleep time. There is low‐certainty evidence (downgraded one level each for risk of bias and imprecision) that physical activities may slightly increase total nocturnal sleep time compared with usual care (after 7 weeks: MD 11.80 minutes, 95% CI −16.14 to 39.74; 102 participants; Richards 2011; after 2 months: MD 11.80 minutes, 95% CI −28.63 to 52.23; 65 participants; McCurry 2011; Analysis 2.1).
2.1. Analysis.

Comparison 2: Physical activity, Outcome 1: Total nocturnal sleep time (minutes)
Sleep efficiency
Two studies (167 participants) reported sleep efficiency. We found low‐certainty evidence (downgraded one level each for risk of bias and imprecision) that physical activities may slightly increase sleep efficiency (after 7 weeks: MD 2.60%, 95% CI −1.29% to 6.49%; 102 participants; Richards 2011; after 2 months: MD 4.90%, 95% CI −0.43% to 10.23%; 65 participants; McCurry 2011; Analysis 2.2).
2.2. Analysis.

Comparison 2: Physical activity, Outcome 2: Sleep efficiency
Total wake time at night
One study reported total wake time at night (McCurry 2011). We found low‐certainty evidence (downgraded one level each for risk of bias and imprecision) that physical activities may reduce total wake time at night after two months in comparison with an attention control group (MD −33.20 minutes, 95% CI −65.11 to −1.29; 65 participants; Analysis 2.3).
2.3. Analysis.

Comparison 2: Physical activity, Outcome 3: Total wake time at night (minutes)
Number of nocturnal awakenings
One study reported number of nocturnal awakenings (McCurry 2011). We found low‐certainty evidence (downgraded one level each for risk of bias and imprecision) that physical activities may slightly reduce the number of nocturnal awakenings after two months in comparison with an attention control group (MD −3.30, 95% CI −6.77 to 0.17; 65 participants; Analysis 2.4).
2.4. Analysis.

Comparison 2: Physical activity, Outcome 4: Number of nocturnal awakenings
Sleep onset latency
For sleep latency, Richards 2011 found no changes after six months (no further information reported).
Other night‐time sleep‐related outcomes
No studies reported the effect of physical activities on consolidated sleep time at night, ratio of daytime sleep to night‐time sleep, or ratio of night‐time sleep to total sleep over 24 hours.
Adverse events
Richards 2011 reported unexpected and serious adverse events. One participant had substernal chest pain 15 hours after exercising, but was negative for myocardial infarction; one participant had back, hip, and leg pain; and one participant had multifocal premature ventricular contractions or non‐specific t‐wave changes in their electrocardiogram. The other studies reported no adverse events.
Secondary outcomes
Subjective sleep‐related outcomes
Two studies reported further sleep‐related outcomes. Richards 2011 found an effect in favour of the intervention for non‐rapid eye movement sleep after six months (P = 0.001; no further information was reported). McCurry 2011 assessed sleep quality and found nearly very little in sleep quality in both groups (change from baseline: MD −0.6 (standard error (SE) 0.2) with intervention versus MD −0.2 (SE 0.2) with control; 65 participants).
Other secondary outcomes
No studies reported the effect of physical activities on behavioural and psychological symptoms of dementia, quality of life, functional status, institutionalisation, compliance with the intervention, attrition rates, or carer outcomes.
Social activities
We presented results for all primary outcomes in Table 3.
Primary outcomes
Objective sleep‐related outcomes
Total nocturnal sleep time
Two studies (236 participants) reported total nocturnal sleep time at 21 days (Richards 2005), and at seven weeks (Richards 2011). They were sufficiently similar to allow for meta‐analysis despite some differences in the social activities.
We found low‐certainty evidence (downgraded one level each for risk of bias and imprecision) that social activities may slightly increase total nocturnal sleep time using actigraphy in comparison with usual care (MD 16.78 minutes, 95% CI −7.78 to 41.34; I2 = 0%; Analysis 3.1; Figure 6).
3.1. Analysis.

Comparison 3: Social activity, Outcome 1: Total nocturnal sleep time (minutes)
6.

Forest plot of comparison: 1 Social Activity, outcome: 1.2 Night‐time total sleep (minutes).
Sleep efficiency
Two studies (236 participants) reported sleep efficiency at 21 days (Richards 2005), and at seven weeks (Richards 2011). They were sufficiently similar to allow for meta‐analysis despite some differences in the social activities.
We found low‐certainty evidence (downgraded one level each for risk of bias and imprecision) that social activities may slightly increase sleep efficiency in comparison with usual care (MD 2.65%, 95% CI −1.79% to 7.09%; I2 = 23%; Analysis 3.2; Figure 7).
3.2. Analysis.

Comparison 3: Social activity, Outcome 2: Sleep efficiency
7.

Forest plot of comparison: 1 Social activity, outcome: 1.1 Sleep efficiency.
Other night‐time sleep‐related outcomes
No studies reported the effect of social activities on consolidated sleep time at night, total wake time at night, number of nocturnal awakenings, sleep onset latency, ratio of daytime sleep to night‐time sleep, or ratio of night‐time sleep to total sleep over 24 hours.
Richards 2005 found a lower day/night sleep ratio in the intervention group, indicating that the proportion of daytime‐to‐night‐time sleep had decreased (day/night sleep ratio: intervention: baseline 0.66 (SD 0.81), follow‐up 0.48 (SD 0.58); control: baseline 0.59 (SD 0.46), follow‐up 0.64 (SD 0.80) with control; P = 0.03). Sleep latency decreased in both study groups, but there was no difference between the groups (intervention: baseline 43.40 (SD 52.94) minutes, follow‐up 33.09 (SD 43.15) minutes; control: baseline 38.28 (SD 48.31) minutes, follow‐up 34.02 (SD 35.35) minutes; P = 0.37).
Adverse events
No studies reported any adverse events or serious adverse events.
Secondary outcomes
Subjective sleep‐related outcomes
In Richards 2011, the duration of REM sleep increased in the intervention group and decreased in the control group (intervention group: baseline 40.3 (SD 24.2) minutes, follow‐up 46.1 (SD 26.3) minutes; control group: baseline 52.0 (SD 31.3) minutes, follow‐up 39.2 (SD 26.3) minutes). The duration of non‐REM sleep increased in both groups (intervention group: baseline 272.6 (SD 73.5) minutes, follow‐up 292.8 (SD 64.5) minutes; control group baseline 289.8 (SD 73.2) minutes, follow‐up 301.2 (SD 67.8) minutes).
Other secondary outcomes
No studies reported the effect of social activities on behavioural and psychological symptoms of dementia, quality of life, functional status, institutionalisation, compliance with the intervention, attrition rates, or carer outcomes.
Carer interventions
Studies assessed different sleep‐related outcomes for carer interventions. We presented results for all primary outcomes in Table 4.
Primary outcomes
Objective sleep‐related outcomes
Total nocturnal sleep time
One study reported total nocturnal sleep time (McCurry 2012). We found low‐certainty evidence (downgraded one level each for risk of bias and imprecision) that carer interventions may modestly increase total nocturnal sleep time using actigraphy in comparison with usual care after six months (MD 108.00 minutes, 95% CI 10.60 to 205.40; 33 participants, Analysis 4.1).
4.1. Analysis.

Comparison 4: Carer interventions, Outcome 1: Total nocturnal sleep time (minutes)
Sleep efficiency
One study reported sleep efficiency (McCurry 2012). We found low‐certainty evidence (downgraded one level each for risk of bias and imprecision) that carer interventions may slightly increase sleep efficiency using actigraphy in comparison with usual care after six months (MD 8.40%, 95% CI −1.55% to 18.35%; 33 participants; Analysis 4.2).
4.2. Analysis.

Comparison 4: Carer interventions, Outcome 2: Sleep efficiency
Total wake time at night
One study reported total wake time at night (McCurry 2012). We found low‐certainty evidence (downgraded one level each for risk of bias and imprecision) that carer interventions may modestly decrease the total awake time at night using actigraphy in comparison with usual care after six months (MD −24.00 minutes, 95% CI −79.01 to 31.01; 33 participants; Analysis 4.3).
4.3. Analysis.

Comparison 4: Carer interventions, Outcome 3: Total wake time at night (minutes)
Other night‐time sleep‐related outcomes
No studies reported the effect of carer interventions on consolidated sleep time at night, number of nocturnal awakenings, sleep onset latency, ratio of daytime sleep to night‐time sleep, or ratio of night‐time sleep to total sleep over 24 hours.
Adverse events
No studies reported any adverse events or serious adverse events with carer interventions.
Secondary outcomes
Subjective sleep‐related outcomes
Two studies assessed other sleep‐related outcomes. McCurry 2012 found that total daytime sleep time was unchanged in the intervention group, but increased in the control group (intervention: baseline 318 (SD 156) minutes, follow‐up 312 (SD 168) minutes; control: 264 (SD 132) minutes, follow‐up 348 (SD 150) minutes). In Gattinger 2017, there was no clear difference between groups in daytime sleepiness (MD −6.00 minutes, 95% CI −107.79 to 95.79; 44 participants; Analysis 4.4), and no differences in quality of sleep between groups (no further details reported).
4.4. Analysis.

Comparison 4: Carer interventions, Outcome 4: Sleepiness during daytime
Other secondary outcomes
No studies reported the effect of carer interventions on behavioural and psychological symptoms of dementia, quality of life, functional status, institutionalisation, compliance with the intervention, attrition rates, or carer outcomes.
Multimodal interventions
Studies assessed different sleep‐related outcomes for multimodal interventions. We presented results for all primary outcomes in the Table 5.
We rated the certainty of this evidence as low because of serious risk of bias and imprecision or inconsistency.
Primary outcomes
Objective sleep‐related outcomes
Total nocturnal sleep time
Three studies (272 participants) reported total nocturnal sleep time. We found low‐certainty evidence (downgraded one level each for risk of bias and one imprecision) that multimodal interventions may modestly increase total nocturnal sleep time (after 32 days: MD 24.00 minutes, 95% CI −3.51 to 51.51; 118 participants; Alessi 2005; after 7 weeks: MD 35.30 minutes, 95% CI 7.99 to 62.61; 88 participants; Richards 2011; after 2 months: MD 29.4 minutes, 95% CI −25.90 to 84.70; 66 participants; McCurry 2011; Analysis 5.1).
5.1. Analysis.

Comparison 5: Multimodal interventions, Outcome 1: Total nocturnal sleep time (minutes)
Sleep efficiency
Five studies (485 participants) reported sleep efficiency. We found very low‐certainty evidence (downgraded one level each for risk of bias, imprecision, and inconsistency). The evidence is very uncertain about the effects of multimodal interventions on sleep efficiency. In three studies, sleep efficiency slightly increase in the intervention groups (MD 4.00%, 95% CI −1.42% to 9.42%; 118 participants; Alessi 2005; MD 2.30%, 95% CI −5.08% to 9.68%; 66 participants; McCurry 2011; MD 4.80%, 95% CI 0.47% to 9.13%; 88 participants; Richards 2005; Analysis 5.2). In one study, sleep efficiency slightly increased in the control group (noise reduction night‐time programme) (MD −3.80%, 95% CI −17.96% to 10.36%; 29 participants; Alessi 1999; Analysis 5.2). In Schnelle 1999, there was no difference between groups (MD 0%, 95% CI −4.61% to 4.61%: 184 participants; Analysis 5.2).
5.2. Analysis.

Comparison 5: Multimodal interventions, Outcome 2: Sleep efficiency
Total wake time at night
Two studies (102 participants) reported total wake time at night. We found low‐certainty evidence (downgraded one level each for risk of bias and imprecision) that multimodal interventions may modestly reduce the total wake time at night after two months (MD −36.00 minutes, 95% CI −89.66 to 17.66; 36 participants; McCurry 2005; MD −7.00 minutes, 95% CI −52.90 to 38.90; 66 participants, McCurry 2011; Analysis 5.3).
5.3. Analysis.

Comparison 5: Multimodal interventions, Outcome 3: Total wake time at night (minutes)
Number of nocturnal awakenings
Four studies (404 participants) reported number of awakenings. We found low‐certainty evidence (downgraded one level each for risk of bias and inconsistency) that multimodal interventions may result in little to no difference in number of awakenings. Two studies found no difference in number of awakenings between groups (MD 0.10, 95% CI −5.25 to 5.45: 118 participants; duration of follow‐up 32 days; Alessi 2005; MD −0.30, 95% CI −0.76 to 0.16; 184 participants; duration of follow‐up not reported; Schnelle 1999; Analysis 5.4). In two studies the number of night‐time awakenings slightly decreased in the multimodal intervention groups after two months (MD −4.00, 95% CI −10.10 to 2.10; 36 participants; McCurry 2005; MD −4.7, 95% CI −9.29 to −0.11; 66 participants; McCurry 2011; Analysis 5.4).
5.4. Analysis.

