Abstract
Background
Of the 200 million patients worldwide affected by peripheral arterial disease (PAD), 4% will inevitably require major limb amputation. Previous systematic reviews presented a conflicting body of evidence in terms of vascular endothelial growth factor (VEGF) family member effects upon PAD natural progression. Despite that, modulation of intrinsic angiogenesis mechanisms targeting the VEGF family members still confers an attractive therapeutic target. The aim of the present study was to evaluate current evidence of VEGF modulation in the context of PAD.
Methods
This is a systematic literature review conducted according to the PRISMA guidelines and registered under PROSPERO database [CRD42021285988]. Independent literature search was performed up to April 1, 2022, on six databases. A total of 22 eligible studies were identified [N: 3, interventional patient studies; N: 19, animal studies]. Animal studies were appraised by the SYRCLE risk of bias tool, while human participant studies were assessed by the Newcastle Ottawa scale. Overall, quality of evidence was deemed fair for both animal and human studies. Main study outcomes were percentage change of injured vessel lumen stenosis and neointimal area formation upon VEGF modulation (inhibition or activation) in comparison with control group.
Findings
Nineteen animal models and three human participant studies were included in the systematic review and assessed separately. Positive modulation of VEGF-A in animal models resulted in a median decrease of 65.58% [95% CI 45.2; 71.87] in lumen stenosis [14 studies]. Furthermore, positive modulation of VEGF-A was found to reduce neointimal area proliferation by a median decrease of 63.41% [95% CI 41.6; 79.59] [14 studies]. Median end of study duration was 28 days [range: 14–84 days]. Data were insufficient to assess these outcomes with respect to VEGF-B or VEGF-C modulation. The limited number of available human studies presented inadequate outcome assessment despite their overall fair NOS grading.
Interpretation
VEGF-A-positive modulation decreases lumen stenosis and neointimal hyperplasia in PAD simulation animal models. Previously identified variability among outcomes was found to strongly stem from the variability of experimental designs. Clinical applicability and safety profile of VEGF-A in the context of PAD remain to be defined by a robust and uniformly designed body of further animal model-based experiments.
Keywords: Peripheral arterial disease, Vascular endothelial growth factor, Lumen stenosis, Neointimal hyperplasia, Animal models
Introduction
Peripheral artery disease (PAD) is a progressive condition driven by gradual stenosis and ultimately occlusion of large and medium-sized arteries, other than coronary and cerebral [1, 2, 3]. It is associated with a wide range of devastating effects which include asymptomatic lumen narrowing (20–50%), classic intermittent claudication complaints (10–35%), and critical limb ischemia features, the latter comprising rest pain and tissue loss (5–15%) [4, 5]. Nonoperative therapeutic options for PAD have classically aimed to control modifiable risk factors such as nonatherogenic diet and daily activity promotion, medical management of hypertension, dyslipidemia, diabetes as well as smoking cessation. In addition, antiplatelet therapy has been adopted as secondary prevention strategy and is now recommended in patients with diabetes and a history of atherosclerotic cardiovascular disease [6, 7]. However, it seems inevitable that 16% of these patients who suffer from intermittent claudication will deteriorate so that, eventually, 7% will require a lower extremity revascularization procedure and 3–4% a major limb amputation [1, 3, 8, 9]. Literature reported rates between revascularization attempt and the requirement for a major limb amputation within the year vary between 6.3% and 16.2% [10, 11].
Despite significant advances in the endovascular and operative revascularization field, with rapidly evolving new technologies striving for reduction of immediate complications and making surgical management of the frail patient possible, feasibility and efficacy limiting caveats, such as calcification, microvascular disease, and restenosis, are yet to be overcome [9]. Modulation of intrinsic angiogenesis mechanisms has offered an attractive therapeutic target not only in patients with limited revascularization options, but also in those who have undergone endovascular or open PAD surgical management in view of stent or graft restenosis prevention. Angiogenesis and arteriogenesis are umbrella terms for highly complex physiological process which is under continuous positive and negative feedback control mechanisms [12, 13]. Although several related proteins have been identified as drivers of angiogenesis, including vascular endothelial growth factor-B (VEGF-B), VEGF-C, and placental growth factor [14], VEGF-A due to its key role in angiogenesis regulation has been the most extensively studied. Hand in hand with the VEGF family members, the VEGF receptors play a pivotal role in the modulation of the downstream intracellular signals. Dimeric VEGF binding to the VEGFR extracellular domains promotes receptor dimerization and mutual receptor subunit tyrosine transphosphorylation of the intracellular domains facilitating downstream pathway activation. VEGF ligands act through specific binding to three different cell membrane-bound receptors: VEGFR-1 (Flt-1), VEGFR-2 (Flk/KDR), and VEGFR-3 (Flt-4) [15]. Targeting, and more specifically inhibiting the action of VEGFRs, has been an attractive target especially in cancer pharmacology with vatalanib, sunitinib, and sorafenib being developed as universal inhibitors of all VEGF receptor tyrosine kinases while other monoclonal antibodies such as semaxanib and ZD6474 displaying selective receptor inhibition [15].
Pathophysiological processes that merge to create the picture of symptomatic PAD split under three main categories: vessel collateralization, plaque formation and inflammatory remodeling and subsequent lumen stenosis, and finally lumen neointima formation following surgical treatment. To successfully provide distal limb flow, without any surgical intervention, two options remain accessible, either increased collateralization and physiological bypassing of the diseased vessel section or reduction of the vessel lumen stenosis to a degree to improve flow. Despite its well-known proangiogenic function, gene therapy based on VEGF-A has had limited success in clinical trials for PAD [16, 17]. Intriguingly, patients with PAD have been shown to have impaired revascularization despite increased endogenous levels of VEGF-A isoforms, namely, VEGF-A165. Recently, it has been suggested that this may be due to further alternative splicing of VEGF-A165 RNA giving rise to two further isoforms in humans, VEGF-A165a and VEGF-A165b, and while VEGF-A165a is a potent stimulator of vascular growth, VEGF-A165b displays strong antiangiogenic effects by competitively binding to the VEGF-A receptor and inhibiting angiogenesis [18]. In terms of lumen stenosis, it has been stipulated that VEGF-A vessel treatment may promote vasodilation in a nitric oxide-dependent manner in peripheral artery models as well as balancing vessel permeability and endothelial cell growth promoting controlled vessel repair process in coronary artery models [19, 20].
Despite the promising evidence, VEGF-A increase has also been implicated with promotion rather than reduction of lumen stenosis in peripheral, coronary artery as well as arteriovenous fistula models [14, 21, 22]. Whether this is a result of experimental variability, inability to control endogenous VEGF levels, locoregional differences between PAD models (carotid vs. femoral vs. iliac arteries), or a fine balance between pro- and anti-angiogenic alternative splice variants as previously discussed remains to be delineated. A limited number of previous reviews have attempted to delineate the relationship between exogenous VEGF upon neointimal hyperplasia and subsequent arterial lumen stenosis, augmenting the ongoing controversy of findings in this field [23, 24]. Herein, we attempt to comparatively analyze available data regarding modulation, either increase or decrease, of VEGF family members and its effects upon neointimal hyperplasia and lumen stenosis in order to highlight the reasons behind these controversial findings with focus on peripheral arterial disease. We further aim to provide updates on our current understanding of the VEGF pathway in the context of PAD therapeutics. Thus, the present review aims to propose future research directions to clarify and enable safe VEGF applications in humans.
