Abstract
Listeria monocytogenes, a facultative intracellular bacterium, has been used extensively to study innate immune responses. Macrophages act as hosts for this bacterium as well as a major defense against it. Using mice homozygous for a null mutation (Csf1op) in the gene for the mononuclear phagocytic growth factor colony-stimulating factor 1 (CSF-1), we have demonstrated that CSF-1-regulated macrophages were essential to defend against a listerial infection. In the absence of CSF-1, monocytes were not recruited to the sites of infection due to the lack of synthesis of the macrophage chemoattractant chemokine MCP-1. In addition, there was no burst of interleukin-10 (IL-10) synthesis that has been shown to result in the egress of neutrophils from sites of infection. Consequently, neutrophils were not replaced by macrophages, and numerous neutrophil-filled microabscesses developed, followed by tissue destruction and death of the mice. In the CSF-1 nullizygous mice compared to wild-type mice, there was also a very low synthesis of gamma interferon (IFN-γ), resulting in reduced macrophage activation. However, the concentrations of the IFN-γ-inducing cytokines IL-12 and IL-18 at this bacterial load were similar in these mutant mice. In contrast, IL-6 concentrations were dramatically reduced. Administration of IL-6 to Csf1op/Csf1op mice significantly increased the synthesis of IFN-γ and reduced the bacterial burden to a greater extent than treatment with IFN-γ alone. These data indicate that IL-6 occupies a central role in the CSF-1-regulated macrophage response to L. monocytogenes.
Listeria monocytogenes has been a preferred experimental model to study the host defense to intracellular bacteria. It is a gram-positive intracellular bacterium that replicates primarily within macrophages; therefore, infection is restricted to macrophage-rich organs such as the liver and spleen. L. monocytogenes can also infect other cells, especially hepatocytes in the liver and epithelial cells, which may provide the portal of entry through the intestine.
L. monocytogenes provokes a powerful innate immune response that requires interactions between macrophages, NK cells, and neutrophils. However, for sterile eradication, T cells need to be activated (4). Most of these cellular interactions are mediated by cytokines, and studies, particularly with neutralizing antibodies or mice carrying null mutations in cytokine and cytokine receptor genes, have established roles for interleukin-1 (IL-1), IL-4, IL-6, IL-12, IL-18, tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ) in host response. For instance, macrophage synthesis of IL-12 and TNF-α early after infection results in a synergistic stimulation of NK cells to synthesize IFN-γ (51, 53). This IFN-γ activates resident macrophages to become listeriocidal, particularly through the production of NO, and also induces Th0 cells to undergo differentiation to CD4+ Th1 cells. These Th1 cells synthesize additional IFN-γ, thus positively amplifying the host response. The activated macrophages now have an increased ability to present listerial antigens to these T cells, resulting in their differentiation into armed effector cells and the sterile eradication of the bacteria (26, 53).
Colony-stimulating factor 1 (CSF-1) is the major regulator of cells of the mononuclear phagocytic lineage (46). It regulates their survival, proliferation, and differentiation and is a major chemoattractant for these cells (46, 54). Studies on the biology of CSF-1 were significantly enhanced when the recessive osteopetrotic mutation Csf1op (formerly Csfmop or op) was shown to be a null mutation in the CSF-1 gene (56, 61). The phenotype of Csf1op/Csf1op mice confirmed the central role of CSF-1 in macrophage biology since analysis with a wide range of markers showed these mice to be depleted in monocytes and many populations of macrophages in many tissues (8, 25, 47, 56, 58, 59). However, these studies also indicated a further level of complexity in the mononuclear phagocytic system since some macrophage populations, particularly those associated with the immune system such as those in the spleen and lymph nodes, are relatively unaffected by the absence of CSF-1 (8, 39).
Surprisingly, given the macrophage deficiency, early studies of immune function in Csf1op/Csf1op mice showed that they can mount effective humoral and cell-mediated responses to T-cell-dependent antigens (9, 55). The noncompromised ability to present antigens might be due to the normal numbers of dendritic cells in these mice (59), the previously mentioned normal macrophage density in immune organs, or antigen presentation by other immune cells. Furthermore, in studies using an intraperitoneal infection of mice with fecal bacteria, although Csf1op/Csf1op mice were slower to resolve the infection than wild-type mice, they mounted an effective immune response and eradicated the bacteria (57). However, recent studies with the intracellular bacterium Mycobacterium tuberculosis indicate that the mutant mice are more susceptible to challenge than control mice, indicating defects in macrophage function (48). To further study macrophage functions and because L. monocytogenes replicates in macrophages but also requires macrophage functions for an effective immune response to be mounted, we challenged Csf1op/Csf1op mice with L. monocytogenes. Csf1op/Csf1op mice were considerably more susceptible to L. monocytogenes than wild-type mice. These studies confirmed the central role of CSF-1-dependent macrophages in immune response to L. monocytogenes and indicated an important role for IL-6 in this response that is at least partially mediated by IFN-γ.
MATERIALS AND METHODS
Mice.
Female homozygous (Csf1op/Csf1op) and heterozygous (+/Csf1op) mice (referred to as controls) were maintained on a C3H/C57BL/6 background in a closed, randomly bred colony. Both age-matched (8- to 12-week-old) and weight-matched (18- to 20-g) mice were used for the study. Heterozygous mice were chosen as control mice since their systemic concentrations of CSF-1 and tissue macrophage densities were not different from those of homozygous wild-type mice (8). Details of their housing and care have been given elsewhere (11). All experiments were performed under institutional animal care guidelines and an approved protocol.
Bacteria and infections of mice.
L. monocytogenes strain EGD was grown to log phase in tryptic phosphate broth (Difco Laboratories, Detroit, Mich.) and stored in aliquots at −70°C. One tube was thawed and serially diluted, and dilutions were plated on tryptic phosphate agar plates in duplicate in order to quantitate the bacteria. Colonies were counted 24 h later. Mice were infected intravenously (i.v.) via the lateral tail vein with titers of L. monocytogenes as indicated below. However, in most experiments 104 CFU per mouse was used. The mice were monitored for survival and mortality over 6 days. Other groups of mice were sacrificed at 1, 24, 48, and 72 h postinfection (p.i.), and their livers and spleens were taken. These tissues were homogenized in phosphate-buffered saline (PBS), and serial dilutions of tissue homogenates were plated onto tryptic soy agar. The number of CFU of L. monocytogenes was counted after 24 h of incubation.
Preparation of HKLM.
A suspension of heat-killed L. monocytogenes (HKLM) was prepared by growing bacteria in tryptic soy broth overnight. Cultures in log phase growth were harvested, centrifuged, and washed in PBS. An aliquot from this suspension was used to enumerate the number of viable units of bacteria. The recovered bacteria were heat inactivated by incubation at 80°C for 1 h. The absence of viable colonies was confirmed by lack of growth on tryptic soy agar plates.
Spleen cell and T-cell preparation.
Spleens taken at 24, 48, and 72 h p.i. were teased through a cell strainer (70-μm mesh; Beckton Dickinson and Co., Franklin Lakes, N.J.) to obtain single-cell suspensions, and erythrocytes were lysed by treatment with ammonium chloride. Spleen cells were enriched for T cells by 2× passage over nylon wool columns (44), resulting in >95% pure T cells. Splenocytes or T cells were resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum–2 mM glutamine, 100 U of penicillin-streptomycin per ml, and 5 × 10−5 β2-mercaptoethanol. Syngeneic irradiated spleen cells served as antigen-presenting cells (ACs) in T-cell assays. Since Csf1op/Csf1op mice have a mixed background, F1 generation of a cross between strain C3H and strain C57BL/6 was used to prepare ACs. HKLM (108 and 107) antigens served as specific antigens to restimulate splenocytes or T cells in vitro. Mitogen controls were performed by stimulation with concanavalin A (Con A) (Sigma Chemical Co., St. Louis, Mo.) for T-cell assays and by lipopolysaccharide (LPS) (Sigma Chemical Co.) for splenocyte assays. Anti-mouse CD4 (L3T4), clone H129.19 (Pharmingen, San Diego, Calif.) (37), or anti-mouse CD8 (Ly-2), clone 53-6.7 (Pharmingen) (29), along with the isotype-matched control antibody rat immunoglobulin G2a (IgG2a), was used for blocking experiments. Splenocytes were used to analyze cytokine synthesis by macrophages.
Cell proliferation assay.
For T-cell proliferation assay, 105 T cells/well were cultured with 105 ACs in the presence of HKLM bacteria (108/well or 107/well) or in the presence of the ConA (2.0 μg/ml) in 96-well plates. Cells were harvested 4 to 5 days later, after an 8-h pulse with [3H]thymidine (1 μCi/well), and the incorporated thymidine was measured as previously described (44).
Cytokine analysis.
T cells (in the presence of ACs) or splenocytes were cultured in the presence of specific (HKLM) antigens or with mitogen (ConA/LPS). After 24 and 48 h, supernatants were collected and frozen at −70°C for cytokine analysis. For measuring IFN-γ, MCP-1, IL-4, and IL-5, matched antibody pairs from Pharmingen were used in a sandwich enzyme-linked immunosorbent assay (ELISA) along with recombinant murine cytokine to construct a standard curve. IL-6, IL-10, KC, macrophage inflammatory protein 2 (MIP-2), and MIP-1α were measured using R&D System's (Minneapolis, Minn.) immunoassay kits. TNF-α was measured using an ELISA kit from Endogen. IL-18 was measured with an immunoassay kit from MBL International (Nagoya, Japan). IFN-γ secretion was measured from T-cell/splenocyte culture supernatants. Other cytokines were detected from culture supernatants of splenocytes. Where indicated, anti-CD4 (1, 5, or 10 μg/ml) or anti-CD8 (1, 5, or 10 μg/ml) was added to splenocytes to block signaling through the CD4 or CD8 receptor. In some cultures equal concentrations of the isotype-matched antibody rat IgG2a were added. All cultures were set up in triplicate, and the culture supernatants were tested for IFN-γ production after 24 and 48 h. For analysis in homogenates, spleens and livers were homogenized in 1 ml of PBS with 1% (vol/vol) Triton. They were clarified by centrifugation at 11,750 × g. The supernatants were aliquoted and stored at −70°C for cytokine analysis. To confirm the quantitative recovery of cytokines, a range of known concentrations of recombinant cytokine was added to tissue homogenates and measured by ELISA. These experiments showed that the concentrations recovered were not significantly different from those of cytokines that had been diluted in PBS and assayed in the absence of homogenate.
