Abstract
Background
Cough is a common symptom of interstitial lung disease (ILD) and negatively impacts health-related quality of life (QOL). Previous studies have shown that among patients with idiopathic pulmonary fibrosis, cough may predict progression of lung disease and perhaps even respiratory hospitalizations and mortality.
Research Question
Does cough-specific QOL predict disease progression, respiratory hospitalization, lung transplantation, and death among patients with ILD?
Study Design and Methods
We analyzed data from the Pulmonary Fibrosis Foundation Registry, which comprises a multicenter population of well-characterized patients with ILD. We first examined associations between patient factors and baseline scores on the Leicester Cough Questionnaire (LCQ), a cough-specific QOL tool, using a proportional odds model. Next, we examined associations between baseline LCQ scores and patient-centered clinical outcomes, as well as pulmonary function parameters, using a univariable and multivariable proportional hazards model that was adjusted for clinically relevant variables, including measures of disease severity.
Results
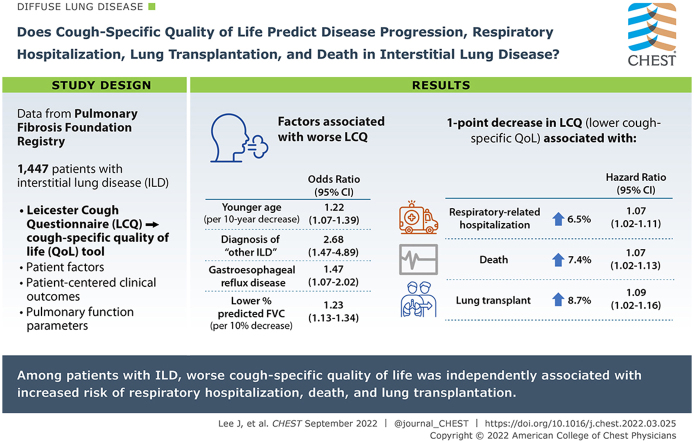
One thousand four hundred forty-seven patients with ILD were included in our study. In the multivariable proportional odds model, we found that the following patient factors were associated with worse cough-specific QOL: younger age, diagnosis of “other ILD,” gastroesophageal reflux disease, and lower FVC % predicted. Multivariable Cox regression models, adjusting for several variables including baseline disease severity, showed that a 1-point decrease in LCQ score (indicating lower cough-specific QOL) was associated with a 6.5% higher risk of respiratory-related hospitalization (hazard ratio [HR], 1.065; 95% CI, 1.025-1.107), a 7.4% higher risk of death (HR, 1.074; 95% CI, 1.020-1.130), and an 8.7% higher risk of lung transplantation (HR, 1.087; 95% CI, 1.022-1.156).
Interpretation
Among a large population of well-characterized patients with ILD, cough-specific QOL was associated independently with respiratory hospitalization, death, and lung transplantation.
Key Words: cough, disease progression, health-related quality of life, idiopathic pulmonary fibrosis, interstitial lung disease, Leicester cough questionnaire, mortality
Abbreviations: ACE, angiotensin-converting enzyme; Dlco, diffusing capacity of the lungs for carbon monoxide; GERD, gastroesophageal reflux disease; HP, hypersensitivity pneumonitis; HR, hazard ratio; IIP, idiopathic interstitial pneumonia; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; LCQ, Leicester Cough Questionnaire; PFT, pulmonary function test; PRO, patient-reported outcome; PFFR, Pulmonary Fibrosis Foundation Registry; QOL, quality of life
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 501
Take-home Points.
Study Question: Does cough-specific quality of life (QOL), as measured by the Leicester Cough Questionnaire (LCQ), predict disease progression, respiratory hospitalization, lung transplantation, and death among patients with interstitial lung disease (ILD)?
Results: Multivariable Cox regression models showed that, after adjusting for several variables, including baseline disease severity, a one-point decrease in LCQ score (correlated with worse cough-specific QOL) was associated with a 6.5% higher risk of respiratory-related hospitalization (hazard ratio [HR], 1.065 [95% CI, 1.025-1.107]), a 7.4% higher risk of death (HR, 1.074 [95% CI, 1.020-1.130]), and an 8.7% higher risk of lung transplantation (HR, 1.087 [95% CI 1.022-1.156]).
Interpretation: Among patients with ILD, worse cough-specific QOL was associated independently with increased risk of respiratory hospitalization, death, and lung transplantation.
Interstitial lung disease (ILD) describes a wide range of pulmonary conditions, diverse in their pathophysiologic features, disease course, and treatment response. Although patients with ILD are likely to cite dyspnea as their primary symptom, an equally vexing and widespread symptom is cough.1 Cough frequency is higher among patients with IPF when compared with patients with asthma or COPD.2, 3, 4 Additionally, cough negatively impacts health-related quality of life (QOL) among patients with ILD5, 6, 7 and decreases workplace productivity.8
Much remains to be learned about the mechanism of cough in ILD. In IPF, postulated cough mechanisms include increased cough reflex sensitivity,9 architectural distortion of the airways resulting from fibrosis,10 and overexpression of airway mediators such as mucin11 and neurotrophins.9 Complicating this further is the fact that many patients with ILD have comorbidities such as COPD, gastroesophageal reflux disease (GERD), OSA, and asthma, which also can influence cough.3,12, 13, 14
Cough may predict prognosis among patients with IPF. This was described first by Ryerson et al,15 who, in their single-center tertiary cohort, identified cough as a risk factor for disease progression, independent of the severity of pulmonary fibrosis. At least three subsequent studies echoed these findings. In an IPF cohort, Zaman et al16 found cough to be associated negatively with transplant-free survival, but only among male patients. In their analysis of the Australian IPF registry, Jo et al17 found that a visual analog scale to measure cough severity predicted mortality, even after adjustment for demographic factors and disease severity. Finally, in a post hoc analysis of data from the INPULSIS trials,18 patients with a worse baseline cough frequency showed a faster decline in pulmonary function.19 Furthermore, multiple studies showed that treatment of IPF and scleroderma-associated ILD results in decreased cough,20, 21, 22 underscoring cough as a meaningful clinical end point.
