Abstract
Introduction
Sodium zirconium cyclosilicate (SZC) is a selective potassium (K+) binder for hyperkalemia management that provides rapid and sustained correction of hyperkalemia. The NEUTRALIZE study is investigating whether SZC, in addition to correcting hyperkalemia and maintaining normal serum K+, can provide sustained increases in serum bicarbonate (HCO<sub>3</sub>−) in patients with hyperkalemia and metabolic acidosis associated with chronic kidney disease (CKD).
Methods
This is a prospective, randomized, double-blind, placebo-controlled phase 3b study of US adults with stage 3–5 CKD not on dialysis with hyperkalemia (K+ >5.0–≤5.9 mmol/L) and low-serum HCO<sub>3</sub>− (16–20 mmol/L). In the open-label correction phase, all eligible patients receive SZC 10 g three times daily for up to 48 h. Patients who achieve normokalemia (K+ ≥3.5–≤5.0 mmol/L) are then randomized 1:1 to once-daily SZC 10 g or placebo for a 4-week, double-blind, placebo-controlled maintenance phase. The primary endpoint is the proportion of patients with normokalemia at the end of treatment (EOT) without rescue therapy for hyperkalemia. Key secondary endpoints include mean change in serum HCO<sub>3</sub>−, the proportion of patients with an increase in serum HCO<sub>3</sub>− of ≥2 or ≥3 mmol/L without rescue therapy for metabolic acidosis, and the proportion of patients with serum HCO<sub>3</sub>− ≥22 mmol/L at EOT.
Conclusions
NEUTRALIZE will establish whether SZC can provide sustained increases in serum HCO<sub>3</sub>− while lowering serum K+ in patients with hyperkalemia and CKD-associated metabolic acidosis and may provide insights on the mechanism(s) underlying the increased serum HCO<sub>3</sub>− with SZC treatment.
Keywords: Ammonium, Hyperkalemia, Metabolic acidosis, Sodium zirconium cyclosilicate, Study design
Introduction
Patients with chronic kidney disease (CKD) often develop metabolic acidosis, largely because of the kidney's diminished capacity to excrete acid in the form of ammonium (NH4+) [1]. In the Chronic Renal Insufficiency Cohort study conducted in the USA, the estimated prevalence of metabolic acidosis (i.e., serum bicarbonate [HCO3−] <22 mmol/L) was 13% in patients with stage 3 CKD, 37% in stage 4 CKD, and 80% in stage 5 CKD [2]. Metabolic acidosis associated with CKD is an independent risk factor for CKD progression [3]. In a French observational study of patients with stage 1–4 CKD, those in the lowest tertile of venous total carbon dioxide (CO2) were significantly more likely to have a fast decline in measured glomerular filtration rate (GFR) [4]. Metabolic acidosis can also be associated with an increased risk of metabolic bone disease, cardiovascular events, and mortality [1].
In addition to metabolic acidosis, patients with CKD commonly develop hyperkalemia, primarily as a result of a reduction in potassium (K+) excretion as GFR declines [5, 6]. According to a US retrospective study, the estimated annual prevalence of metabolic acidosis in patients with CKD and hyperkalemia (K+ >5.0 mmol/L) ranged between 25% and 29% from 2014 to 2017 [7]. Hyperkalemia is also associated with an increased risk of all-cause mortality, particularly in patients with CKD, heart failure, or diabetes [8]. More recently, hyperkalemia was shown to be independently associated with an increased risk of all-cause mortality, cardiovascular events, hospitalizations, and intensive care unit admissions in a Canadian retrospective study [9].
Sodium zirconium cyclosilicate (SZC) is a novel selective K+ binder that is currently approved for the treatment of hyperkalemia [10]. SZC is a nonabsorbed, nonpolymeric, crystalline agent that preferentially entraps K+ in exchange for hydrogen (H+) [10] and sodium (Na+) throughout the gastrointestinal tract [11]. In phase 3 clinical studies, SZC effectively lowered serum K+ (median time to K+ decline 2.2 h) and provided sustained normokalemia (K+ 3.5–5.0 mmol/L) during 12 months of follow-up [12, 13, 14, 15, 16].
In addition to its effects on serum K+, in post hoc analyses, SZC had acute effects on serum HCO3− during correction of hyperkalemia, with rapid dose-dependent placebo-adjusted increases in serum HCO3− of 1.02 mmol/L with SZC 5 g three times daily (TID) to 1.78 mmol/L with SZC 10 g TID within 48 h [17]. The rise in serum HCO3− during the 48-h correction phase was independent of CKD stage [17] and was sustained during the 29-day maintenance phase with SZC 10–15 g once daily (QD) [17] and for 11 months of continued open-label treatment [15]. Furthermore, SZC was associated with dose-dependent decreases in blood urea nitrogen (BUN) during correction (first 48 h) and maintenance (21 days) treatment compared with placebo in patients with CKD [13, 18, 19]. The mechanism(s) underlying the increase in serum HCO3− with SZC are not fully understood but may be due to direct binding and removal of NH4+ by SZC in the gastrointestinal tract and/or an increase in renal ammonia (NH3) production with correction of hyperkalemia, which would allow for increased renal acid excretion [17].
The post hoc analyses of previous clinical studies showing dose-dependent increases in serum HCO3− with SZC did not systematically examine patients with CKD with a combination of hyperkalemia and low serum HCO3− [17, 19]. In addition, the effect of SZC on serum HCO3− may have been confounded by patients receiving sodium bicarbonate therapy, although it should be noted that increases in serum HCO3− were also seen in patients not receiving sodium bicarbonate. Moreover, the impact of SZC on urine NH4+ excretion was not evaluated [17, 19]. Thus, further study is needed to elucidate if SZC causes clinically meaningful increases in serum HCO3− in patients with CKD-associated hyperkalemia and metabolic acidosis. Here, we describe the rationale and design of the NEUTRALIZE study (NCT04727528), which aims to investigate whether SZC can: (1) correct hyperkalemia and maintain normal serum K+ levels and (2) provide sustained increases in serum HCO3− in patients with CKD-associated hyperkalemia and metabolic acidosis.