Flow cytometric analysis.
To determine splenic immune cell populations, single cell suspensions of splenocytes from Csf1op/Csf1op and +/Csf1op mice were prepared as described above. Cells were incubated with 10 μg of murine IgG per ml for 15 min on ice to block Fc receptor binding. Subsequently the cells were stained with various antibodies for 30 min at 4°C. Cells were stained with phycoerythrin-conjugated anti-CD4 (Caltag, South San Francisco, Calif.) and fluorescein isothiocyanate-conjugated anti-CD8 (Caltag), anti-pan-NK cells (Pharmingen), and anti-polymorphonuclear leukocyte (PMN) cells (RB6-8C5 antibody) (Pharmingen). In order to analyze the expression of class II major histocompatibility complex (MHC) (Ia) on macrophages, spleen cells were costained with phycoerythrin-conjugated F4/80 and fluorescein isothiocyanate-conjugated Ia antigen (Pharmingen) and sorted according to these two parameters in a two-color analysis. Isotype-matched antibodies were used as negative controls. All cell sorting was performed on a FACScan II (Becton Dickinson, Inc.).
Natural killer cell function assay.
The Cyto Tox 96 nonradioactive cytotoxicity assay (Promega, Madison, Wis.), a colorimetric alternative to the 51Cr release cytotoxicity assay, was used to study the ability of splenocytes from L. monocytogenes-infected mice to lyse target cells. This assay measures lactate dehydrogenase (LDH), a stable cytosolic enzyme that is released upon cell lysis. A constant number of target cells (Yac1 cells) were added to a 96-well culture plate followed by various numbers of effector cells added to triplicate sets of wells to test several effector/target ratios. The effector/target ratios used were 12.5:1, 6.25:1, and 3.12:1. The plate was centrifuged to ensure effector and target cell contact. After 4 h of incubation at 37°C, the plate was centrifuged, and the supernatant was tested for LDH release with a coupled enzymatic assay (Promega), which results in conversion of a tetrazolium salt into a red formazan product. The amount of color formed is proportional to the number of lysed cells. The necessary analyses of effector cell-spontaneous, target cell-spontaneous, and target cell-maximum LDH release as well as volume correction control and culture medium control were performed. Spontaneous LDH release did not exceed 10% of the maximum release as determined by lysis of target cells with 10% Triton X-100. The percentage of specific lysis was calculated as [(experimental - effector spontaneous - target spontaneous)/(target maximum - target spontaneous)] × 100.
Histology.
Periodate-lysine-paraformaldehyde-glutaraldehyde-fixed tissues were embedded in low-melting-temperature wax as previously described (8). Five-micrometer sections were stained with hematoxylin and eosin or immunostained with anti-F4/80 rat monoclonal antibody specific for macrophages (24), anti-Mac1 (45), or RB6-8C5 anti-Gr 1 antibody specific for neutrophils (49). Immunoreactive material was detected using a peroxidase detection kit (Vector Laboratories, Burlingame, Calif.). Sections were also stained with Gram's stain (Sigma Diagnostics) for the detection of gram-positive L. monocytogenes.
Isolation of total RNA and competitive-quantitative reverse transcription-PCR.
Total RNA from the spleen and liver tissues of L. monocytogenes-infected or uninfected Csf1op/Csf1op and +/Csf1op mice was extracted with Trizol (Gibco-BRL, Gaithersberg, Md.). This RNA was reverse transcribed with Superscript II RNase H− reverse transcriptase (RT) (Gibco-BRL) using random primers. As a negative control, the reaction was performed in the absence of RT.
Primers used were IL-10 5′ CCAGTTTTACCTGGTAGAAGTGATC, 3′ TGTCTAGGTCCTGAGTCCAGCAGACTCAA, iNOS 5′ TGGGAATGGAGACTGTCCCAG, 3′ GGGATCTGAATGTGATAGTTTG, TNF-α 5′ GTTCTATGGCCCAGACCCTCACA, and 3′ TCCCAGGTATAGGGTTCATACC. For a positive-control housekeeping gene hypoxanthine-guanine phosphoribosyl transferase (HPRT), the primers used were 5′ GTTGGATACAGGCCAGACTTTGTTG and 3′ GAGGTAGGCTGGCCTATGGCT.
An aliquot of cDNA was amplified by PCR in a reaction mix containing 1.0 U of Taq Gold (Perkin Elmer, Foster City, Calif.); 1 × PCR buffer; 1.5 mM MgCl2 (Gibco-BRL); 50 μM sense and antisense primers; and 0.2 mM concentration each of dATP, dTTP, dCTP, and dGTP (Gibco-BRL); to this mixture was added 8.0 μl of competitor, diluted to concentrations ranging from 0.4 to 0.04 ng/ml, and nuclease-free water was also added to bring the total volume of the mixture to 50 μl (40). Cycling conditions included initial denaturation at 94°C for 1 min followed by 34 cycles of 94°C for 40 s, 60°C for 70 s, 72°C for 40 s, and final elongation at 72°C for 10 min. Taq Gold is inactive at room temperature; therefore, the samples were heated at 94°C for 12 min before starting the PCR cycle. Amplified products over the range of competitor dilutions were separated on 0.8% agarose gels and stained with ethidium bromide.
Reconstitution of mice with IFN-γ, CSF-1, or IL-6.
Csf1op/Csf1op mice were given a single injection of IFN-γ (104 IU) (Pharmingen) i.v. 2 h prior to infection with 104 L. monocytogenes cells, or the mice were given 106 U of CSF-1 sub cutaneously for 3 days (8) before and during the course of infection with L. monocytogenes. CFU recovered from tissue homogenates were enumerated at 48 h p.i. In another set of experiments, Csf1op/Csf1op mice were injected with 12.5 μg of recombinant murine IL-6 (rmIL-6) (R&D System) subcutaneously at days −1, 0, and +1 of Listeria infection. Csf1op/Csf1op mice injected with saline and infected with the same titer of L. monocytogenes served as controls. At 48 h p.i., mice were sacrificed, and their livers and spleens were removed. Livers were used for colony counts of Listeria cells and spleens were used for in vitro studies. Liver homogenates were assayed for viable listeriae as described earlier. Splenocytes were used to study the production of IFN-γ after in vitro stimulation with HKLM.
Statistical analysis.
Numbers of CFU in tissues were compared between different groups of mice by using the Mann-Whitney U test. Statistical significance between the cytokines and chemokines of different groups of mice was analyzed by Student's t test.
RESULTS
Increased susceptibility of Csf1op/Csf1op mice to L. monocytogenes.
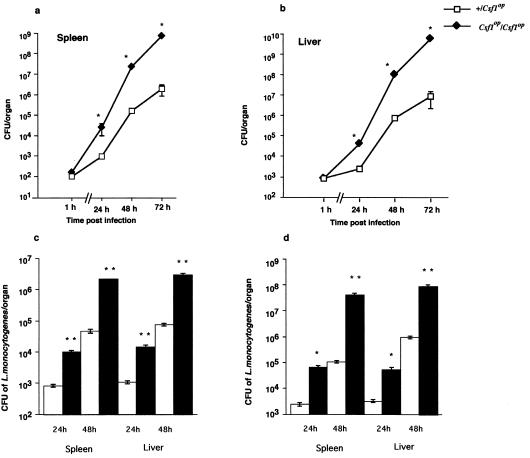
Heterozygous controls (+/Csf1op) and homozygous mutant (Csf1op/Csf1op) mice were infected i.v. with 104 CFU of L. monocytogenes, and CFUs were determined in the main infected organs, the liver and spleen. Initial titers in these organs at 1 h p.i. were similar, showing that the dosage was comparable despite the ∼30% smaller size of Csf1op/Csf1op mice. However, the titers in Csf1op/Csf1op mice were 1 to 3 logs higher (P < 0.001, Mann-Whitney U-test) than those detected in wild-type mice over the 72 h of infection (Fig. 1a, b). This resulted in a more rapid rate of death of Csf1op/Csf1op mice with 50% viability by 2 to 3 days p.i., relative to 50% viable mice at 4 to 5 days p.i. for controls (data not shown). A similar differential survival between Csf1op/Csf1op and control mice was found after infection with 5 × 103, 5 × 104, or 1 × 105 CFU/mouse (data not shown). Furthermore, even at a sublethal dose of 103 CFU, there was a 1- to 2-log difference in recovered CFU in both liver and spleen tissues of Csf1op/Csf1op mice compared to that recovered in controls over the first 48 h of infection (Fig. 1c). To ensure that the effects observed were not due to the small differences in size resulting in a differential inoculum. weight-matched mice of both genotypes were infected with 104 CFU i.v. followed by determination of the bacterial titers in liver and spleen over the 48 h of infection. In a similar fashion to that observed in age-matched mice, there was also a 1- to 3-log-higher bacterial titer in both the livers and spleens of Csf1op/Csf1op mice than that found in control mice (Fig. 1d).
FIG. 1.