Prior cough studies have been limited by small size or single-center design. Also, the aforementioned analyses included only patients with IPF, limiting their generalizability to patients with other forms of ILD. In contrast, the present study used data from a large and well-characterized multicenter ILD population, the Pulmonary Fibrosis Foundation Registry (PFFR).23 Additionally, although previous studies focused on the presence or absence of cough, we measured cough more precisely using the Leicester Cough Questionnaire (LCQ),24 a guideline-recommended tool13 that not only measures cough-specific QOL, but also correlates well with objective cough frequency.2
Using the PFFR, we analyzed the associations of patient factors and disease severity on cough-specific QOL. We also sought to determine whether cough-specific QOL serves as an important predictor of pulmonary function and clinical outcomes in patients with ILD. Some of these results previously were presented in abstract form.25
Study Methods and Design
Participants and Study Design
The present study used data from the PFFR, a prospective cohort of well-characterized patients with ILD from multiple centers across the United States. It was first established in March 2016 and includes > 2,000 patients (ClinicalTrials.gov Identifier: NCT02758808). Specific details of this registry have been published.23 Participants were eligible for enrollment if they had been seen at one of 42 Pulmonary Fibrosis Foundation Care Center Network sites, distinguished by their ability to provide multidisciplinary care in the diagnosis and treatment of patients with ILD. Institutional review board approval was obtained at all sites. Additional eligibility criteria included a diagnosis of ILD, age older than 18 years, and informed consent. The most commonly represented ILD diagnoses included IPF, collagen-vascular disease ILD, non-IPF idiopathic interstitial pneumonia (IIP), and hypersensitivity pneumonitis (HP). Non-IPF IIP included the following diagnoses: fibrotic or cellular nonspecific interstitial pneumonia, respiratory bronchiolitis ILD, desquamative interstitial pneumonia, cryptogenic organizing pneumonia, acute interstitial pneumonia, lymphocytic interstitial pneumonia, pleuroparenchymal fibroelastosis, lymphocytic interstitial pneumonia, and unclassifiable IIP. Less common ILD diagnoses were grouped as “other ILD” and included the following: drug or treatment induced, occupational exposure, pulmonary Langerhans’ cell histiocytosis, chronic aspiration, and chronic eosinophilic pneumonia. Exclusion criteria included a history of lung transplantation, sarcoidosis, lymphangioleiomyomatosis, pulmonary alveolar proteinosis, cystic fibrosis, or amyloidosis. At its inception, the PFFR targeted a 60% enrollment of patients with IPF into the registry.
The first set of data was captured at enrollment and included demographic and physical examination information, smoking status, comorbidities, medications, and oxygen use. Testing data included pulmonary function test (PFT) results, 6-min walk test results, CT scan findings, and lung biopsy information if they were obtained within 24 months of enrollment. At enrollment and at subsequent clinic visits, patients were asked to complete the following four patient-reported outcome (PRO) surveys: Rand Short Form 6D, LCQ, University of San Diego Shortness of Breath Questionnaire, and Fatigue Severity Scale.24,26, 27, 28 This information was uploaded into a secure system, and follow-up data were added every 6 months. This included updates to diagnoses, medications, testing, clinical outcomes, and PRO data. If patients had not been seen for follow-up within 12 months, attempts were made to contact them by phone or mail to complete the PRO surveys.
Measurements
Our primary measure of interest was the LCQ, a PRO survey designed to measure the impact of cough on health-related QOL.24 Consisting of 19 questions, this questionnaire provides a numeric total score ranging between 3 and 21, representing the sum of three separate subscales measuring the physical, psychological, and social impacts of cough. A higher score is indicative of a better cough-specific QOL.24 The minimal important difference indicative of clinically meaningful change related to treatment previously was reported to be 1.3.29 The LCQ score was categorized as mild (≥ 14), moderate (10 to < 14), and severe (< 10); these cutoff values were obtained from a recently published abstract.30 Of note, although this publication separated the latter group into a severe (7 to < 10) and very severe (< 7) group,30 we combined them into one severe group because of the small number of patients in the very severe category.
We first sought to identify associations between patient characteristics and LCQ score at enrollment. Next, we evaluated associations between LCQ score at enrollment and time to event for the following clinical outcomes in the 12-month time frame after consent: respiratory-related hospitalizations, lung transplantation, death, FVC decline of ≥ 10%, and diffusing capacity of the lungs for carbon monoxide (Dlco) decline of ≥ 15%.
Patient characteristics of interest included sex, race, smoking status, BMI, ILD type, comorbidities, medications, supplemental oxygen use, PFT data, and baseline LCQ score. Comorbidities were reported by the site investigators and included anxiety or depression, arrhythmia, asthma, congestive heart failure, coronary artery disease, COPD, GERD, hypertension, OSA, pulmonary hypertension, and respiratory or thoracic cancer. GERD was patient reported, determined by the site investigator, or both; criteria included having symptoms of GERD or positive objective testing results (pH manometry, endoscopy, esophagography, or manometry).23 Supplemental oxygen and medication use also were reported by the site investors; medications of interest included angiotensin-converting enzyme (ACE) inhibitors, antifibrotics, histamine-2 blockers, N-acetylcysteine, narcotic cough suppressants, nonnarcotic cough suppressants, and proton pump inhibitors. PFT data included 6-min walk distance, FVC (% predicted based on Hankinson et al31 predicted values), and Dlco (%predicted based on Crapo and Morris32 predicted values).
Statistical Analyses
Frequencies with proportions were reported for categorical variables and means with SDs for continuous variables. To take advantage of the ordinality of LCQ scores, proportional odds models were used to examine the univariable and multivariable associations with the dependent variable of baseline LCQ score. For the purposes of the proportional odds model, baseline LCQ score was stratified into mild, moderate, and severe groups, as described previously.30 Proportional hazards models were used for univariable and multivariable associations with time-to-event outcomes. Estimated OR, 95% CI, and P values were reported for the proportional odds model, and estimated hazard ratios (HRs), 95% CI, and P values were reported for the proportional hazards models. The proportional odds assumption was tested and verified using the score test. The proportionality assumption was reviewed for each association through a combination of Kaplan-Meier curves, tests, and graphs based on the Schoenfeld residuals and through testing time-dependent covariates in the Cox model. All models were evaluated for multicollinearity using variance inflation factors, and no significant multicollinearity was found. Variables chosen for inclusion in the multivariable analysis were based on clinical relevance to the outcome. For the proportional odds model, we included the following variables (obtained at time of consent): age, sex, race, ILD type, presence of coronary artery disease, presence of COPD, presence of GERD, presence of pulmonary hypertension, smoking status, ACE inhibitor use, FVC % predicted, and Dlco % predicted. The same covariates were included in the Cox model, with the addition of LCQ score at enrollment as a covariate.