Materials and Methods
Study Design
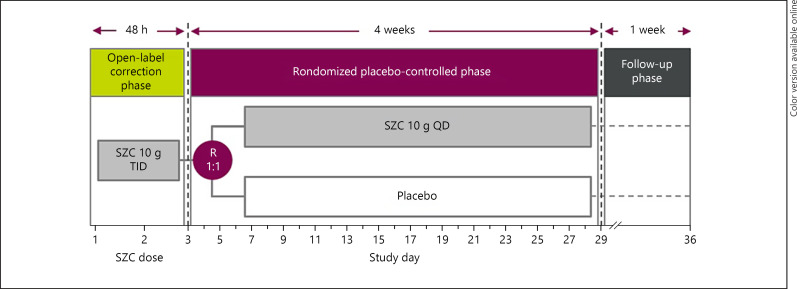
NEUTRALIZE is an ongoing prospective, randomized, double-blind, placebo-controlled, parallel-group, multicenter phase 3b study that is being conducted in US adults with hyperkalemia and metabolic acidosis associated with CKD. The study includes a screening visit, a 4-week treatment period, and an off-treatment follow-up visit 1 week after the end of treatment (EOT). The treatment period consists of an open-label correction phase of up to 48 h for all eligible participants, followed by a randomized, placebo-controlled maintenance phase from study days 3–29 (Fig. 1).
Fig. 1.
Design of the NEUTRALIZE study. Patients with stage 3–5 CKD (eGFR ≤59 mL/min/1.73 m2) not on dialysis, hyperkalemia (2× consecutive point-of-care K+ levels >5.0–≤5.9 mmol/L), and metabolic acidosis (2× point-of-care HCO3− levels 16–20 mmol/L taken 1 h apart) are eligible to enter the open-label correction phase. Patients who achieve normokalemia (K+ ≥3.5–≤5.0 mmol/L) during the open-label correction phase will enter the randomized placebo-controlled phase. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HCO3−, bicarbonate; K+, potassium; QD, once daily; R, randomization; SZC sodium zirconium cyclosilicate; TID, three times daily.
Study Oversight and Eligibility
The study is being conducted in accordance with the approved protocol and with international guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Science International Ethical Guidelines, applicable International Council for Harmonisation Good Clinical Practice guidelines, and all applicable local and national regulations. Study sites must comply with the approved protocol and the requirements of the Institutional Review Board/Independent Ethics Committee. The study is ongoing at approximately 35 sites across the USA.
The inclusion and exclusion criteria for the study are summarized in Table 1. Patients aged ≥18 years with stage 3–5 CKD (estimated GFR ≤59 mL/min/1.73 m2) not on dialysis are eligible to participate in the study if they have two consecutive serum K+ levels >5.0–≤5.9 mmol/L and two serum HCO3− levels 16–20 mmol/L (taken approximately 1 h apart). Patients requiring dialysis are excluded from the study.
Table 1.
Summary of the inclusion and exclusion criteria for the NEUTRALIZE study
| Inclusion criteriaa | Exclusion criteriab |
|---|---|
| Age ≥18 years | Pseudohyperkalemia |
|
| |
| Stage 3–5 CKD (eGFR ≤59 mL/min/1.73 m2) and not on dialysis | Dialysis requirement within 3 months |
|
| |
| Two consecutive serum K+ levels (taken 1 h apart) > 5.0–≤5.9 mmol/L | History of kidney transplantation or acute/chronic worsening of kidney function (i.e., ≥30% decline in eGFR within 3 months) |
|
| |
| Two serum HCO3– levels (taken 1 h apart) between 16 and 20 mmol/L, inclusive | Cardiac arrhythmias requiring immediate treatment; current acute decompensated HF or hospitalization for HF within 4 weeks; MI, UA, stroke, or TIA within 12 weeks; coronary revascularization within 12 weeks; or symptomatic hypotension, symptomatic or uncontrolled AF, asymptomatic VT, or QT prolongation |
|
| |
| Able to undergo repeated blood draws or effective venous catheterization | Low HCO3– requiring emergency intervention or treatment |
|
| |
| Informed consent | Active or suspected diabetic ketoacidosis; history of diabetic gastroparesis; bariatric surgery; bowel obstruction; or swallowing disorders |
| Current exacerbation of COPD/asthma or hospitalization for COPD/asthma within 4 weeks | |
| Active malignancy requiring treatment | |
| Life expectancy of <3 months | |
| Active treatment (within 7 days prior to screening) with a K+ binder (i.e., SZC, SPS, or patiromer), sodium bicarbonate, or lactulose | |
| Prior or concurrent participation in another clinical study with the administration of study drug within 1 month | |
| Known hypersensitivity to SZC | |
| Current pregnancy | |
| Evidence of COVID-19 within 2 weeks prior to screening | |
AF, atrial fibrillation; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; HCO3–, bicarbonate; HF, heart failure; K+, potassium; MI, myocardial infarction; SPS, sodium polystyrene sulfonate; SZC, sodium zirconium cyclosilicate; TIA, transient ischemic attack; UA, unstable angina; VT, ventricular tachycardia.
Eligible participants must meet all of the inclusion criteria.
Participants are excluded if any of the exclusion criteria are met.
Study Treatment
Following a short screening period (Study Day 1), all eligible patients will enter the open-label correction phase in which they receive SZC 10 g TID for up to 48 h (Fig. 1). Patients who achieve normokalemia (serum K+ ≥3.5–≤5.0 mmol/L) after 24 h (Day 2) will enter the randomized, placebo-controlled maintenance phase, whereas patients with serum K+ ≥5.1 mmol/L will continue SZC TID for another 24 h. Participants who do not achieve normokalemia will be discontinued from the study.
Patients who achieve normokalemia during the open-label correction phase will be randomized 1:1 to receive SZC 10 g QD or placebo QD during the 4-week, double-blind, placebo-controlled maintenance phase. Patients with serum K+ <3.5 mmol/L at any time during the open-label correction phase or serum K+ ≥5.1 mmol/L at the end of the open-label correction phase will be discontinued from the study drug. During the first 2 weeks of the maintenance phase, the SZC/placebo dose will be titrated as needed to maintain serum K+ levels by increasing or decreasing the dose by 5-g increments at 1-week intervals to between 5 g every other day to 15 g QD. A stable SZC/placebo dose is recommended during the last 2 weeks of the maintenance phase.
