Abstract
Background
Atrial fibrillation (AF) is a common complication of sepsis. It is unclear whether norepinephrine, an α- and β-agonist, and phenylephrine, an α-agonist, are associated with different heart rates among patients with sepsis and AF.
Research Question
Among patients with sepsis and AF, what is the difference in heart rate after phenylephrine initiation vs norepinephrine initiation?
Study Design and Methods
With the use of an extensive database, we identified patients with sepsis and AF at the time of norepinephrine or phenylephrine initiation. We estimated the difference in heart rate between patients who received phenylephrine or norepinephrine 1 and 6 h after vasopressor initiation with the use of multivariable-adjusted linear regression, tested for effect modification by heart rate, and stratified by baseline heart rate ≥ 110 or < 110 beats/min. Secondary outcomes included conversion to sinus rhythm, bradycardia, vasopressor duration, ICU and hospital length of stay, and hospital death. Exploratory analyses were adjusted for practices that occurred after vasopressor initiation; sensitivity analyses used interrupted time series to estimate the difference in average heart rate between patients who received phenylephrine or norepinephrine.
Results
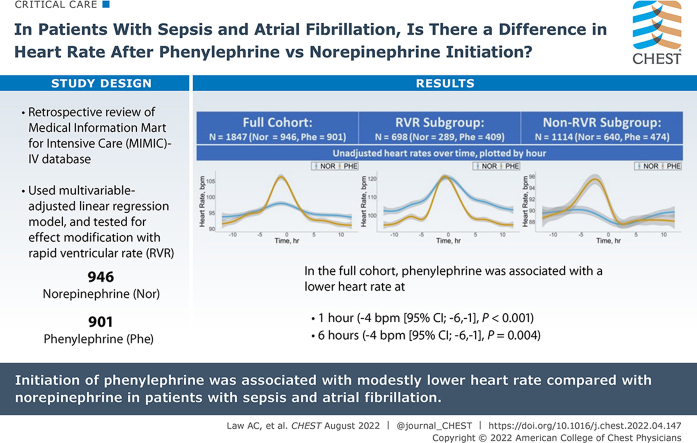
Among 1847 patients with sepsis and AF, 946 patients (51%) received norepinephrine, and 901 patients (49%) received phenylephrine. After multivariable adjustment, phenylephrine was associated with a lower heart rate at 1 h (−4 beats/min; 95% CI, −6 to −1; P < .001) and 6 h (−4 beats/min; 95% CI, −6 to −1; P = .004). Higher heart rate before vasopressor administration was associated with larger heart rate reduction in patients who received phenylephrine compared with norepinephrine. There were no differences in secondary outcomes. Results were similar in exploratory and sensitivity analyses.
Interpretation
In patients with sepsis and AF, the initiation of phenylephrine was associated with modestly lower heart rate compared with norepinephrine. Heart rate at vasopressor initiation appeared to be an important effect modifier. Whether modest reductions in heart rate are associated with clinical outcomes requires further study.
Key Words: atrial fibrillation, sepsis, vasopressor
Abbreviations: AF, atrial fibrillation; MIMIC, Medical Information Mart for Intensive Care; RVR, rapid ventricular rate
Graphical Abstract
Take-home Points.
Study Question: Among patients with sepsis and AF, what is the difference in heart rate after phenylephrine initiation vs norepinephrine initiation?
Results: Among patients with sepsis and AF, receipt of phenylephrine (compared with norepinephrine) was associated with a lower heart rate at 1 h (−4 beats/min; 95% CI, −6 to −1) and 6 h (−4 beats/min; 95% CI, −6 to −1) after vasopressor initiation. Reductions in heart rate were larger among patients in rapid ventricular rate at time of vasopressor initiation. There were no differences in secondary outcomes (conversion to sinus, bradycardia, vasopressor duration, ICU/hospital length of stay, and death).
Interpretation: In patients with sepsis and AF, the initiation of phenylephrine was associated with modestly lower heart rates compared with norepinephrine. Heart rate at vasopressor initiation appears to be an important modifier, because the association between vasopressor choice and subsequent heart rate was greater in patients with rapid ventricular response at the time of vasopressor initiation. Larger, randomized, controlled studies are needed to determine whether the association between phenylephrine and decreased heart rate leads to different clinically important outcomes.