Materials and Methods
Search Strategy and Selection Criteria
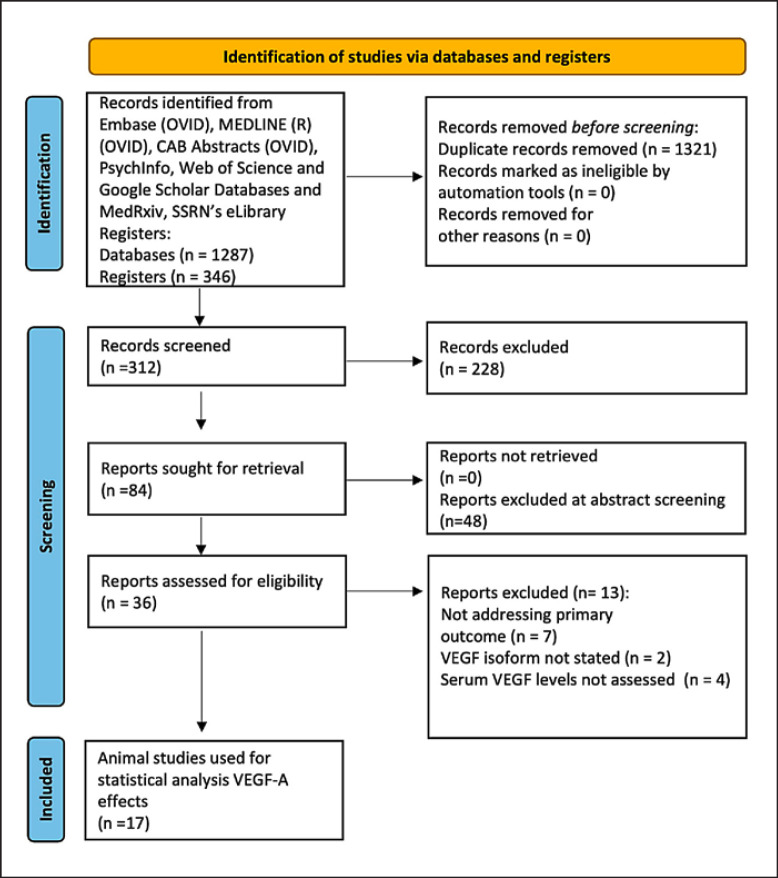
A systematic literature review was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA; Population Intervention Comparison Outcome (PICO) chart] (Fig. 1; Table 1). Independent literature search for relevant studies was performed up to September 20, 2021, and updated on the April 1, 2022, on six databases: Embase, Medline, CAB Abstracts, PsychINFO, Web of Science, and Google Scholar. Additional records were identified through other sources, including SSRNs eLibrary, Research Square, and medRxiv. The medRxiv search was simplified according to database search functionality. The references of the included studies were scrutinized for additional relevant studies. Search limitations included articles in English language. The following search strategy was employed: (VEGF or Vascular endothelial factor) and (*stenosis OR neointimal*).ti,ab,mh., limit to English language and adapted as per individual database requirements. The present study was registered under the international database of prospectively registered systematic reviews in health and social care (PROSPERO) with accession number CRD42021285988.
Fig. 1.
PRISMA chart. *One study, Szabo et al., 2007, removed from analysis following consideration of reviewers' comments.
Table 1.
PICO chart
| Manuscript | Date | Organism | Anatomical site/mode of vessel injury | Delivery construct | Gene/protein levels measured | VEGF isomer examined | VEGF-A/B/C effects | Lumen stenosis | Neointimal area proliferation mm2 | Neovascularization | Adventitial lymphatics | Maximum day of assessment, days |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal studies [N:19] | ||||||||||||
| Asahara et al. [27] | 1996 | Rabbit | Femoral Balloon injury | VEGF165 plasmid | Gene/protein | VEGF-165 | VEGF-A activation | ↓ 60% | ↓ 79.71% | Not assessed | Not assessed | 28 |
| Hutter et al. [28] | 2004 | Mouse | Femoral Guide wire | VEGF165/adenovirus and trap-conditioned medium | Protein | VEGF-165 | VEGF-A activation | ↓ 10.62% | ↓ 66.6% | Not assessed | Not assessed | 28 |
| Koga et al., [29] | 2009 | Mouse | Femoral Guide wire | Flt1 knock-out | Protein | VEGF-B | VEGF-B inhibition | ↑ 39.2% | ↑ 8.3% | Not assessed | Not assessed | 28 |
| Kondoh et al. [30] | 2004 | Rabbits | Femoral Balloon injury | VEGF 165/adenovirus | Gene/protein | VEGF-165 | VEGF-A activation | ↓ 18.3% | Not assessed | Not assessed | Not assessed | 28 |
| Laitinen et al. [31] | 1997 | Rabbit | Carotid Ligation | VEGF164/cytomegalovirus | Gene/protein | VEGF 164 | VEGF-A activation | ↓ 45.2% | Not assessed | Not assessed | Not assessed | 14 |
| Leppanen et al. [32] | 2004 | Rabbit | Carotid Balloon injury | VEGF-C/adenovirus | Gene/protein | VEGF-C | VEGF-C activation | ↓ 42.85% | ↓ 20% | Not assessed | Not assessed | 42 |
| Liu et al. [33] | 2004 | Rabbit | Carotid Balloon injury | VEGF-165/adenovirus | Gene | VEGF-165 | VEGF-A activation | Not assessed | ↓ 71.4% | Not assessed | Not assessed | 21 |
| Ohtani et al. [34] | 2004 | Rabbit | Carotid Balloon injury | Silence RNA Flt-1/adenovirus | Gene/protein | VEGF-B | VEGF-B inhibition | ↓ 41.25% | ↓ 47% | Not assessed | Not assessed | 28 |
| Paul et al. [35] | 2012 | Dog | Femoral Balloon injury | DNA nanoparticles of Ang1/Vegf | Gene | VEGF-165 | VEGF-A activation | ↓ 65.45% | ↓ 41.6% | Not assessed | Not assessed | 42 |
| Sun et al. [36] | 2018 | Rat | Carotid Balloon injury | Thalidomide as VEGF inhibitor | Gene/protein | VEGF-A | VEGF-A inhibition | ↑ 22.5% | ↓ 46.1% | Not assessed | Not assessed | 14 |
| Tang et al. [37] | 2011 | Rabbit | Aorta Stenting | VEGF121-adenovirus HUVE cells | Gene/protein | VEGF-121 | VEGF-A activation | ↓ 67% | ↓ 65.48% | Not assessed | Not assessed | 28 |
| Tsai et al. [38] | 2012 | Mouse | Carotid Decellularized vessel grafting | Local application of VEGF | Protein | VEGF-A | VEGF-A activation | ↓ 83.3% | Not assessed | Not assessed | Not assessed | 28 |
| Van Belle et al. [39] | 1997 | Rabbit | Iliac Balloon injury and stent | VEGF-165 plasmid | Gene/protein | VEGF-165 | VEGF-A activation | ↓ 65.7% | ↓ 61.33% | Not assessed | Not assessed | 28 |
| Walter et al. [40] | 2004 | Rabbit | Iliac Stenting | VEGF-2 plasmid | Gene/protein | VEGF -2R | VEGF-A activation | ↓ 54.2% | Not assessed | Not assessed | Not assessed | 84 |
| Whitlock et al. [41] | 2004 | Mouse | Iliac Ligation | VEGF (121, 165, 189)/adenovirus | Gene/protein | VEGF-121 | VEGF-A activation | ↓ 71.87% | Not assessed | Not assessed | Not assessed | 21 |
| Wu et al. [42] | 2016 | Rabbit | Aorta Balloon injury | VEGF121 lentivirus and siRNA control | Gene/protein | VEGF-121 | VEGF-A activation VEGF-A inhibition (siRNA) |
↓ 71.42% ↑ 11.11% |
↓ 57.1% ↑ 29% Not assessed | Not assessed | 84 | |
| Xie et al. [43] | 2015 | Rabbit | Aorta Balloon injury | VEGF nanoparticle | Protein | VEGF-165 | VEGF-A activation | Not assessed | ↓ 79.59% | Not assessed | Not assessed | 28 |
| Ye et al. [44] | 2019 | Rabbit | Aorta Stent | VEGF165 plasmid | Gene/protein | VEGF-165 | VEGF-A activation | Not assessed | ↓ 46% | Not assessed | Not assessed | 28 |
| Zhang et al. [45] | 2016 | Rabbit | Femoral Balloon injury | VEGF165 plasmid versus VEGF165 microsphere delivery |
Protein | VEGF-165 | VEGF-A activation | Not assessed | ↓ 14.7% | Not assessed | Not assessed | 28 |
| Human participant studies [N:3] | ||||||||||||
| Baumgartner et al. [46] |
1998 | Human [N:9] |
Calf/thigh | VEGF165 plasmid | Gene/protein | VEGF-165 | VEGF-A activation | Not assessed | Not assessed | ↑ [% not available] | Not assessed | 56 |
| Kim et al. [47] | 2004 | Human [N:9] |
Calf/thigh | VEGF165 plasmid | Gene | VEGF-165 | VEGF-A activation | Not assessed | Not assessed | ↑ [% not available] | Not assessed | 252 |
| Rajagopalan et al. [48] | 2003 | Human [N:105] |
Calf/thigh | VEGF121 plasmid | Protein | VEGF-121 | VEGF-A activation | Not assessed | Not assessed | No change | ? ↑ 61% (tissue edema) | 182 |
Inclusion and Exclusion Criteria
Full-text inclusion criteria were defined as below.