Study Assessments
Patient demographics (e.g., age, sex, race, and ethnicity), comprehensive medical and surgical history, and baseline laboratory results, including hematology and clinical chemistry, will be collected at screening and at specified study visits (Table 2). In order to exclude pseudohyperkalemia, the study requires two consecutive K+ levels from two blood samples, taken approximately 1 h apart, to fall within the inclusion criteria. All study staff will be trained on proper blood sample collection. Patients will be instructed to relax the muscles of their forearm throughout the blood drawing procedure to minimize hemolysis. Standard laboratory procedures will be used to determine whether blood samples contain clots or show signs of hemolysis in the plasma. Point-of-care K+ and HCO3− will be used to screen patients for study inclusion, determine eligibility for the randomized, placebo-controlled phase and to titrate the SZC/placebo dose. Central laboratory serum K+ and point-of-care HCO3− levels will be used for study endpoint assessments. Spot urine tests will be conducted during the randomized phase and include central laboratory measurement of urinary albumin, NH4+, citrate, pH, creatinine, Na+, chloride, and K+ (to enable calculation of the urine anion gap) and calculated urinary albumin-to-creatinine, citrate-to-creatinine, and NH4+-to-creatinine ratios. We consider direct measurement of urinary NH4+ to be a more accurate measurement of urinary acid excretion than other surrogate measurements, such as the urinary anion gap or osmolality gap. All samples will be stored consistent with standard operating procedures to preserve sample integrity for analysis.
Table 2.
Schedule of key study assessments in the NEUTRALIZE study
| Procedure | Screening | Open-label phase | Randomized phase (visit 2 or 3–7) | Follow-up | |||||
|---|---|---|---|---|---|---|---|---|---|
| Visit | 1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Study day | 1 | 1 | 2 | 2 or 3 | 8 (±1) | 15 (±1) | 22 (±1) | 29 (±1) | 36 (±3) |
| Routine clinical procedures | |||||||||
| Demographics | X | ||||||||
| Physical exam | X | X | |||||||
| Medical history | X | ||||||||
| Concomitant medications | At every visit and may be conducted by phone (if not part of a visit) | ||||||||
| Vital signs | X | X | X | X | X | X | X | ||
| Weight | X | X | X | X | X | X | X | ||
| Routine safety measurements | |||||||||
| AEs | At every visit and may be conducted by phone (if not part of a visit) | ||||||||
| Urine pregnancy test | X | ||||||||
| Clinical safety laboratory testsa | X | X | X | X | |||||
| ECG | X | X | X | X | |||||
| Efficacy laboratory measurementsb | |||||||||
| Central laboratory K+, HCO3–, Cl– | (X)c | (X)c | X | X | X | (X)c | (X)c | ||
| i-STAT testsd | X, X | X | X | X | X | X | X | X | |
| Spot urine testse | X | X | X | X | X | X | X | ||
| Serum aldosterone | X | X | X | X | |||||
| Optional genomics blood sample | X | ||||||||
| Study drug administration | |||||||||
| Drug dispensation (open-label phase) | X | X | |||||||
| Drug dispensation (randomized phase) | X | X | X | X | |||||
| Randomization | X | ||||||||
| Dose titration (if needed) | X | X | X | ||||||
AE, adverse event; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Ca2+, calcium; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; Cl–, chloride; CO2, carbon dioxide; ECG, electrocardiography; eGFR, estimated glomerular filtration rate; HCO3–, bicarbonate; K+, potassium; Mg2+, magnesium; Na+, sodium; NH4+, ammonium; PO4, phosphate; UACR, urinary albumin-to-creatinine ratio.
Includes hematology (hemoglobin, hematocrit, leukocyte count, and platelet count), urinalysis by dipstick (urinary hemoglobin/erythrocytes/blood, protein/albumin, and glucose), and serum chemistry (serum Na+, K+, HCO3– [total CO2], Cl–, glucose, creatinine, BUN, BUN-to-creatinine ratio, eGFR [using CKD-EPI formula], anion gap [blood from i-STAT], albumin, total protein, Ca2+, Mg2+, PO4, total bilirubin, ALP, ALT, and AST).
Measurements are taken after the patient has fasted for 4 h (meals can transiently change acid-base status).
When measured on Visits 1, 3, 7, and 8, these results were also included as part of the clinical safety assessment.
Includes HCO3– as total CO2, K+, creatinine, and anion gap; at screening, 2 measurements will be taken 1 h (±15 min) apart.
Central laboratory measurement of urinary albumin, NH4+, citrate, pH, and creatinine, and calculated anion gap (based on urinary Na+, K+, and Cl–), UACR, and NH4+-to-creatinine ratio.
Study Endpoints
A summary of the primary, secondary, and exploratory endpoints is provided in Table 3. Consistent with the primary indication for SZC in the USA [10], the primary endpoint is the proportion of patients with normokalemia (central laboratory serum K+ ≥3.5–≤5.0 mmol/L) at the EOT (Day 29), without the need for rescue treatment for hyperkalemia (i.e., serum K+ >6.0 mmol/L) at any point during the maintenance phase. The safety assessments include the occurrence, frequency, and severity of adverse effects and their relationship to treatment, as assessed by the investigator, and monitoring of vital signs, clinical laboratory assessments (e.g., hematology, urinalysis by dipstick, and serum chemistry), and electrocardiography (Table 1).
Table 3.
Summary of primary, secondary, and exploratory endpoints for the NEUTRALIZE study
| Endpoint | Outcome |
|---|---|
| Primary | Proportion of patients with normokalemia (central laboratory serum K+ ≥3.5-≤5.0 mmol/L) at the EOT (Day 29), without the need for rescue treatment for hyperkalemia (i.e., serum K+ >6.0 mmol/L) at any point during the maintenance phase |
|
| |
| Secondary | Mean change from baseline in serum HCO3– at EOT |
| Proportion of patients with ≥2 or ≥3 mmol/L increase in serum HCO3– from baseline (Day 1) to EOT, without the need for rescue treatment for metabolic acidosis (i.e., serum HCO3– ≤15 mmol/L) |
|
| Proportion of patients with serum HCO3– ≥22 mmol/L |
|
| Proportion of patients with normokalemia and ≥3 mmol/L increase from baseline in serum HCO3– at EOT, without the need for rescue treatment for hyperkalemia or metabolic acidosis |
|
| Proportion of patients with normokalemia and serum HCO3– ≥22 mmol/L at EOT, without the need for rescue treatment for hyperkalemia or metabolic acidosis |
|
| Proportion patients needing rescue treatment for metabolic acidosis at any time during the maintenance phase | |
|
| |
| Exploratory | Mean change from baseline in spot urine NH4+, citrate, pH, anion gap, creatinine, urine NH4+-to-creatinine ratio, urine citrate-to-creatinine ratio, and urine albumin-to-creatinine ratio at EOT |
| Mean change from baseline in serum creatinine, anion gap, serum chloride, and serum aldosterone at EOT | |
EOT, end of treatment; HCO3–, bicarbonate; K+, potassium; NH4+, ammonium.