Comparison 5: Multimodal interventions, Outcome 4: Number of nocturnal awakenings
Other night‐time sleep‐related outcomes
No studies reported the effect of multimodal interventions on consolidated sleep time at night, sleep onset latency, ratio of daytime sleep to night‐time sleep, or ratio of night‐time sleep to total sleep over 24 hours.
Adverse events
Only Richards 2011 reported adverse events (see under 'Physical activities' above).
Secondary outcomes
Subjective sleep‐related outcomes
Li 2009 assessed sleep quality and found a decrease in scores in the intervention group compared with the control group (mean score: intervention: 13.63 at baseline to 2.19 at follow; control: 13.01 at baseline to 4.85 at follow‐up). Alessi 2005 found a decrease in daytime sleeping in the intervention group after 32 days (32% at baseline, 30% at follow‐up; P < 0.001). McCurry 2011 found little change in sleep quality in both groups (mean: intervention: baseline 1.1 (SE 0.2), follow‐up 0.8 (SE 0.2); control: 0.8 (SE 0.2), follow‐up 0.5 (SE 0.1); 66 participants).
In Schnelle 1999, noise was reduced from a mean of 83 intervals per night with peak noises recorded above 50 dB to a mean of 58 intervals per night in the group that received the initial intervention, whereas there was little change in noise in the control group. Light changes were reduced from a mean of four per night per resident to two per night.
In Richards 2011, the duration of REM sleep slightly decreased in the intervention group and decreased in the control group (intervention: baseline 40.5 (SD 31.0) minutes, follow‐up 38.7 (SD 21.3) minutes; control: baseline 52.0 (SD 31.3) minutes, follow‐up 39.2 (SD 26.3) minutes). The duration of non‐REM sleep increased in the intervention group and modestly increased in the control group (intervention: baseline 262.1 (SD 93.2) minutes, follow‐up 322.6 (SD 45.0) minutes; control: baseline 289.8 (SD 73.2) minutes, follow‐up 301.2 (SD 67.8) minutes).
Behavioural and psychological symptoms of dementia
Alessi 1999 assessed agitation. There was a decrease in the number of observations with agitation in the intervention group and an increase in the control group (mean number of observations with agitation: intervention: baseline 9.4 (SD 15.4), follow‐up 7.3 (SD 14.0); control: baseline 5.9 (SD 9.7), follow‐up 14.7 (SD 19.7); 39 participants).
Other secondary outcomes
No studies reported the effect of light therapy interventions on quality of life, functional status, institutionalisation, compliance with the intervention, attrition rates, or carer outcomes.
Daytime sleep restriction
Primary outcomes
Objective sleep‐related outcomes
No studies reported the effects of daytime sleep restriction on total nocturnal sleep time, consolidated sleep time at night, sleep efficiency, total wake time at night, number of nocturnal awakenings, sleep onset latency, ratio of daytime sleep to night‐time sleep, or ratio of night‐time sleep to total sleep over 24 hours.
One study assessed time awake over the daytime (Ancoli‐Israel 2003). The time awake over the daytime improved slightly in the intervention group (baseline 65%, follow‐up 68%; P < 0.074) indicating that the daytime sleep restrictions were successfully implemented. There was no significant changes in night‐time sleep quality and in the circadian activity rhythm parameters in the intervention or control group. Thus, we found low‐certainty evidence (downgraded one level each for risk of bias and imprecision) from this single study that daytime sleep restrictions may have little to no effect on night‐time sleep quality and the circadian activity rhythm parameters.
Adverse events
The study did not report any adverse events or serious adverse events with daytime sleep restriction.
Secondary outcomes
No studies did not report the effect of daytime sleep restriction on subjective sleep‐related outcomes, behavioural and psychological symptoms of dementia, quality of life, functional status, institutionalisation, compliance with the intervention, attrition rates, or carer outcomes.
Slow‐stroke back massage
Primary outcomes
Objective sleep‐related outcomes
Total nocturnal sleep time
Harris 2012 assessed night‐time sleep. We found low‐certainty evidence (downgraded one level each for risk of bias and imprecision) that slow‐stroke back massage may result in little to no difference in night‐time sleep (P = 0.18).
Sleep efficiency
Harris 2012 assessed sleep efficacy. We found low‐certainty evidence (downgraded one level each for risk of bias and imprecision) that slow‐stroke back massage may result in little to no difference in sleep efficiency (P = 0.26).
Sleep onset latency
Harris 2012 assessed sleep latency. We found low‐certainty evidence (downgraded one level each for risk of bias and imprecision) that slow‐stroke back massage may result in little to no difference in sleep latency (P = 0.99).
Other night‐time sleep‐related outcomes
The study did not report the effect of slow‐stroke back massage on consolidated sleep time at night, total wake time at night, number of nocturnal awakenings, ratio of daytime sleep to night‐time sleep, or ratio of night‐time sleep to total sleep over 24 hours.
Harris 2012 assessed wake after sleep onset. We found low‐certainty evidence (downgraded one level each for risk of bias and imprecision) that slow‐stroke back massage may result in little to no difference in wake after sleep onset (P = 0.65).
Adverse events
Harris 2012 reported no unexpected or serious adverse events due to slow‐stroke back massage.
Secondary outcomes
The study did not report the effect of slow‐stroke back massage on subjective sleep‐related outcomes, behavioural and psychological symptoms of dementia, quality of life, functional status, institutionalisation, compliance with the intervention, attrition rates, or carer outcomes.
The study assessed daytime inactivity and found no differences between groups. None of our other secondary outcomes were reported.
Transcranial electrostimulation
Primary outcomes
Objective sleep‐related outcomes
No studies reported the effects of transcranial electrostimulation on total nocturnal sleep time, consolidated sleep time at night, sleep efficiency, total wake time at night, number of nocturnal awakenings, sleep onset latency, ratio of daytime sleep to night‐time sleep, or ratio of night‐time sleep to total sleep over 24 hours.
Hozumi 1996 assessed sleep disorder. We found low‐certainty evidence from one study (downgraded one level each for risk of bias and imprecision) that transcranial electrostimulation may result in little to no difference in sleep disorder (intervention: baseline 1.13 (SD 1.24), follow‐up 0.77 (SD 0.92); control: baseline 1.47 (SD 1.24), follow‐up 1.06 (SD 1.08); 27 participants; Hozumi 1996).
Adverse events
Adverse events were only reported in one participant, who complained of a dull pain in the head during active treatment.
Secondary outcomes
The study did not report the effect of transcranial electrostimulation on subjective sleep‐related outcomes, behavioural and psychological symptoms of dementia, quality of life, functional status, institutionalisation, compliance with the intervention, attrition rates, or carer outcomes.
Discussion
Summary of main results
Despite the relatively large number of studies included, we were unable to perform meta‐analyses for most interventions due to marked clinical and methodological heterogeneity and results remain inconclusive. We performed only one meta‐analysis for effects of social activities. The most frequently examined intervention was some form of light therapy in seven studies and there were four studies on physical or social activities (or both). Two studies assessed different carer interventions and one study each applied daytime sleep restriction, slow‐stroke back massage, or transcranial electrostimulation. Seven studies examined multimodal interventions consisting of at least two intervention components (Figure 3).
We are uncertain about the effects of light therapy on sleep‐related outcomes due to the very low‐certainty evidence. For physical activity interventions, we found differences favouring activities for total nocturnal sleep time, sleep efficiency (percentage of night‐time sleep), night‐time total wake time, and the number of night‐time awakenings (low‐certainty evidence). For social activity interventions, we found improvements regarding total nocturnal sleep time and sleep efficiency (low‐certainty evidence). For carer interventions, we found small differences in favour of the interventions on total nocturnal sleep time, sleep efficiency, night‐time total wake, and night‐time number of awakenings (low‐certainty evidence). We found small differences in favour of multimodal interventions on the total nocturnal sleep time and total wake time at night, but no clear improvement on the number of awakenings (low‐certainty evidence). We were uncertain about the effects of multimodal interventions on sleep efficiency (very low‐certainty evidence). For daytime‐sleep restriction, slow‐stroke back massage, and transcranial electrostimulation, we found no improvement on sleep‐related outcomes (all low‐certainty evidence). Only one study applying physical activity interventions reported adverse events. No unexpected or serious adverse events were attributed to any other intervention.
In summary, we found that neither single nor multimodal interventions consistently improved sleep with sufficient certainty, but we found some positive effects of physical and social activities as well as carer interventions; however, the certainty of evidence was low.
Overall completeness and applicability of evidence
We included 19 RCTs with 1335 participants evaluating different non‐pharmacological interventions to avoid sleep disturbances in people with dementia. Studies were very heterogeneous. Methodological deficiencies were frequent, as was missing information on study methodology. Therefore, we can draw no firm conclusions. In addition, the number of studies on specific interventions contributing to the different outcomes of interest in this review was relatively small. Adverse events were frequently not reported, but the nature of most interventions means that adverse events are not likely.
We identified nine ongoing studies, three investigating therapeutic light, two multimodal interventions, two sleep education, and two studies an activity intervention (see Characteristics of ongoing studies table), which might challenge the reported results. We rated three studies as awaiting classification, for which we were unable to receive at least some relevant information on study design, conduction, outcomes, or a combination of these (see Characteristics of studies awaiting classification table).
Most studies were conducted in nursing home residents and therefore, we are unable to draw conclusions about other settings (i.e. home care or hospitals), although care providers of these settings are also confronted with important challenges concerning sleep disturbances of people with dementia. As most studies in this review included patients similar to those seen in clinical practice (i.e. with moderate‐to‐severe dementia and a variety of common sleep problems at baseline), results should be principally applicable. Still, due to marked clinical heterogeneity of studies and important methodological limitations, results must be interpreted with caution and additional high‐quality studies are needed.
Quality of the evidence
Fifteen studies measured objective sleep‐related outcomes using actigraphy, while one study used polysomnography. Actigraphy or polysomnography (or both) are considered as gold standard to assess sleep‐related outcomes and there is evidence showing a good correlation between actigraphy and polysomnography measurement in general and in people with dementia (Ancoli‐Israel 1997; Quante 2018). Principally, the measures allow for blinding assessment, which most studies achieved. Only for two studies, we considered the risk of detection bias as high, as research staff who performed the outcome assessment were not blinded. There were significant problems with attrition in two studies, as these only reported completers without mentioning attrition. Two studies had a high risk of reporting bias. We evaluated the certainty of evidence using the GRADE approach (Guyatt 2011) and judged the overall certainty of evidence predominantly as low or very low due to several methodological limitations in the studies. The risk of bias in the included studies was very often unclear and there were various areas of incomplete reporting.
Five studies used the PSQI to measure sleep quality. The PSQI covers seven domains indicating sleep problems by participants self‐rating their sleeping behaviour during a one‐month interval. Originally, the PSQI was developed for people with psychiatric disorders and for research activities (Buysse 1989). Currently, it is the most used generic instrument to assess sleep quality in clinical and research settings (Mollayeva 2016). Due to the nature of dementia, participants' abilities for self‐rating sleep for a full month is limited. In practice, the assessment is often performed by proxy‐ratings of carers. Unfortunately, items such as 'having bad dreams' or 'feeling too hot/ too cold' (Buysse 1989) cannot be answered precisely by others. This probably leads to inaccurate results of the assessments, which should be considered when interpreting PSQI results.
The SSS is used to assess daytime sleepiness, and consists of a single item to be answered by the participant. It requires reading seven full sentences, which can also pose a difficulty for people with dementia or sensory impairment (or both).
We included one study in which one intervention group received a placebo tablet. We are aware of the possible effect from the intake of a placebo, but considering the likely polypharmacy in the study population, we considered that an additional tablet would not lead to relevant placebo effects. Therefore, we consider the placebo administration as control group receiving usual care.
Potential biases in the review process
We identified no potential biases in the review process. We followed the preplanned methods described in the review protocol (Wilfling 2015), and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We conducted a comprehensive literature search including several sources (e.g. databases and trial registers) guided by the CDCIG's Information Specialist. Two review authors independently performed study selection, data extraction, and quality assessment. However, based on the small number of studies per outcome, we were unable to assess the risk of publication bias.
Agreements and disagreements with other studies or reviews
At present there is no other systematic review comprehensively summarising the evidence on non‐pharmacological interventions for sleep disturbances in people with dementia. Earlier reviews frequently focused on a number of dementia‐related disturbances as, for example, the review by Livingston 2014 focusing on "managing agitation in older adults with dementia". O'Neil 2011 included sleep disturbances as one of several behavioural symptoms associated with dementia. Therefore, neither review specifically addressed the effectiveness of interventions on improving sleep in people with dementia.
Two more‐recently published systematic reviews targeted non‐pharmacological interventions to improve night‐time sleep among residents of long‐term care settings (Capezuti 2018; Shang 2019). Capezuti 2018 included 54 studies comprising 25 different interventions. Thirty included studies were RCTs. The authors categorised interventions into environmental (including light therapy), complementary health practices, defined as "touch and oral supplements", social/physical stimulation (including physical exercise), clinical care practices (including warm foot baths), mind–body practices (including relaxation), and multimodal interventions. Compared to our review, this review included all study designs beyond RCTs and focussed on residents in long‐term care settings, irrespective of cognitive status and presence of baseline sleep disturbances. Despite the relatively large number of included studies, the authors concluded that there is a need for further research as results were judged insufficient to clearly answer the review question. However, three interventions were considered as showing "the most promising results" (i.e. increased daytime light exposure, night‐time use of melatonin, and acupressure) (Capezuti 2018). Shang 2019 included 28 studies targeting nursing home residents, describing five intervention types: physical activity, light therapy, mind–body practices, complementary, and alternative therapy, and multicomponent interventions. The review included RCTs in residents aged over 60 years irrespective of sleep problems at baseline. The authors also found a wide range of interventions, although physical activity, mind–body practices, acupressure, and chamomile extract capsules were considered to demonstrate positive effects on sleep quality and night‐time sleep.
O'Caoimh 2019 summarised the evidence on non‐pharmacological treatments for sleep disturbance in people with mild cognitive impairment and mild dementia, and identified 48 eligible studies. Compared to our review, inclusion criteria differed as study designs beyond RCTs were included as well as participants without sleep problems at baseline. Interventions were categorised into light therapy, multimodal interventions, electrotherapy stimulation, physical exercises, acupressure/acupuncture, and cognitive behavioural therapy. The authors conducted a meta‐analysis of data from RCTs showing statistically significant improvements in sleep efficiency for multimodal interventions.
Although there are several recent systematic reviews both on behavioural disturbances and on sleep disturbances in older people or people with dementia, the reviews' scopes and methodologies considerably differ from our review, and it seems unfeasible to directly compare results. Still, the challenges, such as synthesising diverse and complex interventions, were comparable to our review in all reviews.
To account for this problem and to add value to the existing evidence, Wilfling 2021 conducted a systematic review analysing every single component of multicomponent interventions based on TIDieR (Hoffmann 2014) and CReDECI 2 criteria (Möhler 2015). All evaluation studies investigating multicomponent, non‐pharmacological interventions aiming to avoid or reduce sleep disturbances in nursing home residents were eligible for inclusion, except case studies. The review identified nine different interventions, categorised into daytime activities, night‐time activities, staff training, and light exposure. The analysis identified positive effects for sleep‐related outcomes, although interventions differed in terms of procedures, materials, modes of delivery, intervention provider, and intervention period. Analyses also showed that challenges in developing, evaluating, and implementing complex interventions were not sufficiently considered in the primary publications.