Increased susceptibility of Csf1op/Csf1op mice to L. monocytogenes infection. Mice (+/Csf1op and Csf1op/Csf1op) were infected with 104 L. monocytogenes i.v. Groups of infected mice were sacrificed at 1, 24, 48, and 72 h p.i., and the numbers of CFU of L. monocytogenes recovered from spleen (a) and liver (b) were determined by plating serial dilutions of tissue homogenate on tryptic soy agar plates. In another set of experiments, either (c) mice were infected with 103 L. monocytogenes cells i.v. or (d) weight-matched (18- to 20-g) mice were challenged with 104 L. monocytogenes cells i.v. and the CFU from liver and spleen were enumerated. Each data point represents the mean and standard error of the mean of 8 mice in panels a and b and of 5 mice in panels c and d. The experiment was repeated two times with similar results (∗, P < 0.001; ∗∗, P < 0.05; Mann-Whitney U test).
The Csf1op mutation is maintained on a mixed C3H and C57BL/6 background by interbreeding in a closed colony. To rule out that future results would be significantly influenced by the segregation of different strain susceptibilities to L. monocytogenes, inbred C3H and C57BL/6 mice were infected individually with 104 CFU i.v., and their viability was followed. Both strains showed identical kinetics of death at this inoculum concentration, and these results were indistinguishable from the viability curve of control (+/Csf1op) mice (data not shown). Thus, background effects appeared minimal at this infectivity. Consequently and given the results described above, 104 CFU administered i.v. was used for all of the studies in this report.
Delayed macrophage recruitment and aberrant neutrophil response in Csf1op/Csf1op mice.
The Csf1op mutation results in a depletion of many populations of mononuclear phagocytes. However, not all populations are affected equally by the mutation, with F4/80+ splenic macrophages relatively unaffected, while MOMA1+ splenic macrophages are completely absent and F4/80+ liver macrophages are significantly depleted (30% of normal) (8, 35). Therefore, we determined the responses of splenic and liver macrophage populations after infection in control and Csf1op/Csf1op mice by immunohistochemistry with anti-F4/80 and Mac1 antibody as previously described (8). In the livers of control mice, there was a dramatic increase in macrophage density within 24 h of infection (Table 1; Fig. 2a), with changes that persisted for another 24 h (Table 1). In contrast, in the livers of Csf1op/Csf1op mice, there were fewer F4/80+ cells prior to infection, and this population did not expand over the first 2 days of infection (Table 1; Fig. 2b). However, at 72 h p.i. there was a dramatic increase in F4/80+ cell density (Table 1; Fig. 2d), but at this stage the liver architecture was already severely disrupted as indicated by numerous lesions (Fig. 2d and f). In the spleens of control mice, there was also an increase in F4/80+ macrophage density at 24 h p.i., which decreased to some degree by 72 h (Table 1). In contrast, in the spleens of Csf1op/Csf1op mice, despite the similar starting population of F4/80+ cells (8), there was an absence of recruitment in the first 24 h, and the populations showed little change in density up to 48 h (Table 1). At this time, spleen architecture is also severely disrupted (data not shown). Using Mac1 (45) as a marker for recruited monocytes, Csf1op/Csf1opmice showed a slower recruitment of monocytes in the spleen through the first 24 h of infection, although by 48 h p.i. significant numbers of Mac1-positive cells could be observed, confirming the data using F4/80+ as a marker (data not shown).
TABLE 1.
F4/80 immunostaining of tissues from +/Csf1op and Csf1op/Csf1op mice challenged with L. monocytogenesa
| Time of quantitation (p.i.) | Macrophage densityb in tissue from strain
|
|||
|---|---|---|---|---|
| Liver
|
Spleen
|
|||
| +/Csf1op | Csf1op/Csf1op | +/Csf1op | Csf1op/Csf1op | |
| Day 0 | ++ | + | ++ | ++ |
| 24 h | ++++ | + | +++++ | ++ |
| 48 h | +++ | + | ++++ | +++ |
| 72 h | + | +++ | +++ | +++ |
Tissue sections from uninfected (day 0) and L. monocytogenes-infected (24, 48, and 72 h p.i.) +/Csf1op and Csf1op/Csf1op mice were stained with antibody to macrophage surface marker F4/80.
Macrophage density was recorded by independent observers semiquantitatively on a scale + (lowest) to +++++ (highest). The experiment was repeated twice with similar results.
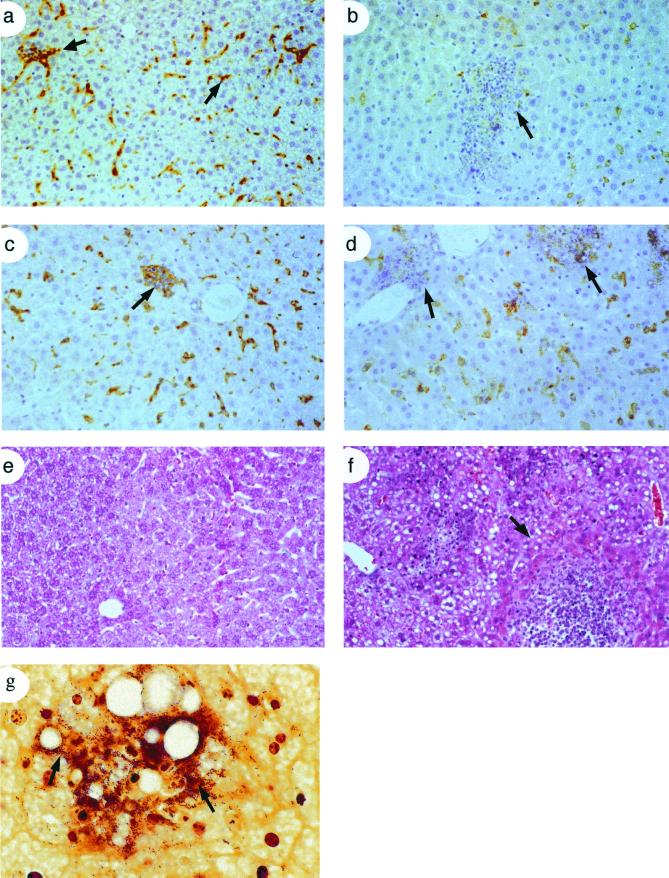
FIG. 2.
Delayed recruitment of macrophages and loss of tissue integrity in Csf1op/Csf1op mice. Liver sections from +/Csf1op (a and c) and Csf1op/Csf1op mice infected with L. monocytogenes were immunostained at 24 h (a and b) and 72 h p.i. (c and d) using antibody against the macrophage-specific surface marker F4/80. F4/80+ macrophages stain brown with the antibody. Arrows (a and c) indicate foci containing F4/80+ macrophages with granulocytes and lymphocytes in +/Csf1op mice, and arrows (b and d) indicate leukocytic infiltrates leading to microabscess formation in Csf1op/Csf1op mice. (e and f) Sections were also stained with hematoxylin-eosin to show the histology of liver lesions and the extensive tissue necrosis in Csf1op/Csf1op mice (f) compared to the relatively normal morphology in +/Csf1op mice (e). An arrow (f) shows the boundary of a typical lesion. (g) Some sections were stained with Gram's stain to show the enormous number of bacilli (arrows) seen in the liver lesions of Csf1op/Csf1op mice.
In the livers of infected heterozygous control mice, we observed macrophages associated with few granulocytes and surrounded by lymphocytes (Fig. 2c). In contrast, livers of Csf1op/Csf1op mice contained large leukocytic infiltrates at 24 h resulting in microabscesses by 72 h (Fig 2d and f). These micro abscesses harbored numerous gram-positive bacilli, many being extracellular but some apparently replicating within hepatocytes (Fig. 2g). In control mice, Gram staining revealed few bacteria, and those that were present were contained within macrophages (data not shown).
Neutrophils play an important role in the early immune response to L. monocytogenes and can be identified by histological analysis and immunostaining with the anti-neutrophil antibody RB6-8C5 (anti-Gr-1) (49). In the livers of infected control mice, neutrophils were detected in small foci randomly distributed throughout the tissue at 24 h p.i. (Fig. 3a and c) but were largely gone by 48 h. However, numerous microabscesses were observed throughout the livers of Csf1op/Csf1op mice, and they showed heavy neutrophil infiltration (Fig. 3b and d) that persisted for the entire infection.
FIG. 3.
Massive infiltration of neutrophils leading to microabscess formation following Listeria infection in Csf1op/Csf1op mice. (a through d) Liver sections from 24- and 48-h-postinfected mice were immunostained with RB6-8C5 antibody to stain neutrophils in tissues. There were fewer neutrophils in +/Csf1op mice (a) compared to the massive accumulation of neutrophils seen in Csf1op/Csf1op mice (b). Staining at higher magnification is shown (c and d) with arrowheads indicating RB6-8C5+ neutrophils in +/Csf1op mice (c) and a typical micro abscess filled with RB6-8C5+ cells in Csf1op/Csf1op mice (d). Data shown are from 24 h p.i. Similar results were obtained at 48 h p.i.
Analysis of splenic immune cell populations.
B cells play only a small role in the immune response to L. monocytogenes (16). However, most other classes of immune cells have important roles in the host response to this pathogen. Therefore, we analyzed the changes in these populations in the spleen at 4, 8, 24, 48, and 72 h p.i. We did not observe any significant differences between infected control and mutant mice with respect to the CD4/CD8 ratio and γδ T-cell and NK cell counts. In contrast, RB6-8C5+ neutrophils declined by 48 h from initially high levels induced by listerial infection in both control and mutant mice (data not shown).
Macrophage and T-cell interactions are essential for an effective immune response against L. monocytogenes. Therefore, we determined T-cell proliferation in response to HKLM. Upon T-cell stimulation with accessory cells and 107 HKLM, there was a similar proliferation rate between +/Csf1op and Csf1op/Csf1op mice, while at 108 HKLM the proliferation was suppressed to a comparable extent (Fig. 4a). Specific T-cell proliferation therefore seemed unaffected by the absence of CSF-1-regulated macrophage functions.