Statistical Considerations
Approximately 400 patients will be screened and approximately 200 patients will be enrolled in the open-label correction phase in order to achieve 182 evaluable patients randomly assigned to the study intervention. A sample size of 91 patients each in the SZC and placebo arms will be needed to have >90% power to detect an absolute difference in HCO3− of 20% between baseline and EOT, using a 2-sided t test at a significance level of 5%. This calculation assumes an 8% drop-out rate between the open-label correction phase and the randomized maintenance phase and is based on the proportion of patients with serum HCO3− 16–20 mmol/L in previous studies who had a placebo-adjusted increase in serum HCO3− of ≥3 mmol/L with SZC [12, 15, 16].
Continuous variables will be summarized by study arm using the number of observations (n), mean, standard deviation, median, interquartile range, and minimum and maximum values. Categorical variables will be summarized by study arm using the number of patients and percentage. For all logistic regression analyses, odds ratios, 95% confidence intervals, and 2-tailed t test p values (significance level <0.05) will be presented. All statistical analyses will be conducted using SAS® statistical software, version 9.3 (or later).
The primary, secondary, and exploratory endpoints will be analyzed in the full analysis set (defined as all patients who achieve normokalemia during the open-label correction phase and enter the randomized maintenance phase). Patients will be analyzed on an intent-to-treat basis. Safety endpoints will be analyzed in the open safety set (defined as all patients enrolled in the open-label correction phase who received ≥1 dose of the study drug and analyzed as a single group) and in the randomized safety set (defined as all patients who achieve normokalemia during the open-label correction phase and enter the randomized maintenance phase and analyzed according to randomized treatment). Sensitivity analyses will also be conducted in the per-protocol set (defined as all patients in the full analysis set without any important protocol deviations and analyzed according to randomized treatment).
In the primary endpoint analysis, the null hypothesis is that there is no difference between SZC and placebo in the proportion of patients with normokalemia at EOT without the need for rescue treatment for hyperkalemia at any time during the maintenance phase. The primary endpoint will be analyzed by logistic regression, with the proportion of patients with normokalemia as the dependent variable and the randomized study arm as the independent variable.
For each secondary endpoint, the null hypothesis is that there is no difference between SZC and placebo in the outcome measured. To control for type I error, a hierarchical testing procedure will be followed when formally testing secondary endpoints. Two-sided p values <0.05 will be considered statistically significant. Exploratory endpoints will be analyzed using analysis of covariance models with the randomized study arm as the main effect and the baseline variable as the covariate. The last observation carried forward approach will be used to manage missing data.
Discussion
NEUTRALIZE is a randomized, double-blind, placebo-controlled phase 3b study designed to investigate whether SZC provides clinically meaningful increases in serum HCO3−, in addition to correcting hyperkalemia and maintaining normokalemia, in patients with CKD-associated hyperkalemia and metabolic acidosis. This is the first known interventional study to prospectively evaluate a K+ binder in patients with metabolic acidosis.
The standard of care for patients with CKD-associated metabolic acidosis is currently sodium bicarbonate therapy. Sodium bicarbonate increases serum HCO3− and may lower serum K+ while decreasing urinary NH4+ excretion in patients with CKD and metabolic acidosis [20, 21]. Gastrointestinal intolerance and the high pill burden needed to correct serum HCO3− are limitations of sodium bicarbonate [22]. Moreover, treatment of metabolic acidosis with sodium bicarbonate- or citrate-containing liquids is often limited by poor palatability [23]. In patients with advanced CKD, adequate correction of metabolic acidosis (i.e., serum HCO3− levels >22 mmol/L) with sodium bicarbonate or sodium citrate is associated with a significant increase in Na+ load [24] and is often not achieved, most likely due to poor adherence [25]. Therefore, alternative therapeutic options for the management of CKD-associated metabolic acidosis with hyperkalemia would be useful.
SZC was shown to provide dose-dependent increases in serum HCO3− in post hoc analyses of phase 3 studies of patients with hyperkalemia [17, 19] and may provide an alternative to sodium bicarbonate, or at least allow for a reduced dose, in patients with CKD-associated hyperkalemia and metabolic acidosis. Therefore, SZC has the potential to provide clinically meaningful improvements in serum HCO3− in these patients.
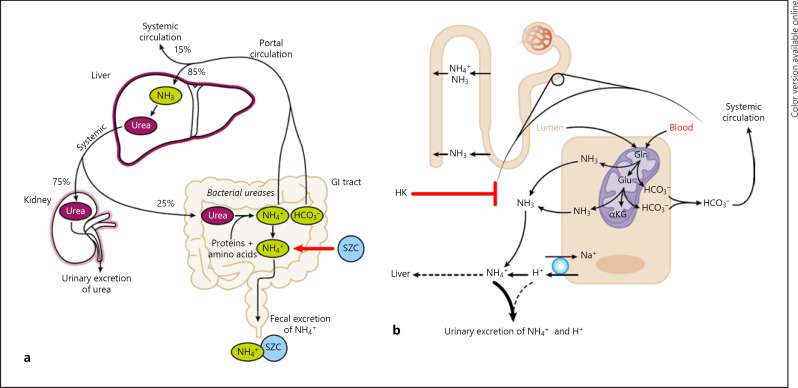
Two general possible mechanisms are proposed to contribute to the serum HCO3− increases observed with SZC: (1) SZC directly binding and removing NH4+ from the gastrointestinal tract (Fig. 2a) and (2) augmentation of renal ammoniagenesis and normalization of altered NH4+ transport as a result of hyperkalemia correction (Fig. 2b).