Authors' conclusions
Implications for practice.
Due to the largely inconclusive results, implications for practice remain limited. The evidence reviewed suggests some positive effects of physical and social activities as well as multimodal interventions. Carer interventions, such as carer education and management strategies also showed some positive effects. It seems obvious that intervention approaches have to be tailored to the situation and to the setting as e.g. for people with dementia cared for at home as opposed to nursing home residents. Despite the limitations of the evidence, practitioners should be encouraged to discuss and apply non‐pharmacological interventions aiming to improve sleep in people with dementia before using drug therapies, as there is no clear evidence for their superiority, and adverse events are more likely. Guidelines and information materials are needed to inform professional and informal carers about different interventions to enhance sleep. Although a recent qualitative study has shown that carers are already aware of different interventions, they still need education and counselling (Sagha 2018).
Implications for research.
Due to the lack of high‐quality evidence for the effectiveness of pharmacological and non‐pharmacological interventions, no firm conclusions can be drawn, and no recommendations can be derived concerning interventions to improve sleep in nursing home residents with dementia. More research is clearly needed to develop, evaluate, and implement effective interventions. From the studies included in this review, it seems likely that multimodal or "complex" interventions have the strongest potential to be effective in improving sleep in people with dementia, which is supported by a current analysis of multicomponent, non‐pharmacological interventions to avoid or reduce sleep disturbances in nursing home residents (Wilfling 2021). The review also shows a lack of well‐developed theory‐based complex interventions based on adequate theoretical models and frameworks. Ideally, effective interventions should target both carers and care recipients and should be adaptable to individual and institutional settings. When developing interventions, recommendations for complex interventions (Craig 2008) have to be considered and the evaluation of interventions' effectiveness need to be evaluated in RCTs with a mixed‐methods process evaluation following specific guidance (Moore 2015). Choice of intervention components has to be theoretically and empirically founded and reported (Hoffmann 2014; Möhler 2015). Conduction of feasibility studies (Eldridge 2016) before piloting the intervention and subsequently conducting an RCT are also important. As studies especially in institutional settings will usually be cluster‐randomised trials, the challenges of cluster‐randomisation must be acknowledged in the development, the conduct, the analysis, and the reporting of the study (Lorenz 2018).
History
Protocol first published: Issue 9, 2015
Acknowledgements
We thank the Cochrane Dementia and Cognitive Improvement Group, especially Sue Marcus, Jenny McCleery, and Anna Noel‐Storr.
We acknowledge Nora Eisemann and Anne Junghans (Institute of Social Medicine and Epidemiology, University of Lübeck, Germany) and Lisa Marshall (University Hospital Schleswig‐Holsein, Campus Lübeck, Germany) for their contribution to the review protocol.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| CDCIG Register (crsweb.cochrane.org/login.html) [Date of most recent search: 13 January 2022] |
(SLE OR sleep* OR circadian OR nocturnal OR insomnia* OR hypersomnia or parasomnia) AND (RCT OR CCT) AND (non‐pharmacological) | Jul 2011: Jun 2015: 237 Mar 2016: 3 Nov 2016: 0 Jan 2018: 2 Dec 2018: 9 Dec 2019: 13 Oct 2020: 6 Jan 2022: 20 |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1946‐present (OvidSP) [Date of most recent search: 13 January 2022] |
1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*.mp. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (creutzfeldt or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. "cognit* impair*".mp. 21. neurodegenerat*.mp. 22. cerebrovascular.mp. 23. neuropsychiatric.mp. 24. neurobehavioral.mp. 25. or/1‐24 26. exp Sleep/ 27. sleep*.ti,ab. 28. "Sleep Initiation and Maintenance Disorders"/ 29. insomnia.mp. 30. exp sleep disorders, circadian rhythm/ or "disorders of excessive somnolence"/ 31. (hypersomnia or parasomnia).mp. 32. circadian.mp. 33. or/26‐32 34. 25 and 33 35. randomized controlled trial.pt. 36. controlled clinical trial.pt. 37. randomized.ab. 38. placebo.ab. 39. drug therapy.fs. 40. randomly.ab. 41. trial.ab. 42. groups.ab. 43. or/35‐42 44. (animals not (humans and animals)).sh. 45. 43 not 44 46. 34 and 45 |
Jul 2011: Jun 2015: 94 Mar 2016: 232 Nov 2016: 185 Jan 2018: 463 Dec 2018: 198 Dec 2019: 463 Oct 2020: 466 Jan 2022: 758 |
| Embase (OvidSP) 1974‐present [Date of most recent search: 13 January 2022] |
1. exp Dementia/ 2. dement*.mp. 3. alzheimer*.mp. 4. (lewy* adj2 bod*).mp. 5. (chronic adj2 cerebrovascular).mp. 6. ("organic brain disease" or "organic brain syndrome").mp. 7. (cerebr* adj2 deteriorat*).mp. 8. (cerebral* adj2 insufficient*).mp. 9. (pick* adj2 disease).mp. 10. (creutzfeldt or jcd or cjd).mp. 11. huntington*.mp. 12. binswanger*.mp. 13. korsako*.mp. 14. or/1‐13 15. exp Sleep/ 16. sleep*.ti,ab. 17. sleep disorder/ 18. insomnia.mp. 19. circadian rhythm/ or circadian rhythm sleep disorder/ 20. circadian.mp. 21. (hypersomnia or parasomnia).mp. 22. somnolence/ 23. somnolence.mp. 24. or/15‐23 25. randomized controlled trial/ 26. controlled clinical trial/ 27. randomly.ab. 28. randomi?ed.ab. 29. groups.ab. 30. RCT.ti,ab. 31. "double‐blind*".ti,ab. 32. "single blind*".ti,ab. 33. placebo.ab. 34. randomi?ed.ti. 35. or/25‐34 |
Jul 2011: Jun 2015: 134 Mar 2016: 815 Nov 2016: 213 Jan 2018: 487 Dec 2018: 431 Dec 2019: 552 Oct 2020: 468 Jan 2022: 569 |
| PSYCINFO 1806‐present (OvidSP) [Date of most recent search: 13 January 2022] |
1. exp Dementia/ 2. dement*.mp. 3. alzheimer*.mp. 4. (lewy* adj2 bod*).mp. 5. (chronic adj2 cerebrovascular).mp. 6. ("organic brain disease" or "organic brain syndrome").mp. 7. (cerebr* adj2 deteriorat*).mp. 8. (cerebral* adj2 insufficient*).mp. 9. (pick* adj2 disease).mp. 10. (creutzfeldt or jcd or cjd).mp. 11. huntington*.mp. 12. binswanger*.mp. 13. korsako*.mp. 14. or/1‐13 15. exp Sleep Treatment/ or exp Sleep/ or exp Sleep Disorders/ 16. sleep*.ti,ab. 17. insomnia.mp. 18. exp Sleep Wake Cycle/ 19. circadian.mp. 20. (hypersomnia or parasomnia).mp. 21. exp Hypersomnia/ 22. somnolence.mp. 23. or/15‐22 24. exp Clinical Trials/ 25. randomly.ab. 26. randomi?ed.ab. 27. groups.ab. 28. RCT.ti,ab. 29. "double‐blind*".ti,ab. 30. "single blind*".ti,ab. 31. placebo.ab. 32. randomi?ed.ti. 33. or/24‐32 34. 14 and 23 and 33 |
Jul 2011: Jun 2015: 19 Mar 2016: 45 Nov 2016: 30 Jan 2018: 77 Dec 2018: 30 Dec 2019: 72 Oct 2020: 67 Jan 2022: 92 |
| CINAHL (EBSCOhost) [Date of most recent search: 13 January 2022] |
Jul 2011: Jun 2015: 12 Mar 2016: 45 Nov 2016: 19 Jan 2018: 85 Dec 2018: 129 Dec 2019: 231 Oct 2020: 198 Jan 2022: 238 |
|
| Web of Science – all databases (Clarivate) [Date of most recent search: 13 January 2022 |
(dement* OR alzheimer* OR AD OR VCI OR VaD OR "vascular cognitive impairment" OR "lew* bod*" OR CADASIL) ANDTOPIC: (sleep* OR circadian* OR insomnia* OR hypersomnia OR parasomnia OR somnolence) ANDTOPIC: (randomized OR randomised OR randomly OR "random allocat*" OR RCT OR "double‐blind*" OR "single‐blind*") Timespan: All years. Search language=Auto |
Jul 2011: Jun 2015: 31 Mar 2016: 109 Nov 2016: 83 Jan 2018: 206 Dec 2018: 89 Dec 2019: 175 Oct 2020: 162 Jan 2022: 217 |
| LILACS (BIREME) [Date of most recent search: 13 January 2022] |
dementia OR demencia OR alzheimer$ [Words] and sleep OR insomnia OR circadian OR hypersomnia OR parasomnia OR sueño OR dorme [Words] and randomised OR randomized OR trial OR randomly OR groups [Words] | Jul 2011: Jun 2015: 0 Mar 2016: 0 Nov 2016: 0 Jan 2018: 0 Dec 2018: 0 Dec 2019: 0 Oct 2020: 12 Jan 2022: 0 |
| CENTRAL (the Cochrane Library) [Date of most recent search: 13 January 2022] |
#1 MeSH descriptor: [Dementia] explode all trees #2 dement* #3 alzheimer* #4 lewy* near/2 bod* #5 chronic near/2 cerebrovascular #6 "organic brain disease" or "organic brain syndrome" #7 cerebr* near/2 deteriorat* #8 cerebral* near/2 insufficient* #9 pick* near/2 disease #10 creutzfeldt or jcd or cjd #11 huntington* #12 binswanger* #13 korsako* #14 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 #15 sleep* #16 insomnia* #17 circadian #18 hypersomnia #19 parasomnia #20 MeSH descriptor: [Sleep] explode all trees #21 #15 or #16 or #17 or #18 or #19 or #20 #22 #14 and #21 |
Jul 2011: Jun 2015: 4 Mar 2016: 39 Nov 2016: 18 Jan 2018: 148 Dec 2018: 132 Dec 2019: 392 Oct 2020: 120 Jan 2022: 255 |
| ClinicalTrials.gov (www.clinicaltrials.gov) [Date of most recent search: 13 January 2022] |
sleep OR circadian OR nocturnal OR insomnia OR hypersomnia OR parasomnia | Interventional Studies | dementia OR alzheimers OR alzheimer OR lewy OR “vascular cognitive impairment” [Recruitment status: all] |
Jul 2011: Jun 2015: 4 Mar 2016: 27 Nov 2016: 2 Jan 2018: 8 Dec 2018: 33 Dec 2019: 92 Oct 2020: 92 Jan 2022: 117 |
| ICTRP Search Portal (apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrials.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [Date of most recent search: 13 January 2022] |
sleep OR circadian OR nocturnal OR insomnia OR hypersomnia OR parasomnia | Interventional Studies | dementia OR alzheimers OR Alzheimer OR lewy OR “vascular cognitive impairment” [Recruitment status: all] |
Jul 2011: Jun 2015: 0 Mar 2016: 40 Nov 2016: 5 Jan 2018: 5 Dec 2018: 2 Dec 2019: 22 Oct 2020: n/a Jan 2022: 15 |
| TOTAL before deduplication | Jul 2011: 415 Jun 2015: 535 Mar 2016: 1355 Nov 2016: 555 Jan 2018: 1481 Dec 2018: 1053 Dec 2019: 2012 Oct 2020: 1591 Jan 2022: 2281 TOTAL: 11,278 |
|
| TOTAL after deduplication and first assessment (if performed) by Cochrane Dementia and Cognitive Improvement Group Information Specialists | Jul 2011: 69 Jun 2015: 247 Mar 2016: 43 Nov 2016: 40 Jan 2018: 72 Dec 2018: 136 Dec 2019: 1557 Oct 2020: 1231 Jan 2022: 1709 TOTAL: 5104 |
|
Data and analyses
Comparison 1. Light therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Total nocturnal sleep time (minutes) | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2 Sleep efficiency | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3 Total wake time at night (minutes) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4 Number of nocturnal awakenings | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5 Sleep onset latency | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6 Sleep quality | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6.1 Pittsburgh Sleep Quality Index | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6.2 Sleep Disorders Inventory | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Physical activity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Total nocturnal sleep time (minutes) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Sleep efficiency | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3 Total wake time at night (minutes) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4 Number of nocturnal awakenings | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Social activity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Total nocturnal sleep time (minutes) | 2 | 236 | Mean Difference (IV, Random, 95% CI) | 16.78 [‐7.78, 41.34] |
| 3.2 Sleep efficiency | 2 | 236 | Mean Difference (IV, Random, 95% CI) | 2.65 [‐1.79, 7.09] |
Comparison 4. Carer interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Total nocturnal sleep time (minutes) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2 Sleep efficiency | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.3 Total wake time at night (minutes) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.4 Sleepiness during daytime | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Multimodal interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Total nocturnal sleep time (minutes) | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2 Sleep efficiency | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.3 Total wake time at night (minutes) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.4 Number of nocturnal awakenings | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alessi 1999.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: week 14 |
|
| Participants |
Country: USA, Los Angeles Setting: 1 long‐term care facility Inclusion criteria:
Exclusion criteria:
Number of participants completing the study: 29 (IG 15, CG 14) Baseline characteristics:
Group differences:
|
|
| Interventions |
Intervention: daily physical activity for 14 weeks, noise reduction night‐time programme for the last 5 days the of intervention, education for staff, reminding signs. Intervention consisted of functional incidental training, performed during daily nursing care routines (e.g. toileting). Training included arm and leg exercises, sit‐to‐stands, and walking or wheelchair propulsion, depending on participants' abilities. Trained research staff conducted the training sessions every 2 hours between 8.00 a.m. and 16.00 p.m. (maximum 5 sessions a day). Intervention performed 5 days a week, for 14 weeks in total. After the 14 weeks, the additional night‐time programme was introduced for 5 nights. This aimed to minimise light, noise, and sleep‐disruptive nursing care interventions at night. Control: noise reduction night‐time programme |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Of the remaining 29 participants, 15 were randomised to receive the combined daytime physical activity plus night‐time environmental programme (IG), and 14 were randomised to receive the night‐time programme alone (CG). |
| Allocation concealment | Unclear risk | Unknown. |
| Blinding of participants and personnel All outcomes | Unclear risk | Probably not blinded, but risk of bias unclear. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unknown. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unknown. |
| Blinding of outcome assessors Objective outcome measures | Low risk | Blinded assessment of actigraphy results (night‐time), observers blinded. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | Low risk | No information about 4 dropouts. |
| Selective outcome reporting | Unclear risk | No protocol identified. |
| Other sources of bias | Low risk | None. |
Alessi 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: days 3–5 |
|
| Participants |
Country: USA, Los Angeles Setting: 4 long‐term care facilities Inclusion criteria:
Exclusion criteria:
Number of participants completing the study: 118 (IG 62, CG 56) Baseline characteristics:
Group differences: comparable at baseline |
|
| Interventions | Quote: "Intervention research staff provided the intervention for five consecutive days and nights to five to six participants at a time." Intervention: 5 days of: 1. keeping residents out of bed between 8.00 a.m. and 18.00 p.m., and a minimum duration of 30 minutes of sunlight exposure a day (at 10,000 lux); 2. participating in a low‐level physical activity programme 3 times a day; and 3. an individualised bedtime routine (between 20.00 p.m. and 22.00 p.m.), including personal care and reduced light and noise. The study aimed to minimise night‐time noise and light for the whole night (22.00 p.m. to 6.00 a.m.). All aspects of the intervention were documented in detail. Control: usual care |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Quote: "Participants were randomly allocated to intervention or control groups within each site using a random sequence, without blocking or stratification. The generated sequence was used to randomly allocate 53% of the enrolled sample to intervention and 47% to control, because of the a priori expectation of greater drop‐out of intervention participants (20% expected dropout rate) than of controls (10% expected dropout rate)." |
| Allocation concealment | Unclear risk | No information. |
| Blinding of participants and personnel All outcomes | Unclear risk | Unknown. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unknown. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unknown. |
| Blinding of outcome assessors Objective outcome measures | High risk | Quote: "To minimize bias in assessment, independent research staff completed the assessment and intervention aspects of the study. Research staff who performed outcome assessments could not be adequately blinded to study condition at follow‐up because the characteristics of the intervention were directly observable, although they were blinded to study research questions." Different for outcomes: daytime observations: high; night‐time actigraphy: low. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | Low risk | Fairly balanced. |
| Selective outcome reporting | Unclear risk | Protocol not identified. |
| Other sources of bias | High risk | Delayed time series? Follow‐up time in CG twice as long as IG. Also IG received intervention at different points. Contamination as risk? Not possible? |
Ancoli‐Israel 2003.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: day 11–15 |
|
| Participants |
Country: USA Setting: unclear number of long‐term care facilities Inclusion criteria:
Exclusion criteria:
Number of participants completing the study: 92 (IG1 30, IG2 31, CG3 31) Baseline characteristics: not reported Group differences: no group differences |
|
| Interventions |
Intervention 1: morning bright light: given from 9:30 a.m. to 11:30 a.m., bright light resulted in an exposure of 2500 lux for 10 days Intervention 2: evening bright light: given from 5:30 p.m. to 7:30 p.m., bright light resulted in an exposure of 2500 lux for 10 days Intervention 3: morning dim red light: given from 9:30 a.m. to 11:30 a.m., dim red light resulted in an exposure of < 300 lux for 10 days Intervention 4: residents were accompanied by staff members for 6 hours during the day for 10 days, ensuring that residents would not fall to sleep |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | In a personal communication (email 17 July 2022) the first author Dr Ancoli‐Israel confirmed that the 2 publications refer to the same study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Quote: "Block‐stratified randomization using preassignment by order of entry was used. Stratification was by gender and by quartiles of the categorical SSS distribution. This information was used to assign patients randomly to one of four treatments: evening bright light, morning bright light, evening dim red light, and daytime sleep restriction (DSR)." |
| Allocation concealment | Unclear risk | Participants were randomly assigned to 1 of 3 treatment groups: morning bright light (30 participants), morning dim red light (31 participants), or evening bright light (31 participants), by block stratified randomisation using preassignment by order of entry within strata. Participants were stratified by type of agitation (i.e. agitated primarily in the morning, agitated primarily in the evening, or agitated all day based on nurses' ratings). |