Atrial fibrillation (AF) is a common arrhythmia in patients with sepsis. Multiple physiologic perturbations that occur in sepsis may trigger the onset of AF, including electrolyte abnormalities, autonomic dysfunction, and inflammation.1, 2, 3, 4 The already-tenuous hemodynamic state in patients with sepsis may be exacerbated further by the loss of atrial systole and the onset of rapid ventricular rates (RVR) in AF.
Patients with AF who require vasopressors for septic shock often present a clinical dilemma for clinicians. Although the 2021 Surviving Sepsis Guidelines strongly recommend norepinephrine as the first-line vasopressor in patients with septic shock,5 clinicians may seek to avoid the chronotropic and arrhythmogenic β-1-agonist effects of norepinephrine (and second-line agents epinephrine and dopamine6) in patients with AF. Prior studies have investigated vasopressin’s catecholamine-sparing effect and found a decreased risk of tachyarrhythmias in patients who receive vasopressin alone or in combination with norepinephrine.7, 8, 9 Whether phenylephrine, which is a titratable catecholamine vasopressor without β-agonism, may be associated with lower heart rates than norepinephrine in critically ill patients with AF and shock is unknown.
Study Design and Methods
Data Source and Study Cohort
We conducted a retrospective cohort study using electronic health records data from the Medical Information Mart for Intensive Care (MIMIC)-IV database,10,11 which contains comprehensive clinical data from nine ICUs at a single center. We identified adult patients (≥ 18 years old) with sepsis, defined per sepsis-3 criteria, as suspicion of infection (receipt of antibiotics and sampling of bodily fluids for microbiologic culture) and organ dysfunction (acute increase in Sequential Organ Failure Assessment score ≥ 2).12,13 Nurse-documented cardiac rhythm is available on an approximate hourly basis in MIMIC (more frequently if cardiac rhythm changes); the identification of AF has been validated previously.14,15 Among patients with sepsis, we included the first episode in which a patient was initiated on either norepinephrine or phenylephrine therapy alone (Time 0) while in AF (and AF was the only heart rhythm documented in the hour that the vasopressor was initiated); vasopressors must have been initiated in the ICU (not receiving vasopressors in the first hour of ICU admission) and after a vasopressor-free period of ≥ 1 h. Although only patients initiated on monotherapy (norepinephrine or phenylephrine alone) were included, there were no restrictions on other vasopressor use ≥ 1 h before Time 0 or any time after Time 0. The maximum heart rate for each hour from 12 h before and after Time 0 were collected.
Primary outcomes were the heart rate at 1 and 6 h after Time 0. Secondary outcomes included conversion into sinus rhythm during 6-h follow up (high adrenergic tone may drive AF in critical illness; norepinephrine’s β-1-agonist effects may be associated with reduced probability of conversion to sinus rhythm), occurrence of bradycardia (heart rate, < 60 beats/min) during the 6-h follow up, vasopressor duration (hours from Time 0 to first hour without any vasopressor use), ICU and hospital length of stay, and hospital death.
Analysis
In primary analysis, we compared heart rates between patients who received phenylephrine and patients who received norepinephrine at 1 and 6 h after vasopressor initiation using multivariable linear regression models, adjusting for variables available at Time 0 that were deemed a priori to be confounders (ie, associated both with vasopressor choice and outcomes): demographics (age, sex, race) and clinical factors (type of ICU; history of heart failure; use of rate or rhythm control agents in the 6 h before vasopressor initiation; use of vasopressors of any kind in the 2 to 6 h before vasopressor initiation; heart rate change in the 6 h before Time 0; duration of AF; and the following Time 0 measurements: vasopressor dose in norepinephrine-equivalent dose (phenylephrine dose/10, μg/kg/min),16 heart rate, mean arterial pressure, mechanical ventilation status, and Sequential Organ Failure Assessment score). After testing for effect modification by heart rate (as a continuous variable) and vasopressor type on 1- and 6-h heart rate, we repeated analyses (adjusting for same covariables) stratified by those with rapid ventricular response (RVR subgroup; ie, maximum heart rate during the hour of vasopressor initiation > 110 beats/min) at the time of vasopressor initiation and by those without RVR (non-RVR subgroup). Similarly, we tested for effect modification by the presence of AF on ICU admission on association of vasopressor type with heart rate outcomes. Models that were analogous to the primary analysis were created for secondary outcomes.