1. In vivo studies.
2. Assessing VEGF as a result of a molecular or chemical intervention.
3. Assessing either neointimal area proliferation (mm2 or intimal: media depth ratio) or lumen stenosis (either as percentage change from control or mm2) as primary outcomes. Assessing either lymphangiogenesis or neovascularization (assessed quantitatively through imaging evidence or ankle/brachial pressure index change) as secondary outcomes with crude measurement numbers provided.
Full-text exclusion criteria were defined as below.
1. Nonperipheral vascular ischemia model (e.g., coronary or cerebral artery).
2. Not assessing any of the primary or secondary outcomes of the present study.
3. VEGF-specific protein (A, B, C, D, E) or isoform not stated.
4. Studies conducted in in vitro models.
Studies were included in the final analysis if at least one of the primary outcomes was addressed as per inclusion criteria statement. Excluded studies, with justifications, are presented in online supplementary Table S1 (for all online suppl. material, see www.karger.com/doi/10.1159/000527079).
Study Outcomes
The primary outcome of the present study was to assess the effect of VEGF sub-type modulation either increase/promotion or decrease/inhibition in protein levels upon vascular lumen stenosis and neointimal hyperplasia promotion. Secondary outcomes included the assessment of VEGF family sub-types with respect to lymphangiogenesis and neovascularization.
Data Extraction
After removing duplicates, citations were screened by title and abstract, and then full texts were appraised to determine their eligibility by three authors (S.L.K., J.E., and M.G.) (Fig. 1). Three authors (S.L.K., J.E., and M.G.) independently conducted the abstract and full-text screening. Disagreements were resolved by the fourth author (R.M.). Study design, model organism, delivery construct, VEGF isoform sub-types assessed, effects on neointimal area proliferation, lumen stenosis, neovascularization and lymphangiogenesis, and duration of study were extracted per manuscript. Risk of bias was assessed using the Systematic Review Centre for Laboratory Animal Experimentation Risk of Bias tool (SYRCLE RoB) for animal studies and the Newcastle Ottawa scale for human studies (online suppl. Table S2, S3) [25, 26].
Data Analysis
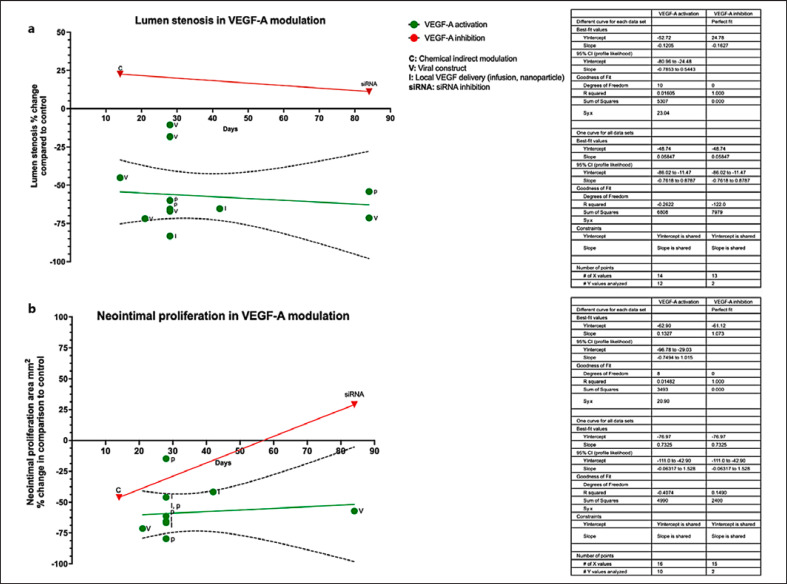
Statistical analysis and histogram plots were performed using GraphPad Prism (v. 9). Linear regression (best fit line and 95% confidence intervals [95% CIs]) was calculated from original data. Change in restenosis and neointimal proliferation area (mm2) was depicted as percentages where percentage change was defined as (control reported value-experimental reported value/control reported value *100) (Fig. 2). Percentage change was calculated to normalize data in comparison with control group values as time point of data collection as stated in each individual study. Best fit lines of available data were calculated with GraphPad Prism V. 9. VEGF pathway illustration (Fig. 3) was created with the BioRender.com., scientific image illustration online platform.
Fig. 2.
a Percentage change of arterial lumen stenosis and of neointimal proliferation area across animal studies, histogram, and best fit lines. X-axis depicts the percentage change in arterial lumen stenosis following VEGF modulation (increase or decrease) observed in the experimental group versus the matched control group per study. Data displayed here reflect experimental data stemming from animal studies only. Green dots represent the results of studies attempting activation of VEGF-A, while those attempting inhibition are represented by red dots. Percentage change was calculated in relevance to the control group of each study. Y-axes depict duration of experiment and day of data reporting (days) per study. Mechanism of VEGF modulation is depicted as initial letters, where C is chemical modulation (activation or inhibition) of VEGF; V delivery of VEGF by viral transfection construct; I local infusion of VEGF protein; siRNA inhibition of VEGF by silence RNA (RNA interference). Linear regression and 95% CI asymptotic slopes (dashed lines) are depicted, where applicable. a VEGF-A activation; Y intercept −60.92 (slope 95% CI [asymptotic] −0.51 to 0.93), VEGF-A inhibition Y intercept (line) 11.97. b VEGF-A activation Y intercept −64.37 (line) (slope 95% CI [asymptotic] −0.58 to 0.67), VEGF-A inhibition Y intercept (line) 51.74. Graph was generated with GraphPad Prism V. 9.