| Blinding of participants and personnel All outcomes | Unclear risk | Unknown. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unknown. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unknown. |
| Blinding of outcome assessors Objective outcome measures | Low risk | Although nursing staff and research staff could not be kept blind to light treatment condition, all were told that both white and red light conditions were expected to show improvement and the study was examining which light colour would be better. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | Unclear risk | Of the 92 participants, 8 (8.7%) refused to wear the Actillume. 1 initially agreed, but then took the Actillume off and lost it. 9.5% of data were loss due to either human or device error. Overall, of 368 data files, 84% were usable. For 12 participants, 1 of the 4 files (baseline, treatment days 1–5, treatment days 6–10, or post‐treatment follow‐up) was missing; therefore, data for those 12 participants could not be included in all analyses. Complete analyses were performed on the remaining 72 participants. Judgement comment: 72/92 participants not assessed with no information about allocation. |
| Selective outcome reporting | Unclear risk | Protocol not identified. |
| Other sources of bias | Low risk | None. |
Chan 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: 2 months |
|
| Participants |
Country: China Setting: 2 community centres Inclusion criteria:
Exclusion criteria:
Number of participants completing the study: 52 (IG 27, CG 25) Baseline characteristics:
Group differences:
|
|
| Interventions |
Intervention: 60‐minute Tai Chi Qigong session twice a week for 2 months Control: usual care consisting of weekly health talk in community centre for 2 months |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Participants were randomly allocated to either IG (27 participants) or CG (25 participants) by computer‐generated random numbers. The grouping sequence list was password protected and stored on a computer. |
| Allocation concealment | Unclear risk | The grouping sequence list was password protected and stored on a computer. Only the authorised staff responsible for group allocation were allowed access to the list. |
| Blinding of participants and personnel All outcomes | Unclear risk | Unknown. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unknown. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unknown. |
| Blinding of outcome assessors Objective outcome measures | Low risk | To minimise researcher bias, the research assistants responsible for data collection were blinded to the study. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | Unclear risk | A "GEE model" was used to (quote) "account for intra‐correlated repeated measures data and accommodate missing data caused by dropouts, provided the data are missing at random, and thus is particularly suitable for intention‐to‐treat analysis, without the need for imputation of missing data." As dropout rates were high but did not differ markedly between groups at 6 months (IG 17/27, CG 14/25), risk of bias remained unclear. |
| Selective outcome reporting | Unclear risk | The protocol stated, "An objective measure of sleep pattern using sleep tracker" as first secondary endpoint which is neither reported nor discussed in the paper (www2.ccrb.cuhk.edu.hk/registry/public/287). |
| Other sources of bias | Low risk | None. |
Dowling 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: week 10 |
|
| Participants |
Country: USA, San Francisco Setting: 2 long‐term care facilities Inclusion criteria:
Exclusion criteria:
Number of participants completing the study: 46 (IG 29, CG 17) Baseline characteristics: not reported Group differences: no differences reported |
|
| Interventions |
Intervention: bright light exposure (≥ 2500 lux in gaze direction) from 9.30 a.m. to 10.30 a.m. (Monday to Friday) for 10 weeks Control: usual indoor light (150–200 lux) for 10 weeks |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | No information provided. Imbalances in the number of participants (IG 29, CG 17) due to different phases. |
| Allocation concealment | Unclear risk | No information. |
| Blinding of participants and personnel All outcomes | Unclear risk | Unknown. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unknown. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unknown. |
| Blinding of outcome assessors Objective outcome measures | Low risk | Possibly unblinded, but actigraphy. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | Low risk | Possibly all data considered although ITT not clearly described. |
| Selective outcome reporting | Unclear risk | No protocol identified. |
| Other sources of bias | Low risk | None. |
Figueiro 2019.
| Study characteristics | ||
| Methods |
Study design: RCT (cross‐over) Follow‐up: 12 weeks (phase 1: 4 weeks, washout period: 4 weeks, phase 2: 4 weeks) |
|
| Participants |
Country: USA, New York Capital District and Bennington Setting: 4 assisted‐living facilities and 4 long‐term care facilities Inclusion criteria:
Exclusion criteria:
Number of participants completing the study: total 46, useable data for both study periods: 41 (for questionnaire data); 32 (for actigraphy data) Baseline characteristics (described by gender): Female: (65.2%)
Male: (34.8%)
Group differences: comparable at baseline |
|
| Interventions |
Intervention: an active lighting intervention that provided high circadian stimulus for 4 weeks. Consisted of floor luminaires (550 lux or 600 lux), light boxes (350 lux), and light tables (750 lux). Control: provided low circadian stimulus for 4 weeks |
|
| Outcomes |
|
|
| Funding | National Institute on Aging (grant # R01AG034157) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Insufficient information. Quote: "Block randomization (block size of 4) was used to randomize participants into each of the study groups …" |
| Allocation concealment | Unclear risk | Insufficient information. Quote: "The participant blocks were then sequentially assigned to receive the active or the control intervention first." |
| Blinding of participants and personnel All outcomes | Low risk | Quote: "Facility staff were not informed of any differences between the lighting interventions and were told that the study’s goal was to determine which type of light was more effective." |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Low risk | Facility staff were not informed of any differences between the lighting interventions and were told that the study's goal was to determine which type of light was more effective. |
| Blinding of participants and personnel Objective sleep measures | Low risk | Actigraphy. |
| Blinding of outcome assessors Objective outcome measures | Low risk | Actigraphy. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Low risk | Facility staff were not informed of any differences between the lighting interventions and were told that the study's goal was to determine which type of light was more effective. |
| Incomplete outcome data All outcomes | Unclear risk | 52 participants randomised, 46 received intervention. Actigraphy data available for 42, but only 32 for both interventions. PSQI data available for 46, but only 41 for both interventions. Unclear numbers for each intervention. |
| Selective outcome reporting | High risk | Primary outcome PSQI not mentioned in initial study registration as well as actigraphy outcomes not matching between registration and publication. URL: clinicaltrials.gov/ct2/show/NCT01816152 |
| Other sources of bias | Low risk | None. |
Fontana Gasio 2003.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: 4–6 weeks |
|
| Participants |
Country: Switzerland, Basel Setting: 2 long‐term care facilities and 1 hospital Inclusion criteria:
Exclusion criteria:
Number of participants completing the study: 13 (IG 9, CG 4) Baseline characteristics:
Group differences: no means reported |
|
| Interventions |
Intervention: dawn‐dusk simulation for 1 week. An overhead halogen lamp behind a diffusing membrane was placed behind the participant's bed. A computer algorithm controlled this lamp, exposing the participant to light ranging from 0.001 lux to a maximum of 400 lux, simulating a dusk, dawn, and dark period. Control: placebo dim red light (white light replaced with a "placebo" 15 W red‐light bulb yielding 5 lux) for 1 week |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | 13 inpatients with the diagnosis of dementia and with nurse‐reported sleep disturbances were randomly assigned to a regimen of DDS (9 women, aged 86.8 (SD 4.5) years, MMSE: 13.8 (SD 5.9)) or 'placebo' dim red light (5 lux; 4 participants (3 women and 1 man), aged 83.0 (SD 5.2) years, MMSE: 14.3 (SD 4.1)). No further information given. |
| Allocation concealment | Unclear risk | No information given. |
| Blinding of participants and personnel All outcomes | Unclear risk | Unknown. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unknown. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unknown. |
| Blinding of outcome assessors Objective outcome measures | Low risk | Actimetry. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | Low risk | Arose by chance from the original randomisation scheme for 40 participants. Judgement comment: no information, but seemingly all 13 randomised were still there at follow‐up. Imbalance in participant numbers between groups explained by initial sample size of 40. |
| Selective outcome reporting | Unclear risk | No protocol identified. |
| Other sources of bias | High risk | The low number in the second group arose by chance from the original randomisation scheme for 40 participants. The group size was not balanced by the time they realised that the required number of participants could not be recruited. All wore an activity/lux monitor continuously. Sample size calculation, 40 participants, recruited 13. Group imbalance (IG 9, CG 4) with unclear relevance |
Gattinger 2017.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT Follow‐up: 10 weeks |
|
| Participants |
Country: Switzerland Setting: 3 long‐term care facilities Inclusion criteria:
Exclusion criteria: not reported Number of participants completing the study: 44 (IG 22, CG 22) Baseline characteristics:
Group differences:
|
|
| Interventions | Open 2‐phase RCT: duration of first phase 10 weeks, second phase 3 months. Intervention: implementation of motion monitoring system, education (sleep and dementia, monitoring system), 3 sleep case conferences led by an advanced nurse practitioner and 2 sleep case conferences led by an internal registered nurse Control: education (sleep and dementia), 3 sleep case conferences led by an advanced nurse practitioner, 2 sleep case conferences led by an internal registered nurse |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Simple randomisation used to assign the wards to IG and CG. |
| Allocation concealment | Unclear risk | Not reported, but seemingly recruitment of residents before randomisation. |
| Blinding of participants and personnel All outcomes | Unclear risk | Obviously not blinded, but unclear relevance. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unknown. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unknown. |
| Blinding of outcome assessors Objective outcome measures | High risk | Staff assessed primary outcome measure. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | Low risk | 2/24 participants (IG) and 5/27 participants (CG) lost to follow‐up mostly due to death which seems expectable in this population. For 'sleep quality', results were only available for only 15/22 residents in the IG (no reason given). |
| Selective outcome reporting | High risk | The study registration listed the Swiss version of neuropsychiatric inventory for nursing home as primary outcome, which was not mentioned in the study. |
| Other sources of bias | High risk | Contamination possible as wards in the same nursing homes belonged to both IG and CG. |
Harris 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: day 3–4 |
|
| Participants |
Country: USA, southeast Setting: 4 long‐term care facilities Inclusion criteria:
Exclusion criteria:
Number of participants completing the study: 40 (IG 20, CG 20) Baseline characteristics:
Group differences:
|
|
| Interventions |
Intervention: a certified geriatric advanced practice nurse was trained in slow‐stroke back massage and performed 3 minutes of massage for 2 nights at bedtime in residents' rooms. Control: usual bedtime care |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Participants sleeping < 7 hours (420 minutes) based on actigraphy data were randomly assigned to IG or CG. The Number Crunching Statistical Software was used to generate a table of random numbers and a randomisation schedule for the allocation sequence as participants were enrolled in the study (Hintze 2005). |
| Allocation concealment | Unclear risk | A randomisation schedule for the allocation sequence as participants were enrolled in the study. After consensus with Ralph Möhler. |
| Blinding of participants and personnel All outcomes | Unclear risk | Unknown. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unknown. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unknown. |
| Blinding of outcome assessors Objective outcome measures | Low risk | Actigraphy. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | Low risk | No attrition. |
| Selective outcome reporting | Unclear risk | No protocol identified. |
| Other sources of bias | Low risk | Short duration: 3‐minute slow‐stroke back massage at bedtime for 2 nights. |
Hozumi 1996.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: day 14 |
|
| Participants |
Country: Japan Setting: 1 hospital Inclusion criteria:
Exclusion criteria:
Number of participants completing the study: 27 (IG 14, CG 13) Baseline characteristics:
Group differences: no differences reported or identified |
|
| Interventions |
Intervention: 20 minutes of daily transcranial electrostimulation using a HESS‐100 device (electrodes attached through a headband) for 2 weeks at 10:00 a.m. (rectangular monophasic pulses of 0.2 ms duration and 6–8 V at increasing frequencies from 6 to 80 Hz, with a root mean square value of 256–530 µA). Control: placebo therapy without electric current |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | The participants were randomly assigned to the 2 subgroups, the active therapy group (14 participants) and the placebo therapy group (13 participants). |
| Allocation concealment | Unclear risk | No information given. |
| Blinding of participants and personnel All outcomes | Unclear risk | Unknown. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unknown. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unknown. |
| Blinding of outcome assessors Objective outcome measures | Unclear risk | All evaluations were made by the same doctor and nurse throughout the experimental period. No information other than "double‐blind" and unclear if clinicians performing assessment were blinded. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | High risk | People who reacted with obvious anxiety and distress at any time during the study were excluded. No information about the number of randomised participants or participants excluded during the study. |
| Selective outcome reporting | Unclear risk | No protocol identified. |
| Other sources of bias | Low risk | None. |
Li 2009.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: week 15 |
|
| Participants |
Country: China, Yangpu District of Shanghai City Setting: 1 mental health centre Inclusion criteria:
Exclusion criteria:
Baseline characteristics: not reported Number of participants completing the study: 68 (IG 34, CG 34) Group differences: no differences between IG and CG in age, year, sex, disease course, education level, and PSQI score (P > 0.05). |
|
| Interventions |
Intervention: 12 weeks of 1. morning exercise from 8.00 a.m. to 9.00 a.m. outdoor activities for 60 minutes, 2. afternoon activities according to participants' interest, such as painting, games, and music; 3. no napping: participants were allowed to go to bed only when sleepy without reading or television in bed as well as limited food intake 15–30 minutes before going to bed, and 4. getting up at 6.30 a.m. every morning. Control: usual care Co‐intervention: before study start, both groups received oral estazolam 1 mg (no further information available) |
|
| Outcomes |
|
|
| Funding | Sponsorship source: unclear | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Study mentioned "randomization", but no details given on the method. |
| Allocation concealment | Unclear risk | Study mentioned "randomization", but no details given on the method. |
| Blinding of participants and personnel All outcomes | Unclear risk | Unknown. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unknown. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unknown. |
| Blinding of outcome assessors Objective outcome measures | Unclear risk | No information given. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | Low risk | Data for all outcomes provided. |
| Selective outcome reporting | Unclear risk | No study protocol available and the study was not registered. |
| Other sources of bias | Low risk | None. |
McCurry 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: 2 months |
|
| Participants |
Country: USA Setting: independent living (with informal carers) Inclusion criteria:
Exclusion criteria:
Baseline characteristics: Patients:
Carers:
Number of participants completing the study: 36 (IG 17, CG 19) Group differences: no differences reported |
|
| Interventions |
Intervention: night‐time insomnia treatment (education about sleep hygiene, daily walks, decreased daytime sleep/in bed and increased daylight exposure) for 2 months. This consisted of 1. the development of an individual sleep hygiene programme for participants by carers; 2. participant walked daily for 30 minutes; and 3. increased daytime light exposure via a SunRay light box (2500 lux). Light intervention performed within a 3‐hour window before participants went to bed. Interventions performed over 3 weekly treatment sessions by a gerontopsychologist experienced in behavioural interventions with people with dementia. Control: non‐directive, supportive approaches and provided information about general dementia care. This consisted of 1. offering sleep‐related reading materials at baseline; the interventionist was available for questions about the materials during the intervention period, offered general information and support, information about general dementia care and community resources if requested, but no specific recommendations about hygiene‐related issues; 3. carers were encouraged spending 1 hour per day with their participants and engaging them in pleasant activities of their choice to control for the increased carer attention in the intervention group. |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Patient–carer dyads were randomly assigned to NITE‐AD or to a contact control condition. Dyads were randomised after the baseline assessment using a random numbers table that blocked groups of 8–12 participants. |