Missing covariate data patterns were examined along with group means across missing data patterns. Review of missing data showed that 6-min walk distance was missing for 544 of the 1,447 patients, BMI was missing for six patients, and both were missing for two patients. Because neither BMI nor 6-min walk distance were used in the models, the missing data were ignored. Average LCQ score among patients with and without walk distance data was 16.45 and 16.52, respectively. Analyses were performed using SAS version 9.4 software (SAS Institute).
Results
Patient Characteristics
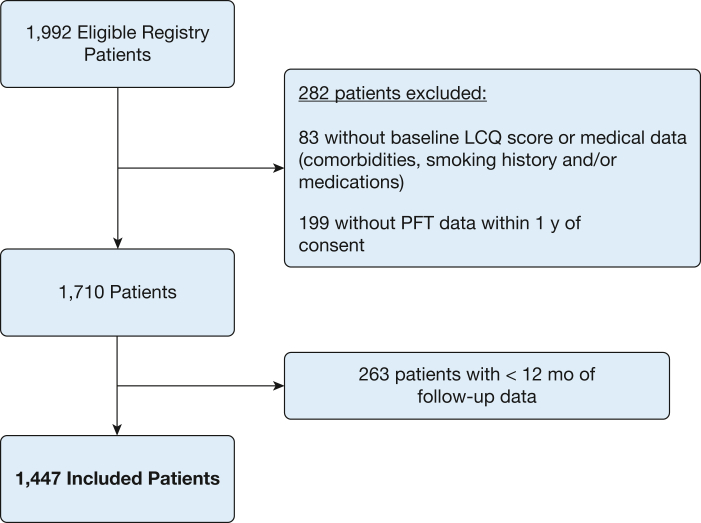
At the time of the analysis, data from 2,003 registry patients were available. Of these patients, 11 were considered ineligible because they there were enrolled after lung transplantation. Of the remaining 1,992 eligible patients, a total of 282 patients were excluded because of missing baseline data: 83 were missing either the LCQ score at enrollment or medical data (including comorbidities, smoking history, or medications) and the remaining 199 lacked PFT data within 1 year of consent. Of the remaining 1,710 patients, an additional 263 were excluded for having fewer than 12 months of follow-up data; this was almost entirely because of inadequate data collection. Thus, our final data analysis included a total of 1,447 patients (Fig 1).
Figure 1.
Study flow diagram showing patients from the Pulmonary Fibrosis Foundation Registry included in this study. LCQ = Leicester Cough Questionnaire; PFT = pulmonary function test.
Table 1 shows the characteristics of the study population. The mean ± SD age was 68 ± 10 years. The population consisted of mostly male patients (63%) or White patients (91%). A range of ILD diagnoses was represented: 61% of patients had a diagnosis of IPF, 17% of patients had a diagnosis of collagen-vascular disease ILD, 10% of patients had a diagnosis of non-IPF IIP, 8% of patients had a diagnosis of HP, and 3% of patients had a diagnosis of other ILD. The average ± SD LCQ score for the study population was 16.5 ± 3.7 points. At baseline, 75.2% of patients showed an LCQ score of ≥ 14 (mild disease), 18.2% of patients showed an LCQ score of 10 to < 14 (moderate disease), and 6.6% of patients showed an LCQ score of < 10 (severe disease).
Table 1.
Patient Characteristics (N = 1,447)
| Characteristic | Data |
|---|---|
| Demographics | |
| Age, y | 67.6 ± 10.1 |
| Female sex | 534 (36.9) |
| Race | |
| White | 1,313 (90.7) |
| Black | 70 (4.8) |
| Other | 64 (4.4) |
| BMI, n = 1,439, kg/m2 | 29.5 ± 5.9 |
| ILD type | |
| IPF | 887 (61.3) |
| IIP non-IPFa | 149 (10.3) |
| CVD ILD | 245 (16.9) |
| HP | 120 (8.3) |
| Otherb | 46 (3.2) |
| Medical history | |
| Anxiety or depression | 374 (25.9) |
| Arrhythmias | 188 (13.0) |
| Asthma | 137 (9.5) |
| Congestive heart failure | 88 (6.1) |
| Coronary artery disease | 341 (23.6) |
| COPD | 136 (9.4) |
| GERD | 235 (16.2) |
| Hypertension | 726 (50.2) |
| OSA | 389 (26.9) |
| Pulmonary hypertension | 167 (11.5) |
| Respiratory or thoracic cancer | 20 (1.38) |
| Tobacco use | |
| Current or previous | 841 (58.1) |
| Medications | |
| ACE inhibitor | 316 (21.8) |
| Antifibrotic | 595 (41.1) |
| Pirfenidone | 318 (22.0) |
| Nintedanib | 245 (16.9) |
| Both | 32 (2.2) |
| Histamine 2 blocker | 144 (10.0) |
| N-acetylcysteine | 22 (1.5) |
| Narcotic cough suppressant | 69 (4.8) |
| Nonnarcotic cough suppressant | 175 (12.1) |
| Proton pump inhibitor | 801 (55.4) |
| Supplemental oxygen | 886 (61.2) |
| Pulmonary function | |
| FVC, % predicted | 68.5 ± 17.9 |
| Dlco, % predicted | 43.1 ± 17.0 |
| 6-min walk distance, n= 901, m | 364.8 ± 128.8 |
| LCQ total score | |
| Mean ± SD | 16.5 ± 3.7 |
| Mild disease (≥ 14) | 1,088 (75.2) |
| Moderate disease (10 to < 14) | 264 (18.2) |
| Severe disease (< 10) | 95 (6.6) |
Data are presented as No. (%) or mean ± SD. ACE = angiotensin-converting enzyme; CVD = collagen vascular disease; Dlco = diffusing capacity of the lungs for carbon monoxide; GERD = gastroesophageal reflux disease; HP = hypersensitivity pneumonitis; IIP = idiopathic interstitial pneumonia; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; LCQ = Leicester Cough Questionnaire.