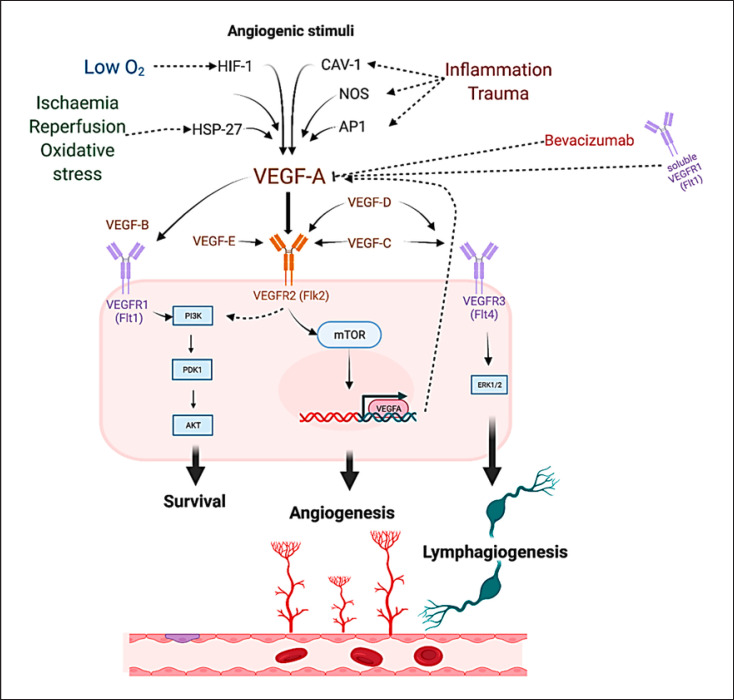
Fig. 3.
Angiogenic stimuli such as hypoxia through hypoxia-inducible factor 1 (HIF-1); reperfusion ischemia and oxidative stress through heat shock protein 27 (HSP27); tissue trauma, and inflammation though activator protein 1 (AP-1), caveolin-1 (Cav-1), or nitric oxide synthase (NOS) [66, 67] promote VEGF-A activation. Bevacizumab and soluble VEGFR1 exert inhibitory effects upon VEGF-A [14, 71]. Binding of VEGF-A to VEGFR2 activates downstream proangiogenic cascades [14, 51, 52]. VEGF-B is the main substrate of the membrane-bound VEGFR-1, activation of which leads to promotion of cell survival via the phosphatidylinositol 3-kinase/Ak strain transforming (PI3K/AKT) pathway with little effect upon angiogenesis [68, 69]. VEGF-C and VEGF-D are primary substrates of the VEGFR3 receptor, activation of which leads to lymphangiogenic promotion via the extracellular signal-regulated kinases 1 and 2 (ERK1/2) pathway [70, 71, 72]. Cross-activation of the VEGF receptors is possible by multiple VEGF family members with variable effects still to be fully elucidated [14].
Results
Following the PRISMA guidelines on systematic review search, we identified 36 eligible studies for data extraction. Full-text screening excluded 13 studies (online suppl. Table S1). A total of 22 studies remained all of which were included in this systematic review (Fig. 1; Table 1) [27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48].
Model Organism
Thirteen studies were conducted in rabbits [27, 30, 31, 32, 33, 34, 37, 39, 40, 42, 43, 44, 45], four studies in mice [28, 29, 38, 41], one in canine models [35], and a further one in rats [36]. Finally, three studies included human subjects (Table 1) [46, 47, 48].
VEGF Delivery System
Of the included animal studies, nine studies used viral constructs to achieve VEGF gene transfer to native tissue [28, 30, 31, 32, 33, 34, 37, 41, 42]. Eight employed plasmid constructs for VEGF delivery, of which five in animals [12, 39, 40, 44, 45] and three in human participants [4, 46, 47, 48]. Two studies implemented nanoparticle delivery of VEGF [35, 43]. One further study used chemical blockade of VEGF with thalidomide [36]. One study used local application of VEGF solution [38], and finally, the last study included double gene knock-out models of the Fms-related receptor tyrosine kinase 1 gene (Flt1−/−) (Table 1) [28].
VEGF Sub-Types or Isoform Examined
Vast variation was also observed in the VEGF-A isoforms used as well as the individual VEGF sub-types which were examined across studies. Twenty studies effectively analyzed decrease or increase of VEGF-A [36, 38] or its isoforms (VEGF121, 164, 165) [27, 28, 30, 31, 33, 35, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48]. One of them employed membrane-bound, VEGF receptor 2 expression plasmid (binding site of VEGF-A), effectively increasing the action of VEGF-A [40]. Regarding VEGF-B, one study attempted the modulation of VEGF-B by employing a double Flt1 gene knock-out (Flt1−/−) murine model [29], while another employed silence RNA against Flt1 (VEGF receptor-1), thus inhibiting VEGF-B activity [34]. Lastly, one study focused on VEGF-C modulation [32].
Study Duration
Study end time was also a significant variable across reported results. Median time across included animal studies was 28 days [min: 14; max: 84] [27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45]. Duration of human participant studies was extended to 217 days [range: 56–392 days] [46, 47, 48].
Study Outcomes
Sixteen animal model studies [27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 45] reported change in arterial lumen post-VEGF modulation. Fourteen studies reported change in neointimal area proliferation (mm2) [27, 28, 29, 32, 33, 34, 35, 36, 37, 39, 42, 43, 44, 45].
Human Studies
Three studies were conducted on human participants and concentrated on VEGF effects upon peripheral arterial vessels [46, 47, 48]. Two studies suggested increase of neovascularization, as observed through ABPI and angiographic imaging, following intramuscular injection of VEGF165 in calf/thigh muscles of ischemic limbs [46, 47]. The latter did not report significant differences among control and experimental groups, in terms of neovascularization [48], but identified significant increase (61%) of limb edema following intramuscular injection of VEGF121.
Animal Studies
Accepting the recognized inter-species variability [49], we attempted to clarify whether previously identified contradictions among VEGF animal model derived data from overall study duration. Crude numbers (mm2) for neointimal hyperplasia and percentage of change in lumen stenosis were translated in percentage change in comparison with each individual study control group at matched time points to enable comparability across studies (Table 1; Fig. 2). Twelve studies assessed the VEGF-A effects upon lumen stenosis while eleven on neointimal area proliferation. Given the lack of adequate number of studies [3 studies] to assess the effects of other VEGF sub-types upon the defined outcomes, analysis was not feasible for VEGF-B or VEGF-C (Fig. 2).
Regarding VEGF-A treatment effects upon vessel lumen stenosis (Fig. 2a), the twelve studies that attempted to increase VEGF-A levels congruently suggested a decrease on vessel lumen stenosis even at 84 days. Equally, two studies which attempted to decrease VEGF-A levels concluded an exactly opposing result, i.e., an increase on vessel lumen stenosis. The inhibitory effect of VEGF-A increase upon lumen stenosis did not appear to reduce even in lengthier study durations (Fig. 2a). An examination of the interpolation curve indicated that the maximal effects of VEGF-A upregulation upon lumen stenosis prevention are exerted at day 51 across studies, resulting in 68.04% reduction of lumen stenosis in comparison with control (Fig. 2a).
Regarding neointimal area proliferation percentage change, nine studies employed VEGF-A upregulation to assess this outcome, one conducted both up- and downregulation of VEGF-A to augment results [42], while one focused on its negative regulation solely (Fig. 2b) [36]. All studies indicated that positive regulation of VEGF-A, regardless of upregulation mechanism, resulted in decreased neointimal area proliferation and suppressed hyperplasia. Data interpolation suggested that optimal results upon neointimal hyperplasia suppression were noted on day 21 with 71.23% prevention in a sustainable fashion in terms of duration. On the other hand, VEGF-A inhibition resulted in promotion of neointimal proliferation, albeit conclusions could not be safely drawn due to the limited number of studies and data variability (Fig. 2b) [36, 42].