In exploratory analysis, we evaluated whether the association between vasopressor choice and heart rate might be mediated by subsequent practice patterns. We repeated the aforementioned multivariable models for heart rate at 1 and 6 h and additionally included adjustment for direct current cardioversion, use of rate/rhythm control agents, the addition of any other vasopressor agents, and maximum norepinephrine or phenylephrine dose during the 1- and 6-h follow-up periods, respectively.
We conducted a sensitivity analysis using interrupted time series analysis with segmented regression,17,18 which allowed an alternative, quasi-experimental approach to assess the difference in heart rates between patients who received norepinephrine and patients who received phenylephrine after vasopressor initiation, accounting for heart rate trends before the initiation of the vasopressor. We assessed the average hourly heart rate among patients who received phenylephrine and patients who received norepinephrine, respectively, in the 12 h before and 12 h after vasopressor initiation. We modeled the difference in average hourly heart rates between patients who received phenylephrine and patients who received norepinephrine (phenylephrine-norepinephrine) and estimated the change in the difference in heart rate between cohorts after vasopressor initiation. Models included variables for time observed (ie, number of hours from start of observation, 0 to 24), onset of vasopressor (ie, before vs after vasopressor initiation), and hours after vasopressor initiation (ie, 0 to 12). Polynomial models (with quadratic and cubic terms for time-based variables) were explored for best fit by comparing adjusted-R2 values. Statistical testing was two-tailed, with an α = .05 with R (version 4.0.2; R Project for Statistical Computing). This study was deemed not human subjects research by the Boston University Medical Campus institutional review board.
Results
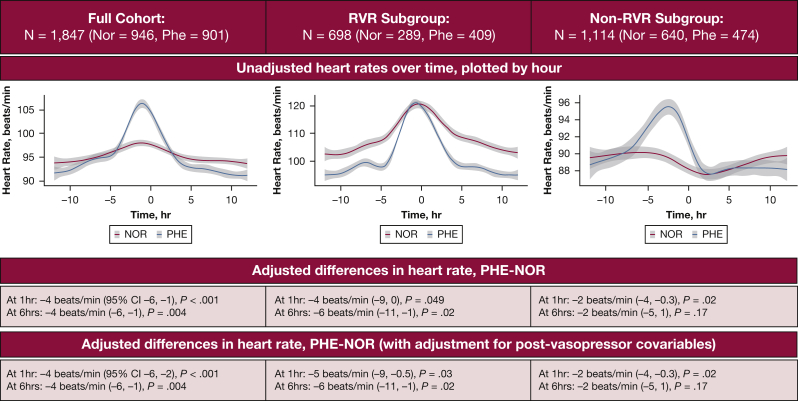
Among 27,139 patients admitted to an ICU with sepsis, 8,780 patients had AF; 1,847 patients were initiated on norepinephrine (946 [51%]) or phenylephrine (901 [49%]) alone while in AF. Demographics and baseline characteristics are shown in Table 1 (mean age: phenylephrine, 74.4 [SD, 11.2] years; norepinephrine, 75.6 [SD, 11.1] years; female patients: phenylephrine, 38%; norepinephrine, 41%). Patients who received phenylephrine had lower rates of prior congestive heart failure and a larger increase in heart rate in the 6 h before vasopressor initiation compared with the cohort that received norepinephrine. Unadjusted heart rates were higher before vasopressor initiation but lower after vasopressor initiation in patients who received phenylephrine compared with patients who received norepinephrine (Fig 1).
Table 1.