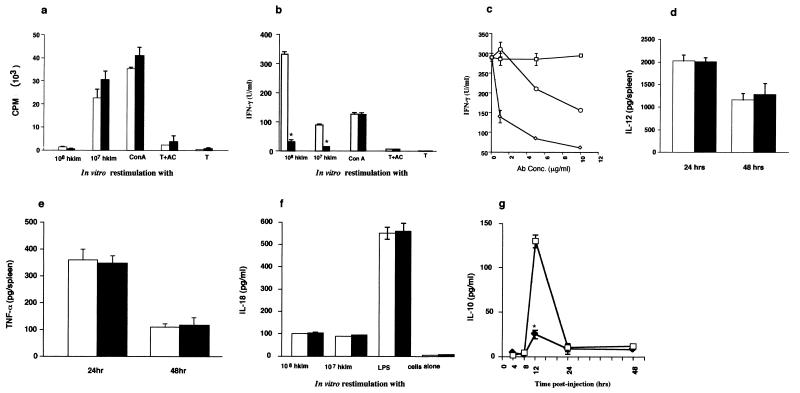
FIG. 4.
T-cell proliferative responses and cytokine production in spleens of L. monocytogenes-infected mice. +/Csf1op (open histograms) and Csf1op/Csf1op (filled histograms) mice were infected i.v. with 104 L. monocytogenes. (a through c) At 48 h p.i., spleens from eight mice were pooled. T cells were purified from splenocytes as indicated in Materials and Methods. Cells were stimulated in vitro with HKLM (108 or 107) or ConA (2.0 μg/ml) in the presence of ACs. (a) T cells were cultured for 5 days, pulsed with 1 μCi of [3H]thymidine for the last 8 h, and harvested, and 3H incorporation was assessed by scintillation counting. Data are presented as mean cpms of thymidine incorporation in triplicate cultures. The results are representative of two separate experiments. (b) T-cell culture supernatants after 48 h of culture were assessed for IFN-γ production (∗, P < 0.001). (c) At 48 h p. i., splenocytes from +/Csf1op mice were cultured with HKLM in the presence of anti-CD4 (◊, 1, 5, and 10 μg/ml) or anti-CD8 (○, 1, 5, and 10 μg/ml) or isotype-matched control (□, 1, 5, and 10 μg/ml), and IFN-γ production after 24 to 48 h was checked in the culture supernatants. (d and e) Spleen homogenates (eight mice per group) were tested at indicated time points for quantitative measurement of (d) IL-12 and (e) TNF-α. (f and g) Culture supernatants from splenocytes (five mice per group) were restimulated with HKLM or mitogen for measurement of (f) IL-18 and (g) IL-10 (□, +/Csf1op; ⧫, Csf1op/Csf1op). In order to study IL-10 production by splenocytes rather than T cells (T cells did not produce IL-10 in our assays), splenocytes from infected mice were cultured at 4, 8, 12, 24, and 48 h p.i. They were restimulated with HKLM or LPS. At 12 h p.i., splenocytes from +/Csf1op mice, on restimulation with HKLM (108), produced significantly (∗, P < 0.001, Student's t test) higher levels of IL-10 than Csf1op/Csf1op mice. IL-10 production upon restimulation with LPS was similar between genotypes (data not shown). All data shown are means ± standard errors of the means of triplicate cultures. Results are representative of three experiments in each panel except panel c, where the data are representative of two experiments.
Early during infection, natural killer cells are an important source of IFN-γ production. To determine if there is any difference in NK cell activity between +/Csf1op and Csf1op/Csf1op mice, splenocytes from these mice were challenged with L. monocytogenes and were tested for their ability to lyse Yac1 cells. The results showed a similar level of cytotoxic activity of NK cells from +/Csf1op and Csf1op/Csf1op at several different effector/target ratios (data not shown).
Cytokine production by splenocytes and splenic T cells from control and Csf1op/Csf1op mice.
IFN-γ is a major cytokine in the host defense to L. monocytogenes, serving to regulate T-cell differentiation and to activate macrophages. Therefore, we measured T-cell production of IFN-γ by ELISA upon in vitro stimulation of purified T cells with HKLM in the presence of accessory cells, from 48 h and 72 h postinfected mice. T cells from control mice produced high levels of IFN-γ upon stimulation with either 107 (∼100 U/ml) or 108 (∼330 U/ml) HKLM (Fig. 4b). This level was significantly (P < 0.001, Student's t test) reduced to ∼30 U/ml in Csf1op/Csf1op T-cell cultures at both doses of HKLM. However, similar concentrations of IFN-γ between genotypes were produced in response to the nonspecific mitogen ConA (Fig. 4b), suggesting that the reduced IFN-γ production in Csf1op/Csf1op mice was specifically due to a failure of a T-cell response to L. monocytogenes. IFN-γ production was blocked by antibodies to CD4 or CD8 (Fig. 4c), with the effect of anti-CD4 being greater.
IL-12 is the major cytokine reported to regulate IFN-γ during listerial infection. Surprisingly, at both 24 and 48 h p.i., similar but significant concentrations of IL-12 in splenic homogenates were detected in both +/Csf1op and Csf1op/Csf1op mice (Fig. 4d). TNF-α can synergize with IL-12 to enhance IFN-γ synthesis and is important in the immune responses to L. monocytogenes (51). Therefore, we measured TNF-α in spleen cell culture medium. However, no differences in synthesis of TNF-α could be detected between +/Csf1op and Csf1op/Csf1op mice at either 24 or 48 h (Fig. 4e). These data were confirmed by competitive RT-PCR of splenic RNA using primers specific for TNF-α (data not shown). More recently it has been shown that IL-18 has IFN-γ-inducing properties (36). Splenocytes from both genotypes produced low but similar concentrations of IL-18 (∼100 pg) on in vitro restimulation with listerial antigens (Fig. 4f). However, IL-18 levels in response to LPS were high and similar in both genotypes (Fig. 4f). Levels of Th2 cytokines known to down-regulate IFN-γ, IL-4, IL-5, and IL-10 were essentially below the level of detection in T-cell culture supernatants, and they are thus unlikely to play a role in down-regulating IFN-γ synthesis in Csf1op/Csf1op mice. Nevertheless, when splenocytes were tested for IL-10 production, those from +/Csf1op mice produced significantly higher (P < 0.001, Student's t test) levels of IL-10 than those from Csf1op/Csf1op mice (Fig. 4g). However, this production showed a sharp peak at 12 h p.i., and it was only at this time that there was a difference or that significant concentrations of IL-10 were detected.
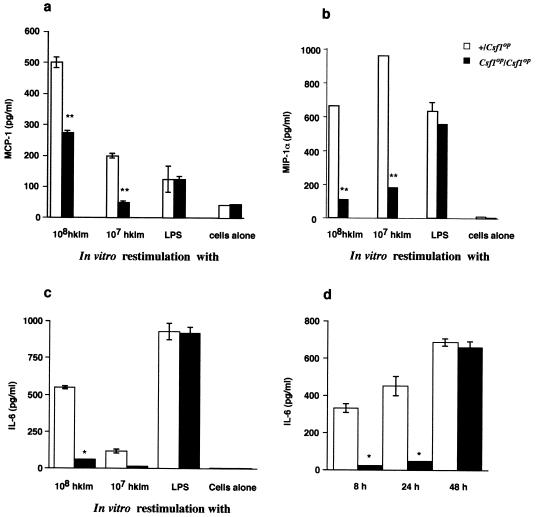
In addition to cytokines, certain chemokines such as MCP-1 and MIP-1α have been reported to be important in the early response to L. monocytogenes. MCP-1 and MIP-1α are thought to be important for monocyte/macrophage recruitment. Since this recruitment appears to be delayed in spleens of Csf1op/Csf1op mice, we first measured MCP-1 production in culture supernatants of splenocytes isolated from infected mice at 8, 24, and 48 h p.i. and stimulated with 107 or 108 HKLM cells. No MCP-1 could be detected at 8 and 48 h p.i. in either genotype (data not shown). However, at 24 h p.i. with +/Csf1op splenocytes, we observed a robust HKLM-induced synthesis of MCP-1 at both stimulating doses (Fig. 5a). In Csf1op/Csf1op mice, this production was significantly reduced such that with 107 HKLM stimulation, the level was barely above that detected with cells alone, while upon stimulation with 108 HKLM, the concentration was approximately half of that in the control mice. In contrast, upon LPS stimulation, similar levels of MCP-1 were detected for both genotypes, suggesting a specificity of this response for L. monocytogenes. Levels of a second macrophage-attracting chemokine, MIP-1α, were low in both groups of mice at 8 h, but greater production could be detected at 24 h p.i., and this was significantly higher in heterozygous controls than Csf1op/Csf1op mice (Fig. 5b).
FIG. 5.
Impaired MCP-1, MIP-1α, and IL-6 production by splenocytes from Csf1op/Csf1op mice. Mice were infected i.v. with 104 L. monocytogenes cells. Splenocytes were cultured in the presence of HKLM or LPS. Supernatants were collected after 8, 24, and 48 h p.i. MCP-1 (a) and MIP-1α (b) levels were significantly reduced in Csf1op/Csf1op mice (∗∗, P < 0.005, Student's t test) compared to +/Csf1op mice at 24 h p.i. No MCP-1 was detected at 8 and 48 h p.i. Low but similar levels of MIP-1α were detected at 8 h between the two genotypes, and no MIP-1α was detectable at 48 h. (c) IL-6 levels in culture supernatants were significantly reduced (∗, P < 0.001, Student's t test) at 24 h p.i. in Csf1op/Csf1op mice compared to control mice. (d) IL-6 levels in spleen homogenates at 8, 24, and 48 h p.i. are also shown. Significant differences (∗, P < 0.001) were observed at 8 and 24 h p.i. Results from a representative experiment are shown (mean ± standard error of mean). Similar data were obtained in three independent experiments.