| Allocation concealment | Unclear risk | No information. |
| Blinding of participants and personnel All outcomes | Unclear risk | Unknown. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unknown. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unknown. |
| Blinding of outcome assessors Objective outcome measures | Low risk | Actigraphy. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | Low risk | No information. |
| Selective outcome reporting | Unclear risk | No protocol identified. |
| Other sources of bias | Low risk | None. |
McCurry 2011.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: 2 months |
|
| Participants |
Country: USA Setting: independent living (with informal carers) Inclusion criteria:
Exclusion criteria:
Baseline characteristics: Patients:
Carer:
Number of participants completing the study: 132 (IG1 32, IG2 34, IG3 33, CG 33) Group differences: no pretreatment group differences in any participant or carer demographic variables or any group differences in any baseline actigraphic or subjective measurements of participant sleep or in any other covariate measures. |
|
| Interventions | Interventions duration 2 months. Participants in all groups received 3 × 1‐hour in‐home training visits and 2 brief telephone calls to reinforce caregiver use of the daily log. Intervention 1: walking and sleep hygiene recommendations Intervention 2: SunRay light box (equal to approximately 2500 lux) for 1 hour/day and sleep hygiene recommendations Intervention 3: guided sleep education, walking, light box. The carer sleep education consisted of 6 training sessions. In session 1, carers learned to develop an individualised sleep plan for residents, aiming to reduce daytime napping, establish bedtime routine, and identify reasons for night‐time awakenings. In session 2, carers were trained about implementing the daily light exposure programme. Sessions 3–6 was on identifying reasons of night‐time awakenings as well as challenges in implementing the sleep, walking, and light exposure plans. Control: non‐directive dementia care support during the 3 in‐home training visits, including non‐directive dementia care support, but provided no training about sleep‐ or dementia‐related issues. |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | The random allocation sequence was obtained from a computer program that blocked in groups of 12 participants (p.1394). |
| Allocation concealment | Low risk | A research co‐ordinator assigned treatment conditions using sealed envelopes containing the random assignment (p.1394). |
| Blinding of participants and personnel All outcomes | Unclear risk | Unknown. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | High risk | Carers unblinded (see below). |
| Blinding of participants and personnel Objective sleep measures | Low risk | Not blinded, but without influence on outcomes due to method (actigraphy) (p.1395). |
| Blinding of outcome assessors Objective outcome measures | Low risk | Interviewers blind to treatment assignment conducted assessments at baseline, 2‐month (immediately after treatment) follow‐up, and 6‐month follow‐up (p.1395). Sleep–wake activity was measured at each assessment using a Micro‐Mini Motionlogger actigraph (p.1395). |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | Carers unblinded due to setting (independent community‐living) and type of intervention (e.g. daily walks) (both p.1393). It remains unclear if carers knew if they were part of the IG or CG but maybe this had no effect on bias. |
| Incomplete outcome data All outcomes | Low risk | Dropout rate reported (p.1396), ITT conducted (p.1397). |
| Selective outcome reporting | Low risk | Registered: ClinicalTrials.gov (Identifier: NCT00183378). Missing outcomes: residential status, carer sleep (not relevant here). |
| Other sources of bias | Low risk | None. |
McCurry 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: 1 month |
|
| Participants |
Country: USA Setting: 37 long‐term care facilities Inclusion criteria:
Exclusion criteria:
Number of participants completing the study: 47 (IG 31, CG 16) Baseline characteristics: Residents:
Carers:
Group differences: not reported |
|
| Interventions |
Intervention: 4 sessions of sleep education programme for carer‐staff (in 1 month). Consisted of 1. 30‐minute in‐service education on general sleep issues and the intervention; 2. verbal and visual feedback (noise levels recorded in nursing home were presented and verbal feedback about noise levels and sources of noise given); 3. noise abatement: implementation of procedures to reduce noise (e.g. turn off unwatched televisions); 4. individualised incontinence care: research staff provided incontinence care during hourly rounds when residents were awake. Otherwise, frequency of waking residents up for incontinence care was based on residents' risk for skin problems. During incontinence care, staff attempted to reduce noise and light exposure. Control: usual care |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Quote: "Residents were randomly assigned after the baseline assessment […] according to a 2:1 simple allocation ratio …" |
| Allocation concealment | Unclear risk | Not reported. |
| Blinding of participants and personnel All outcomes | Unclear risk | Unknown. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unknown. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unknown. |
| Blinding of outcome assessors Objective outcome measures | Low risk | Assessors blinded to treatment assignment. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | Low risk | 3 (6%) residents lost to follow‐up after 1 month. |
| Selective outcome reporting | Low risk | Analysis followed published study protocol. |
| Other sources of bias | Low risk | No adjustment for cluster effects as none were detected. Quote: "Twenty‐seven AFHs [adult family homes] (73%) had only one resident study participant, and 10 AFHs had two resident participants. Analysis of variance components indicated that including site effects did not enhance the explanatory power of models." |
Nowak 2008.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: day 15–19 |
|
| Participants |
Country: USA, Southeastern Michigan Setting: long‐term care facility (unclear number) Inclusion criteria:
Exclusion criteria:
Number of participants completing the study: 20 (IG 10, CG 10) Baseline characteristics:
Group differences: none for sleep measures (others not reported) |
|
| Interventions |
Intervention: blue–green light exposure to 12,000 lux for 30 minutes between 6 a.m. and 7 a.m. for 14 consecutive days via cap visors. Control: dim red light exposure to 5 lux for 30 minutes between 6 a.m. and 7 a.m. for 14 consecutive days via cap visors. |
|
| Outcomes |
|
|
| Funding |
|
|
| Notes | PhD thesis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Quote: "… randomized to either the experimental condition or control group utilizing a five‐block randomized block design (Appendix F)" (p.54). |
| Allocation concealment | Unclear risk | Not reported. |
| Blinding of participants and personnel All outcomes | Unclear risk | All interventions and outcome assessment actions were performed by the principal investigator. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | High risk | Quote: (post‐test and follow‐up) "The SSS was completed by the PI [principal investigator] as noted in Phase 2 three times per day (at meal times) for five consecutive days beginning on day 1 of the follow‐up period." (by telephone or by direct observation) (p.56/57). Unblinded staff assessed the outcomes. |
| Blinding of participants and personnel Objective sleep measures | Low risk | Actigraphy. |
| Blinding of outcome assessors Objective outcome measures | Low risk | Actigraphy. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | High risk | Quote: (post‐test and follow‐up) "The SSS was completed by the PI as noted in Phase 2 three times per day (at meal times) for five consecutive days beginning on day 1 of the follow‐up period" (by telephone or by direct observation) (p.56/57). Unblinded staff assessed these outcomes. |
| Incomplete outcome data All outcomes | Low risk | Dropout rate: 1/21 (p.105). |
| Selective outcome reporting | Unclear risk | Study was not registered and no protocol was published. |
| Other sources of bias | Low risk | None. |
Richards 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: day 17–21 |
|
| Participants |
Country: USA, central Southeast Setting: 7 long‐term care facilities Inclusion criteria:
Exclusion criteria: not reported Number of participants completing the study: 139 (IG 71, CG 68) Baseline characteristics: not reported Group differences: not reported |
|
| Interventions |
Intervention: 21 days of individualised social activities for 1–2 hours daily Control: usual care |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Participants randomly assigned to IG or CG. |
| Allocation concealment | Unclear risk | No information. |
| Blinding of participants and personnel All outcomes | Unclear risk | Unknown. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unknown. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unknown. |
| Blinding of outcome assessors Objective outcome measures | Unclear risk | Not blinded, but probably not relevant (maybe determination of "time in bed" by nursing assistants). |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | Low risk | Outcomes available for most. No information about group differences. |
| Selective outcome reporting | Unclear risk | No protocol identified. |
| Other sources of bias | Low risk | None. |
Richards 2011.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: week 8 |
|
| Participants |
Country: USA Setting: 10 long‐term care facilities and 3 assisted living facilities Inclusion criteria:
Exclusion criteria:
Baseline characteristics:
Number of participants completing the study: 193 (IG1 55, IG2 50, IG3 41, CG 47) Group differences: no differences |
|
| Interventions |
Intervention 1: exercise. Consisted of high‐intensity physical resistance strength training and walking programme. Hypothesised that the combination of both activities would have positive effects on total physical activity. Strength training consisted of hip extensions on a hip‐extension/leg‐press chair plus arm extensions from a seated position in a chest‐press chair. Exercises supervised by trained nurses. High‐intensity physical resistance strength training performed 3 days a week and on 2 further days, participants walked for up to 45 minutes. Intervention 2: social activity. Consisted of individualised social activities for 1 hour a day, 5 days a week. Nursing assistants in the research project performed social activities. They received 40 hours of training to be able to plan and guide activities for residents. Intervention 3: exercise plus social activity Control: usual care Interventions duration 7 weeks. |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Sealed envelopes with participants' group assignments prepared by a research team member otherwise not involved with the study to enact randomisation. Inside the envelope was the participant's group assignment determined using a random number generator with random block sizes to balance the assignments across the 4 groups. |
| Allocation concealment | Low risk | Sealed envelopes with participants' group assignments were prepared by a research team member otherwise not involved with the study to enact randomisation. The project director opened the envelopes after baseline data collection. |
| Blinding of participants and personnel All outcomes | Unclear risk | Unknown. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unknown. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unknown. |
| Blinding of outcome assessors Objective outcome measures | Low risk | Because of the nature of the intervention and control conditions, only the sleep technicians and registered polysomnography technologist were blinded to group assignment. Participants, investigators, project staff, and residential staff were not blinded. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | Low risk | Clearly number of dropouts related to interventions (9 each, while only 1 in CG), but possibly adequate imputation used. Quote: "Using the intention‐to‐treat approach, regression imputation was performed for the missing postintervention variables using a Stata regression algorithm." |
| Selective outcome reporting | Low risk | In line with study registration: NCT00888706. |
| Other sources of bias | Low risk | None. |
Schnelle 1999.
| Study characteristics | ||
| Methods |
Study design: RCT Follow‐up: not reported |
|
| Participants |
Country: USA Setting: 8 nursing homes Inclusion criteria:
Exclusion criteria:
Baseline characteristics:
Number of participants completing the study: 184 (IG 90, CG 94) Group differences: no significant differences |
|
| Interventions | Interventional/ control phase duration not reported. Intervention: in‐service education for 30 minutes and brief sessions before each shift (5–10 minutes), verbal/visual feedback during each night shift, noise reduction (e.g. closing bedroom door), less disruptive nursing practices in the night‐time Control: usual care |
|
| Outcomes |
|
|
| Funding |
Sponsorship source:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Study mentioned 'randomization' but no details about the method reported. |
| Allocation concealment | Unclear risk | No information reported. |
| Blinding of participants and personnel All outcomes | Unclear risk | Obviously not blinded, but unclear relevance. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | Unclear relevance. |
| Blinding of participants and personnel Objective sleep measures | Unclear risk | Unclear relevance. |
| Blinding of outcome assessors Objective outcome measures | Low risk | Assessors not blinded, but objective measurements via actigraphy. When the participant was in bed, a bedside monitor and a wrist activity monitor were activated. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | Unclear relevance. |
| Incomplete outcome data All outcomes | Unclear risk | Of initially 267 participants who consented to take part, only 184 (IG 90, CG 94) were analysed. It is unclear, how many participants were excluded from the 2 groups and also for what reason. |
| Selective outcome reporting | Unclear risk | No protocol identified. |
| Other sources of bias | Low risk | None. |
Sloane 2015.
| Study characteristics | ||
| Methods |
Study design: RCT (cross‐over) Follow‐up: 16 weeks (phase 1: 6 weeks, washout period: 4 weeks, phase 2: 6 weeks) |
|
| Participants |
Country: USA Setting: private home/apartment Inclusion criteria:
Exclusion criteria:
Number of participants completing the study: 14, number per group not reported Baseline characteristics
Group differences: no differences reported |
|
| Interventions | Intervention duration 6 weeks, followed by a washout period of 4 weeks Intervention: 13,000 K (blue–white) compact fluorescent light bulbs and an LED light box at the area where the individual ate breakfast and lunch Control: 2700 K (yellow–white) compact fluorescent light bulbs and a red LED light box at the area where the individual ate breakfast and lunch |
|
| Outcomes |
|
|
| Funding |
Neither Philips Lighting nor the study sponsors had input into the experimental design, data analysis, or manuscript preparation. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Quote: "A stratified permuted block randomization scheme was used that employed a block size of four participants early in the study and two later in the study, with stratification by participant gender." |
| Allocation concealment | Unclear risk | No information. |
| Blinding of participants and personnel All outcomes | Unclear risk | No information. |
| Blinding of participants and personnel Subjective sleep quality (carer ratings) | Unclear risk | No information. |
| Blinding of participants and personnel Objective sleep measures | Low risk | Actigraphy. |
| Blinding of outcome assessors Objective outcome measures | Low risk | Actigraphy. |
| Blinding of outcome assessors Subjective sleep quality (carer ratings) | Unclear risk | No information available. |
| Incomplete outcome data All outcomes | Low risk | 15/18 dyads completed both phase, 1 dropped out completely, 1 completed only the intervention phase, but not the control phase, 1 completed a part of the intervention phase, but not the control phase. |
| Selective outcome reporting | Unclear risk | No protocol identified. |
| Other sources of bias | Low risk | None. |
C‐PSQI: Chinese Pittsburgh Sleep Quality Index; CG: control group; CIRS‐G: Cumulative Illness Rating Scale – Geriatric; DDS: dawn–dusk simulation; EFAS: Essener Fragebogen Alter und Schläfrigkeit (Essen questionnaire on age and sleepiness); ESS: Epworth Sleepiness Scale; IG: intervention group; ITT: intention to treat; LED: light‐emitting diode; MMSE: Mini‐Mental State Examination; MOS: Medical Outcomes Study; NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke‐Alzheimer's Disease and Related Disorders Association; PSQI: Pittsburgh Sleep Quality Index; RCT: randomised controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12615000212550 | Sleep problem not obligatory |
| ACTRN12617000056392 | Study not conducted |
| ACTRN12618001402235 | Study not conducted |
| Allen 2003 | Sleep not a primary outcome |
| Asiret 2018 | < 80% of participants with dementia |
| Bademli 2019 | < 80% of participants with dementia |
| Blytt 2018 | Pharmacological intervention |
| Bromundt 2016 | Sleep problem not obligatory |
| Burns 2009 | Sleep not a primary outcome |
| Chan 2011 | < 80% of participants with dementia |
| Chen 2021 | Sleep problem not obligatory |
| Cibeira 2021 | Sleep problem not obligatory |
| Cimenser 2021 | Sleep problem not obligatory |
| Colenda 1997 | Not an RCT design |
| Connell 2007 | Not an RCT design |
| Cremascoli 2022 | Sleep problem not obligatory |
| Dowling 2008 | Wrong study design (historical control group) |
| Falck 2020 | < 80% of participants with dementia |
| Friedman 2012 | < 80% of participants with dementia |
| Hanson 2013 | Sleep problem not obligatory |
| Hjetland 2020 | Sleep problem not obligatory |
| ISRCTN30488204 | Study not conducted |
| Judge 2011 | Not an RCT design |
| Jøranson 2021 | Sleep problem not obligatory |
| Kobayashi 2011 | < 80% of participants with dementia |
| Koyama 1999 | Not an RCT design |
| Kuck 2014 | < 80% of participants with dementia |
| Lee 2008b | Not an RCT design |
| Lee 2018a | Not an RCT design |
| Li 2013 | Not an RCT design |
| Li 2017 | Sleep problem not obligatory |
| Liu 2022 | Not an RCT design |
| Livingston 2019 | Sleep not a primary outcome |
| Lyketsos 1999 | Sleep problem not obligatory |
| Mailloux 2019 | Not an RCT design |
| Mishima 1994 | Not an RCT design |
| Mishima 1998 | Exclusion due to data loss |
| Most 2010 | < 80% of participants with dementia |
| Moyle 2018 | Sleep problem not obligatory |
| Naismith 2019 | < 80% of participants with dementia |
| Nascimento 2014 | Sleep problem not obligatory |