Demographic and Clinical Characteristics
| Variable | All Patients (N = 1,847) | Phenylephrine (n = 901) | Norepinephrine (n = 946) |
|---|---|---|---|
| Baseline variables, at time of vasopressor initiation | |||
| Age, mean (SD), y | 75.0 (11.2) | 74.4 (11.2) | 75.6 (11.1) |
| Female, No. (%) | 727 (39.4) | 339 (37.6) | 388 (41.0) |
| Race/ethnicity, No. (%) | |||
| White | 1,340 (72.6) | 660 (73.3) | 680 (71.9) |
| Black | 122 (6.6) | 46 (5.1) | 76 (8.0) |
| Hispanic | 36 (1.9) | 19 (2.1) | 17 (1.8) |
| Asian | 51 (2.8) | 25 (2.8) | 26 (2.7) |
| American Indian/Alaska Native | 3 (0.2) | 0 (0.0) | 3 (0.3) |
| Other | 295 (16.0) | 151 (16.8) | 144 (15.2) |
| ICU type, No. (%) | |||
| Medical | 757 (41.0) | 243 (27.0) | 514 (54.3) |
| Surgical | 441 (23.9) | 263 (29.2) | 178 (18.8) |
| Neurologic | 38 (2.1) | 20 (2.2) | 18 (1.9) |
| Cardiac | 611 (33.1) | 375 (41.6) | 236 (24.9) |
| History of congestive heart failure, No. (%) | 1,016 (55.0) | 391 (43.4) | 625 (66.1) |
| Mechanically ventilated, No. (%) | 878 (47.5) | 402 (44.6) | 476 (50.3) |
| Sequential Organ Failure Assessment score, mean (SD) | 6.6 (3.4) | 5.1 (2.9) | 8.0 (3.2) |
| Atrial fibrillation present on admission to ICU, No. (%) | 849 (46.0) | 375 (41.6) | 474 (50.1) |
| Duration of atrial fibrillation, median (interquartile range), hr | 8 (1-41) | 7 (2-41) | 9 (1-41) |
| Heart rate, mean (SD), beats/min | 100.0 (22.6) | 103.8 (22.6) | 96.4 (22.1) |
| Heart rate change in 6 h before vasopressors, mean (SD), beats/min | 8.4 (25.6) | 13.2 (27.8) | 3.3 (21.9) |
| Mean arterial pressure, mean (SD), mm Hg | 69.8 (13.1) | 70.6 (13.0) | 68.9 (13.2) |
| Vasopressor dose in the norepinephrine-equivalent dose, mean (SD), μg/kg/min | 0.10 (0.13) | 0.10 (0.15) | 0.10 (0.11) |
| Use of rate/rhythm control agents 6 h before vasopressors, No. (%) | |||
| Amiodarone | 87 (4.7) | 44 (4.9) | 43 (4.5) |
| Beta-blocker | 163 (8.8) | 87 (9.7) | 76 (8.0) |
| Calcium channel blocker | 35 (1.9) | 23 (2.6) | 12 (1.3) |
| Digoxin | 13 (0.7) | 6 (0.7) | 7 (0.7) |
| Use of any vasopressors in the 6 h before inclusion, No. (%) | |||
| Norepinephrine | 280 (15.2) | 19 (2.1) | 261 (27.6) |
| Phenylephrine | 168 (9.1) | 162 (18.0) | 6 (0.6) |
| Vasopressin | 30 (1.6) | 8 (0.9) | 22 (2.3) |
| Any vasopressor | 468 (25.3) | 189 (21.0) | 279 (29.5) |
| Variables recorded after vasopressor initiation: direct current cardioversion, No. (%) | 57 (3.1) | 35 (3.9) | 22 (2.3) |
| During 1-h follow up | |||
| Maximum vasopressor dose in the norepinephrine-equivalent dose, mean (SD), μg/kg/min | 0.12 (0.14) | 0.12 (0.16) | 0.12 (0.12) |
| Addition of other vasopressors (beyond initial vasopressor) in the first hour after inclusion, No. (%) | |||
| Norepinephrine | 15 (0.8) | 15 (1.7) | N/A |
| Phenylephrine | 18 (1.0) | N/A | 18 (1.9) |
| Vasopressin | 18 (1.0) | 7 (0.8) | 11 (1.2) |
| Any vasopressor | 54 (2.9) | 24 (2.7) | 30 (3.2) |
| Use of rate/rhythm control agents, No. (%) | |||
| Amiodarone | 105 (5.7) | 51 (5.7) | 54 (5.7) |
| Beta-blocker | 175 (9.5) | 94 (10.4) | 81 (8.6) |
| Calcium channel blocker | 47 (2.5) | 29 (3.2) | 18 (1.9) |
| Digoxin | 14 (0.8) | 7 (0.8) | 7 (0.7) |
| During 6-h follow up | |||
| Maximum vasopressor dose in the norepinephrine-equivalent dose, mean (SD), μg/kg/min | 0.15 (0.18) | 0.15 (0.19) | 0.15 (0.17) |
| Addition of other vasopressors (beyond initial vasopressor) in the 6 h after inclusion, No. (%) | |||
| Norepinephrine | 67 (3.6) | 67 (7.4) | N/A |
| Phenylephrine | 51 (2.8) | N/A | 51 (5.4) |