Includes fibrotic nonspecific interstitial pneumonia, cellular nonspecific interstitial pneumonia, respiratory bronchiolitis ILD, desquamative interstitial pneumonia, cryptogenic organizing pneumonia, acute interstitial pneumonia, lymphocytic interstitial pneumonia, pleuroparenchymal fibroelastosis, lymphocytic interstitial pneumonia, and unclassifiable IIP.
Includes drug or treatment induced, occupational exposure, pulmonary Langerhans’ cell histiocytosis, chronic aspiration, and chronic eosinophilic pneumonia.
Relationship Between Patient Factors and Baseline Cough-Specific Quality of Life: Univariable and Multivariable Analyses
Using a proportional odds model, we identified several patient factors associated with worse baseline cough-specific QOL or lower LCQ score (Table 2). Decreased age was associated with higher odds of experiencing a worse cough-specific QOL; specifically, in the multivariable analysis, for every decade in age, the estimated odds of lower LCQ score were 1.216 times higher (P < .0032). In the unadjusted analysis, a race difference seemed to appear, with non-White, non-Black patients showing the worst cough-specific QOL; however, this association did not remain significant in the multivariable analysis. Compared with patients with IPF, those with other ILD experienced 2.68 times higher odds of lower cough-specific QOL (P = .0013). The only comorbidity that was associated significantly with worse baseline LCQ scores was GERD, which in a multivariable analysis was associated with 1.469 times higher odds of worse LCQ score (P = .0172). Compared with patients with a history of smoking, patients who never smoked had 1.296 times higher odds (P = .0329) of worse LCQ scores; however, this relationship was not statistically significant in the multivariable analysis. We did not find an association between sex, COPD, or ACE inhibitor use with baseline LCQ scores. For every 10% decrease in FVC % predicted, the odds of worse LCQ score were > 1.2-fold (P < .0001) in both univariable and multivariable analyses. In the univariable analysis, for every 15% decrease in Dlco % predicted, 1.25 times higher odds (P = .0001) of worse baseline LCQ score appeared; this trend was not significant in the multivariable analysis.
Table 2.
ORs for Factors Predicting Worse Cough-Specific Quality of Life
| Characteristic | Univariable Analysis |
Multivariable Analysisa |
||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Demographics | ||||
| Age (per 10-y decrease) | 1.292 (1.155-1.445) | < .0001b | 1.216 (1.068-1.385) | .0032b |
| Sex | ||||
| Male | 0.942 (0.738-1.203) | .6309 | 1.013 (0.773-1.336) | .9080 |
| Female | Reference | |||
| Race | ||||
| Black | 1.623 (0.981-2.685) | .0593 | 1.467 (0.852-2.527) | .1667 |
| Other | 1.873 (1.122-3.126) | .0164b | 1.656 (0.974-2.815) | .0626 |
| White | Reference | |||
| Diagnosis | ||||
| IIP non-IPFc | 1.350 (0.920-1.980) | .1249 | 1.206 (0.802-1.813) | .3684 |
| CVD ILD | 1.116 (0.806-1.546) | .5090 | 0.811 (0.553-1.190) | .2848 |
| HP | 1.055 (0.677-1.643) | .8143 | 0.875 (0.549-1.395) | .5745 |
| Otherd | 2.701 (1.511-4.829) | .0008b | 2.680 (1.470-4.886) | .0013b |
| IPF | Reference | |||
| Presence of medical condition | ||||
| Coronary artery disease | 1.135 (0.863-1.493) | .3648 | 1.208 (0.895-1.629) | .2164 |
| COPD | 1.105 (0.743-1.644) | .6223 | 1.200 (0.786-1.833) | .3991 |
| GERD | 1.351 (0.995-1.834) | .0539 | 1.469 (1.071-2.017) | .0172b |
| Pulmonary hypertension | 1.143 (0.797-1.640) | .4664 | 0.986 (0.668-1.454) | .9416 |
| Tobacco use | ||||
| Never | 1.296 (1.021-1.644) | .0329b | 1.262 (0.973-1.634) | .0790 |
| Current or previous | Reference | |||
| Medication use | ||||
| ACE inhibitor | 0.865 (0.645-1.159) | .3313 | 0.860 (0.632-1.171) | .3383 |
| Pulmonary function | ||||
| FVC % predicted (per 10% decrease) | 1.283 (1.194-1.379) | < .0001b | 1.232 (1.134-1.337) | < .0001b |
| Dlco % predicted (per 15% decrease) | 1.250 (1.115-1.401) | .0001b | 1.116 (0.981-1.270) | .0958 |
ACE = angiotensin-converting enzyme; CVD = collagen vascular disease; Dlco = diffusing capacity of the lungs for carbon monoxide; GERD = gastroesophageal reflux disease; HP = hypersensitivity pneumonitis; IIP = idiopathic interstitial pneumonia; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis.
Adjusted for baseline Dlco % predicted, baseline FVC % predicted, age at time of consent, presence of coronary artery disease, presence of COPD, presence of GERD, presence of pulmonary hypertension, ILD diagnosis type, race, sex, ACE inhibitor use, and smoking history.
Denotes statistical significance for α < 0.05.
Includes fibrotic nonspecific interstitial pneumonia, cellular nonspecific interstitial pneumonia, respiratory bronchiolitis ILD, desquamative interstitial pneumonia, cryptogenic organizing pneumonia, acute interstitial pneumonia, lymphocytic interstitial pneumonia, pleuroparenchymal fibroelastosis, IIP, and unclassifiable IIP.
Includes drug or treatment induced, occupational exposure, pulmonary Langerhans’ cell histiocytosis, chronic aspiration, and chronic eosinophilic pneumonia.