Regarding the remaining VEGF sub-types, two studies assessed the effects of VEGF-B inhibition upon lumen stenosis (increase by 39.2% [29] and decrease by 41.25% [34]) and neointimal hyperplasia (8.3% increase and 47% decrease) by day 28 with contradictory results (Table 1). Of note, two different animal model organisms were employed to draw associations, respectively, mouse and rabbit, that may suggest inter-species variation of the VEGF-B downstream pathway [28, 34]. Lastly, only one study investigated the effects of VEGF-C modulation, noting a 42.85% decrease of lumen stenosis progression at day 42 [32]. Taken collectively, these data support that VEGF-A-positive modulation (increase) inhibits arterial lumen stenosis and neointimal hyperplasia, while data remain inconclusive regarding the other sub-types of the VEGF family.
Discussion
In the present work, we have collectively assessed manuscripts addressing VEGF modulation in the context of peripheral arterial disease treatment to clarify the ongoing controversy concerning VEGF use in therapeutic angiogenesis and post-operative vessel restenosis [50]. To the best of our knowledge, this is the first systematic review to address this specific topic by highlighting the variability of experimental design published in the literature. Factors introducing variability across studies have been investigated, and speculations as to the sources of outcome heterogeneity have been raised [24, 31, 50]. A total of 22 studies were included in the final analysis, overall supporting the hypothesis that VEGF-A-positive modulation inhibits both arterial lumen stenosis and neointimal hyperplasia.
VEGF-A remains the most studied and pharmacologically targeted member of the VEGF sub-types for therapeutic angiogenesis and graft patency studies. Among its direct cellular actions lie endothelial cell mitogenesis and proliferation, survival promotion, control of vascular permeability, and angiogenesis regulation [14, 51]. Despite the importance of VEGF-A in cellular integrity, survival, and angiogenesis promotion, there remains at a complex interplay with the other members of the protein family, namely, VEGF-B, VEGF-C, VEGF-D, placental growth factor [52], and cross-binding cellular receptors such as VEGFR-1; VEGFR-2; VEGFR-3 (Fig. 3). Furthermore, human VEGF-A is known to undergo alternative exon splicing leading to a plethora of isoforms, namely, VEGF121, VEGF165 (VEGF164 in murine models), VEGF189, and VEGF206, which nonetheless do not have the same physiological efficacy in driving VEGF-A cellular targets and biological functions [53]. Additionally, soluble forms of VEGF receptors may exert strong inhibitory effects upon VEGF-A, in contrast to their membrane-bound counterparts [54, 55]. This evidence strongly highlights the complexity associated with VEGF signaling, its essential role to vascular homeostasis, and the different affinities of its key receptors. On a clinical level, tissue ischemia due to critical vessel stenosis and subsequent hypoxia drives symptomatic PAD. These factors stimulate endogenous VEGF production which in turn drives angiogenesis. A vital step in this process is the sprouting of endothelial cells from pre-existing capillary beds and their subsequent migration, controlled proliferation, which leads to the formation of new collateral lumens and intussusception of existing capillaries [56, 57]. This process enables the incorporation of circulating endothelial progenitors to the pre-existing, diseased vessels promoting healing [58, 59, 60]. Overall, a fine balance of the proangiogenic VEGF-A splice variants (VEGFA165a) and downregulation of antiangiogenic ones (VEGFA165b), which commonly are found elevated in PAD patients, drives controlled lumen restoration and successful collateralization instead of deregulated growth and neointimal hyperplasia [61, 62].
Previous attempts to clarify preclinical data and inform the applicability of VEGF-A as a treatment option not only for patients noneligible for revascularization options, but also for those that have undergone endovascular or open procedures, accentuated a significant disparity between reported outcomes. Past systematic literature reviews of preclinical PAD models have produced contradictory summative outcomes regarding VEGF-A exogenous increase and its subsequent benefits upon lumen stenosis prevention and angio- and arteriogenesis. Ganta et al. [61], in their systematic review, conclude that inhibiting a VEGF-A isoform in ischemic muscle promotes perfusion recovery in preclinical PAD models whereas Gabhann et al. [63] suggested that activation of VEGF-A promotes a similar perfusion recovery. Differences between across studies may stem from a spectrum of experimental variables. The instability of preceding transfection construct expression, employed to modulate VEGF-A levels, necessitates the use of contemporary, durable expression vectors in future experiments [64]. Furthermore, soluble decoy receptors may have unpredictable effects, given the complexity of the VEGF pathway interplay and thus resulting in contradictory evidence (Fig. 3) [65]. Another important variability factor among studies may also stem from the induction of alternative VEGF-A splice variants, which as recently shown may promote or in fact inhibit angiogenesis [18].
Other sources of variability may lie within the multiple angiogenic stimuli which have been identified as positive regulators of VEGF-A in response to hypoxia, tissue trauma, and inflammation as well as tissue ischemia (Fig. 3) [66, 67, 68]. While VEGF-A mainly exerts its effects upon binding to the VEGFR-2, encoded by the Flk2 gene, other receptors accommodate VEGF-A binding with differential effects. For example, VEGFR-2 also acts as a binding site for VEGF-C, -D, and -E with variable downstream target activation (Fig. 3) [14]. For simplicity, canonical activation of the VEGFR-2 by VEGF-A results in angiogenesis, activation of VEGFR-1 by VEGF-B promotes cell survival through the PI3K/AKT cascade (Fig. 3) [69, 70, 71], while VEGFR-3 activation by VEGF-C and -D leads to lymphangiogenesis (Fig. 3) [72, 73, 74]. Of note, the complex interaction of the VEGF protein network may result in synergistic or antagonistic effects via either direct binding or initiation of feedback loops, in response to variable exogenous stimuli, thus enabling fine-tuning of cellular responses (Fig. 3) [14, 67, 68].
Moving from the molecular to an organism level, inter-species variation and overall experimental design discrepancies were notable across studies [27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45]. While differential experimental designs may be overall beneficial in assessing holistically the VEGF pathway interaction, they may cloud outcome clarity, creating controversy in the clinical applicability of VEGF-A in PAD management. The VEGF pathway differences across animal models and humans are not unexpected given the widely reported variability of other major morphogenesis and proliferation pathways such as the mammalian target of rapamycin, PI3K/Akt, and the rat sarcoma [75, 76, 77]. VEGF species variability in both structure and function emphasizes the need for identification of the optimal animal model to enable comparable VEGF modulation outcomes with human counterparts. Given the pathway transferability between swine (Sus scrofa) models and humans in muscle integrity modulation pathways, swine models prove superior for the study of VEGF effects on PAD, in comparison with murine or rabbit ones [76]. Intriguingly, among model organisms VEGF-A proteins compared against the Homo sapiens reference sequence, Sus scrofa VEGF-A isoform demonstrated the highest sequence cover (100%) with an 86.84% sequence identity in Basic Local Alignment Search Tool (BLAST) analysis [78]. Taking into account the experimental cost-effectiveness as well as all ethical and ecological considerations associated with the use of swine models [62, 79], human xenografts may also be considered as a viable, more clinically relevant alternative [80, 81].
Regarding selection of VEGF isoforms, VEGF165 is the most frequently expressed isoform in tissues as well as the most physiologically active [14]. In view of the observed isoform selection variability across studies, we suggest measurement of VEGF165, rather than other VEGF isoforms, in PAD-oriented experimental designs. More importantly, a human study employing VEGF165 plasmid vectors as tissue delivery system reported VEGF expression in local but also distant areas at 10 weeks post-injection. Consequently, the local but also the systemic effects of VEGF165 conveying plasmid vector administration should be thoroughly investigated [46]. Lastly, whether the activation of VEGF-A alone or congruent activation of downstream or parallel pathways is responsible for the beneficial effects observed upon neointimal hyperplasia diminution and consequent decrease in lumen stenosis remains to be delineated. Overall, variability of outcomes originates from the variability of experimental designs. Therefore, the applicability of VEGF-A in PAD management clinical practice remains to be clarified by a uniform body of further animal model experiments.