Fig. 2.
Hypothetical mechanisms for the increase in serum HCO3− with SZC. a Binding of NH4+ ions by SZC in the gastrointestinal tract (indicated by the red arrow) blocks NH4+ reabsorption into the portal circulation, reducing urea resynthesis in the liver and allowing for increased levels of HCO3− in the systemic circulation. b Correction of HK by SZC restores ammoniagenesis in the renal proximal tubule, allowing for increased excretion of NH4+ and H+ ions, as well as increased HCO3− production [38]. The HCO3− produced by ammoniagenesis is reabsorbed into the systemic circulation, while a small proportion of the NH3 is transported to the liver as NH4+, where it is converted to urea (consuming two HCO3−). αKG, α-ketoglutarate; GI, gastrointestinal; Gln, glutamine; Glu, glutamate; H+, hydrogen; HCO3−, bicarbonate; HK, hyperkalemia; Na+, sodium; NH3, ammonia; NH4+, ammonium; SZC, sodium zirconium cyclosilicate.
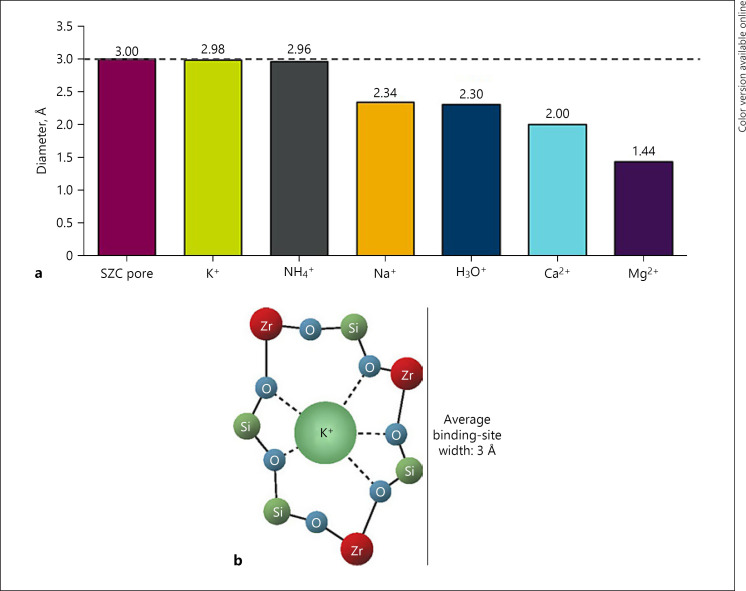
Under normal physiologic conditions, the main mechanism for increasing renal acid excretion is by augmentation of urinary NH4+ excretion [26]. NH4+ production and subsequent excretion lead to increased HCO3− reabsorption [27]. Urinary NH4+ is produced primarily in the proximal tubule from systemically derived glutamine, which is metabolized by proximal cells to glutamate, then α-ketoglutarate, producing two NH4+ and two HCO3− ions per glutamate molecule [26]. HCO3− is subsequently reabsorbed into the systemic circulation, whereas NH4+ is mostly excreted in the urine, with a small proportion undergoing metabolism to urea in the liver (consuming two HCO3− ions) [28, 29]. NH4+ produced in proximal cells is transported into the urinary space of the proximal tubule, either by the apical Na+/H+ exchanger (in place of H+) or as NH3 or both. Subsequently, NH4+ is reabsorbed by the thick ascending limb and is trapped in the interstitium, where NH3 is then available to diffuse into the collecting tubule and interact with H+, secreted mainly by H+-ATPase, such that NH4+ is formed in the lumen of the collecting tubule and excreted in the urine [28]. However, in patients with CKD, NH4+ excretion is markedly diminished and hyperkalemia, when present, further decreases NH4+ excretion [28, 29, 30]. It is conceivable that SZC raises serum HCO3− in part by directly binding and removing NH4+ in the gastrointestinal tract. The SZC binding site is similar in size to the ionic diameters of NH4+ and K+ in an aqueous solution (both approximately 3 Å; Fig. 3). As such, it is postulated that SZC binds not only K+ but also NH4+ in exchange for H+ or Na+ in the gastrointestinal tract [10, 11]. Direct removal of NH4+ from the gastrointestinal tract by SZC would additionally explain why dose-dependent decreases in BUN are also observed with SZC [17, 19].
Fig. 3.
SZC average pore size relative to the ionic diameters of several common cations (a) and illustration of the SZC-binding site showing the average binding site diameter of ∼3 Å (b). With similar nonhydrated ionic diameters, K+ and NH4+ fit best within the SZC binding site. Ca2+, calcium; H3O+, hydronium; K+, potassium, Mg2+, magnesium; Na+, sodium; NH4+, ammonium; SZC, sodium zirconium cyclosilicate.
To understand how direct binding of NH4+ by SZC in the gastrointestinal tract may result in increased serum HCO3−, an explanation of urea enterohepatic cycling is warranted. Urea is synthesized in the liver following the breakdown of amino acids from protein-rich foods [29]. Although urea is mostly removed from the body via urinary excretion, a fraction (approximately 25%) passes from the systemic circulation into the gastrointestinal tract, most likely by passive diffusion [31, 32]. As patients with CKD have elevated BUN levels [33], diffusion of urea from the systemic circulation into the gastrointestinal tract is likely to be proportionately increased compared with that of healthy individuals. In the gastrointestinal tract, urea is hydrolyzed by bacterial ureases to release NH4+ and HCO3−, which are normally absorbed through the gastrointestinal epithelium [34]. NH4+ is transported via the portal vein to the liver, where it is metabolized to urea by combining with HCO3− [28, 29, 34].
The binding of NH4+ by SZC in exchange for Na+ and subsequent increased removal of NH4+ in the feces likely interrupt this enterohepatic loop of urea nitrogen salvage, allowing HCO3− in the portal vein to bypass the liver, and decreasing urea resynthesis in the liver. Because dose-dependent decreases in BUN have been observed with SZC [17, 19], the overall expected effect is an increase in serum HCO3− and a reduction in BUN [35]. This effect would be additive and independent of any K+-lowering effect of SZC on renal ammoniagenesis (described in the following paragraphs).