Relationship Between Baseline LCQ Score and Clinical Outcomes: Univariable and Multivariable Analyses
During the study period, the following clinical outcomes were observed: 179 respiratory-related hospitalizations, 74 lung transplantations, and 113 deaths. Univariable and multivariable HRs for LCQ score as a predictor of clinical outcomes over 12 months are shown in Table 3. In the univariable analysis, a lower baseline LCQ score was associated significantly with respiratory hospitalization, death, and lung transplantation. After adjusting for several variables, including disease severity, a one-point decrease or worsening in LCQ score was associated with a 6.5% higher risk of respiratory-related hospitalization (HR, 1.065 [95% CI, 1.025-1.107]; P = .0016), a 7.4% higher risk of death (HR, 1.074 [95% CI, 1.020-1.130]; P = .0060), and an 8.7% higher risk of lung transplantation (HR, 1.087 [95% CI, 1.022-1.156]; P = .0079). Notably, although baseline LCQ score seemed to predict clinical outcomes consistently, no observed association was found between baseline LCQ score and pulmonary function decline.
Table 3.
Association of Worse Cough-Specific Quality of Life with Clinical Outcomes at 12 Months
| Outcome at 12 Months | Univariable Analysis |
Multivariable Analysisa |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Respiratory hospitalization | 1.105 (1.067-1.144) | < .0001b | 1.065 (1.025-1.107) | .0016b |
| Death | 1.079 (1.031-1.129) | .0011b | 1.074 (1.020-1.130) | .0060b |
| Lung transplantation | 1.139 (1.080-1.200) | < .0001b | 1.087 (1.022-1.156) | .0079b |
| Any outcome (first of those stated above) | 1.102 (1.063-1.142) | < .0001b | 1.066 (1.024-1.099) | .0021b |
| Decrease in FVC ≥ 10% from baseline | 1.004 (0.962-1.047) | .8597 | 1.032 (0.986-1.080) | .1715 |
| Decrease in Dlco ≥ 15% from baseline | 1.000 (0.881-1.135) | .9984 | 1.033 (0.907-1.175) | .6265 |
Dlco = diffusing capacity of the lungs for carbon monoxide.
Adjusted for baseline Dlco % predicted, baseline FVC % predicted, baseline Leicester Cough Questionnaire score, age at time of consent, presence of coronary artery disease, presence of COPD, presence of gastroesophageal reflux disease, interstitial lung disease diagnosis type, pulmonary arterial hypertension, race, sex, angiotensin-converting enzyme inhibitor use, and smoking history.
Denotes statistical significance for α < 0.05.
Kaplan-Meier Estimates for Clinical Outcomes, Stratified by LCQ Score Severity
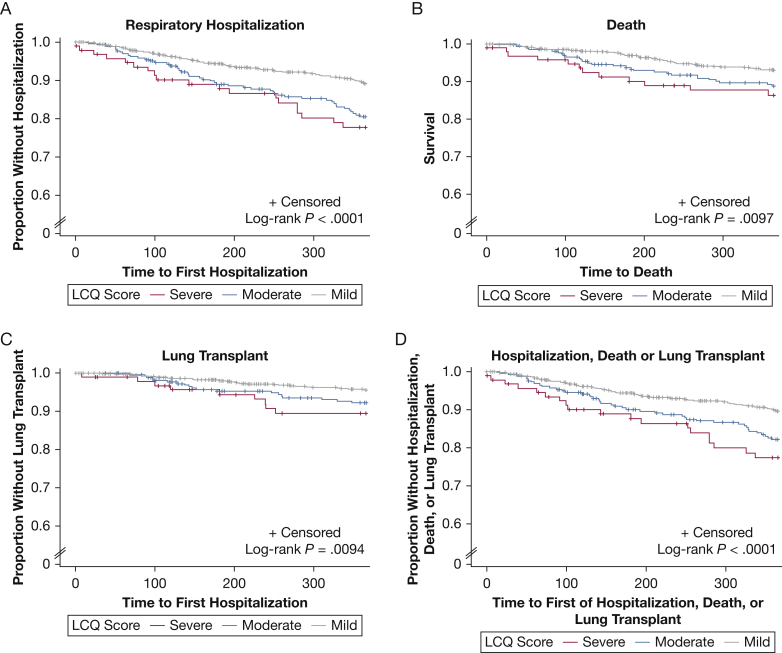
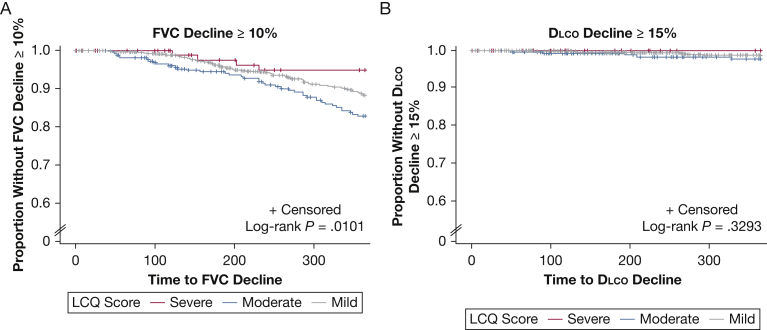
Kaplan-Meier curves for the aforementioned clinical outcomes and pulmonary function decline, stratified by LCQ score severity, are depicted in Figures 2 and 3, respectively. For respiratory hospitalization, death, lung transplantation, and the composite outcome, a consistent and statistically significant difference was found between groups when stratified by cough severity (Fig 2). Patients with a low LCQ score (< 10), indicative of severe impairment in cough-specific QOL, showed an increased likelihood of respiratory hospitalization, death, or lung transplantation when compared with patients with only mild (≥ 14) or moderate (10 to < 14) impairment in the cough-specific QOL. For pulmonary function decline, neither a consistent nor significant difference between groups was found (Fig 3).
Figure 2.
Kaplan-Meier estimates for respiratory hospitalization, death, and lung transplant by LCQ score severity over time. Patients were stratified into groups based on LCQ score as follows: mild disease (≥ 14), moderate disease (10 to < 14), and severe disease (< 10). LCQ = Leicester Cough Questionnaire.
Figure 3.
Kaplan-Meier estimates for FVC and Dlco decline by LCQ score severity over time. Patients were stratified into groups based on LCQ score as follows: mild disease (≥ 14), moderate disease (10 to < 14), and severe disease (<10). Dlco = diffusing capacity of the lungs for carbon monoxide; LCQ = Leicester Cough Questionnaire.