The observed concomitant reduction of IFN-γ and MCP-1 in Csf1op/Csf1op mice led us to hypothesize that it might be due to a deficiency in IL-6, a pleiotropic cytokine reported to induce IFNγ and MCP-1 following bacterial infection (5, 31). Splenocytes from +/Csf1op mice harvested at either 8 (data not shown) or 24 h p.i. upon restimulation with HKLM synthesized very high concentrations of IL-6 (Fig. 5c). In contrast, splenocytes from Csf1op/Csf1op mice produced small amounts of IL-6 either at 8 (not shown) or 24 h p.i. (Fig. 5c). On stimulation with LPS, splenocytes from Csf1op/Csf1op mice produced similar levels of IL-6 to those of control mice (Fig. 5c). Measurement of IL-6 in splenic homogenates confirmed these results (Fig. 5d). At 8, 24, and 48 h p.i., high concentrations of IL-6 could be measured in control spleens. However, in Csf1op/Csf1op spleens at 8 and 24 h p.i., only low concentrations could be detected, and these were significantly (P < 0.001) below those of control mice. It is interesting that, by 48 h p.i., splenic IL-6 concentrations were similar in splenic homogenates from control and mutant mice (Fig. 5d).
Liver cytokines.
Early during the course of i.v. listerial infection, most of the pathogen is cleared by Kupffer cells or hepatocytes of the liver (19, 34, 38, 41). Therefore, we analyzed cytokine production in this tissue (Table 2). In the liver, the patterns of cytokine and chemokine expression and their deficiencies in Csf1op/Csf1op mice were very similar, although not identical to those found for splenocytes. Liver homogenates from +Csf1op mice contained sixfold higher concentrations of IFN-γ than homogenates from Csf1op/Csf1op mice. The levels of IL-12 in liver homogenates of the two genotypes however were similar. Significant concentrations of neither TNF-α nor IL-18 could be detected in liver homogenates. IL-6 levels in liver homogenates from +/Csf1op mice were high (Table 2) but significantly (P < 0.001, Student's t test) lower in Csf1op/Csf1op mice. The Th2 cytokines IL-4, IL-5, and IL-10 were not detectable in the liver homogenates of either control or Csf1op/Csf1op mice (Table 2).
TABLE 2.
Cytokine levels in liver homogenates of +/Csf1op and Csf1op/Csf1op mice following L. monocytogenes infectiona
| Cytokine/chemokine | Cytokine levelb in liver homogenate of mouse strain
|
|
|---|---|---|
| +/Csf1op | Csf1op/Csf1op | |
| IFN-γ | 115 ± 10 | 20 ± 2* |
| TNF-α | ND | ND |
| IL-4 | ND | ND |
| IL-5 | ND | ND |
| IL-6 | 144 ± 24 | 33 ± 2* |
| IL-10 | ND | ND |
| IL-12 | 893 ± 135 | 1,026 ± 235+ |
| IL-18 | ND | ND |
| MIP-2 | 702 ± 14 | 752 ± 20+ |
| KC | 736 ± 26 | 800 ± 30+ |
| MCP-1 | 785 ± 65 | 114 ± 14* |
| MIP-1α | 235 ± 25 | 36 ± 3* |
Mice (8 per group) were infected with L. monocytogenes at 104 cells i.v. Livers were taken at 24 and 48 h p.i. and homogenized in 2 ml of PBS with 1% Triton. Tissue homogenates were centrifuged to remove particulate matter. Cytokine levels in supernatants were quantitated by ELISA.
Data (mean ± SEM) shown for cytokines are from 48-h-p.i. mice; similar results were obtained at 24 h p.i. Data shown for chemokines are from 24 h p.i. At 48 h p.i, chemokine levels were low to undetectable. The experiment was repeated twice with similar results. ND, not detectable; ∗, statistically different (P < 0.001, Student's t test) +, not statistically different.
Because liver macrophage recruitment was severely retarded in nullizygous mice, we measured concentrations of the chemokines MCP-1 and MIP-1α in liver homogenates. The concentration at 24 h p.i. of both of these chemokines was significantly lower in Csf1op/Csf1op mice (about sevenfold) as compared to that of in controls (Table 2). Because of the abundant accumulation of neutrophils, we determined concentrations of the neutrophil chemoattractants KC and MIP-2, the mouse IL-8 homologues (3). It is interesting that there was no difference in the concentration of either of these chemokines between control and mutant mice at 24 and 48 h p.i. (Table 2). KC and MIP-2 were detected as early as 8 h p.i. at similar levels in the livers and spleens of both +/Csf1op mice and Csf1op/Csf1op mice (data not shown).
Reduced macrophage activation in Csf1op/Csf1op mice.
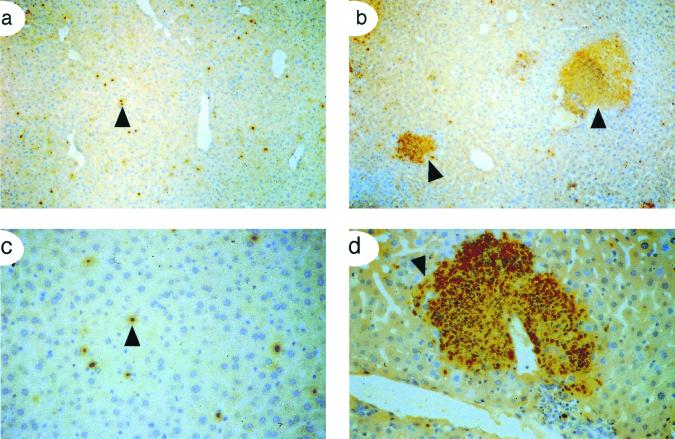
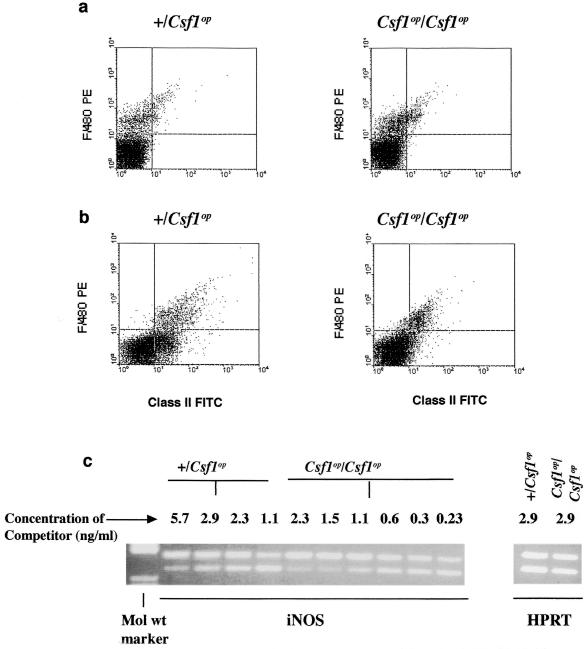
IFN-γ plays a major role in activating macrophages during infection with L. monocytogenes. Since IFN-γ production was significantly lower in Csf1op/Csf1op mice, the activation state of splenic macrophages, identified by F4/80 expression, was determined by measuring the surface expression of MHC class II. The population of F4/80+ macrophages was similar in the spleens of +/Csf1op and Csf1op/Csf1op mice (Fig. 6a). This is consistent with previous results in uninfected animals and has been confirmed by a wide variety of other macrophage markers (8) and the similar sizes of spleens in these mice (data not shown). In +/Csf1op mice at 24 and 48 p.i., F4/80+ macrophages were activated as assessed by a marked increase in class II MHC expression (Fig. 6b). However, in Csf1op/Csf1op mice, fewer F4/80+ cells showed high MHC class II expression (Fig. 6b), with levels very similar to those of the uninfected control (data not shown).
FIG. 6.
Reduced macrophage activation in Csf1op/Csf1op mice. Splenocytes were pooled from five mice each at 24 and 48 h p.i. and were analyzed (a) for a macrophage surface marker (F4/80) only and (b) for class II MHC expression on the F4/80+ macrophages. Data shown are from 48-h-p.i. mice. The experiment was repeated once with similar results. (c) Quantitative RT-PCR showing another marker for macrophage activation, iNOS, in RNA isolated from the spleens of infected mice. The point of equivalence of the experimental (lower) compared to competitor (upper) band is the iNOS message in +/Csf1op (2.3-ng) and Csf1op/Csf1op (0.3-ng) mice. Controls using HPRT showed comparable levels of mRNA expression between +/Csf1op and Csf1op/Csf1op mice, indicating an equivalent input of mRNA into the assay. The experiment was repeated once with similar results.
NO synthesized by macrophages, hepatocytes, and other cells is utilized as one mechanism of restricting the replication of L. monocytogenes. Inducible nitric oxide synthase (iNOS) is the major enzyme responsible for producing this NO, and macrophages are its major source after activation (18, 60). In the spleen, iNOS mRNA expression as assessed by competitive-quantitativeRT-PCR, was significantly (about sevenfold) lower in Csf1op/Csf1op compared to that of control mice (Fig. 6c). Similar differences were found for iNOS mRNA expression in the liver (data not shown). This result, together with the finding of reduced MHC class II expression, indicates a relative failure of these mice to produce appreciable concentrations of IFN-γ.
Reduced bacterial load following CSF-1, IFN-γ, and IL-6 treatment of Csf1op/Csf1op mice.