Strengths
The present work reflects the first systematic review conducted according to the PRISMA guideline criteria, to collectively assess manuscripts addressing VEGF modulation in the context of PAD, while highlighting experimental design and model organism variability and effects upon reproducibility in human participants. Here, we also provide guidance as to selection of model organism, specific VEGF isoform assessment, and delivery system a to generate clinically relevant data.
Limitations
The variability of animal model employed, overall experimental design, VEGF sub-types, and VEGF-A isoform measured as well as timing of outcome measurement was highlighted throughout this study, but inevitably may have introduced significant data bias. Only three studies were undertaken in human participants with noncomparable outcomes. Of note, among human studies, only one was randomized [48].
Conclusion and Future Directions
Herein, we demonstrated that VEGF-A-positive modulation inhibits arterial lumen stenosis and neointimal hyperplasia. We also suggested that modulation of the VEGF165 isoform in swine models, with an extended experimental period, e.g., 58 days for neointimal proliferation or 98 days for lumen stenosis as measurement outcomes, may be the most physiologically relevant experimental option in the context of delineating the PAD and VEGF association. Further fundamental and clinical research is required to enable applicability of VEGF-A therapy in the clinical management of PAD.
Statement of Ethics
The authors have no ethical conflicts to disclose. The present work reflects a systematic review of published data.
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Funding Sources
The authors declare that no funds, grants, or other support were received during the preparation of the manuscript.
Author Contributions
Stavroula L. Kastora: conceptualization, data collection and analysis, critical appraisal, manuscript drafting and editing, final corrections, submission, and final approval. Jonathan Eley: data collection, critical appraisal, manuscript editing, and final approval. Martin Gannon and Ross Melvin: critical appraisal, manuscript editing, and final approval. Euan Munro: expert opinion, manuscript editing, and final approval. Sotirios Makris: supervision, manuscript editing, and final approval.
Data Availability Statement
All supporting and associated data are available as online supplementary material.
Supplementary Material
Supplementary data
Acknowledgments
The first author would like to thank Mr. Michael Sharp for his ongoing guidance and support.
Funding Statement
The authors declare that no funds, grants, or other support were received during the preparation of the manuscript.
References
- 1.Shu J, Santulli G. Update on peripheral artery disease: epidemiology and evidence-based facts. Atherosclerosis. 2018 Aug 1;275:379–381. doi: 10.1016/j.atherosclerosis.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006 Mar 7;47((5)):921–929. doi: 10.1016/j.jacc.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 3.Hiatt WR, Fowkes FG, Heizer G, Berger JS, Baumgartner I, Held P, et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017 Jan 5;376:32–40. doi: 10.1056/NEJMoa1611688. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JL, Halperin JL, Albert N, Bozkurt B, Brindis RG, Curtis LH, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 Apr 9;61((14)):1555–1570. doi: 10.1016/j.jacc.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies MG. Criticial limb ischemia: epidemiology. Methodist DeBakey Cardiovascular J. 2012 Oct;8((4)):10–4. doi: 10.14797/mdcj-8-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996 Dec 1;94((11)):3026–3049. doi: 10.1161/01.cir.94.11.3026. [DOI] [PubMed] [Google Scholar]
- 7.Wrobel JS, Mayfield JA, Reiber GE. Geographic variation of lower-extremity major amputation in individuals with and without diabetes in the Medicare population. Diabetes Care. 2001 May 1;24((5)):860–864. doi: 10.2337/diacare.24.5.860. [DOI] [PubMed] [Google Scholar]
- 8.Shammas NW. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Vasc Health Risk Manag. 2007 Apr;3((2)):229–234. doi: 10.2147/vhrm.2007.3.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckman JA, Schneider PA, Conte MS. Advances in revascularization for peripheral artery disease: revascularization in PAD. Circ Res. 2021 Jun 11;128((12)):1885–912. doi: 10.1161/CIRCRESAHA.121.318261. [DOI] [PubMed] [Google Scholar]
- 10.Lin JH, Jeon SY, Romano PS, Humphries MD. Rates and timing of subsequent amputation after initial minor amputation. J Vasc Surg. 2020 Jul;72((1)):268–275. doi: 10.1016/j.jvs.2019.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meloni M, Morosetti D, Giurato L, Stefanini M, Loreni G, Doddi M, et al. Foot revascularization avoids major amputation in persons with diabetes and ischaemic foot ulcers. J Clin Med. 2021 Sep 2;10((17)):3977. doi: 10.3390/jcm10173977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, et al. Vascular endothelial growth factor165 gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000 Jun 23;86((12)):1198–202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 13.Forster R, Liew A, Bhattacharya V, Shaw J, Stansby G. Gene therapy for peripheral arterial disease. Cochrane Database Syst Rev. 2018;10((10)):CD012058. doi: 10.1002/14651858.CD012058.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019 Mar 7;176((6)):1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist. 2006 Jul;11((7)):753–764. doi: 10.1634/theoncologist.11-7-753. [DOI] [PubMed] [Google Scholar]
- 16.Shimamura M, Nakagami H, Koriyama H, Morishita R. Gene therapy and cell-based therapies for therapeutic angiogenesis in peripheral artery disease. BioMed Res Int. 2013;2013:186215. doi: 10.1155/2013/186215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stehr A, Töpel I, Müller S, Unverdorben K, Geissler EK, Kasprzak PM, et al. VEGF: a surrogate marker for peripheral vascular disease. Eur J Vasc Endovasc Surg. 2010 Mar 1;39((3)):330–332. doi: 10.1016/j.ejvs.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Boucher JM, Bautch VL. Antiangiogenic VEGF-A in peripheral artery disease. Nat Med. 2014;20((12)):1383–1385. doi: 10.1038/nm.3767. [DOI] [PubMed] [Google Scholar]
- 19.Luo Z, Asahara T, Tsurumi Y, Isner JM, Symes JF. Reduction of vein graft intimal hyperplasia and preservation of endothelium-dependent relaxation by topical vascular endothelial growth factor. J Vasc Surg. 1998 Jan;27((1)):167–173. doi: 10.1016/s0741-5214(98)70304-0. [DOI] [PubMed] [Google Scholar]
- 20.Cherian AM, Joseph J, Nair MB, Nair SV, Maniyal V, Menon D. Successful reduction of neointimal hyperplasia on stainless steel coronary stents by titania nanotexturing. ACS omega. 2020 Jul 7;5((28)):17582–91. doi: 10.1021/acsomega.0c02045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang X, Guan J, Sheng Z, Wang M, Xu T, Guo G, et al. Effect of local anti-vascular endothelial growth factor therapy to prevent the formation of stenosis in outflow vein in arteriovenous fistula. J Transl Int Med. 2021 Dec 1;9((4)):307–317. doi: 10.2478/jtim-2021-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhardwaj S, Roy H, Heikura T, Ylä-Herttuala S. VEGF-A, VEGF-D and VEGF-D (DeltaNDeltaC) induced intimal hyperplasia in carotid arteries. Eur J Clin Invest. 2005 Nov;35((11)):669–676. doi: 10.1111/j.1365-2362.2005.01555.x. [DOI] [PubMed] [Google Scholar]