| Vasopressin | 91 (4.9) | 29 (3.2) | 62 (6.6) |
| Any vasopressor | 208 (11.3) | 90 (10.0) | 118 (12.5) |
| Use of rate/rhythm control agents, No. (%) | |||
| Amiodarone | 133 (7.2) | 66 (7.3) | 67 (7.1) |
| Beta-blocker | 213 (11.5) | 111 (12.3) | 102 (10.8) |
| Calcium channel blocker | 47 (2.5) | 29 (3.2) | 18 (1.9) |
| Digoxin | 21 (1.1) | 11 (1.2) | 10 (1.1) |
Primary models were adjusted for variables that were present at the time of vasopressor initiation; models in exploratory analyses included adjustment for factors that were recorded during follow-up periods after vasopressor initiation. N/A = not applicable.
Figure 1.
Heart rates in patients with sepsis who began norepinephrine vs phenylephrine therapy while in atrial fibrillation. Unadjusted heart rates before and after vasopressor initiation are plotted (red = norepinephrine; blue = phenylephrine) for the full cohort and subgroups of patients with or without rapid ventricular response (heart rate, < or ≥ 110 beats/min) at time of vasopressor initiation. Unadjusted heart rates were higher before vasopressor initiation but lower after vasopressor initiation in patients who received phenylephrine compared with patients who received norepinephrine. The adjusted association between phenylephrine (compared with norepinephrine) and heart rates at 1 and 6 h after vasopressor initiation are shown in the graphs. Results are similar after adjustment for postvasopressor covariables. Nor = norepinephrine; Phe = phenylephrine; RVR = rapid ventricular response.
In the full, unstratified cohort, receipt of phenylephrine was associated with a lower heart rate at 1 h (−4 beats/min [95% CI, −6 to −1; P < .001]) and 6 h (−4 beats/min [95% CI, −6 to −1; P = .004]) after adjustment for all covariables available at Time 0. Because effect modification by baseline heart rate was detected (Pinteraction = .02 and < .001, for effect modification by baseline heart rate on association between vasopressor type and 1- and 6-h follow up, respectively), we conducted subgroup analyses stratified by RVR, again adjusting for all covariables available at Time 0. Phenylephrine was associated with larger reductions in heart rate in patients with RVR at vasopressor initiation (−4 beats/min [95% CI, −9 to 0; P = .049] at 1 h and −6 beats/min [95% CI, −1 to −1; P = .02] at 6 h) than in patients not in RVR at vasopressor initiation (−2 beats/min [95% CI, −4 to −0.3; P = .02] and −2 beats/min [95% CI, −5 to 1; P = .17]) (Fig 1). Effect modification by AF present on admission was not detected (Pinteraction = .81 and .06 for 1-h and 6-h follow up, respectively). There were no significant differences in secondary outcomes between patients who received phenylephrine and patients who received norepinephrine in the total cohort after multivariable adjustment. There was also no effect modification of secondary outcomes by RVR status, thereby precluding stratified analyses (Table 2).
Table 2.
Secondary Outcomes
| Outcomea | Full Cohortb |
|
|---|---|---|
| Adjusted OR (95% CI) | P value | |
| Conversion to sinusc | 1.1 (0.8 to 1.5) | .7 |
| Bradycardiac | 1.1 (0.7 to 1.6) | .7 |
| Vasopressor duration, hr | −3.4 (−7.4 to 0.7) | .1 |
| Length of stay, d | ||
| ICU | −0.3 (−1.5 to 0.9) | .6 |
| Hospital | −1.7 (−3.8 to 0.4) | .1 |
| Hospital deaths | 1.0 (0.7 to 1.4) | .9 |
Receipt of phenylephrine (compared with norepinephrine) was not associated significantly with any secondary outcomes; there was no significant effect modification by baseline heart rate (Pinteraction > .05 for all outcomes).