Discussion
To our knowledge, this is the first study demonstrating that independent of disease severity, poorer cough-specific QOL, as measured by the LCQ, is associated with increased risk of respiratory hospitalization, death, and lung transplantation at 12 months among a large and diverse population of patients with ILD. Although prior studies15, 16, 17 have demonstrated a relationship between cough and patient outcomes, our findings are more generalizable because of inclusion of a multicenter population of patients with heterogeneous forms of ILD beyond IPF and use of a nonbinary and validated questionnaire to measure the impact of cough on QOL. Moreover, our study adds to the current evidence showing that PRO tools can predict mortality among patients with IPF independently.17,33, 34, 35, 36 This was underscored in a recent publication by Case et al34 using data from the IPF prospective outcomes patient registry, which showed that worse scores on the St. George’s Respiratory Questionnaire, a measure of general health-related QOL, were associated with higher risk of death or lung transplantation over 1 year.
Although we found that worse cough-specific QOL was associated significantly with a higher risk of important clinical outcomes (respiratory hospitalizations, death, and lung transplantation), we did not find a significant association between cough-specific QOL and a decline in FVC or Dlco at 12 months. This seemingly paradoxical finding highlights the limitations of the current testing methods to predict outcomes. As an example, in a clinical setting, patients with ILD are followed up routinely with serial PFTs and chest imaging; decrements in pulmonary function and new or worsening changes on chest radiography or CT imaging may prompt the clinician to escalate therapy and revisit goals of care. Conversely, despite increasing knowledge of cough as a meaningful measurement, serial cough measurements are performed rarely in the clinical setting. Our study supports integration of routine quantitative assessment of cough alongside other measures of disease status because patients with worse cough-specific QOL may experience a deterioration in clinical status in the absence of obvious decline in their PFT results.
We identified several potentially important associations between patient characteristics and baseline cough-specific QOL scores in the ILD population. As expected, a correlation was found between worse percent predicted FVC and Dlco and cough-specific QOL, arguing that disease progression in ILD may manifest as cough. Likely reflective of survivorship bias, younger age was associated strongly with worse cough-specific QOL. We did not observe a significant disease-specific difference in cough scores among patients with IPF, non-IPF IIP, collagen-vascular disease ILD, and HP. Although patients with other ILD showed significantly worse cough-specific QOL, this proportion of patients comprised only 3% of the study population. A recent cross-sectional study of a Japanese ILD cohort analyzed cough among a population with IIP, HP, and connective-tissue disease ILD. They also did not find a significant difference in scores on the LCQ, but patients with IIP reported worse cough severity and frequency when using the cough visual analog scale.37 Another cross-sectional study measuring cough severity on a visual analog scale found that patients with IPF and chronic HP reported worse cough severity compared with patients with systemic sclerosis ILD.6 We found that GERD was associated significantly with worse cough-specific QOL. This is an interesting finding in light of the postulated pathogenic role of excessive GERD-related microaspiration perpetuating progression of ILD.38, 39, 40
We notably did not observe an association between ACE inhibitor use and LCQ score. Likewise, although studies in the general population previously identified female sex and tobacco use to be risk factors for chronic cough,41 we did not identify sex-specific differences, and tobacco use seemed to be protective against worse cough scores. These results are consistent with prior findings among patients with IPF,15 suggesting that the mechanism of cough in ILD may be distinct from causes of chronic cough in a general population.
Our study has several limitations. First, it is important to acknowledge that although the LCQ is a valuable and frequently used tool to measure cough-specific QOL,24 difficulties are associated with using it as the sole measure of cough. Indeed, although LCQ scores have shown good correlation with objective cough frequency, cough reflex sensitivity, and measures of cough severity,42, 43, 44 they cannot be used interchangeably with these other cough measures. Another issue with the use of the LCQ is that although it has been validated in some pulmonary conditions including COPD,45 noncystic fibrosis bronchiectasis,46 and nontuberculous mycobacterial lung disease,47 it has not been validated formally in IPF or the ILD population. Second, although our study has the advantage of including patients with multiple types of ILD, some characteristics of the PFFR limit generalizability of our results: (1) the PFFR was prespecified to target an enrollment of 60% patients with IPF, (2) only patients from academic centers with multidisciplinary infrastructures were included in this study, and (3) patients with sarcoidosis, a population with frequent reports of cough, was excluded from the registry, per protocol.
In conclusion, we were able to identify several interesting correlations with potential impact related to cough-specific QOL in a varied ILD population. This study adds evidence to the existing literature supporting the value of measuring and assessing cough not only in patients with IPF, but also in the broader ILD population. Future studies are needed urgently to understand better the underlying mechanisms of cough in ILD, the means by which cough may lead to poorer outcomes in ILD, as well as its treatment.
Interpretation
Among patients with interstitial lung disease, worse cough-specific QOL was associated independently with increased risk of respiratory hospitalization, death, and lung transplantation.
Acknowledgments
Author contributions: N. M. P. is the guarantor of the manuscript and had final responsibility for all content of the manuscript. E. W. and E. F. performed the statistical analysis. J. L. wrote the first draft of the manuscript, and all authors critically reviewed, edited, and approved of the final version of the manuscript.
Funding/support: This study was funded by the Pulmonary Fibrosis Foundation. A. P. is supported by the National Institutes of Health [Grant K23HL140199].
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. B. S. is on the advisory board for Veracyte, Genentech, and United Therapeutics; is a speaker for Boehringer-Ingelheim, Veracyte, and United Therapeutics; and is a primary investigator for Boehringer-Ingelheim, Galapago, the National Institutes of Health, and Nitto Denko. A. P. is on the advisory board for Boehringer-Ingelheim and reports consulting fees from Regeneron, Roche, and Imvaria unrelated to the submitted work. M. E. S. is a primary investigator for institutional studies with Boehringer Ingelheim, Galapagos, and the National Institutes of Health; reports involvement with Boehringer Ingelheim medical writing assistance; and is a member of the Fibrogen Endpoint Adjudication Committee. N. M. P. is employed at Boehringer-Ingelheim. None declared (J. L., E. W., E. F.).