- 23.Ganta VC, Annex BH. Peripheral vascular disease: preclinical models and emerging therapeutic targeting of the vascular endothelial growth factor ligand-receptor system. Expert Opin Ther Targets. 2021 May 4;25((5)):381–391. doi: 10.1080/14728222.2021.1940139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isner JM. Still more debate over VEGF. Nat Med. 2001 Jun;7((6)):639–641. doi: 10.1038/88966. [DOI] [PubMed] [Google Scholar]
- 25.Hooijmans CR, Rovers MM, De Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014 Dec;14((1)):43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses
- 27.Asahara T, Chen D, Tsurumi Y, Kearney M, Rossow S, Passeri J, et al. Accelerated restitution of endothelial integrity and endothelium-dependent function after phVEGF165 gene transfer. Circulation. 1996 Dec 15;94((12)):3291–302. doi: 10.1161/01.cir.94.12.3291. [DOI] [PubMed] [Google Scholar]
- 28.Hutter R, Carrick FE, Valdiviezo C, Wolinsky C, Rudge JS, Wiegand SJ, et al. Vascular endothelial growth factor regulates reendothelialization and neointima formation in a mouse model of arterial injury. Circulation. 2004 Oct 19;110((16)):2430–2435. doi: 10.1161/01.CIR.0000145120.37891.8A. [DOI] [PubMed] [Google Scholar]
- 29.Koga JI, Matoba T, Egashira K, Kubo M, Miyagawa M, Iwata E, et al. Soluble Flt-1 gene transfer ameliorates neointima formation after wire injury in flt-1 tyrosine kinase–deficient mice. Arterioscler Thromb Vasc Biol. 2009 Apr 1;29((4)):458–464. doi: 10.1161/ATVBAHA.109.183772. [DOI] [PubMed] [Google Scholar]
- 30.Kondoh K, Koyama H, Miyata T, Takato T, Hamada H, Shigematsu H. Conduction performance of collateral vessels induced by vascular endothelial growth factor or basic fibroblast growth factor. Cardiovasc Res. 2004 Jan 1;61((1)):132–142. doi: 10.1016/j.cardiores.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Laitinen M, Zachary I, Breier G, Pakkanen T, Häkkinen T, Luoma J, et al. VEGF gene transfer reduces intimal thickening via increased production of nitric oxide in carotid arteries. Hum Gene Ther. 1997 Oct 10;8((15)):1737–1744. doi: 10.1089/hum.1997.8.15-1737. [DOI] [PubMed] [Google Scholar]
- 32.Leppänen O, Rutanen J, Hiltunen MO, Rissanen TT, Turunen MP, Sjöblom T, et al. Oral imatinib mesylate (STI571/gleevec) improves the efficacy of local intravascular vascular endothelial growth factor-C gene transfer in reducing neointimal growth in hypercholesterolemic rabbits. Circulation. 2004 Mar 9;109((9)):1140–1146. doi: 10.1161/01.CIR.0000117234.08626.7C. [DOI] [PubMed] [Google Scholar]
- 33.Liu Q, Lu Z, Yue Y, Lin L, Zhang W, Yan J. Experimental study of adenovirus vector mediated-hVEGF165 gene on prevention of restenosis after angioplasty. J Huazhong Univ Sci Technolog Med Sci. 2004 Mar;24((2)):132–133. doi: 10.1007/BF02885410. [DOI] [PubMed] [Google Scholar]
- 34.Ohtani K, Egashira K, Hiasa KI, Zhao Q, Kitamoto S, Ishibashi M, et al. Blockade of vascular endothelial growth factor suppresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Circulation. 2004 Oct 19;110((16)):2444–2452. doi: 10.1161/01.CIR.0000145123.85083.66. [DOI] [PubMed] [Google Scholar]
- 35.Paul A, Shao W, Shum-Tim D, Prakash S. The attenuation of restenosis following arterial gene transfer using carbon nanotube coated stent incorporating TAT/DNA(Ang1+ Vegf) nanoparticles. Biomaterials. 2012 Oct 1;33((30)):7655–7664. doi: 10.1016/j.biomaterials.2012.06.096. [DOI] [PubMed] [Google Scholar]
- 36.Sun S, Zhang Q, Wang Q, Wu Q, Xu G, Chang P, et al. Local delivery of thalidomide to inhibit neointima formation in rat model with artery injury. Pathol Res Pract. 2018 Sep 1;214((9)):1303–1308. doi: 10.1016/j.prp.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Tang C, Wang G, Wu X, Li Z, Shen Y, Lee JCM, et al. The impact of vascular endothelial growth factor-transfected human endothelial cells on endothelialization and restenosis of stainless steel stents. J Vasc Surg. 2011 Feb 1;53((2)):461–471. doi: 10.1016/j.jvs.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Tsai TN, Kirton JP, Campagnolo P, Zhang L, Xiao Q, Zhang Z, et al. Contribution of stem cells to neointimal formation of decellularized vessel grafts in a novel mouse model. Am J Pathol. 2012 Jul 1;181((1)):362–373. doi: 10.1016/j.ajpath.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 39.Van Belle E, Tio FO, Chen D, Maillard L, Chen D, Kearney M, et al. Passivation of metallic stents after arterial gene transfer of phVEGF165 inhibits thrombus formation and intimal thickening. J Am Coll Cardiol. 1997 May;29((6)):1371–1379. doi: 10.1016/s0735-1097(97)00049-1. [DOI] [PubMed] [Google Scholar]
- 40.Walter DH, Cejna M, Diaz-Sandoval L, Willis S, Kirkwood L, Stratford PW, et al. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: an alternative strategy for inhibition of restenosis. Circulation. 2004 Jul 6;110((1)):36–45. doi: 10.1161/01.CIR.0000133324.38115.0A. [DOI] [PubMed] [Google Scholar]
- 41.Whitlock PR, Hackett NR, Leopold PL, Rosengart TK, Crystal RG. Adenovirus-mediated transfer of a minigene expressing multiple isoforms of VEGF is more effective at inducing angiogenesis than comparable vectors expressing individual VEGF cDNAs. Mol Ther. 2004 Jan 1;9((1)):67–75. doi: 10.1016/j.ymthe.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Zhao Y, Tang C, Yin T, Du R, Tian J, et al. Re-endothelialization study on endovascular stents seeded by endothelial cells through up-or downregulation of VEGF. ACS Appl Mater Inter. 2016 Mar 23;8((11)):7578–7589. doi: 10.1021/acsami.6b00152. [DOI] [PubMed] [Google Scholar]
- 43.Xie H, Yang J, Han Y, Zhu X, Fang Q. Inhibition of intimal hyperplasia via local delivery of vascular endothelial growth factor cDNA nanoparticles in a rabbit model of restenosis induced by abdominal aorta balloon injury. Exp Ther Med. 2015 Jul 1;10((1)):55–61. doi: 10.3892/etm.2015.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye W, Chen Y, Tang W, Zhang N, Li Z, Liu Z, et al. Reduction-responsive nucleic acid delivery systems to prevent in-stent restenosis in rabbits. ACS Appl Mater Interfaces. 2019 Jun 25;11((31)):28307–16. doi: 10.1021/acsami.9b08544. [DOI] [PubMed] [Google Scholar]
- 45.Zhang T, Qu G. Magnetic nanosphere-guided site-specific delivery of vascular endothelial growth factor gene attenuates restenosis in rabbit balloon-injured artery. J Vasc Surg. 2016 Jan 1;63((1)):226–33.e1. doi: 10.1016/j.jvs.2014.11.068. [DOI] [PubMed] [Google Scholar]
- 46.Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998 Mar 31;97((12)):1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- 47.Kim HJ, Jang SY, Park JI, Byun J, Kim DI, Do YS, et al. Vascular endothelial growth factor-induced angiogenic gene therapy in patients with peripheral artery disease. Exp Mol Med. 2004 Aug;36((4)):336–344. doi: 10.1038/emm.2004.44. [DOI] [PubMed] [Google Scholar]
- 48.Rajagopalan S, Mohler ER, III, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003 Oct 21;108((16)):1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 49.Voelkl B, Altman NS, Forsman A, Forstmeier W, Gurevitch J, Jaric I, et al. Reproducibility of animal research in light of biological variation. Nat Rev Neurosci. 2020 Jul;21((7)):384–393. doi: 10.1038/s41583-020-0313-3. [DOI] [PubMed] [Google Scholar]