| NCT01123993 | Sleep problem not obligatory |
| NCT01816152 | Not an RCT design |
| NCT01894620 | Sleep is no primary outcome |
| NCT03445299 | Not an RCT design |
| NCT04364191 | < 80% of participants with dementia |
| Nguyen 2012 | Sleep not a primary outcome |
| NL1422 | Sleep not a primary outcome |
| Ouslander 2006 | < 80% of participants with dementia |
| Pa 2014 | < 80% of participants with dementia |
| Page 2014 | Not an RCT design |
| Pu 2021 | Sleep problem not obligatory |
| Richards 2020 | Pharmacological intervention |
| Riemersma‐van der Lek 2008 | Sleep not a primary outcome |
| Rodríguez‐Mansilla 2013 | Sleep problem not obligatory |
| Sloane 2007 | Sleep problem not obligatory |
| Sun 2013 | < 80% of participants with dementia |
| Tanaka 2012 | < 80% of participants with dementia |
| van Os 2012 | Study not conducted |
| Warburton 2013 | Not an RCT design |
| Wilhelmsen‐Langeland 2013 | < 80% of participants with dementia |
| Wolfe 1996 | Not an RCT design |
| Zeng 2016 | < 80% of participants with dementia |
RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Katagi 2018.
| Methods | RCT |
| Participants | 23 female residents living in 2 group homes for elderly people with dementia |
| Interventions | Combined regimen of a short‐term nap and light physical exercise |
| Outcomes | Sleep efficacy, total nocturnal wake time, frequencies of nocturnal episodes of urination, frequencies of nocturnal behavioural or psychological symptoms associated with dementia |
| Notes | Unclear if participants had sleep problems at baseline. No response after several attempts to contact authors. |
NCT02502045.
| Methods | RCT |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Change in sleep characteristics and rest–activity rhythm |
| Notes | Awaiting response from author. |
Petrovsky 2020.
| Methods | RCT |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | Listening to tailored calming music at bedtime for 30 minutes every night for 4 weeks (28 sessions total), provided by a carer |
| Outcomes | Feasibility and acceptability study, sleep latency, wake after sleep onset, total sleep duration, sleep diary, Neuropsychiatric Inventory sleep item, PROMIS sleep‐related impairment version SF 8a, sleep disorder inventory |
| Notes | Results will not be published until second half of 2022. |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR2000039991.
| Study name | The effectiveness of light therapy on sleep quality, cognitive function, BPSD, and depression in patients with mild cognitive impairment and dementia: a randomized controlled trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
Aged: 65–100 years Gender: both |
| Interventions |
Intervention: light therapy Control: standard light group |
| Outcomes | Sleep quality; cognitive function; behavioural and psychological symptom associated with dementia, BPSD; depression |
| Starting date | 8 February 2020 |
| Contact information | kueiru@tmu.edu.tw |
| Notes | Authors did not respond after several contact attempts. |
Dichter 2021.
| Study name | Evaluation of a multi‐component, non‐pharmacological intervention to prevent and reduce sleep disturbances in people with dementia living in nursing homes (MoNoPol‐sleep): study protocol for a cluster‐randomized exploratory trial |
| Methods | Cluster‐randomised exploratory trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: multicomponent intervention consists of 6 components: 1. assessment of established sleep‐promoting interventions and an appropriate environment in the participating nursing homes; 2. implementation of 2 'sleep nurses' as change agents per nursing home; 3. basic education course for nursing staff: "Sleep problems in dementia"; 4. an advanced education course for nursing staff: "Tailored problem‐solving" (2 workshops); 5. workshops: "Development of an institutional sleep‐promoting concept" (2 workshops with nursing management and sleep nurses); and 6. written information and education material (e.g. brochure and "One Minute Wonder" poster). Intervention will be performed over 16 weeks. Control: usual care |
| Outcomes | Primary outcome: prevalence of sleep problems Secondary outcomes: quality of life, quality of sleep, daytime sleepiness, agitated behaviour, psychotropic medication, falls, and physical restraints |
| Starting date | November 2020 |
| Contact information | martin.dichter@uk‐koeln.de |
| Notes |
Hodgson 2021.
| Study name | A timed activity protocol to address sleep‐wake disorders in home dwelling persons living with dementia: the healthy patterns clinical trial. |
| Methods | Randomised controlled trial 2‐group parallel design of 200 people living with dementia and their carers (dyads) |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: 1‐month home‐based activity intervention designed to improve sleep–wake disorders and quality of life. Involves 4 in‐home visits and 1. assessing individuals' functional status and interests; 2. educating carers on environmental cues to promote activity and sleep; and 3. training carers in using timed morning, afternoon, and evening activities based on circadian needs across the day. Control: attention control group |
| Outcomes | Quality of life; sleep assessed by objective and subjective indicators including actigraphy, subjective sleep quality; and presence of neuropsychiatric symptoms. |
| Starting date | September 2018 |
| Contact information | hodgsonn@nursing.upenn.edu |
| Notes |
ISRCTN13072268.
| Study name | A parallel multi‐centre randomised controlled trial to determine the clinical and cost‐effectiveness of DREAMS START (Dementia RElAted Manual for Sleep; STrAtegies for RelaTives) for people living with dementia and their carers |
| Methods | Randomised controlled trial |
| Participants |
Study population: 370 people living with dementia at home and experiencing sleep difficulties and their family carers Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: multimodal intervention. 6 sessions over approximately 3 months (with sessions offered flexibly weekly to fortnightly) for intervention group. Sessions will be delivered to family carers alone or where appropriate to the family carer and the person living with dementia together. Control: usual care |
| Outcomes | Resident sleep at 8 months measured using Sleep Disorders Inventory |
| Starting date | 1 February 2021 |
| Contact information | p.rapaport@ucl.ac.uk |
| Notes |
NCT03455569.
| Study name | A dyadic sleep intervention for Alzheimer's disease patients and their caregivers. |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions |
Intervention: behavioural sleep education: manual‐based sleep hygiene recommendations and a behavioural sleep intervention including sleep compression therapy Control: active control group |
| Outcomes | Sleep efficiency, total wake time, sleep quality |
| Starting date | February 2018 |
| Contact information | Yeonsu Song, University of California, Los Angeles, USA |
| Notes |
NCT03777722/NCT03933696.
| Study name | Light and the effect on metabolic syndrome and Alzheimer's disease |
| Methods | Crossover randomised controlled trial |
| Participants |
Inclusion criteria:
|
| Interventions |
Intervention: tailored lighting intervention will provide high circadian stimulation during the day produced by light sources that provide moderate light levels of spectra that are tuned to the sensitivity of the circadian system. The active lighting intervention will be in place for 8 weeks. Following an 8‐week washout period, the participants will receive the placebo control intervention for 8 weeks. Control: placebo lighting intervention designed to have no effect on circadian system. The control intervention will be in place for 8 weeks. Following an 8‐week washout period, the participants will receive the active tailored lighting intervention for 8 weeks. |
| Outcomes | Change in glucose tolerance, change in sleep disturbance, change in depression, sleep efficiency using actigraphy, light exposure using the Daysimeter |
| Starting date | November 2018 |
| Contact information | barbara.plitnick@mountsinai.org |
| Notes | Estimated study completion date: 31 August 2023. |
NCT04073628.
| Study name | The long‐term impact of a light intervention on sleep and cognition in mild cognitive impairment |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: active lighting intervention Control: standard light |
| Outcomes | Sleep quality in the participant with mild cognitive impairment (PSQI); sleep quality in the carer (PSQI) |
| Starting date | April 2020 |
| Contact information | Mariana Figueiro |
| Notes | Estimated date of study completion: April 2024 |
NCT04533815.
| Study name | Enhancing sleep quality for nursing home residents with dementia |
| Methods | Stepped‐wedge design |
| Participants | People with Alzheimer disease, dementia, sleep disorder, and sleep disturbance |
| Interventions |
Intervention: LOCK sleep intervention: nursing home staff are trained to use a collaborative problem‐solving approach to sleep quality improvement using front‐line huddling Control: usual care |
| Outcomes | Total sleep time (total minutes asleep each night‐time period from 10 p.m. to 6 a.m.) |
| Starting date | August 2020 |
| Contact information | A Lynn Snow |
| Notes | Estimated date of completion: 31 August 2022 |
UMIN000042051.
| Study name | The effectiveness of Tai Chi on the sleep, physical and mental health for the elderly with dementia: 3‐arm randomized controlled trial |
| Methods | Cluster‐randomised controlled trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Tai Chi group: 1 hour after breakfast, a professional instructor with > 5 years of teaching experience or a qualification will teach the Type 8 Simple Tai Chi Movement in a group. Researchers and research assistants watch over the movement to ensure the safety of elderly people with dementia. Tai Chi intervention performed 3 times a week for 50 minutes. Warm‐up exercise for 5 minutes (turn the ankle and wrist, turn the waist, lightly loosen, such as flexion of the knee). It is to expand the range of motion further while moving. From dynamic stretching to close to the movement of the main movement, Tai Chi is performed for 40 minutes. Adjust the range and strength you can to match the level of individual physical function and activity ability and cool down exercise for 5 minutes (stretch slowly to loosen muscles). Stretch with the strength to be comfortable slowly without overdoing it. Stretch not only the lower body centred on the foot, but also the upper body Conventional exercise group: 1 hour after breakfast, instructors with training guidance history of ≥ 5 years to teach conventional exercise, such as aerobic exercise in a group. Researchers and research assistants watch over the movement to ensure the safety of elderly people with dementia. Intervention by conventional motion is carried out 3 times a week for 50 minutes. Warm‐up exercise for 5 minutes (same as Tai Chi group), conventional exercise such as aerobic exercise for 40 minutes, for elderly people, less burden, it is said that the effect of loosening the muscle and improvement of blood flow in a simple exercise can be expected. Strength training performed sitting on a chair is performed for 10 minutes, the strength training performed standing for 10 minutes, the radio gymnastics first and second for 10 minutes, and the walk according to the music for 10 minutes. Adjust the range and intensity to match the level of individual physical function and activity ability, and perform a cool down exercise for 5 minutes (same as Tai Chi group) Control: to continue the same life as usual without intervention. After the research is complete, the elderly people are free to choose Tai Chi or conventional exercise and conduct it for 12 weeks |
| Outcomes | Primary outcome: objective sleep status measured by activity meter (ActiGraphGT9X+) or sleep scan to measure the sleep efficiency and activity, Short Physical Performance Battery Secondary outcome: Quality of Life in Alzheimer's Disease |
| Starting date | October 2020 |
| Contact information | 176k056k@stu.kobe‐u.ac.jp |
| Notes |
BPSD: psychological symptoms of dementia; ICD‐10: International Classification of Diseases 10th Edition; MMSE: Mini‐Mental State Examination; MoCA: Montreal Cognitive Assessment; PSQI: Pittsburgh Sleep Quality Index.
Differences between protocol and review
Three review authors involved in the protocol were not involved in conducting the review.
Two review authors joined the review team.
In addition to including studies with people with dementia, we also included studies with participants with an MMSE score lower than 24 as this was chosen as criterion for cognitive impairment in some studies.
Sensitivity analyses were planned to examine the effect of inclusion or exclusion of low‐quality studies as well as studies using or not using validated outcome instruments. Due to the lack of high‐quality studies, no sensitivity analyses were performed.
Due to heterogeneity of included studies we were unable to perform meta‐analyses and subgroup analyses for all but one intervention.
We had planned to analyse results at the level of individuals while accounting for cluster effects using either reported direct estimate of effect measure from cluster randomised controlled trials or use calculated or estimated intracluster correlation coefficient (ICC). Since no ICC was reported, we were unable to conduct such an analysis.
Contributions of authors
DW: study selection, data extraction, risk of bias assessment, reviewing relevant literature, and drafting the review.
SC: study selection, data extraction, risk of bias assessment, content review, drafting the review.
MD: study selection, drafting the review.
GM: content review and drafting the review.
RM: data extraction, content review and drafting the review.
SK: developing the main concept, study selection, data extraction, risk of bias assessment, content review and drafting the review.
Sources of support
Internal sources
-
University of Lübeck, Germany
Institute for Social Medicine and Epidemiology
External sources
-
NIHR, UK
This review was supported by the National Institute for Health and Care Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health
Declarations of interest
DW: none.
SC: none.
MD: none.
GM: none.
RM: none.
SK: none.
New
References
References to studies included in this review
Alessi 1999 {published data only}
- Alessi CA, Yoon EJ, Schnelle JF, Al-Samarrai NR, Cruise PA. A randomized trial of a combined physical activity and environmental intervention in nursing home residents: do sleep and agitation improve? Journal of the American Geriatrics Society 1999;47(7):784-91. [DOI] [PubMed] [Google Scholar]
Alessi 2005 {published data only}
- Alessi CA, Martin J, Marler M, Harker J, Jesphson K. A multicomponent nonpharmacological intervention improves activity rhythms among nursing home residents with disrupted sleep/wake patterns. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2007;62A(1):67-72. [DOI] [PubMed] [Google Scholar]
- Alessi CA, Martin JL, Webber AP, Kim EC, Harker JO, Josephson KR. Randomized, controlled trial of a nonpharmacological intervention to improve abnormal sleep/wake patterns in nursing home residents. Journal of the American Geriatrics Society 2005;53(5):803-10. [DOI] [PubMed] [Google Scholar]
Ancoli‐Israel 2003 {published data only}
- Ancoli-Israel S, Gehrman P, Martin JL, Shochat T, Marler M, Corey-Bloom J, et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer's disease patients. Behavioral Sleep Medicine 2003;1(1):22-36. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. Journal of the American Geriatrics Society 2002;50(2):282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2016 {published and unpublished data}
- Chan AW, Yu DS, Choi KC, Lee DT, Sit JW, Chan HY. Tai Chi Qigong as a means to improve night-time sleep quality among older adults with cognitive impairment: a pilot randomized controlled trial. Clinical Interventions in Aging 2016;11:1277-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowling 2005 {published data only}
- Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Someren EJ. Effect of morning bright light treatment for rest–activity disruption in institutionalized patients with severe Alzheimer's disease. International Psychogeriatrics 2005;17(2):221-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling GA, Luxenberg JS, Davids HR, Davidson A. Light therapy for sleep-activity disruption in Alzheimer's disease. World Alzheimer Congress; 2000 Jul 9-18; Washington (DC).
- Dowling GA, Luxenberg JS, Davids HR. Light therapy in Alzheimer's disease. Gerontologist 1999;39(Suppl 1):330-1. [Google Scholar]
- Dowling GA, Mastick J, Hubbard EM, Luxenberg JS, Burr RL. Effect of timed bright light treatment for rest–activity disruption in institutionalized patients with Alzheimer's disease. International Journal of Geriatric Psychiatry 2005;20(8):738-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Figueiro 2019 {published data only}
- Figueiro MG, Plitnick B, Roohan C, Sahin L, Kalsher M, Rea MS. Effects of a tailored lighting intervention on sleep quality, rest–activity, mood, and behavior in older adults with Alzheimer disease and related dementias: a randomized clinical trial. Journal of Clinical Sleep Medicine 2019;15(12):1757-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fontana Gasio 2003 {published data only}
- Fontana Gasio P, Kräuchi K, Cajochen C, Someren EJ, Amrhein I, Pache M, et al. Dawn–dusk simulation light therapy of disturbed circadian rest–activity cycles in demented elderly. Experimental Gerontology 2003;38(1-2):207-16. [DOI] [PubMed] [Google Scholar]
Gattinger 2017 {published data only}
- DRKS00006829. E-health information system for an advanced nursing care process [Einsatz eines E-Health Informationssystems für einen fortgeschrittenen Pflegeprozess]. drks.de/search/de/trial/DRKS00006829 (first received 2 October 2014).
- Gattinger H, Hantikainen V, Ott S, Stark M. Effectiveness of a mobility monitoring system included in the nursing care process in order to enhance the sleep quality of nursing home residents with cognitive impairment. Health Technology 2017;7:161-71. [Google Scholar]
Harris 2012 {published data only}
- Harris M, Richards KC, Grando VT. The effects of slow-stroke back massage on minutes of nighttime sleep in persons with dementia and sleep disturbances in the nursing home: a pilot study. Journal of Holistic Nursing 2012;30(4):255-63. [DOI] [PubMed] [Google Scholar]
Hozumi 1996 {published data only}
- Hozumi S, Hori H, Okawa M, Hishikawa Y, Sato K. Favorable effect of transcranial electrostimulation on behavior disorders in elderly patients with dementia: a double-blind study. International Journal of Neuroscience 1996;88(1-2):1-10. [DOI] [PubMed] [Google Scholar]
Li 2009 {published data only}
- Li X, Sun Y, Yan Y. Observation on effect of application of sleep restriction therapy for Alzheimer's disease patients with sleep disorders. Chinese Nursing Research 2009;23(34):6f. [Google Scholar]
McCurry 2005 {published data only}