Since Csf1op/Csf1op mice are deficient in CSF-1, we investigated the effectiveness of CSF-1 given immediately before infection and concurrent with it to confer protection to Csf1op/Csf1op mice from L. monocytogenes. CSF-1 was administered subcutaneously at doses that had previously been established, to elevate circulating concentrations to control level (8). This treatment reduced L. monocytogenes burden in both liver (33-fold) and spleen (14-fold) compared to the saline-treated control group (Table 3). This result is similar to previously reported data in other wild-type mice (27).
TABLE 3.
Increased resistance of Csf1op/Csf1op mice to L. monocytogenes following CSF-1, IFN-γ, or IL-6 administration
| Cytokine treatment | Mean (±SEM) CFU of L. monocytogenes ind:
|
|
|---|---|---|
| Spleen | Liver | |
| CSF-1a | ||
| No CSF-1 | 4.5 × 105 ± 8.9 × 104 | 2.6 × 106 ± 4.6 × 105 |
| CSF-1 | 3.2 × 104 ± 3.4 × 103* | 7.9 × 104 ± 1.0 × 104* |
| IFN-γb | ||
| No IFN-γ | 3.3 × 106 ± 3.9 × 105 | 1.0 × 106 ± 5.0 × 104 |
| IFN-γ | 4.1 × 105 ± 7.9 × 104* | 1.2 × 105 ± 2.9 × 104* |
| IL-6c | ||
| No IL-6 | Not done | 2.0 × 107 ± 3.0 × 106 |
| IL-6 | Not done | 4.1 × 105 ± 6.8 × 104* |
Csf1op/Csf1op mice (six per group) were given CSF-1 for 3 days before infection with 104 L. monocytogenes. At 48 h. p.i., mice were sacrificed and CFU recovered from liver and spleen were enumerated.
Mice were given IFN-γ in a single injection (104 IU) i.v. 2 h before infection with 104 L. monocytogenes. Mice were grouped as in a above. CFU enumeration was performed as for the CSF-1 group.
Mice were injected with rIL-6 at day −1, 0, and +1 p.i., and CFU recovered from liver were enumerated 48 h p.i. The experiment was repeated once with similar results.
∗, P < 0.001, Student's t test;Not done, spleens were used for in vitro studies.
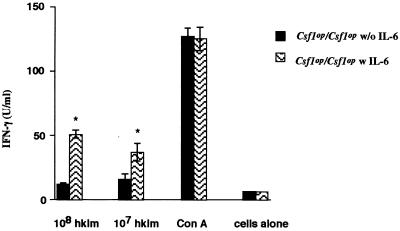
A major deficiency in the L. monocytogenes-infected Csf1op/Csf1op mice was the reduced ability to synthesize IFN-γ. To determine whether this is the cause of at least part of the observed increased bacterial load, these mice were treated with 104 units of IFN-γ i.v. 2 h prior to infection with L. monocytogenes, and they were sacrificed at 2 days p.i. The IFN-γ treatment significantly reduced the L. monocytogenes CFU, by approximately 1 order of magnitude, in both liver and spleen (Table 3). Because the IFN-γ-inducing cytokines IL-12 and IL-18 were normal in Csf1op/Csf1op mice and because of the report that IL-6 induces IFN-γ production in mice (31), we treated Csf1op/Csf1op mice with IL-6 1 day prior to infection and daily thereafter. This treatment reduced the bacterial burden by 46-fold in the liver 48 h after infection (Table 3). In the spleens of mice treated with IL-6, the synthesis of IFN-γ by cultured splenocytes stimulated by either 107 or 108 HKLM cells was also significantly (P < 0.005, Student's t test) increased 48 h after infection (Fig. 7). These data confirm that the low level of IFN-γ synthesis in Csf1op/Csf1op mice resulted in a failure to activate macrophages to effectively kill L. monocytogenes and that the synthesis of IFN-γ was, at least in part, regulated by IL-6.
FIG. 7.
Recombinant IL-6 treatment of Csf1op/Csf1op mice increases IFN-γ synthesis following Listeria challenge. Csf1op/Csf1op mice were injected with 12.5 μg of rmIL-6 in saline or with the saline vehicle alone subcutaneously at days −1, 0, and +1 of Listeria (104 cells i.v.) infection. At 48 h p.i., splenocytes (5 × 105/well) were pooled from five mice and were stimulated in vitro with HKLM or with ConA. After 24 and 48 h, culture supernatants were collected, and IFN-γ concentrations were measured by ELISA. Data shown are from 48-h-culture supernatant. Each bar represents the mean of triplicate values; error bars, standard errors of mean; ∗, P < 0.005 by Student's t test. Results are representative of two independent experiments.
DISCUSSION
Macrophages play a central role in host immune response to L. monocytogenes, representing a major habitat for the bacteria as well as being major effectors of defense response (26, 32). Mice homozygous for a recessive null mutation (Csf1op) in the mononuclear phagocytic growth factor CSF-1 gene have a severe depletion of macrophages, as assayed with a wide variety of markers and by uptake of India ink (8, 25, 47, 56, 59) in many organs such as the liver, ovary, testis, and kidney. However, in other tissues, particularly those associated with the immune function, such as the spleen, densities of macrophages are relatively normal (8, 39). Macrophages can therefore be divided into two classes, CSF-1 dependent and CSF-1 independent, at least as far as their tissue density is concerned.
Immediately following i.v. listerial infection, bacterial titers in both spleen and liver were similar between each genotype, but thereafter the rate of infection in both tissues progressed more rapidly in the CSF-1-null mutant mice, which suggests that even those macrophages that were present in the spleen were compromised in their immunological function in the absence of CSF-1. This view is supported by the proximate treatment of Csf1op/Csf1op mice with CSF-1 during the course of infection, which significantly reduces the bacterial titer in both spleen and liver in a manner similar to that previously shown for wild-type mice (27). These data suggest that CSF-1-regulated macrophages are essential in the immune response to L. monocytogenes. This is consistent with numerous studies implicating macrophages, including those that depleted macrophages by chemical means (15, 26, 52).
A caveat to this conclusion is that the Csf1op mutation is carried on a mixed C3H and C57BL/6 background, representing strains that are relatively susceptible and resistant to listerial infection, respectively (10). Unfortunately several attempts to bring the Csf1op mutation onto an inbred background have failed (our unpublished data). Therefore, we chose a bacterial titer that overcame the small amount of differential resistance and produced identical rates of mortality in C3H and C57BL/6 mice. Furthermore, the Listeria resistance gene maps to chromosome 2 (Jackson Laboratory, Mouse Genome Informatics [www.informatics.jax.org]), different from either CSF-1 (chromosome 3) or the CSF-1 R (chromosome 18) (Jackson Laboratory, Mouse Genome Informatics [www.informatics.jax.org]). Consequently, under the closed breeding condition of our colony, which has been maintained for 10 years by sibling intermating, there would be independent segregation of the Lisr from the Csf1op allelle. While this might increase the variability of the data, the effect of the Lisr gene is randomly ascribed to both control and Csf1op/Csf1op mice.
Early in listerial infection there is an increase in macrophage density in the liver and spleen in control but not in Csf1op/Csf1op mice. Correlated with this macrophage recruitment in control mice is the synthesis of the monocyte chemoattractant chemokines MCP-1 and MIP-1α in both liver and spleen. Both of these chemokines play an important role in the regulation of leukocyte trafficking by eliciting directional migration of mononuclear phagocytes to inflammatory foci (21, 43). Csf1op/Csf1op mice had a very significantly reduced ability to synthesize MCP-1 and MIP-1α and a concordant inability to recruit monocytes to sites of infection. MCP-1 interacts with the CCR2 chemokine receptor while MIP-1α interacts with CCR5 and CCR1 receptors (2). CCR2−/− mice have defects in the recruitment of peritoneal macrophages in response to thioglycollate, and in in vitro chemotaxis assays, the CCR2−/− leukocytes fail to respond to MCP-1. Furthermore, CCR2−/− mice were unable to clear infection by L. monocytogenes (28). CCR5-deficient mice are also relatively unable to clear L. monocytogenes (62). However, this effect was small, and there appeared to be normal macrophage recruitment to the site of infection in CCR5-deficient mice. In light of these reports and our studies, we concluded that a major function for CSF-1-regulated resident macrophages early in the innate immune response is the synthesis of MCP-1, thereby recruiting more potent macrophages to the inflammatory lesion.
During listerial infection of control mice, macrophages are recruited into the tissue, and the early recruitment of neutrophils is regulated in a controlled manner. However, in Csf1op/Csf1op mice, there develop numerous microabscesses filled with neutrophils. The concentrations of the neutrophil chemoattractant IL-8 homologues KC and MIP-2 are similar in both control and mutant mice. This strongly suggests a role for the newly recruited macrophage in regulating the extent of neutrophil influx. Recently (1), it has been shown that resident macrophages can play a role in PMN accumulation via IL-10-inhibiting action on PMN influx. It is interesting that at 12 h p.i., +/Csf1op mice produced a peak of IL-10 that was significantly higher than the negligible amount found in Csf1op/Csf1op mice. The absence of IL-10 at 12 h could partially explain the massive neutrophil influx in these mice. The IL-10 is likely to be macrophage derived because of its absence in Csf1op/Csf1op mice and the failure to detect IL-10 in T-cell assays.
The pleiotropic cytokine IL-6 is produced by a variety of cell types and is an important factor in host defenses to Listeria infection. Administration of anti-IL-6 antibody to mice increases numbers of bacteria in various organs following Listeria infection, and mice homozygous for a null mutation in the IL-6 are highly susceptible to this infection (12). IL-6 synthesis is almost undetectable in Csf1op/Csf1op spleens and livers early in infection, in contrast to the large amounts synthesized in control mice. In liver, Kupffer cells (liver macrophages) are the main producers of IL-6 (20). These cells are severely depleted in Csf1op/Csf1op mice, providing an explanation for the low IL-6 synthesis in the liver, but in spleen, where macrophage are normal, this depletion suggests a CSF-1-regulated macrophage function.