Reference = norepinephrine.
Total number of patients, 1,847 (norepinephrine, 946; phenylephrine, 901).
Within a 6-h follow-up period.
In exploratory analysis, the additional adjustment for factors introduced after vasopressor initiation (use of rate/rhythm control agents, direct current cardioversion, addition of other vasopressors, and maximum norepinephrine or phenylephrine dose during follow-up periods) did not alter results (Fig 1).
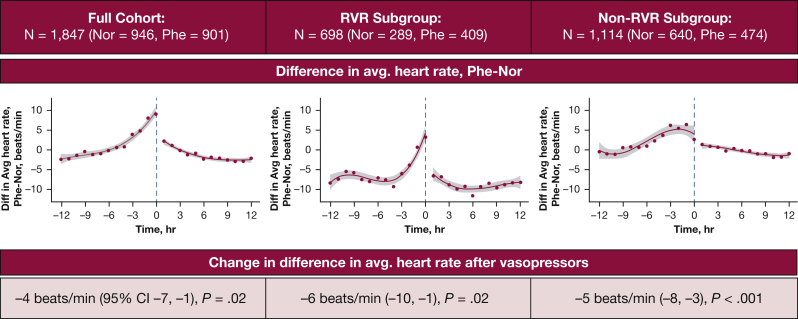
In sensitivity analysis, the controlled interrupted time series model with cubic terms had the best fit (adjusted R2 values: linear, 0.66; quadratic, 0.87; cubic, 0.93). Accounting for trends before vasopressor initiation, the change in difference in average heart (phenylephrine and norepinephrine) was −4 beats/min (95% CI, −7 to −1; P = .02) after vasopressor initiation. Changes in the difference in average heart rate between patients who received phenylephrine and patients who received norepinephrine were similar in the RVR subgroup and non-RVR subgroup (−6 beats/min [95% CI, −10 to −1; P = .02] and −5 beats/min [95% CI, −8 to −3; P < .001], respectively) (Fig 2).
Figure 2.
Controlled interrupted time series analysis that compares average heart rates in patients who received phenylephrine vs norepinephrine therapy. Cubic models of the average hourly difference, phenylephrine and norepinephrine, are shown for the full cohort and for the rapid ventricular response and non-rapid ventricular response subgroups. The change in difference in hourly average heart rates between patients who received phenylephrine and patients who received norepinephrine after vasopressor initiation is shown in each graph. Nor = norepinephrine; Phe = phenylephrine; RVR = rapid ventricular response.
Discussion
In a large retrospective study of patients with septic shock and AF, the initiation of phenylephrine was associated with modestly lower heart rate compared with norepinephrine. Baseline heart rate appeared to be an important effect modifier; the reduction in heart rate associated with phenylephrine use was greater among patients with higher heart rates at time of vasopressor initiation.
We observed almost equal proportions of patients who received phenylephrine and patients who received norepinephrine in our cohort. Despite the common use of phenylephrine in patients with septic shock and AF, there is limited literature that examines the use of phenylephrine in septic shock and even fewer studies that specifically examine the effect of phenylephrine on heart rate compared with norepinephrine during AF. One randomized controlled trial that compared norepinephrine with phenylephrine in patients with septic shock found no difference in heart rate during 12-h follow up; however, only 32 patients were enrolled, and patients did not have tachyarrythmia.19 A retrospective cohort of patients with septic shock complicated by AF with RVR compared time to rate control among patients who remained on norepinephrine with patients who transitioned from norepinephrine to phenylephrine and found no statistically significant difference between groups after covariable adjustment, although the study was similarly limited by small sample size and low power (N = 67).20
Our results add to studies that have demonstrated lower heart rates with catecholamine-sparing strategies. In the Vasopressin vs Norepinephrine Infusion in Patients with Septic Shock Trial, patients who received vasopressin had a lower heart rate than patients who received norepinephrine in the first 4 days of vasopressor administration.9 In meta-analysis, the use of vasopressin as a catecholamine-sparing agent was associated with a reduced risk of AF onset compared with catecholamine use alone.8 Our finding that phenylephrine, a catecholamine with primarily α-1 and α-2 activity, is also associated with reduced heart rates in patients with septic shock provides evidence that avoidance of β-1 stimulation may mediate heart rate differences.