The second hypothesized mechanism by which SZC increases serum HCO3− is that the normalization of serum K+ with SZC leads to the correction of metabolic acidosis. An early case study by Szylman et al. [36] showed that normalization of hyperkalemia with sodium polystyrene sulfonate in a patient with isolated hypoaldosteronism led to resolution of acidosis and restored urinary NH3 excretion. Correction of hyperkalemia could lead to increased NH3 production in the renal proximal tubule and increased final excretion as NH4+, which subsequently leads to increased serum HCO3−. Renal ammoniagenesis is decreased during hyperkalemia [37, 38], as indicated by an in vitro study showing reduced ammoniagenesis with elevated K+ concentrations [39]. Moreover, an inverse relationship between plasma K+ and NH4+ excretion was demonstrated using in vivo experimental models [37, 40]. In addition, hyperkalemia can alter NH4+ transport within the nephron, causing reduced medullary accumulation of NH4+ in the collecting duct [40], as demonstrated in an animal model of chronic hyperkalemia [41]. Based on an animal model of selective aldosterone deficiency, aldosterone enhances H+ secretion and increases ammoniagenesis directly and indirectly by lowering serum K+ [42]. Therefore, patients with CKD and aldosterone deficiency often develop hyperkalemic metabolic acidosis [43].
The exploratory path analysis using data from three phase 3 studies of SZC in patients with hyperkalemia strongly suggested that the SZC-associated rise in serum HCO3− is the result of NH4+ binding in the gastrointestinal tract, based on the association of the increase in HCO3− with the serum urea reduction path but not with the paths via reduction in serum K+ or increases in urine pH [17]. The NEUTRALIZE study may provide further data to support this hypothesis as study assessments will include serum urea, spot urine pH measurement, and urine NH4+-to-creatinine and citrate-to-creatinine ratio calculations, as well as assessment of serum HCO3− and serum K+.
Given that SZC is proposed to bind NH4+ (as well as K+) in exchange for Na+, there is some concern that SZC treatment may be associated with Na+ release and systemic absorption. A phase 1 study in healthy volunteers on a high K+/low Na+ diet previously showed no significant changes in urinary or fecal Na+ excretion with SZC administration [44], and a phase 2 study in patients with CKD and hyperkalemia showed no increase in 24-h urinary Na+ excretion with SZC compared with placebo [18], suggesting that SZC is not associated with significant release and systemic absorption of Na+. In clinical trials, mild-to-moderate edema was observed in patients, not on dialysis, primarily with the 15 g QD SZC dose [10]. In a clinical trial in patients on chronic hemodialysis, most of whom received SZC doses of 5 g–10 g QD on non-dialysis days, there was no difference in interdialytic weight gain between the SZC and placebo groups, suggesting fluid retention was similar [10, 45].
There are several potential limitations of this study. Assessment of 24-h urinary Na+, K+, urea, and NH4+ would have provided better insights regarding the effects of SZC on their urinary excretion than spot urinary analyses, while the amount of NH4+ that is bound by SZC would require 24-h fecal measurements. However, these tests were not included in the study protocol because of practical considerations. Further studies may be needed to examine these parameters in more detail. In addition, SZC will be administered without the requirement for fasting; therefore, acid-base status may be affected in those who receive SZC with meals.
Conclusions
NEUTRALIZE is the first study to prospectively explore the potential benefits and risks of using SZC in patients with CKD-associated hyperkalemia and metabolic acidosis. This study will determine if SZC can provide sustained increases in serum HCO3− while maintaining normokalemia in this patient population. The study will also provide additional evidence for the efficacy and safety of SZC administration in these patients and may provide further information on the mechanism by which SZC increases serum HCO3− concentrations.
Statement of Ethics
This study protocol was reviewed and approved by the central Institutional Review Board (Advarra®, approval number PRO00048231) or local IRBs where applicable. The clinical study protocol is publicly registered (NCT04727528) and the results will be disclosed according to the AstraZeneca Global Policy on Bioethics and in compliance with prevailing laws and regulations. All procedures will be in accordance with the Declaration of Helsinki and informed consent will be obtained from all individuals included in the study.
Conflict of Interest Statement
S.R.A. has received honoraria from AstraZeneca for participation in corporate-sponsored programs but has no current financial ties to AstraZeneca. D.B. has received an investigator-initiated grant from AstraZeneca to study the effect of SZC on fecal NH4+ and has received consulting fees from Tricida, Astra Zeneca, and Relypsa, is the founder and main stakeholder of Angiotensin Therapeutics Inc. and is coinventor of patents entitled “Active Low Molecular Weight Variants of Angiotensin Converting Enzyme 2,” “Active Low Molecular Weight Variants of Angiotensin Converting Enzyme 2 (ACE2) for the Treatment of Diseases and Conditions of the Eye,” and “Shorter Soluble Forms of Angiotensin Converting Enzyme 2 (ACE2) for Treating and Preventing Coronavirus Infection.” J.K. is an advisory board member for AstraZeneca and has received grants from Fresenius Renal Therapies and consulting fees from Tricida and Nephcentric. Y.O., W.P., Y.B., and E.G. are employees of AstraZeneca. L.F. has no conflicts of interest to declare, and as a Veterans Affairs employee, does not receive compensation.
Funding Sources
The NEUTRALIZE study and development of the manuscript were supported by AstraZeneca. None of the authors have received compensation for the current work.
Author Contributions
Yemisi Oluwatosin led the study conception and design and protocol development. Stephen R. Ash, Daniel Batlle, William Pottorf, and Linda Fried contributed to the study design and protocol development. Linda Fried and Daniel Batlle are on the study steering committee, and Linda Fried is a national principal investigator for the study. Jessica Kendrick, Yasmin Brahmbhatt, and Emily Guerrieri have contributed to the study conduct. Stephen R. Ash provided the study hypothesis that the direct binding of NH4+ by SZC in the gastrointestinal tract is the reason for the increase in serum HCO3− during SZC treatment. Stephen R. Ash, Daniel Batlle, Jessica Kendrick, Yemisi Oluwatosin, William Pottorf, Yasmin Brahmbhatt, Emily Guerrieri, and Linda Fried meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, contributed to manuscript drafting and critical review/revision, and have read and approved the final manuscript.
