Abstract
Tallman and colleagues’ review of consolidation studies found that the length of the delay between “recent” and “remote” events is an influential determinant of detecting temporally graded hippocampal activity. Here, we discuss two additional factors—separate analysis of distinct regions within the hippocampus and the use of overt recall methods—that should be considered when testing competing theories of hippocampal contributions to memory.
After considering some potential shortcomings in how standard systems consolidation has been tested using human neuroimaging, Tallman et al. executed an ambitious study to measure retrieval-related activity for items studied across varying levels of remoteness. Although hippocampal connectivity results were consistent with predictions of systems consolidation, the prediction of BOLD activity changing as a function of time was not observed. The authors note that is a common observation in the literature and identify the possibility that it is a result of using too “recent” a “remote” time period. Here, we consider several additional factors of the current research that might also be considered in follow-up investigations in this area, and that would offer the opportunity to test competing predictions of standard systems consolidation and other theories.
Where are specific effects located within the hippocampus?
Although standard systems consolidation does not clearly distinguish between subregions of the hippocampus (e.g., Squire et al., 2015), evidence of distinct anatomical connectivity, functional connectivity, and task activation profiles for anterior and posterior aspects of the hippocampus has steadily mounted over the preceding decades (Poppenk et al., 2013). In part, this may be due to different subfields being more or less prominent in anterior or posterior aspects of the hippocampus (Miller et al., 2020). Therefore, an important consideration is not whether activity in “the” hippocampus responds in a particular way to recent or remote retrieval, but rather, where activity in the hippocampus may or may not respond in particular ways. Treating the hippocampus as a single ROI may obscure effects of interest that would otherwise be theoretically informative. Similarly, it may spuriously suggest effects to be structure-wide when they may be limited only to a particular subregion of the hippocampus. One wonders if some of the confusion and apparent contradiction in the human neuroimaging literature might be reconciled if the hippocampus were less frequently treated as a homogeneous structure.
What, exactly, is being retrieved?
As noted by Tallman et al. (2022), multiple trace theory, trace transformation theory, and contextual binding theory all emphasize memory content as critical to the consideration of the role of the hippocampus in memory. If the amount of detail or its subjective re-experiencing can impact hippocampal activity, then the task selected by researchers should attempt to make this information available. Cued or free recall should be more useful than recognition tests in this case because they provide a window into what kind of information is being retrieved, and when, while remembering. Narratives from freely recalled events can be scored in multiple ways and can be used to estimate the amount of detail present based on overtly recorded behavior. Studies with such approaches are already providing insights into hippocampal function in healthy young adults (e.g., Gilmore et al., 2021; Reagh and Ranganath, 2021). Figure 1, for example, presents data from anterior and posterior hippocampal subregions during an overt recall task. The authors demonstrated temporally graded activity in the posterior (but not the anterior) hippocampus after activity associated with the contents of each memory was accounted for during GLM creation. We believe that these early examples using overt verbal recall illustrate a promising new approach to studying human memory and understanding the role that the hippocampus plays in supporting it.
Figure 1.
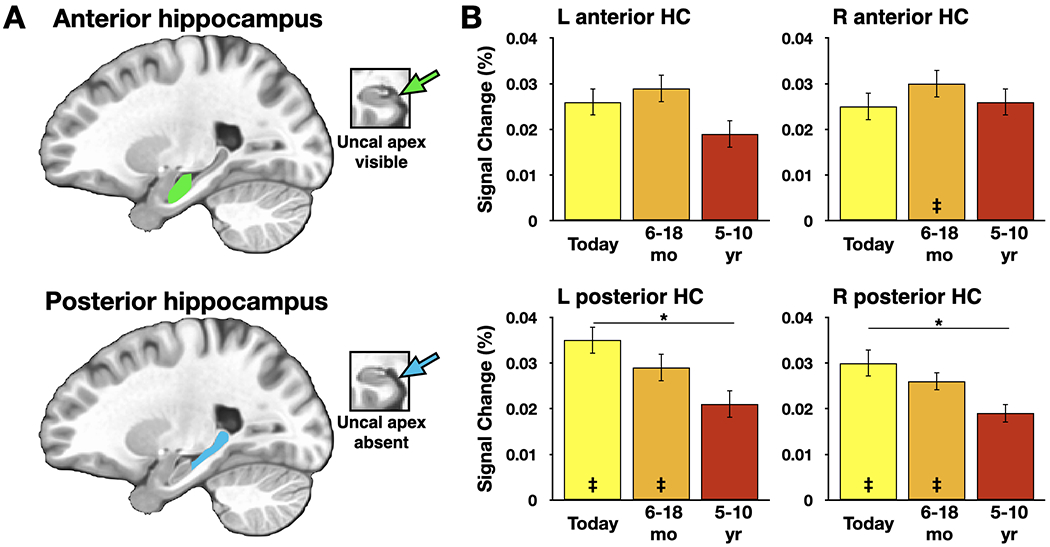
Activity in the hippocampus during autobiographical recall dissociates as a function of hippocampal subregion and temporal distance. A) Participant-specific anterior and posterior hippocampal subregions were segmented using the uncal apex as a landmark. B) We reported three main findings. 1. In the anterior region of the hippocampus there was no temporal gradient and no neural activity above our baseline control condition except for the 6-18 month period in the right hemisphere. 2. In the posterior region of the hippocampus there was a temporal gradient in both the left and right hemispheres. 3. The posterior region of the hippocampus was active for the earliest and 6-18 month time periods in both hemispheres, but not during recall from the most distant time period (5-10 years ago) (see Gilmore et al., 2021 for details). * denotes p < .05; ‡ denotes significant one-sample test vs. a baseline task, (p < .05, corrected for multiple comparisons); HC: hippocampus. Figure adapted from Gilmore et al. (2021).
ACKNOWLEDGEMENTS:
This work was supported by the Intramural Research Program at the National Institute of Mental Health (ZIAMH002920).
REFERENCES
- Gilmore AW, Quach A, Kalinowski SE, González-Araya EI, Gotts SJ, Schacter DL, Martin A. (2021). Evidence supporting a time-limited hippocampal role in retrieving autobiographical memories. Proc Natl Acad Sci USA 118:e2023069118. 10.1073/pnas.2023069118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TD, Chong TT-J, Aimola Davies AM, Johnson MR, Irani SR, Husain M, Ng TW, Jacob S, Maddison P, Kennard C, Gowland PA, Rosenthal CR. (2020). Human hippocampal CA3 damage disrupts both recent and remote episodic memories. eLife 9:e41836. 10.7554/eLife.41836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. (2013). Long-axis specialization of the human hippocampus. Trends Cog Sci 17:230–240. 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Reagh ZM, Ranganath C. (2021). A cortico-hippocampal scaffold for representing and recalling lifelike events. BioRxiv. 10.1101/2021.04.16.439894. [DOI] [Google Scholar]
- Squire LR, Genzel L, Wixted JT, Morris RG. (2015). Memory consolidation. Cold Spring Harb Perspect Biol 7:a021766. 10.1101/cshperspect.a021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallman CW, Clark RE, Smith CN. (2022). Human brain activity and functional connectivity as memories age from one hour to one month. Cognitive Neuroscience. 17588928.2021.2021164. [DOI] [PMC free article] [PubMed] [Google Scholar]