- McCurry SM, Gibbons LE, Logsdon RG, Vitiello MV, Teri L. Nighttime insomnia treatment and education for Alzheimer's disease: a randomized, controlled trial. Journal of the American Geriatrics Society 2005;53(5):793-802. [DOI] [PubMed] [Google Scholar]
McCurry 2011 {published data only}
- McCurry SM, Logsdon RG, Gibbons LE, Vitello MV, Teri L. Behavioral treatment for sleep disturbances in Alzheimer's disease: the NITE-AD study. Research and Practice in Alzheimer's disease 2006;11:341-6. [Google Scholar]
- McCurry SM, Pike KC, Vitiello MV, Logsdon RG, Larson EB, Teri L. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer's disease: results of a randomized, controlled trial. Journal of the American Geriatrics Society 2011;59(8):1393-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry SM. Using behavioral programs to treat sleep problems in individuals with Alzheimer's disease. clinicaltrials.gov/show/NCT00183378 (first received 16 September 2005).
McCurry 2012 {published data only}
- McCurry S, Pike KC, LaFazia DM, Logsdon RG, Teri L. Feasibility and efficacy of a sleep education program targeting persons with dementia living in adult family homes. 23rd Annual Meeting of the Associated Professionals Sleep Societies Seattle; 2009 Jun 6-11; Seattle (WA).
- McCurry SM, LaFazia DM, Pike KC, Logsdon RG, Teri L. Development and evaluation of a sleep education program for older adults with dementia living in adult family homes. American Journal of Geriatric Psychiatry 2012;20(6):494-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry SM. Behavioral sleep intervention in adult family homes. clinicaltrials.gov/ct2/show/NCT00393627 (first received 29 October 2006).
Nowak 2008 {published data only}
- Nowak L, Davis J. Qualitative analysis of therapeutic light effects on global function in Alzheimer's disease. Western Journal of Nursing Research 2011;33(7):933-52. [DOI] [PubMed] [Google Scholar]
- Nowak L. The Effect of Timed Blue-Green Light on Sleep-Wake Patterns in Women with Alzheimer's Disease. Detroit (MI): Wayne State University, 2008. [Google Scholar]
Richards 2005 {published data only}
- Richards KC, Beck C, O'Sullivan PS, Shue VM. Effect of individualized social activity on sleep in nursing home residents with dementia. Journal of the American Geriatrics Society 2005;53(9):1510-7. [DOI] [PubMed] [Google Scholar]
- Richards KC. Effect of activity on sleep of cognitively-impaired veterans. clinicaltrials.gov/show/NCT00013182 (first received 16 March 2001).
Richards 2011 {published data only}
- Richards KC, Lambert C, Beck CK, Bliwise DL, Evans WJ, Kalra GK, et al. Strength training, walking, and social activity improve sleep in nursing home and assisted living residents: randomized controlled trial. Journal of the American Geriatrics Society 2011;59(2):214-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards KC, Lorenz RA, Gooneratne N, Cole CS, Kleban MH, Kalra GK. Exercise and social activity improve everyday function in long-term care residents. American Journal of Geriatric Psychiatry 2012;20(6):468-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards KC. Effect of activities and exercise on sleep in dementia. clinicaltrials.gov/show/NCT00888706 (first received 28 April 2009).
Schnelle 1999 {published data only}
- Schnelle JF, Alessi CA, Al-Samarrai NR, Fricker RD Jr, Ouslander JG. The nursing home at night: effects of an intervention on noise, light, and sleep. Journal of American Geriatrics Society 1999;47:430-8. [DOI] [PubMed] [Google Scholar]
Sloane 2015 {published data only}
- Sloane PD, Figueiro M, Garg S, Cohen LW, Reed D, Williams CS, et al. Effect of home-based light treatment on persons with dementia and their caregivers. Lighting Research & Technology 2015;2:161-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12615000212550 {published data only}
- ACTRN12615000212550. The exterior interior can pseudo-natural environments, produced through video projections, improve dementia related symptoms and behaviours. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12615000212550 (first received 20 February 2015).
ACTRN12617000056392 {published data only}
- ACTRN12617000056392. A feasibility trial comparing a behavioural intervention, melatonin and usual care for people with dementia and sleep disturbance. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617000056392 (first received 15 December 2016).
ACTRN12618001402235 {published data only}
- ACTRN12618001402235. Achieving a good night's sleep using a non-pharmacological approach: a pilot trial in people with dementia and their families. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12618001402235 (first received 17 October 2018).
Allen 2003 {published data only}
- Allen H, Byrne EJ, Sutherland D, Tomenson B, Butler S, Burns A. The effect of bright light therapy on sleep in dementia. International Psychogeriatrics 2003;15(Suppl 2):S092. [Google Scholar]
Asiret 2018 {published data only}
- Asiret GD. Effect of reminiscence therapy on the sleep quality of the elderly living in nursing homes: a randomized clinical trial. European Journal of Integrative Medicine 2018;20:1-5. [Google Scholar]
Bademli 2019 {published data only}
- Bademli K, Lok N, Canbaz M, Lok S. Effects of physical activity program on cognitive function and sleep quality in elderly with mild cognitive impairment: a randomized controlled trial. Perspectives in Psychiatric Care 2019;55(3):401-8. [DOI] [PubMed] [Google Scholar]
Blytt 2018 {published data only}
- Blytt KM, Bjorvatn B, Husebo B, Flo E. Effects of pain treatment on sleep in nursing home patients with dementia and depression – a multicentre placebo-controlled randomised clinical trial. International Psychogeriatrics 2018;33(4):663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bromundt 2016 {published data only}
- Bromundt V, Wirz-Justice A, Boutellier M, Winter S, Terman M, Haberstroh M, et al. The effects of a new dawn–dusk simulator on circadian rest activity cycles sleep, mood and well-being in dementia patients – a pilot study. Journal of Sleep Research 2016;25:294. [Google Scholar]
Burns 2009 {published data only}
- Burns A, Allen H, Tomenson B, Duignan D, Byrne J. Bright light therapy for agitation in dementia: a randomized controlled trial. International Psychogeriatrics 2009;21(4):711-21. [DOI] [PubMed] [Google Scholar]
Chan 2011 {published data only}
- Chan MF. A randomised controlled study of the effects of music on sleep quality in older people. Journal of Clinical Nursing 2011;20(7-8):979-87. [DOI] [PubMed] [Google Scholar]
Chen 2021 {published data only}
- Chen Z, Wang L, Chen K, Mi Q, Zu X, Xu Q, et al. The effects of a multi-disciplinary team on sleep quality assessment in mild-to-moderate Alzheimer's disease patients with sleep disorders. Scottish Medical Journal 2021;66(3):134-41. [DOI] [PubMed] [Google Scholar]
Cibeira 2021 {published data only}
- Cibeira N, Maseda A, Lorenzo-López L, González-Abraldes I, López-López R, Rodríguez-Villamil JL, et al. Bright light therapy in older adults with moderate to very severe dementia: immediate effects on behavior, mood, and physiological parameters. Healthcare (Basel) 2021;9(8):1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04949984. Bright light therapy in older adults with moderate to very severe dementia. clinicaltrials.gov/show/NCT04949984 (first received 2 July 2021).
Cimenser 2021 {published data only}
- Cimenser A, Hempel E, Travers T, Strozewski N, Martin K, Malchano Z, et al. Sensory-evoked 40-Hz gamma oscillation improves sleep and daily living activities in Alzheimer's disease patients. Frontiers in Systems Neuroscience 2021;15:746859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colenda 1997 {published data only}
- Colenda CC, Cohen W, McCall WV, Rosenquist PB. Phototherapy for patients with Alzheimer disease with disturbed sleep patterns: results of a community-based pilot study. Alzheimer Disease and associated Disorders 1997;11:175-8. [DOI] [PubMed] [Google Scholar]
Connell 2007 {published data only}
- Connell BR, Sanford JA, Lewis D. Therapeutic effects of an outdoor activity program on nursing home residents with dementia. Journal of Housing for the Elderly 2007;21(3-4):195-209. [Google Scholar]
Cremascoli 2022 {published data only}
- Cremascoli R, Sparasci D, Giusti G, Cattaldo S, Prina E, Roveta F, et al. Circadian phase tailored light therapy in Alzheimer's disease: preliminary findings on sleep and cognition. European Journal of Neurology 2020;27(Suppl 1):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremascoli R, Sparasci D, Giusti G, Cattaldo S, Prina E, Roveta F, et al. Effects of circadian phase tailored light therapy on sleep, mood, and cognition in Alzheimer's disease: preliminary findings in a pivotal study. Frontiers in Physiology 2022;12:755322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowling 2008 {published data only}
- Dowling GA, Burr RL, Someren EJ, Hubbard EM, Luxenberg JS, Mastick J, et al. Melatonin and bright-light treatment for rest–activity disruption in institutionalized patients with Alzheimer's disease. Journal of the American Geriatrics Society 2008;56(2):239-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Falck 2020 {published data only}
- Falck RS, Davis JC, Best JR, Chan PC, Li LC, Wyrough AB, et al. Effect of a multimodal lifestyle intervention on sleep and cognitive function in older adults with probable mild cognitive impairment and poor sleep: a randomized clinical trial. Journal of Alzheimers Disease 2020;76(1):179-93. [DOI] [PubMed] [Google Scholar]
Friedman 2012 {published data only}
- Friedman LF, Spira AP, Hernandez B, Mather C, Sheikh J, Yesavage JA, et al. Brief morning light treatment for sleep/wake disturbances in older memory-impaired individuals and their caregivers. Sleep Medicine 2012;13(5):546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LF, Spira AP, Hernandez B, Sheikh J, Yesavage JA, Zeitzer J. Differential effects of morning light treatment combined with sleep hygiene therapy on memory-impaired individuals and their caregivers. Sleep 2011;34:A217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanson 2013 {published data only}
- Hanson L, Cagan A, Rinehimer K, Flottemesch T, Clairmont J, Sackett-Lundeen L, et al. Lavender essential oil improves sleep in residents of a memory care assisted living facility. Alzheimer's & Dementia 2013;9:P655-6. [Google Scholar]
Hjetland 2020 {published data only}
- Hjetland GJ, Kolberg E, Pallesen S, Thun E, Nordhus IH, Bjorvatn B, et al. Ambient bright light treatment improved proxy-rated sleep but not sleep measured by actigraphy in nursing home patients with dementia: a placebo-controlled randomised trial. BMC Geriatrics 2021;21(1):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjetland GJ, Kolberg E, Pallesen S, Thun E, Nordhus IH, Flo-Groeneboom E. Ambient bright light treatment improved subjectively measured sleep but not sleep measured by actigraphy in nursing home patients with dementia: a placebo-controlled randomized trial. Journal of Sleep Research 2020;29:230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolberg E, Hjetland GJ, Thun E, Pallesen S, Nordhus IH, Husebo BS, et al. The effects of bright light treatment on affective symptoms in people with dementia: a 24-week cluster randomized controlled trial. BMC Psychiatry 2021;21(1):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03357328. Therapy light rooms for nursing home patients with dementia. clinicaltrials.gov/ct2/show/NCT03357328 (first received 29 November 2017).
ISRCTN30488204 {published data only}
- ISRCTN30488204. A study to evaluate whether light therapy can help people with dementia to sleep better. www.isrctn.com/ISRCTN30488204 (first received 16 December 2015).
Judge 2011 {published data only}
- Judge KS, Bass DM, Snow AL, Wilson NL, Morgan R, Looman WJ, et al. Partners in dementia care: a care coordination intervention for individuals with dementia and their family caregivers. Gerontologist 2011;51(2):261-72. [DOI] [PubMed] [Google Scholar]
Jøranson 2021 {published data only}
- Jøranson N, Olsen C, Calogiuri G, Ihlebæk C, Pedersen I. Effects on sleep from group activity with a robotic seal for nursing home residents with dementia: a cluster randomized controlled trial. International Psychogeriatrics 2021;33(10):1045-56. [DOI] [PubMed] [Google Scholar]
Kobayashi 2011 {published data only}
- Kobayashi T, Yamagata Z, Yoshiyama M, Araki I, Takeda M. The effect of warm footbath before sleep on the improvement of both nocturia and nocturnal polyuria in geriatric Japanese female with bothersome nocturia. A randomized crossover study. International Urogynecology Journal 2011;22(Suppl 2):518-9. [Google Scholar]
Koyama 1999 {published data only}
- Koyama E, Matsubara H, Nakano T. Bright light treatment for sleep-wake disturbances in aged individuals with dementia. Psychiatry and Clinical Neurosciences 1999;53(2):227-9. [DOI] [PubMed] [Google Scholar]
Kuck 2014 {published data only}
- Kuck J, Pantke M, Flick U. Effects of social activation and physical mobilization on sleep in nursing home residents. Geriatric Nursing 2014;6:455-61. [DOI] [PubMed] [Google Scholar]
Lee 2008b {published data only}
- Lee Y, Kim S. Effects of indoor gardening on sleep, agitation, and cognition in dementia patients – a pilot study. International Journal of Geriatric Psychiatry 2008;23(5):485-9. [DOI] [PubMed] [Google Scholar]
Lee 2018a {published data only}
- Lee JH, Kim SJ, Lee SH, Suh IB, Jang J, Jhoo JH. Changes in the sleep quality and caregiver burden by timed blue light in Alzheimer's disease. Sleep 2018;41(Suppl):A379-80. [Google Scholar]
Li 2013 {published data only}
- Li CH, Lai CL, Chen CH. Preliminary report of the sleep effect of music therapy in Alzheimer's disease. Alzheimer's & Dementia 2013;9(4S):P297-8. [Google Scholar]
Li 2017 {published data only}
- Li J, Grandner MA, Chang YP, Jungquist C, Porock D. Person-centered dementia care and sleep in assisted living residents with dementia: a pilot study. Behavioral Sleep Medicine 2017;15(2):97-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2022 {published data only}
- Liu CR, Liou YM, Jou JH. Ambient bright lighting in the morning improves sleep disturbances of older adults with dementia. Sleep Medicine 2022;89:1-9. [DOI] [PubMed] [Google Scholar]
Livingston 2019 {published data only}
- ISRCTN36983298. DREAMS START (Dementia related manual for sleep; strategies for relatives). www.isrctn.com/ISRCTN36983298 (first received 2 November 2016).
- Kinnunen KM, Rapaport P, Webster L, Barber J, Kyle SD, Hallam B, et al. A manual-based intervention for carers of people with dementia and sleep disturbances: an acceptability and feasibility RCT. Health Technology Assessment 2018;22(71):I-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Barber J, Kinnunen KM, Webster LA. A feasibility and acceptability randomised controlled trial of a manual based intervention for sleep difficulties in people living with dementia (DREAMS-START). Alzheimer's and Dementia 2018;14(7):P1390-1. [Google Scholar]
- Livingston G, Barber JA, Kinnunen KM, Webster L, Kyle SD, Cooper C, et al. DREAMS-START (Dementia RElAted Manual for Sleep; STrAtegies for RelaTives) for people with dementia and sleep disturbances: a single-blind feasibility and acceptability randomized controlled trial. International Psychogeriatrics 2019;31(2):251-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lyketsos 1999 {published data only}
- Lyketsos CG, Veiel LL, Baker A, Steele C. A randomized controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. International Journal of Geriatric Psychiatry 1999;14(7):520-5. [PubMed] [Google Scholar]
Mailloux 2019 {published data only}
- Mailloux M, Belzile F, Sasseville B, Bastien C, Hudon C, Vallieres A. Effects of two physical activity programs on sleep quality in MCI individuals: a pilot study. Sleep Medicine 2019;64:S30. [Google Scholar]
Mishima 1994 {published data only}
- Mishima K, Okawa M, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Morning bright light therapy for sleep and behavior disorders in elderly patients with dementia. Acta Psychiatrica Scandinavica 1994;89(1):1-7. [DOI] [PubMed] [Google Scholar]
Mishima 1998 {published data only}
- Mishima K, Hishikawa Y, Okawa M. Randomized, dim light controlled, crossover test of morning bright light therapy for rest–activity rhythm disorders in patients with vascular dementia and dementia of Alzheimer's type. Chronobiology International 1998;15(6):647-54. [DOI] [PubMed] [Google Scholar]
- Okawa M, Mishima K, Hozumi S. Effects of environmental stimulation with bright light a transcranial electrostimulation on sleep and behavior disorders in elderly patients with dementia. Alzheimer's Disease and Related Disorders 1999;87:757-62. [Google Scholar]
Most 2010 {published data only}
- Most EI, Scheltens P, Someren EJ. Prevention of depression and sleep disturbances in elderly with memory-problems by activation of the biological clock with light – a randomized clinical trial. Trials 2010;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moyle 2018 {published data only}
- Moyle W, Beattie E, Draper B, Shum D, Thalib L, Jones C, et al. Robotic seals – what do we know about their impact on people with dementia? International Psychogeriatrics 2015;27:175-6. [Google Scholar]
- Moyle W, Jones C, Murfield J, Thalib L, Beattie E, Shum D, et al. Effect of a robotic seal on the motor activity and sleep patterns of older people with dementia, as measured by wearable technology: a cluster-randomised controlled trial. Maturitas 2018;110:10-7. [DOI] [PubMed] [Google Scholar]
Naismith 2019 {published data only}
- Naismith SL, Pye J, Palmer J, Hoyos C, Duffy SL. How does sleep–wake disturbance relate to neuropsychological and neuroimaging markers in older people at risk for dementia? Alzheimer's and Dementia 2019;15(7):523. [Google Scholar]
Nascimento 2014 {published data only}
- Nascimento CM, Ayan C, Cancela JM, Gobbi LT, Gobbi S, Stella F. Effect of a multimodal exercise program on sleep disturbances and instrumental activities of daily living performance on Parkinson's and Alzheimer's disease patients. Geriatrics & Gerontology International 2014;14(2):259-66. [DOI] [PubMed] [Google Scholar]
NCT01123993 {published data only}
- NCT01123993. The effect of Thai traditional music on cognitive function, psychological health and quality of sleep among Thai older individuals with dementia. clinicaltrials.gov/ct2/show/NCT01123993 (first received 14 May 2010).
NCT01816152 {published data only}
- NCT01816152. Methodology issues in a tailored light treatment for persons with dementia. clinicaltrials.gov/ct2/show/NCT01816152 (first received 22 March 2013).
NCT01894620 {published data only}
- NCT01894620. Investigating the effect of repetitive transcranial magnetic stimulation as a treatment for Alzheimer's disease and on sleep quality. clinicaltrials.gov/ct2/show/NCT01894620 (first received 10 July 2013).
NCT03445299 {published data only}
- NCT03445299. Music for dementia-related sleep problems. clinicaltrials.gov/ct2/show/NCT03445299 (first received 26 February 2018).
NCT04364191 {published data only}