Role of sponsors:The Pulmonary Fibrosis Foundation had no role in the design of the study. The University of Michigan Statistical Analysis of Biomedical and Educational Research unit served as the registry data coordinating center and managed data entered by registry sites. Statistical analysis of the data was performed by the University of Michigan Statistical Analysis of Biomedical and Educational research unit, as well as preparation of the statistical methods and tables for the manuscript.
Collaborators from the Pulmonary Fibrosis Foundation: Pulmonary Fibrosis Foundation Care Center Network site principal investigators: Rodeo Abrenchillo, MD; Rebecca Bascom, MD; Elizabeth Belloli, MD; Nitin Bhatt, MD; Amy Case, MD; Sachin Chaudhary, MD; Gerard Criner, MD; Alpa Desai, MD; Christine Garcia, MD; Craig Glazer, MD; Mridu Gulati, MD; Nishant Gupta, MD; Mark Hamblin, MD; Tristan Huie, MD; Robert Kaner, MD; Daniel Kass, MD; Hyun Kim, MD; Christopher King, MD; Robert Matthew Kottman, MD; Lisa Lancaster, MD; Joseph Lasky, MD; Andrew Limper, MD; Tracy Luckhardt, MD; Sydney Montesi, MD; Joshua Mooney, MD; Lake Morrison, MD; Anoop Nambiar, MD; Rafael Perez, MD; Mary Porteous, MD; Mary Beth Scholand, MD; Adrian Shifren, MD; Danoff Sonye, MD; Mary Strek, MD; Paul Tessy, MD; Nevins Todd, MD; Rade Tomic, MD; Rajat Walia, MD; Stephen Weight, MD; Timothy Whelan, MD; and Paul Wolters, MD.
Contributor Information
Janet Lee, Email: janetlee423@gmail.com.
Nina M. Patel, Email: nina.patel@boehringer-ingelheim.com.
Pulmonary Fibrosis Foundation:
Rebecca Bascom, Elizabeth Belloli, Nitin Bhatt, Sangeeta Bhorade, Amy Case, Richard Castriotta, Gerard Criner, Sonye Danoff, Joao De Andrade, Alpa Desai, Marilyn Glassberg, Craig Glazer, Mridu Gulati, Nishant Gupta, Mark Hamblin, Tristan Huie, Robert Kaner, Daniel Kass, Hyun Kim, Maryl Kreider, Lisa Lancaster, Joseph Lasky, Andrew Limper, Sydney Montesi, Joshua Mooney, Lake Morrison, Anoop Nambiar, Steven Nathan, Bhupinder Natt, Tessy Paul, Rafael Perez, Anna Podolanczuk, Ganesh Raghu, Mary Beth Scholand, Adrian Shifren, Mary Strek, Nevins Todd, Rajat Walia, Stephen Weight, Timothy Whelan, and Paul Wolters
References
- 1.Behr J., Kreuter M., Hoeper M.M., et al. Management of patients with idiopathic pulmonary fibrosis in clinical practice: the INSIGHTS-IPF registry. Eur Respir J. 2015;46(1):186–196. doi: 10.1183/09031936.00217614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Key A.L., Holt K., Hamilton A., Smith J.A., Earis J.E. Objective cough frequency in Idiopathic Pulmonary Fibrosis. Cough. 2010;6:4. doi: 10.1186/1745-9974-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Manen M.J., Birring S.S., Vancheri C., et al. Cough in idiopathic pulmonary fibrosis. Eur Respir Rev. 2016;25(141):278–286. doi: 10.1183/16000617.0090-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sumner H., Woodcock A., Kolsum U., et al. Predictors of objective cough frequency in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(9):943–949. doi: 10.1164/rccm.201211-2000OC. [DOI] [PubMed] [Google Scholar]
- 5.Yount S.E., Beaumont J.L., Chen S.Y., et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis. Lung. 2016;194(2):227–234. doi: 10.1007/s00408-016-9850-y. [DOI] [PubMed] [Google Scholar]
- 6.Cheng J.Z., Wilcox P.G., Glaspole I., et al. Cough is less common and less severe in systemic sclerosis-associated interstitial lung disease compared to other fibrotic interstitial lung diseases. Respirology. 2017;22(8):1592–1597. doi: 10.1111/resp.13084. [DOI] [PubMed] [Google Scholar]
- 7.Glaspole I.N., Chapman S.A., Cooper W.A., et al. Health-related quality of life in idiopathic pulmonary fibrosis: data from the Australian IPF Registry. Respirology. 2017;22(5):950–956. doi: 10.1111/resp.12989. [DOI] [PubMed] [Google Scholar]
- 8.Algamdi M., Sadatsafavi M., Fisher J.H., et al. Costs of workplace productivity loss in patients with fibrotic interstitial lung disease. Chest. 2019;156(5):887–895. doi: 10.1016/j.chest.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Hope-Gill B.D., Hilldrup S., Davies C., Newton R.P., Harrison N.K. A study of the cough reflex in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168(8):995–1002. doi: 10.1164/rccm.200304-597OC. [DOI] [PubMed] [Google Scholar]
- 10.Jones R.M., Hilldrup S., Hope-Gill B.D., Eccles R., Harrison N.K. Mechanical induction of cough in idiopathic pulmonary fibrosis. Cough. 2011;7:2. doi: 10.1186/1745-9974-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholand M.B., Wolff R., Crossno P.F., et al. Severity of cough in idiopathic pulmonary fibrosis is associated with MUC5 B genotype. Cough. 2014;10:3. doi: 10.1186/1745-9974-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilduff C.E., Counter M.J., Thomas G.A., Harrison N.K., Hope-Gill B.D. Effect of acid suppression therapy on gastroesophageal reflux and cough in idiopathic pulmonary fibrosis: an intervention study. Cough. 2014;10:4. doi: 10.1186/1745-9974-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birring S.S., Kavanagh J.E., Irwin R.S., Keogh K.A., Lim K.G., Ryu J.H. Treatment of interstitial lung disease associated cough: CHEST guideline and expert panel report. Chest. 2018;154(4):904–917. doi: 10.1016/j.chest.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Madison J.M., Irwin R.S. Chronic cough in adults with interstitial lung disease. Curr Opin Pulm Med. 2005;11(5):412–416. doi: 10.1097/01.mcp.0000174249.07762.37. [DOI] [PubMed] [Google Scholar]
- 15.Ryerson C.J., Abbritti M., Ley B., Elicker B.M., Jones K.D., Collard H.R. Cough predicts prognosis in idiopathic pulmonary fibrosis. Respirology. 2011;16(6):969–975. doi: 10.1111/j.1440-1843.2011.01996.x. [DOI] [PubMed] [Google Scholar]