We found no differences in secondary outcomes (conversion to sinus rhythm, occurrence of bradycardia, vasopressor duration, hospital or ICU length of stay, or hospital death) between patients who received phenylephrine or norepinephrine. In a small study of surgical patients with septic shock, phenylephrine use similarly was not associated with a difference in 28-day mortality rate or ICU length of stay.21 However, a study of the impact of a national norepinephrine shortage in 2011 (which led to an increase in phenylephrine exposure from 36.2% to 54.4%) found an increase in hospital mortality rate.22 Further studies of clinical outcomes between phenylephrine and norepinephrine are needed.
Our study has several strengths. First, we were able to leverage granular electronic health records data on a sample size of 1,850 patients (> 25 times larger than previous studies), which afforded us the opportunity to adjust for a multitude of time-varying clinical variables to improve estimate precision. Second, our primary results, with the use of traditional linear regression, were robust to sensitivity analyses with the use of quasi-experimental interrupted time series analysis, which showed similar results.
Our study has limitations. First, given the retrospective design, we cannot exclude completely the effect of confounding by indication; that is, patients estimated by clinicians to have a higher chance of RVR or larger component of shock because of AF (relative to sepsis itself) may be started disproportionately on β-1 sparing phenylephrine. In fact, in the hours before vasopressor initiation, patients who ultimately received phenylephrine experienced a larger increase in heart rate than the patients who ultimately received norepinephrine, which suggests that clinicians interpreted a large increase in heart rate as a risk factor for RVR and preferentially started phenylephrine. Patients who received phenylephrine also received rate/rhythm agents more frequently. However, we were able to adjust for heart rate change in the 6 h before vasopressor onset, use of rate/rhythm agents, and the addition of other vasopressors; we also stratified by presence of RVR at the time of vasopressor initiation. Furthermore, confounding by potential unmeasured risk of greater rapid ventricular response with phenylephrine would likely bias our results toward the null; despite this, we detected an association between phenylephrine use and decreased heart rate after vasopressor initiation. Second, we were unable to distinguish chronic AF from AF of critical illness or to adjust for chronic use of (or withdrawal from) rate/rhythm agents, because chronic comorbidities and home medications are not available readily in MIMIC IV. However, we were able to identify (and adjust for) AF that was present on ICU admission15 and found that the majority of our cohort did not have AF present on ICU admission; further, we found no effect modification by the presence of AF on admission on the relationship between vasopressor and heart rate outcomes. Third, AF was identified via hourly nurse documentation and may be subject to misclassification; however, nursing documentation of AF in MIMIC has been validated against electrophysiologist-interpreted continuous ECG waveforms with strong test characteristics and has been used in prior studies.14,15 Fourth, our findings, despite including a large sample size, are limited to in-hospital outcomes at a single center. Our work is hypothesis-generating and should motivate larger-scale studies that will examine longer term outcomes.
Interpretation
In patients with sepsis and AF, the initiation of phenylephrine was associated with modestly lower heart rates compared with norepinephrine. Heart rate at vasopressor initiation appears to be an important modifier, because the association between vasopressor choice and subsequent heart rate was greater in patients with RVR at the time of vasopressor initiation. Larger, randomized, controlled studies are needed to determine whether the association between phenylephrine and decreased heart rate leads to different clinically important outcomes.
Acknowledgments
Author contributions: A. C. L. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A. C. L. and D. P. performed the acquisition, analysis, or interpretation of data. A. C. L. drafted the manuscript. All authors participated in the concept and design and the critical revision of the manuscript for important intellectual content.
Financial/nonfinancial disclosures: None declared.