- NCT04364191. Multicomponent behavioral sleep intervention for insomnia in older adults with mild cognitive impairment. clinicaltrials.gov/show/NCT04364191 (first received 28 April 2020).
Nguyen 2012 {published data only}
- Nguyen MH, Kruse A. A randomized controlled trial of Tai Chi for balance, sleep quality and cognitive performance in elderly Vietnamese. Clinical Interventions in Aging 2012;7:185-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
NL1422 {published data only}
- NL1422. Implementation of 30 minutes walking in daily care of older people with dementia. www.trialregister.nl/trial/1422 (first received 1 November 2008).
Ouslander 2006 {published data only}
- Ouslander JG, Connell BR, Bliwise DL, Endeshaw Y, Griffiths P, Schnelle JF. A nonpharmacological intervention to improve sleep in nursing home patients: results of a controlled clinical trial. Journal of the American Geriatrics Society 2006;54(1):38-47. [DOI] [PubMed] [Google Scholar]
Pa 2014 {published data only}
- Pa J, Goodson W, Bloch A, King AC, Yaffe K, Barnes DE. Effect of exercise and cognitive activity on self-reported sleep quality in community-dwelling older adults with cognitive complaints: a randomized controlled trial. Journal of the American Geriatrics Society 2014;62(12):2319-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2014 {published data only}
- Page A, Manchip S, Nahas H. Treatment for sleep disturbance in dementia. Midlife & Beyond 2014;44(11):33-5. [Google Scholar]
Pu 2021 {published data only}
- Pu L, Moyle W, Jones C, Todorovic M. The effect of a social robot intervention on sleep and motor activity of people living with dementia and chronic pain: a pilot randomized controlled trial. Maturitas 2021;144:16-22. [DOI] [PubMed] [Google Scholar]
Richards 2020 {published data only}
- Richards K, Morrison J, Wang YY, Rangel A, Loera A, Hanlon A, et al. Nighttime agitation and restless legs syndrome in persons with Alzheimer's disease: study protocol for a double-blind, placebo-controlled, randomized trial (NightRest). Research in Gerontological Nursing 2020;13:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riemersma‐van der Lek 2008 {published data only}
- Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA 2008;299(22):2642-55. [DOI] [PubMed] [Google Scholar]
Rodríguez‐Mansilla 2013 {published data only}
- Rodríguez-Mansilla J, González-López-Arza MV, Varela-Donoso E, Montanero-Fernández J, Jiménez-Palomares M, Garrido-Ardila EM. Ear therapy and massage therapy in the elderly with dementia: a pilot study. Journal of Traditional Chinese Medicine 2013;33(4):461-7. [DOI] [PubMed] [Google Scholar]
Sloane 2007 {published data only}
- Sloane PD, Williams CS, Mitchell CM, Preisser JS, Wood W, Barrick AL, et al. High-intensity environmental light in dementia: effect on sleep and activity. Journal of the American Geriatrics Society 2007;55(10):1524-33. [DOI] [PubMed] [Google Scholar]
Sun 2013 {published data only}
- Sun J, Kang J, Wang P, Zeng H. Self-relaxation training can improve sleep quality and cognitive functions in the older: a one-year randomised controlled trial. Journal of Clinical Nursing 2013;22(9-10):1270-80. [DOI] [PubMed] [Google Scholar]
Tanaka 2012 {published data only}
- Tanaka M, Ishii A, Yamano E, Ogikubo H, Okazaki M, Kamimura K, et al. Effect of a human-type communication robot on cognitive function in elderly women living alone. Medical Science Monitor 2012;18(9):CR550-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Os 2012 {published data only}
- Os AJ, Aziz L, Schalkwijk D, Schols JM, Bie RA. Effectiveness of physio acoustic sound (PAS) therapy in demented nursing home residents with nocturnal restlessness: study protocol for a randomized controlled trial. Trials 2012;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warburton 2013 {published data only}
- Warburton K, Simpson L. Non-pharmacological treatment of sleep disturbance in elderly people with dementia: a review of the literature. Age and Ageing 2013;42(Suppl 2):ii12. [Google Scholar]
Wilhelmsen‐Langeland 2013 {published data only}
- Wilhelmsen-Langeland A, Saxvig IW, Pallesen S, Nordhus IH, Vedaa Ø, Lundervold AJ, et al. A randomized controlled trial with bright light and melatonin for the treatment of delayed sleep phase disorder: effects on subjective and objective sleepiness and cognitive function. Journal of Biological Rhythms 2013;28(5):306-21. [DOI] [PubMed] [Google Scholar]
Wolfe 1996 {published data only}
- Wolfe N, Herzberg J. Can aromatherapy oils promote sleep in severely demented patients? International Journal of Geriatric Psychiatry 1996;11(10):926-7. [Google Scholar]
Zeng 2016 {published data only}
- Zeng H, Liu M, Wang P, Kang J, Lu F, Pan L. The effects of acupressure training on sleep quality and cognitive function of older adults: a 1-year randomized controlled trial. Research in Nursing & Health 2016;5:328-36. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Katagi 2018 {published data only}
- Katagi A, Miyai N. Effects of short-term nap and light exercise on sleep among elderly patients with mild-to-moderate dementia in communal living group homes. Nippon Eiseigaku Zasshi – Japanese Journal of Hygiene 2018;73(3):365-72. [DOI] [PubMed] [Google Scholar]
NCT02502045 {unpublished data only}
- NCT02502045. A study to examine the effect of therapeutic light on sleep, circadian rhythm, and global function in women with Alzheimer's disease. clinicaltrials.gov/ct2/show/NCT02502045 (first received 17 July 2015).
Petrovsky 2020 {published data only}
- Petrovsky DV, Gooneratne NS, Bradt J, Gitlin LN, Hodgson NA. Tailored music listening intervention to reduce sleep disturbances in older adults with dementia: research protocol. Research in Nursing & Health 2020;43(6):557-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR2000039991 {published data only}
- ChiCTR2000039991. The effectiveness of light therapy on sleep quality, cognitive function, BPSD, and depression in patients with mild cognitive impairment and dementia: a randomized controlled trial. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2000039991 (first received 16 November 2020).
Dichter 2021 {published data only}
- Dichter MN, Berg A, Hylla J, Eggers D, Wilfling D, Möhler R, et al. Evaluation of a multi-component, non-pharmacological intervention to prevent and reduce sleep disturbances in people with dementia living in nursing homes (MoNoPol-sleep): study protocol for a cluster-randomized exploratory trial. BMC Geriatrics 2021;21(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodgson 2021 {published data only}
- Hodgson NA, Gooneratne N, Perez A, Talwar S, Huang L. A timed activity protocol to address sleep–wake disorders in home dwelling persons living with dementia: the healthy patterns clinical trial. BMC Geriatrics 2021;21(1):451. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN13072268 {published data only}
- ISRCTN13072268. A parallel multi-centre randomised controlled trial to determine the clinical and cost-effectiveness of DREAMS START (Dementia RElAted Manual for Sleep; STrAtegies for RelaTives) for people living with dementia and their carers. www.isrctn.com/ISRCTN13072268 (first received 12 August 2020).
NCT03455569 {published data only}
- NCT03455569. A dyadic sleep intervention for Alzheimer's disease patients and their caregivers. clinicaltrials.gov/ct2/show/NCT03455569 (first received 6 March 2018).
NCT03777722/NCT03933696 {published data only}
- NCT03777722. Light and the effect on metabolic syndrome and Alzheimer's disease. clinicaltrials.gov/ct2/show/NCT03777722 (first received 17 December 2018).
- NCT03933696. Light, metabolic syndrome and Alzheimer's disease – AIM 2. clinicaltrials.gov/ct2/show/NCT03933696 (first received 1 May 2019).
NCT04073628 {published data only}
- NCT04073628. The long-term impact of a light intervention on sleep and cognition in mild cognitive impairment. clinicaltrials.gov/ct2/show/NCT04073628 (first received 29 August 2019).
NCT04533815 {published data only}
- NCT04533815. Enhancing sleep quality for nursing home residents with dementia (40Winks). clinicaltrials.gov/show/NCT04533815 (first received 1 September 2020).
UMIN000042051 {published data only}
- UMIN000042051. The effectiveness of Tai Chi on the sleep, physical and mental health for the elderly with dementia: 3-arm randomized controlled trial. rctportal.niph.go.jp/en/detail?trial_id=UMIN000042051 (first received 1 January 2021).
Additional references
Ancoli‐Israel 1997
- Ancoli-Israel S, Clopton P, Klauber MR, Fell R, Mason W. Use of wrist actrigraphy for monitoring sleep/wake in demented nursing home patients. Sleep 1997;20:24-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ancoli‐Israel 2006
- Ancoli-Israel S, Vitiello MV. Sleep in dementia. American Journal of Geriatric Psychiatry 2006;14:91-4. [DOI] [PubMed] [Google Scholar]
Anderson 2013
- Anderson L, Petticrew M, Chandler J, Grimshaw J, Tugwell P, O'Neill J, et al. Introducing a series of methodological articles on considering complexity in systematic reviews of interventions. Journal of Clinical Epidemiology 2013;66:1205-8. [DOI] [PubMed] [Google Scholar]
Brown 2013
- Brown C, Berry R, Tan M, Khoshia A, Turlapati L, Swedlove F. A critique of the evidence base for non-pharmacological sleep interventions for persons with dementia. Dementia (London, England) 2013;12:210-37. [DOI] [PubMed] [Google Scholar]
Burns 2009
- Burns A, Iliffe S. Dementia. BMJ 2009;338:b75. [DOI] [PubMed] [Google Scholar]
Buysse 1989
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatric Research 1989;28(2):193-213. [DOI] [PubMed] [Google Scholar]
Campbell 2020
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Capezuti 2018
- Capezuti E, Zadeh RS, Pain K, Basara A, Jiang NZ, Krieger AC. A systematic review of non-pharmacological interventions to improve nighttime sleep among residents of long-term care settings. BMC Geriatrics 2018;18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2015
- Cipriani G, Lucetti C, Danti S, Nuti A. Sleep disturbances and dementia. Psychogeriatrics 2015;15:65-74. [DOI] [PubMed] [Google Scholar]
Colton 2006
- Colton HR, Altevogt BM, editor(s). Sleep Disorders and Sleep Deprivation: an Unmet Public Health Problem. Washington (DC): The National Academies Press, 2006. [PubMed] [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. British Medical Journal 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Datta 2013
- Datta J, Petticrew M. Challenges to evaluating complex interventions: a content analysis of published papers. BMC Public Health 2013;13:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dauvilliers 2007
- Dauvilliers Y. Insomnia in patients with neurodegenerative conditions. Sleep Medicine 2007;4(Suppl):S27-34. [DOI] [PubMed] [Google Scholar]
David 2010
- David R, Zeitzer J, Friedman L, Noda A, O'Hara R, Robert P, et al. Non-pharmacologic management of sleep disturbance in Alzheimer's disease. Journal of Nutrition, Health & Aging 2010;14:203-6. [DOI] [PubMed] [Google Scholar]
Eldridge 2016
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al, PAFS Consensus Group. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355:i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferri 2005
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forbes 2014
- Forbes D, Blake CM, Thiessen E, Peacook S, Hawranik P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No: CD003946. [DOI: 10.1002/14651858.CD003946.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibson 2014
- Gibson R, Gander P, Jones L. Understanding the sleep problems of people with dementia and their family caregivers. Dementia (London, England) 2014;13:350-65. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 6 December 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available from gradepro.org.
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Higgins 2020
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Hintze 2005 [Computer program]
- Number Crunching Statistical Software. Hintze J, Version accessed prior to 20 December 2022. Kaysville (UT): Number Crunching Statistical Systems, 2005.
Hjetland 2020
- Hjetland GJ, Pallesen S, Thun E, Kolberg E, Nordhus IH, Flo E. Light interventions and sleep, circadian, behavioral, and psychological disturbances in dementia: a systematic review of methods and outcomes. Sleep Medicine Reviews 2020;52:101310. [DOI] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann T, Glasziou P, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Hurd 2013
- Hurd M, Martorell P, Delavande A, Mullen K, Langa K. Monetary costs of dementia in the United States. New England Journal of Medicine 2013;368:1326-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larson 2013
- Larson E, Yaffe K, Langa K. New insights into the dementia epidemic. New England Journal of Medicine 2013;369:2275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2011
- Lee D, Thomas A. Sleep in dementia and caregiving – assessment and treatment implications: a review. International Psychogeriatrics / IPA 2011;23:190-201. [DOI] [PubMed] [Google Scholar]
Leicht 2013
- Leicht H, König H, Stuhldreher N, Bachmann C, Bickel H, Fuchs A, et al. Predictors of costs in dementia in a longitudinal perspective. PLOS One 2013;8:e70018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2019
- Li P, Fan W, Yu L, Lim AS, Buchman AS, Bennett DA, et al. Interaction between the progression of Alzheimer's dementia and circadian disturbances: a 13-year longitudinal study in community-based older adults. Sleep 2019;42(S1):A123. [Google Scholar]
Li 2020
- Li T, Higgins JP, Deeks JJ. Chapter 5: Collecting Data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Livingston 2014
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technology Assessment 2014;18:1-vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lorenz 2018
- Lorenz E, Köpke S, Pfaff H, Blettner M. Cluster-randomized studies. Deutsches Ärzteblatt International 2018;115:163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCleery 2020
- McCleery J, Sharpley AL. Pharmacotherapies for sleep disturbances in dementia. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD009178. [DOI: 10.1002/14651858.CD009178.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCurry 1999
- McCurry S, Logsdon R, Teri L, Gibbons L, Kukull W, Bowen J, et al. Characteristics of sleep disturbance in community-dwelling Alzheimer's disease patients. Journal of Geriatric Psychiatry and Neurology 1999;12:53-9. [DOI] [PubMed] [Google Scholar]
Möhler 2015
- Möhler R, Köpke S, Meyer G. Criteria for reporting the development and evaluation of complex interventions in healthcare: revised guideline (CReDECI 2). Trials 2015;16:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mollayeva 2016
- Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh Sleep Quality Index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Medicine Reviews 2016;25:52-73. [DOI] [PubMed] [Google Scholar]
Montgomery 2003
- Montgomery P, Dennis JA. Cognitive behavioural interventions for sleep problems in adults aged 60+. Cochrane Database of Systematic Reviews 2003, Issue 1. Art. No: CD003161. [DOI: 10.1002/14651858.CD003161] [DOI] [PubMed] [Google Scholar]
Moore 2015
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350:h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noyes 2019
- Noyes J, Booth A, Moore G, Flemming K, Tunçalp Ö, Shakibazadeh E. Synthesising quantitative and qualitative evidence to inform guidelines on complex interventions: clarifying the purposes, designs and outlining some methods. BMJ Global Health 2019;4(Suppl 1):e000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Caoimh 2019
- O'Caoimh R, Mannion H, Sezgin D, O'Donovan MR, Liew A, Molloy WD. Non-pharmacological treatments for sleep disturbance in mild cognitive impairment and dementia: a systematic review and meta-analysis. Maturitas 2019;127:82-94. [DOI] [PubMed] [Google Scholar]
O'Neil 2011
- O'Neil M, Freeman M, Christensen V, Telerant R, Addleman A, Kansagara D. A Systematic Evidence Review of Non-pharmacological Interventions for Behavioral Symptoms of Dementia. Washington (DC): Department of Veterans Affairs, 2011. [PubMed] [Google Scholar]
Prince 2015
- Prince M, Wu F, Guo Y, Gutierrez Robledo L, O'Donnell M, Sullivan R, et al. The burden of disease in older people and implications for health policy and practice. Lancet 2015;385:549-62. [DOI] [PubMed] [Google Scholar]
Quante 2018
- Quante M, Kaplan ER, Cailler M, Rueschman M, Wang R, Weng J, et al. Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms. Nature and Science of Sleep 2018;10:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Ritchie 1996
- Ritchie K. Behavioral disturbances of dementia in ambulatory care settings. International Psychogeriatrics 1996;8(Suppl 3):439-42. [DOI] [PubMed] [Google Scholar]
Saeed 2017
- Saeed Y, Abbott SM. Circadian disruption associated with Alzheimer's disease. Current Neurology and Neuroscience Reports 2017;17(4):29. [DOI] [PubMed] [Google Scholar]
Sagha 2018
- Sagha Zadeh R, Capezuti E, Eshelman P, Woody N, Tiffany J, Krieger AC. Non-pharmacological solutions to sleep and circadian rhythm disruption: voiced bedside experiences of hospice and end-of-life staff caregivers. BMC Palliative Care 2018;17(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salami 2011
- Salami O, Lyketsos C, Rao V. Treatment of sleep disturbance in Alzheimer's dementia. International Journal of Geriatric Psychiatry 2011;26:771-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Shang 2019
- Shang B, Yin H, Jia Y, Zhao J, Meng X, Chen L, et al. Nonpharmacological interventions to improve sleep in nursing home residents: a systematic review. Geriatric Nursing 2019;40:405-19. [DOI] [PubMed] [Google Scholar]
Song 2010
- Song Y, Dowling G, Wallhagen M, Lee K, Strawbridge W. Sleep in older adults with Alzheimer's disease. Journal of Neuroscience Nursing 2010;42:190-8. [DOI] [PubMed] [Google Scholar]
van Egroo 2019
- Egroo M, Narbutas J, Chylinski D, Villar González P, Ghaemmaghami P, Muto V, et al. Preserved wake-dependent cortical excitability dynamics predict cognitive fitness beyond age-related brain alterations. Communications Biology 2019;2:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2020
- World Health Organization. Dementia 2020. www.who.int/news-room/fact-sheets/detail/dementia (accessed prior to 1 December 2022).
Wilfling 2019
- Wilfling D, Dichter MN, Trutschel D, Köpke S. Sleep disturbances in German nursing home residents with dementia: a multicenter cross-sectional study. Journal of Alzheimer's Disease 2019;69:227-36. [DOI] [PubMed] [Google Scholar]
Wilfling 2021
- Wilfling D, Hylla J, Berg A, Meyer G, Köpke S, Halek M, et al. Characteristics of multicomponent, nonpharmacological interventions to reduce or avoid sleep disturbances in nursing home residents: a systematic review. International Psychogeriatrics 2021;33(3):245-73. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wilfling 2015
- Wilfling D, Junghans A, Marshall L, Eisemann N, Meyer G, Möhler R, et al. Non-pharmacological interventions for sleep disturbances in people with dementia. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011881. [DOI: 10.1002/14651858.CD011881] [DOI] [PMC free article] [PubMed] [Google Scholar]