IL-6 is a potent inducer of MCP-1 expression and is secreted by primary peripheral blood mononuclear cells (5) and brain macrophages (7). The low IL-6 levels following infection of Csf1op/Csf1op mice, together with the reduced numbers of Kupffer cells in these mice, could result in the lower MCP-1 synthesis observed in mutant mice. IL-6 also influences the oxidative burst and degranulation capacity of human neutrophils. Thus, the low IL-6 levels in Csf1op/Csf1op mice could result in neutrophils that are less efficient in killing the Listeria cells. This is also consistent with recent data showing that the early neutrophil response should be superceded by a mononuclear phagocytic influx for effective clearance of Listeria cells and the avoidance of massive tissue damage (14). However, this is the first report implicating CSF-1 rather than the more conventional granulocyte-macrophage CSF in such neutrophil responses (17, 42).
The other major deficiency in the immune response of Csf1op/Csf1op mice to Listeria infection is in the production of IFN-γ predominantly by CD4 but also by CD8 T cells, even though T cells from mutant mice were present in comparable numbers and could respond to HKLM in proliferation assays similarly to T cells from control mice. Also similarly, IFN-γ concentrations in liver homogenates, where IFN-γ is probably synthesized by NK cells, were significantly reduced in Csf1op/Csf1op mice compared to their heterozygous counterparts. The deficiency in IFN-γ was reflected in the reduced activation of macrophages as shown by reduced class II MHC and iNOS expression in Csf1op/Csf1op mice compared to that in control mice. The reduced IFN-γ was at least in part responsible for the increased susceptibility of Csf1op/Csf1op mice to this bacterial pathogen, because treatment of these mice with IFN-γ resulted in a significantly lower bacterial load in both spleen and liver. Indeed, the importance of IFN-γ in the host response has been shown by a number of methods including analyses of the sensitivity of IFN-γ−/− mice to L. monocytogenes (13, 23).
IL-12 and IL-18 are thought to be the major inducers of IFN-γ. Consequently, neutralization of IL-12 decreases resistance to Listeria infection (50), and IL-12 nullizygous mice are significantly more susceptible to sublethal doses of Listeria cells than are wild-type mice (6). It is interesting that we detected high but similar concentrations of IL-12 and IL-18 in Csf1op/Csf1op and control spleen and liver homogenates. Consequently, neither IL-12 nor IL-18 seemed to be responsible for the difference in IFN-γ synthesis between these mice. This is interesting since NK cells and T cells, the targets of IL-12, showed normal numbers and cytotoxic and proliferative responses, respectively, in Csf1op/Csf1op mice. However, these cells appeared to be unable to synthesize IFN-γ at levels found in control mice, suggesting that there must be a cofactor or regulatory molecule required for IFN-γ synthesis that is missing in Csf1op/Csf1op mice.
IL-6 produced early during infection can directly or indirectly induce IFN-γ production by activated T cells (31). To determine the role of IL-6 in our model system, we administered IL-6 to Csf1op/Csf1op mice before and during the subsequent infection. This resulted in a substantial decrease in bacterial burden in the liver and a significant elevation in the ability of splenocytes to synthesize IFN-γ. Our results support the conclusion of Liu et al. (31), but they are in contrast to those observed in IL-6-nullizygous mice that had normal IFN-γ production and macrophage activation following L. monocytogenes infection (12). However, our data strongly argue that IL-6 synthesis is a central component of the CSF-1-regulated response to listerial infection and that IL-6 is necessary for IFN-γ production. IL-12 plays a central role in the development of IFN-γ secreting Th1 cells in response to L. monocytogenes (22, 30, 33, 51). Since IL-12 is required for the induction of IFN-γ and, given its concentration, is not reduced in Csf1op/Csf1op mice, our data suggest a role for IL-6 as a cofactor for IL-12 induction of IFN-γ.
In conclusion, this study has demonstrated the central role of CSF-1-regulated macrophages, particularly resident macrophages in the immune response to L. monocytogenes. In the absence of CSF-1, monocytes are not recruited to sites of infection to replace the neutrophils that were attracted early in the response. Instead, neutrophils become concentrated in microabscesses but are insufficient to control the infection, which becomes rampant in hepatocytes and throughout the spleen. The lack of monocyte recruitment appeared to be due to a failure by resident macrophages to synthesize the chemokine MCP-1. Without this macrophage recruitment, neutrophil egress was not observed, probably because of the absence of macrophage-derived IL-10. The Listeria infection in the CSF-1-nullizygous mice was also characterized by a significantly reduced ability to synthesize IFN-γ that results in poor macrophage activation and an inadequate Th1 response. IL-6, a cytokine that has been shown in other circumstances to regulate both IFN-γ and MCP-1 synthesis, is produced at only very low levels in Csf1op/Csf1op mice compared to control mice. Administration of IL-6 not only dramatically reduced bacterial burden in Csf1op/Csf1op mice but also induced IFN-γ production. Thus, IL-6 appears to be a central player in the CSF-1 regulation of the immune response to L. monocytogenes.
ACKNOWLEDGMENTS
This research was supported by NIH grant HD30280 and the Comprehensive Cancer Center core grant P30-CA13330.
We thank Jim Lee for excellent technical assistance and D. Gebhard of the Comprehensive Cancer Center FACS facility for help with FACS analysis. Our thanks also go to M. Scharff, A. Casadevall, S. Jung, and E. Barber for constructive comments on the manuscript.
REFERENCES
- 1.Ajuebor M, Das A, Virag L, Flower R J, Szabo C, Perretti M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophïl migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J Immunol. 1999;162:1685–1691. [PubMed] [Google Scholar]
- 2.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307:97–101. doi: 10.1016/0014-5793(92)80909-z. [DOI] [PubMed] [Google Scholar]
- 4.Bancroft G J, Bosma M J, Bosma G C, Unanue E R. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell-independent mechanism. J Immunol. 1986;137:4–9. [PubMed] [Google Scholar]
- 5.Biswas P, Delfanti F, Bernasconi S, Mengozzi M, Cota M, Polentarutti N, Mantovani A, Lazzarin A, Sozzani S, Poli G. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood. 1998;91:258–265. [PubMed] [Google Scholar]
- 6.Brombacher F, Dorfmuller A, Magram J, Dai W J, Kohler G, Wunderlin A, Palmer-Lehmann K, Gately M K, Alber G. IL-12 is dispensable for innate and adaptive immunity against low doses of Listeria monocytogenes. Int Immunol. 1999;11:325–332. doi: 10.1093/intimm/11.3.325. [DOI] [PubMed] [Google Scholar]
- 7.Calvo C F, Yoshimura T, Gelman M, Mallat M. Production of monocyte chemotactic protein-1 by rat brain macrophages. Eur J Neurosci. 1996;8:1725–1734. doi: 10.1111/j.1460-9568.1996.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 8.Cecchini M G, Dominguez M G, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard J W, Stanley E R. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 9.Chang M-D Y, Stanley E R, Khalili H, Chisholm O, Pollard J W. Osteopetrotic (op/op) mice deficient in macrophage have the ability to mount a normal T-cell dependent immune response. Cell Immunol. 1995;162:146–152. doi: 10.1006/cimm.1995.1062. [DOI] [PubMed] [Google Scholar]
- 10.Cheers C, Stanley E R. Macrophage production during murine listeriosis: colony-stimulating factor 1 (CSF-1) and CSF-1 binding cells in genetically resistant and susceptible mice. Infect Immun. 1988;56:2972–2978. doi: 10.1128/iai.56.11.2972-2978.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen P E, Chisholm O, Arceci R J, Stanley E R, Pollard J W. Absence of colony stimulating factor-1 in osteopetrotic (csfmop/csfmop) mice results in male fertility defects. Biol Reproduction. 1996;55:310–317. doi: 10.1095/biolreprod55.2.310. [DOI] [PubMed] [Google Scholar]
- 12.Dalrymple S A, Lucian L A, Slattery R, McNeil T, Aud D M, Fuchnio S, Lee F, Murray R. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun. 1995;63:2262–2268. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 14.DiTirro J, Rhoades E R, Roberts A D, Burke J M, Mukasa A, Cooper A M, Frank A A, Born W K, Orme I M. Disruption of the cellular inflammatory response to Listeria monocytogenes infection in mice with disruptions in targeted genes. Infect Immun. 1998;66:2284–2289. doi: 10.1128/iai.66.5.2284-2289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebe Y, Hasegawa G, Takatsuka H, Umezu H, Mitsuyama M, Arakawa M, Mukaida N, Naito M. The role of Kupffer cells and regulation of neutrophil migration into the liver by macrophage inflammatory protein-2 in primary listeriosis in mice. Pathol Int. 1999;49:519–532. doi: 10.1046/j.1440-1827.1999.00910.x. [DOI] [PubMed] [Google Scholar]
- 16.Edelson B, Cossart P, Unanue E. Cutting edge: paradigm revisited: antibody provides resistance to Listeria infection. J Immunol. 1999;163:4087–4090. [PubMed] [Google Scholar]
- 17.Escribano S, Cuenllas E, Gaitan S, Tejero C. Delayed neutrophil apoptosis after total body irradiation in mice: the role of granulocyte-macrophage colony-stimulating factor in neutrophil function. Exp Hematol. 1998;26:942–949. [PubMed] [Google Scholar]