Data Availability Statement
Data generation and analysis methods for this study are included in this article. Further information regarding AstraZeneca's data sharing policy or clinical trials updates may be obtained at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Acknowledgments
Sarah Greig, PhD, and Meri Pozo, PhD, CMPP, of inScience Communications provided medical writing support, funded by AstraZeneca.
ClinicalTrials.gov identifier: CT04727528.
Funding Statement
The NEUTRALIZE study and development of the manuscript were supported by AstraZeneca. None of the authors have received compensation for the current work.
References
- 1.Kraut JA, Madias NE. Metabolic acidosis of CKD: an update. Am J Kidney Dis. 2016 Feb;67((2)):307–17. doi: 10.1053/j.ajkd.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 2.Raphael KL, Zhang Y, Ying J, Greene T. Prevalence of and risk factors for reduced serum bicarbonate in chronic kidney disease. Nephrology. 2014 Oct;19((10)):648–54. doi: 10.1111/nep.12315. [DOI] [PubMed] [Google Scholar]
- 3.Dobre M, Yang W, Chen J, Drawz P, Hamm LL, Horwitz E, et al. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: a report from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2013 Oct;62((4)):670–8. doi: 10.1053/j.ajkd.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallet M, Metzger M, Haymann JP, Flamant M, Gauci C, Thervet E, et al. Urinary ammonia and long-term outcomes in chronic kidney disease. Kidney Int. 2015 Jul;88((1)):137–45. doi: 10.1038/ki.2015.52. [DOI] [PubMed] [Google Scholar]
- 5.Batlle DC, Arruda JA, Kurtzman NA. Hyperkalemic distal renal tubular acidosis associated with obstructive uropathy. N Engl J Med. 1981 Feb;304((7)):373–80. doi: 10.1056/NEJM198102123040701. [DOI] [PubMed] [Google Scholar]
- 6.Betts KA, Woolley JM, Mu F, McDonald E, Tang W, Wu EQ. The prevalence of hyperkalemia in the United States. Curr Med Res Opin. 2018 Jun;34((6)):971–8. doi: 10.1080/03007995.2018.1433141. [DOI] [PubMed] [Google Scholar]
- 7.Cook E, Davis J, Israni R, Mu F, Betts K, Anzalone D, et al. Prevalence of metabolic acidosis among patients with CKD and hyperkalemia [abstract 89] Am J Kidney Dis. 2020 Apr;75((4)):561–2. [Google Scholar]
- 8.Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017 Sep;46((3)):213–21. doi: 10.1159/000479802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hougen I, Leon SJ, Whitlock R, Rigatto C, Komenda P, Bohm C, et al. Hyperkalemia and its association with mortality, cardiovascular events, hospitalizations, and intensive care unit admissions in a population-based retrospective cohort. Kidney Int Rep. 2021 May;6((5)):1309–16. doi: 10.1016/j.ekir.2021.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration Lokelma® (sodium zirconium cyclosilicate) for oral suspension (prescribing information) [cited 2021 Oct 26]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/207078s007lbl.pdf.
- 11.Stavros F, Yang A, Leon A, Nuttall M, Rasmussen HS. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One. 2014 Dec;9((12)):e114686. doi: 10.1371/journal.pone.0114686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosiborod M, Rasmussen HS, Lavin P, Qunibi WY, Spinowitz B, Packham D, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014 Dec;312((21)):2223–33. doi: 10.1001/jama.2014.15688. [DOI] [PubMed] [Google Scholar]
- 13.Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015 Jan;372((3)):222–31. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 14.Roger SD, Spinowitz BS, Lerma EV, Singh B, Packham DK, Al-Shurbaji A, et al. Efficacy and safety of sodium zirconium cyclosilicate for treatment of hyperkalemia: an 11-month open-label extension of HARMONIZE. Am J Nephrol. 2019 Dec;50((6)):473–80. doi: 10.1159/000504078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spinowitz BS, Fishbane S, Pergola PE, Roger SD, Lerma EV, Butler J, et al. Sodium zirconium cyclosilicate among individuals with hyperkalemia: a 12-Month Phase 3 Study. Clin J Am Soc Nephrol. 2019 Jun;14((6)):798–809. doi: 10.2215/CJN.12651018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zannad F, Hsu BG, Maeda Y, Shin SK, Vishneva EM, Rensfeldt M, et al. Efficacy and safety of sodium zirconium cyclosilicate for hyperkalaemia: the Randomized, Placebo-Controlled HARMONIZE-Global Study. ESC Heart Fail. 2020 Feb;7((1)):54–64. doi: 10.1002/ehf2.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roger SD, Spinowitz BS, Lerma EV, Fishbane S, Ash SR, Martins JG, et al. Sodium zirconium cyclosilicate increases serum bicarbonate concentrations among patients with hyperkalaemia: exploratory analyses from three randomized, multi-dose, placebo-controlled trials. Nephrol Dial Transplant. 2021 May;36((5)):871–83. doi: 10.1093/ndt/gfaa158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ash SR, Singh B, Lavin PT, Stavros F, Rasmussen HS. A Phase 2 Study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int. 2015 Aug;88((2)):404–11. doi: 10.1038/ki.2014.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roger SD, Lavin PT, Lerma EV, McCullough PA, Butler J, Spinowitz BS, et al. Long-term safety and efficacy of sodium zirconium cyclosilicate for hyperkalaemia in patients with mild/moderate versus severe/end-stage chronic kidney disease: comparative results from an Open-Label, Phase 3 Study. Nephrol Dial Transplant. 2021 Jan;36((1)):137–50. doi: 10.1093/ndt/gfz285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bovée DM, Roksnoer LCW, van Kooten C, Rotmans JI, Vogt L, de Borst MH, et al. Effect of sodium bicarbonate supplementation on the renin-angiotensin system in patients with chronic kidney disease and acidosis: a randomized clinical trial. J Nephrol. 2020 Oct;34((5)):1737–45. doi: 10.1007/s40620-020-00944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melamed ML, Horwitz EJ, Dobre MA, Abramowitz MK, Zhang L, Lo Y, et al. Effects of sodium bicarbonate in CKD stages 3 and 4: a randomized, placebo-controlled, multicenter clinical trial. Am J Kidney Dis. 2020 Feb;75((2)):225–34. doi: 10.1053/j.ajkd.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Iorio BR, Bellasi A, Raphael KL, Santoro D, Aucella F, Garofano L, et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J Nephrol. 2019 Dec;32((6)):989–1001. doi: 10.1007/s40620-019-00656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahni V, Rosa RM, Batlle D. Potential benefits of alkali therapy to prevent GFR loss: time for a palatable “solution” for the management of CKD. Kidney Int. 2010 Dec;78((11)):1065–7. doi: 10.1038/ki.2010.364. [DOI] [PubMed] [Google Scholar]