- 50.Shiojima I, Walsh K. The role of vascular endothelial growth factor in restenosis: the controversy continues. Circulation. 2004 Oct 19;110((16)):2283–2286. doi: 10.1161/01.CIR.0000146723.23523.47. [DOI] [PubMed] [Google Scholar]
- 51.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438((7070)):967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 52.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016 Jun;15((6)):385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 53.Peach CJ, Mignone VW, Arruda MA, Alcobia DC, Hill SJ, Kilpatrick LE, et al. Molecular pharmacology of VEGF-A isoforms: binding and signalling at VEGFR2. Int J Mol Sci. 2018 Apr;19((4)):1264. doi: 10.3390/ijms19041264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hornig C, Barleon B, Ahmad S, Vuorela P, Ahmed A, Weich HA. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab Invest. 2000 Apr;80((4)):443–454. doi: 10.1038/labinvest.3780050. [DOI] [PubMed] [Google Scholar]
- 55.Saito T, Takeda N, Amiya E, Nakao T, Abe H, Semba H, et al. VEGF-A induces its negative regulator, soluble form of VEGFR-1, by modulating its alternative splicing. FEBS Lett. 2013 Jul 11;587((14)):2179–2185. doi: 10.1016/j.febslet.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 56.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8((6)):464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 57.Mentzer SJ, Konerding MA. Intussusceptive angiogenesis: expansion and remodeling of microvascular networks. Angiogenesis. 2014 Jul;17((3)):499–509. doi: 10.1007/s10456-014-9428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aicher A, Rentsch M, Sasaki KI, Ellwart JW, Fändrich F, Siebert R, et al. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007 Mar 2;100((4)):581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- 59.Rehman J, Li J, Parvathaneni L, Karlsson G, Panchal VR, Temm CJ, et al. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J Am Coll Cardiol. 2004 Jun 16;43((12)):2314–2318. doi: 10.1016/j.jacc.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 60.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003 Mar 4;107((8)):1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 61.Ganta VC, Choi M, Farber CR, Annex BH. Antiangiogenic VEGF165b regulates macrophage polarization via S100A8/S100A9 in peripheral artery disease. Circulation. 2019 Jan 8;139((2)):226–242. doi: 10.1161/CIRCULATIONAHA.118.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mamer SB, Wittenkeller A, Imoukhuede PI. VEGF-A splice variants bind VEGFRs with differential affinities. Sci Rep. 2020 Sep 2;10((1)):14413. doi: 10.1038/s41598-020-71484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mac Gabhann F, Qutub AA, Annex BH, Popel AS. Systems biology of pro-angiogenic therapies targeting the VEGF system. Wiley Interdiscip Rev Syst Biol Med. 2010 Nov;2((6)):694–707. doi: 10.1002/wsbm.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright JF. Quality control testing, characterization and critical quality attributes of adeno-associated virus vectors used for human gene therapy. Biotechnol J. 2021 Jan;16((1)):e2000022. doi: 10.1002/biot.202000022. [DOI] [PubMed] [Google Scholar]
- 65.Hytönen JP, Taavitsainen J, Laitinen JTT, Partanen A, Alitalo K, Leppänen O, et al. Local adventitial anti-angiogenic gene therapy reduces growth of vasa-vasorum and in-stent restenosis in WHHL rabbits. J Mol Cell Cardiol. 2018 Aug 1;121:145–154. doi: 10.1016/j.yjmcc.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 66.Marais E, Genade S, Salie R, Huisamen B, Maritz S, Moolman JA, et al. The temporal relationship between p38 MAPK and HSP27 activation in ischaemic and pharmacological preconditioning. Basic Res Cardiology. 2005 Jan;100((1)):35–47. doi: 10.1007/s00395-004-0495-7. [DOI] [PubMed] [Google Scholar]
- 67.Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013 Feb;273((2)):114–127. doi: 10.1111/joim.12019. [DOI] [PubMed] [Google Scholar]
- 68.Wang S, Lu J, You Q, Huang H, Chen Y, Liu K. The mTOR/AP-1/VEGF signaling pathway regulates vascular endothelial cell growth. Oncotarget. 2016 Aug 16;7((33)):53269–76. doi: 10.18632/oncotarget.10756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jozkowicz A, Cooke JP, Guevara I, Huk I, Funovics P, Pachinger O, et al. Genetic augmentation of nitric oxide synthase increases the vascular generation of VEGF. Cardiovasc Res. 2001 Sep 1;51((4)):773–783. doi: 10.1016/s0008-6363(01)00344-3. [DOI] [PubMed] [Google Scholar]
- 70.Li X, Lee C, Tang Z, Zhang F, Arjunan P, Li Y, et al. VEGF-B: a survival, or an angiogenic factor? Cell Adh Migr. 2009 Oct 1;3((4)):322–327. doi: 10.4161/cam.3.4.9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nash AD, Baca M, Wright C, Scotney PD. The biology of vascular endothelial growth factor-B (VEGF-B) Pulm Pharmacol Ther. 2006 Feb 1;19((1)):61–9. doi: 10.1016/j.pupt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 72.Szuba A, Skobe M, Karkkainen MJ, Shin WS, Beynet DP, Rockson NB, et al. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J. 2002 Dec;16((14)):1985–1987. doi: 10.1096/fj.02-0401fje. [DOI] [PubMed] [Google Scholar]
- 73.Schoppmann SF, Fenzl A, Schindl M, Bachleitner-Hofmann T, Nagy K, Gnant M, et al. Hypoxia inducible factor-1alpha correlates with VEGF-C expression and lymphangiogenesis in breast cancer. Breast Cancer Res Treat. 2006 Sep;99((2)):135–141. doi: 10.1007/s10549-006-9190-3. [DOI] [PubMed] [Google Scholar]
- 74.Alahmari AK, Almalki ZS, Alahmari AK, Guo JJ. Thromboembolic events associated with bevacizumab plus chemotherapy for patients with colorectal cancer: a meta-analysis of randomized controlled trials. Am Health Drug Benefits. 2016 Jun;9((4)):221–232. [PMC free article] [PubMed] [Google Scholar]
- 75.Cohen AA. Aging across the tree of life: the importance of a comparative perspective for the use of animal models in aging. Biochim Biophys Acta Mol Basis Dis. 2018 Sep 1;1864((9)):2680–2689. doi: 10.1016/j.bbadis.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 76.Doncheva NT, Palasca O, Yarani R, Litman T, Anthon C, Groenen MAM, et al. Human pathways in animal models: possibilities and limitations. Nucleic Acids Res. 2021 Feb 26;49((4)):1859–1871. doi: 10.1093/nar/gkab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Striedter GF. Variation across species and levels: implications for model species research. Brain Behav Evol. 2019;93((2–3)):57–69. doi: 10.1159/000499664. [DOI] [PubMed] [Google Scholar]
- 78.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215((3)):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 79.Benavides F, Guenet JL. Murine models for human diseases. Medicina. 2001 Jan 1;61((2)):215–231. [PubMed] [Google Scholar]
- 80.Doyle RE, Garb S, Davis LE, Meyer DK, Clayton FW. Domesticated farm animals in medical research. Ann N Y Acad Sci. 1968 Mar;147((4)):129–204. doi: 10.1111/j.1749-6632.1968.tb45559.x. [DOI] [PubMed] [Google Scholar]
- 81.Finley SD, Dhar M, Popel AS. Compartment model predicts VEGF secretion and investigates the effects of VEGF trap in tumor-bearing mice. Front Oncol. 2013 Jul 30;3:196. doi: 10.3389/fonc.2013.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All supporting and associated data are available as online supplementary material.