- 18.Flesch I E, Hess J H, Kaufmann S H. NADPH diaphorase staining suggests a transient and localized contribution of nitric oxide to host defence against an intracellular pathogen in situ. Int Immunol. 1994;6:1751–1757. doi: 10.1093/intimm/6.11.1751. [DOI] [PubMed] [Google Scholar]
- 19.Gregory S H, Barczynski L K, Wing E J. Effector function of hepatocytes and Kupffer cells in the resolution of systemic bacterial infections. J Leukoc Biol. 1992;51:421–424. doi: 10.1002/jlb.51.4.421. [DOI] [PubMed] [Google Scholar]
- 20.Gregory S H, Wing E J, Danowski K L, van Rooijen N, Dyer K F, Tweardy D J. IL-6 produced by Kupffer cells induces STAT protein activation in hepatocytes early during the course of systemic listerial infections. J Immunol. 1998;160:6056–6061. [PubMed] [Google Scholar]
- 21.Hausmann E H, Berman N E, Wang Y Y, Meara J B, Wood G W, Klein R M. Selective chemokine mRNA expression following brain injury. Brain Res. 1998;788:49–59. doi: 10.1016/s0006-8993(97)01160-8. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 23.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 24.Hume D A, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: identification of resident macrophages in renal medullary and cortical interstitium and the juxtaglomerular complex. J Exp Med. 1983;157:1704–1709. doi: 10.1084/jem.157.5.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito S, Naito M, Kobayashi Y, Takatsuka H, Jiang S, Usuda H, Umezu H, Hasegawa G, Arakawa M, Shultz L, Elomaa O, Tryggvason K. Roles of a macrophage receptor with collagenous structure (MARCO) in host defense and heterogeneity of splenic marginal zone macrophages. Arch Histol Cytol. 1999;62:83–95. doi: 10.1679/aohc.62.83. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 27.Kayashima S, Tsuru S, Shinomiya N, Katsura Y, Motoyoshi K, Rokutanda M, Nagata N. Effects of macrophage colony-stimulating factor on reduction of viable bacteria and survival of mice during Listeria monocytogenes infection: characteristics of monocyte subpopulations. Infect Immun. 1991;59:4677–4680. doi: 10.1128/iai.59.12.4677-4680.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ledbetter J A, Herzenberg L A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Kurlander R J. Analysis of the interrelationship between IL-12, TNF-alpha, and IFN-gamma production during murine listeriosis. Cell Immunol. 1995;163:260–267. doi: 10.1006/cimm.1995.1125. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Simpson R J, Cheers C. Role of IL-6 in activation of T cells for acquired cellular resistance to Listeria monocytogenes. J Immunol. 1994;152:5375–5380. [PubMed] [Google Scholar]
- 32.Mackaness G B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969;129:973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C Y, Ferrante J, Stewart C, Sarmiento U, Faherty D A, Gately M K. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 34.Mitsuyama M, Takeya K, Nomoto K, Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978;106:165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- 35.Naito M, Hayashi S-I, Yoshida H, Nishikawa S-I, Shultz L D, Takahashi K. Abnormal differentiation of tissue macrophage populations in ‘osteopetrosis’ (op) mice defective in the production of macrophage colony-stimulating factor. Am J Pathol. 1991;139:657–667. [PMC free article] [PubMed] [Google Scholar]
- 36.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 37.Pierres A, Naquet P, Van Agthoven A, Bekkhoucha F, Denizot F, Mishal Z, Schmitt-Verhulst A M, Pierres M. A rat anti-mouse T4 monoclonal antibody (H129.19) inhibits the proliferation of Ia-reactive T cell clones and delineates two phenotypically distinct (T4+, Lyt-2, 3-, and T4-, Lyt-2, 3+) subsets among anti-Ia cytolytic T cell clones. J Immunol. 1984;132:2775–2782. [PubMed] [Google Scholar]
- 38.Pinto A J, Stewart D, van Rooijen N, Morahan P S. Selective depletion of liver and splenic macrophages using liposomes encapsulating the drug dichloromethylene diphosphonate: effects on antimicrobial resistance. J Leukoc Biol. 1991;49:579–586. doi: 10.1002/jlb.49.6.579. [DOI] [PubMed] [Google Scholar]
- 39.Pollard J W, Stanley E R. Pleiotropic roles for CSF-1 in development defined by the mouse mutation osteopetrotic (op) Adv Dev Biochem. 1996;4:153–193. [Google Scholar]
- 40.Reiner S L, Zheng S, Corry D B, Locksley R M. Constructing polycompetitor cDNAs for quantitative PCR. J Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. . (Errata, 173:133 and 175:275.) [DOI] [PubMed] [Google Scholar]
- 41.Samsom J N, Annema A, Groeneveld P H, van Rooijen N, Langermans J A, van Furth R. Elimination of resident macrophages from the livers and spleens of immune mice impairs acquired resistance against a secondary Listeria monocytogenes infection. Infect Immun. 1997;65:986–993. doi: 10.1128/iai.65.3.986-993.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seymour J F, Lieschke G J, Grail D, Quilici C, Hodgson G, Dunn A R. Mice lacking both granulocyte colony-stimulating factor (CSF) and granulocyte-macrophage CSF have impaired reproductive capacity, perturbed neonatal granulopoiesis, lung disease, amyloidosis, and reduced long-term survival. Blood. 1997;90:3037–3049. [PubMed] [Google Scholar]
- 43.Shi M M, Chong I W, Long N C, Love J A, Godleski J J, Paulauskis J D. Functional characterization of recombinant rat macrophage inflammatory protein-1 alpha and mRNA expression in pulmonary inflammation. Inflammation. 1998;22:29–43. doi: 10.1023/a:1022391623063. [DOI] [PubMed] [Google Scholar]
- 44.Singh I G, Mukherjee R, Talwar G P, Kaufmann S H. In vitro characterization of T cells from Mycobacterium w-vaccinated mice. Infect Immun. 1992;60:257–263. doi: 10.1128/iai.60.1.257-263.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Springer T, Galfre G, Secher D S, Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979;9:301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- 46.Stanley E R, Guilbert L T, Tushinski R J, Bartelmez S H. CSF-1: a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21:151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi K, Natio M, Shultz L D, Hayashi S-I, Nishikawa S-I. Differentiation of dendritic cell populations in macrophage colony-stimulating factor-deficient mice homozygous for the osteopetrosis (op) mutation. J Leukoc Biol. 1993;53:19–28. doi: 10.1002/jlb.53.1.19. [DOI] [PubMed] [Google Scholar]
- 48.Teitelbaum R, Schubert W, Gunther L, Kress Y, Macaluso F, Pollard J W, McMurray D, Bloom B R. The M cell as a portal of entry to the lung for the bacterial pathogen for Mycobacterium tuberculosis. Immunity. 1999;10:641–650. doi: 10.1016/s1074-7613(00)80063-1. [DOI] [PubMed] [Google Scholar]
- 49.Tepper R I, Coffman R L, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 50.Tripp C S, Gately M K, Hakimi J, Ling P, Unanue E R. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice: reversal by IFN-gamma. J Immunol. 1994;152:1883–1887. [PubMed] [Google Scholar]
- 51.Tripp C S, Wolf S F, Unanue E R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Unanue E R. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr Opin Immunol. 1997;9:35–43. doi: 10.1016/s0952-7915(97)80156-2. [DOI] [PubMed] [Google Scholar]
- 53.Unanue E R. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol Rev. 1997;158:11–25. doi: 10.1111/j.1600-065x.1997.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 54.Webb S E, Pollard J W, Jones G E. Direct observation and quantification of macrophage chemoattraction to the growth factor CSF-1. J Cell Sci. 1996;109:793–803. doi: 10.1242/jcs.109.4.793. [DOI] [PubMed] [Google Scholar]
- 55.Wiktor-Jedrzejczak W, Ansari A A, Szperl M, Urbanowska E. Distinct in vivo functions of two macrophage subpopulations as evidenced by studies using macrophage-deficient op/op mouse. Eur J Immunol. 1992;22:1951–1954. doi: 10.1002/eji.1830220743. [DOI] [PubMed] [Google Scholar]
- 56.Wiktor-Jedrzejczak W, Bartocci A, Ferrante A W, Jr, Ahmed-Ansari A, Sell K W, Pollard J W, Stanley E R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiktor-Jedrzejczak W, Dzwigala B, Szperl M, Maruszynski M, Urbanowska E, Szwech P. Colony-stimulating factor 1-dependent resident macrophages play a regulatory role in fighting Escherichia coli fecal peritonitis. Infect Immun. 1996;64:1577–1581. doi: 10.1128/iai.64.5.1577-1581.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiktor-Jedrzejczak W, Gordon S. Cytokine regulation of the macrophage (Mø) system studied using the colony stimulating factor-1-deficient op/op mouse. Physiol Rev. 1996;76:927–947. doi: 10.1152/physrev.1996.76.4.927. [DOI] [PubMed] [Google Scholar]
- 59.Witmer-Pack M D, Hughes D A, Schuler G, Lawson L, McWilliam A, Inaba K, Steinman R M, Gordon S. Identification of macrophages and dendritic cells in the osteopetrotic (op/op) mouse. J Cell Sci. 1993;104:1021–1029. doi: 10.1242/jcs.104.4.1021. [DOI] [PubMed] [Google Scholar]
- 60.Yap G S, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J Exp Med. 1999;189:1083–1092. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida H, Hayashi S-I, Kunisada T, Ogawa M, Nishikawa S-I, Okamura H, Sudo T, Shultz L D. The murine mutation osteopetrosis in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Y, Kurihara T, Ryseck R P, Yang Y, Ryan C, Loy J, Warr G, Bravo R. Impaired macrophage function and enhanced T cell-dependent immune response in mice lacking CCR5, the mouse homologue of the major HIV-1 coreceptor. J Immunol. 1998;160:4018–4025. [PubMed] [Google Scholar]