- 24.Bushinsky DA. Tolerance to sodium in patients with CKD-induced metabolic acidosis: does the accompanying anion matter? Am J Kidney Dis. 2019 Jun;73((6)):858–65. doi: 10.1053/j.ajkd.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Caravaca-Fontán F, Díaz-Campillejo R, Valladares J, López Arnaldo C, Barroso S, Luna E, et al. Successful correction of metabolic acidosis is difficult to achieve in chronic kidney disease. Nefrologia. 2020 May–Jun;40((3)):328–35. doi: 10.1016/j.nefro.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Curthoys NP, Moe OW. Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol. 2014 Sep;9((9)):1627–38. doi: 10.2215/CJN.10391012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pourafshar N, Pourafshar S, Soleimani M. Urine ammonium, metabolic acidosis and progression of chronic kidney disease. Nephron. 2018 Feb;138((3)):222–8. doi: 10.1159/000481892. [DOI] [PubMed] [Google Scholar]
- 28.Raphael KL. Metabolic acidosis in CKD: core curriculum 2019. Am J Kidney Dis. 2019 Aug;74((2)):263–75. doi: 10.1053/j.ajkd.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 29.Weiner ID, Mitch WE, Sands JM. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin J Am Soc Nephrol. 2015 Aug;10((8)):1444–58. doi: 10.2215/CJN.10311013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batlle D, Ba Aqeel SH, Marquez A. The urine anion gap in context. Clin J Am Soc Nephrol. 2018 Feb;13((2)):195–7. doi: 10.2215/CJN.13791217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart GS, Smith CP. Urea nitrogen salvage mechanisms and their relevance to ruminants, non-ruminants and man. Nutr Res Rev. 2005 Jun;18((1)):49–62. doi: 10.1079/NRR200498. [DOI] [PubMed] [Google Scholar]
- 32.Barrett KE. Gastrointestinal physiology. 2nd ed. New York, NY: McGraw-Hill Education/Medical; 2013. [Google Scholar]
- 33.Vanholder R, Gryp T, Glorieux G. Urea and chronic kidney disease: the comeback of the century? (in uraemia research) Nephrol Dial Transplant. 2018 Jan;33((1)):4–12. doi: 10.1093/ndt/gfx039. [DOI] [PubMed] [Google Scholar]
- 34.Watford M. The urea cycle: teaching intermediary metabolism in a physiological setting. Biochem Mol Biol Educ. 2003 Sep;31((5)):289–97. [Google Scholar]
- 35.McCullough PA, Costanzo MR, Silver M, Spinowitz B, Zhang J, Lepor NE. Novel agents for the prevention and management of hyperkalemia. Rev Cardiovasc Med. 2015 Jul;16((2)):140–55. doi: 10.3909/ricm0782. [DOI] [PubMed] [Google Scholar]
- 36.Szylman P, Better OS, Chaimowitz C, Rosler A. Role of hyperkalemia in the metabolic acidosis of isolated hypoaldosteronism. N Engl J Med. 1976 Feb 12;294((7)):361–5. doi: 10.1056/NEJM197602122940703. [DOI] [PubMed] [Google Scholar]
- 37.Harris AN, Grimm PR, Lee HW, Delpire E, Fang L, Verlander JW, et al. Mechanism of hyperkalemia-induced metabolic acidosis. J Am Soc Nephrol. 2018 May;29((5)):1411–25. doi: 10.1681/ASN.2017111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karet FE. Mechanisms in hyperkalemic renal tubular acidosis. J Am Soc Nephrol. 2009 Feb;20((2)):251–4. doi: 10.1681/ASN.2008020166. [DOI] [PubMed] [Google Scholar]
- 39.Tannen RL, Kunin AS. Effect of potassium on ammoniagenesis by renal mitochondria. Am J Physiol. 1976 Jul;231((1)):44–51. doi: 10.1152/ajplegacy.1976.231.1.44. [DOI] [PubMed] [Google Scholar]
- 40.Knepper MA, Packer R, Good DW. Ammonium transport in the kidney. Physiol Rev. 1989 Jan;69((1)):179–249. doi: 10.1152/physrev.1989.69.1.179. [DOI] [PubMed] [Google Scholar]
- 41.DuBose TD, Jr, Good DW. Chronic hyperkalemia impairs ammonium transport and accumulation in the inner medulla of the rat. J Clin Invest. 1992 Oct;90((4)):1443–9. doi: 10.1172/JCI116011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DuBose TD, Jr, Caflisch CR. Effect of selective aldosterone deficiency on acidification in nephron segments of the rat inner medulla. J Clin Invest. 1988 Nov;82((5)):1624–32. doi: 10.1172/JCI113774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batlle D, Arruda J. Hyperkalemic forms of renal tubular acidosis: clinical and pathophysiological aspects. Adv Chronic Kidney Dis. 2018 Jul;25((4)):321–33. doi: 10.1053/j.ackd.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Någård M, Singh B, Boulton DW. Effects of sodium zirconium cyclosilicate on sodium and potassium excretion in healthy adults: a Phase 1 Study. Clin Kidney J. 2021 Aug;14((8)):1924–31. doi: 10.1093/ckj/sfaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fishbane S, Ford M, Fukagawa M, McCafferty K, Rastogi A, Spinowitz B, et al. A Phase 3b, Randomized, Double-Blind, Placebo-Controlled Study of sodium zirconium cyclosilicate for reducing the incidence of predialysis hyperkalemia. J Am Soc Nephrol. 2019 Sep;30((9)):1723–33. doi: 10.1681/ASN.2019050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generation and analysis methods for this study are included in this article. Further information regarding AstraZeneca's data sharing policy or clinical trials updates may be obtained at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.