Funding/support: A. C. L. and A. J. W. received grants from the National Institutes of Health (NIH) during the conduct of the study and from Boston University School of Medicine Department of Medicine Career Investment Award (A. C. L), US National Heart, Lung, and Blood Institute (grant 1K23HL153482 [A. C. L.] and grant R01 HL136660 [A. J. W.]). A. C. L. is also supported by Doris Duke Charitable Foundation Fund to Retain Clinician Scientists.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
A. C. L. and N. A. B. are co-first authors.
Supplementary Data
References
- 1.Bosch N.A., Cimini J., Walkey A.J. Atrial fibrillation in the ICU. Chest. 2018;154(6):1424–1434. doi: 10.1016/j.chest.2018.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedford J.P., Harford M., Petrinic T., Young J.D., Watkinson P.J. Risk factors for new-onset atrial fibrillation on the general adult ICU: a systematic review. J Crit Care. 2019;53:169–175. doi: 10.1016/j.jcrc.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Andreis D.T., Singer M. Catecholamines for inflammatory shock: a Jekyll-and-Hyde conundrum. Intensive Care Med. 2016;42(9):1387–1397. doi: 10.1007/s00134-016-4249-z. [DOI] [PubMed] [Google Scholar]
- 4.Dünser M.W., Hasibeder W.R. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med. 2009;24(5):293–316. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- 5.Evans L., Rhodes A., Alhazzani W., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11) doi: 10.1097/CCM.0000000000005337. [DOI] [PubMed] [Google Scholar]
- 6.De Backer D., Biston P., Devriendt J., et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 7.Nagendran M., Russell J.A., Walley K.R., et al. Vasopressin in septic shock: an individual patient data meta-analysis of randomised controlled trials. Intensive Care Med. 2019;45(6):844–855. doi: 10.1007/s00134-019-05620-2. [DOI] [PubMed] [Google Scholar]
- 8.McIntyre W.F., Um K.J., Alhazzani W., et al. Association of vasopressin plus catecholamine vasopressors vs catecholamines alone with atrial fibrillation in patients with distributive shock: a systematic review and meta-analysis. JAMA. 2018;319(18):1889–1900. doi: 10.1001/jama.2018.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell J.A., Walley K.R., Singer J., et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 10.Johnson Alistair, Bulgarelli Lucas, Pollard Tom, Horng Steven, Celi Leo Anthony, Mark Roger. MIMIC-IV [Internet] https://physionet.org/content/mimiciv/1.0/
- 11.Goldberger A.L., Amaral L.A., Glass L., et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215–E220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 12.Singer M., Deutschman C.S., Seymour C.W., et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seymour C.W., Liu V.X., Iwashyna T.J., et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding E.Y., Albuquerque D., Winter M., et al. Novel method of atrial fibrillation case identification and burden estimation using the MIMIC-III electronic health data set. J Intensive Care Med. 2019;34(10):851–857. doi: 10.1177/0885066619866172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosch N.A., Massaro J.M., Winter M.R., et al. New-onset atrial fibrillation as a sepsis-defining organ failure. Ann Am Thorac Soc. 2019;16(10):1332–1334. doi: 10.1513/AnnalsATS.201902-176RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goradia S., Sardaneh A.A., Narayan S.W., Penm J., Patanwala A.E. Vasopressor dose equivalence: a scoping review and suggested formula. J Crit Care. 2021;61:233–240. doi: 10.1016/j.jcrc.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Penfold R.B., Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6):S38–S44. doi: 10.1016/j.acap.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Wagner A.K., Soumerai S.B., Zhang F., Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 19.Morelli A., Ertmer C., Rehberg S., et al. Phenylephrine versus norepinephrine for initial hemodynamic support of patients with septic shock: a randomized, controlled trial. Crit Care. 2008;12(6):R143. doi: 10.1186/cc7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haiduc M., Radparvar S., Aitken S.L., Altshuler J. Does switching norepinephrine to phenylephrine in septic shock complicated by atrial fibrillation with rapid ventricular response improve time to rate control? J Intensive Care Med. 2021;36(2):191–196. doi: 10.1177/0885066619896292. [DOI] [PubMed] [Google Scholar]
- 21.Santoriello L., Schweiger K., Aronowitz D., et al. Effect of phenylephrine versus norepinephrine on 28-day mortality and SICU length of stay in septic shock. Int J Surg: Global Health. 2020;3(4) e19-e19. [Google Scholar]
- 22.Vail E., Gershengorn H.B., Hua M., Walkey A.J., Rubenfeld G., Wunsch H. Association between US norepinephrine shortage and mortality among patients with septic shock. JAMA. 2017;317(14):1433–1442. doi: 10.1001/jama.2017.2841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.