- 16.Zaman T., Moua T., Vittinghoff E., Ryu J.H., Collard H.R., Lee J.S. Differences in clinical characteristics and outcomes between men and women with idiopathic pulmonary fibrosis: a multicenter retrospective cohort study. Chest. 2020;158(1):245–251. doi: 10.1016/j.chest.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jo H.E., Glaspole I., Grainge C., et al. Baseline characteristics of idiopathic pulmonary fibrosis: analysis from the Australian Idiopathic Pulmonary Fibrosis Registry. Eur Respir J. 2017;49(2) doi: 10.1183/13993003.01592-2016. [DOI] [PubMed] [Google Scholar]
- 18.Richeldi L., du Bois R.M., Raghu G., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 19.Wuyts W., Vancheri C., Bonella F., et al. Effects of nintedanib in patients with idiopathic pulmonary fibrosis and varying severities of cough. Poster session presented at: American Thoracic Society International Conference. May 2021 virtual conference. [Google Scholar]
- 20.van Manen M.J.G., Birring S.S., Vancheri C., et al. Effect of pirfenidone on cough in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2017;50(4) doi: 10.1183/13993003.01157-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tashkin D.P., Volkmann E.R., Tseng C.H., et al. Improved cough and cough-specific quality of life in patients treated for scleroderma-related interstitial lung disease: results of Scleroderma Lung Study II. Chest. 2017;151(4):813–820. doi: 10.1016/j.chest.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theodore A.C., Tseng C.H., Li N., Elashoff R.M., Tashkin D.P. Correlation of cough with disease activity and treatment with cyclophosphamide in scleroderma interstitial lung disease: findings from the Scleroderma Lung Study. Chest. 2012;142(3):614–621. doi: 10.1378/chest.11-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B.R., Edwards R., Freiheit E.A., et al. The Pulmonary Fibrosis Foundation patient registry. Rationale, design, and methods. Ann Am Thorac Soc. 2020;17(12):1620–1628. doi: 10.1513/AnnalsATS.202001-035SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birring S.S., Prudon B., Carr A.J., Singh S.J., Morgan M.D., Pavord I.D. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58(4):339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J., Scholand M., Moore J., et al. May 21, 2019. Patient factors and disease severity impact cough-specific quality of life in patients with interstitial lung disease. Poster session presented at American Thoracic Society International Conference. Dallas, TX. [Google Scholar]
- 26.Krupp L.B., LaRocca N.G., Muir-Nash J., Steinberg A.D. The fatigue severity scale. application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 27.Eakin E.G., Resnikoff P.M., Prewitt L.M., Ries A.L., Kaplan R.M. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest. 1998;113(3):619–624. doi: 10.1378/chest.113.3.619. [DOI] [PubMed] [Google Scholar]
- 28.Brazier J., Roberts J., Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 29.Raj A.A., Pavord D.I., Birring S.S. Clinical cough IV: what is the minimal important difference for the Leicester Cough Questionnaire? Handb Exp Pharmacol. 2009;187:311–320. doi: 10.1007/978-3-540-79842-2_16. [DOI] [PubMed] [Google Scholar]
- 30.Cho P.S.P., Cho P.S.P., Rhatigan K., et al. Defining health states with visual analogue scale and Leicester Cough Questionnaire in chronic cough. Eur Respir J. 2021;58(suppl 65):PA3144. [Google Scholar]
- 31.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 32.Crapo R.O., Morris A.H. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis. 1981;123(2):185–189. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- 33.Furukawa T., Taniguchi H., Ando M., et al. The St. George’s Respiratory Questionnaire as a prognostic factor in IPF. Respir Res. 2017;18(1):18. doi: 10.1186/s12931-017-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Case A.H., Hellkamp A.S., Neely M.L., et al. Associations between patient-reported outcomes and death or lung transplant in idiopathic pulmonary fibrosis. Data from the Idiopathic Pulmonary Fibrosis Prospective Outcomes Registry. Ann Am Thorac Soc. 2020;17(6):699–705. doi: 10.1513/AnnalsATS.201906-437OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.du Bois R.M., Weycker D., Albera C., et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(4):459–466. doi: 10.1164/rccm.201011-1790OC. [DOI] [PubMed] [Google Scholar]
- 36.Jo H.E., Glaspole I., Moodley Y., et al. Disease progression in idiopathic pulmonary fibrosis with mild physiological impairment: analysis from the Australian IPF registry. BMC Pulm Med. 2018;18(1):19. doi: 10.1186/s12890-018-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato R., Handa T., Matsumoto H., Kubo T., Hirai T. Clinical significance of self-reported cough intensity and frequency in patients with interstitial lung disease: a cross-sectional study. BMC Pulm Med. 2019;19(1):247. doi: 10.1186/s12890-019-1012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raghu G., Rochwerg B., Zhang Y., et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192(2):e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 39.Lee J.S., Collard H.R., Anstrom K.J., et al. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med. 2013;1(5):369–376. doi: 10.1016/S2213-2600(13)70105-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreuter M., Wuyts W., Renzoni E., et al. Antacid therapy and disease outcomes in idiopathic pulmonary fibrosis: a pooled analysis. Lancet Respir Med. 2016;4(5):381–389. doi: 10.1016/S2213-2600(16)00067-9. [DOI] [PubMed] [Google Scholar]
- 41.Çolak Y., Nordestgaard B.G., Laursen L.C., Afzal S., Lange P., Dahl M. Risk factors for chronic cough among 14,669 individuals from the general population. Chest. 2017;152(3):563–573. doi: 10.1016/j.chest.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 42.Birring S.S., Matos S., Patel R.B., Prudon B., Evans D.H., Pavord I.D. Cough frequency, cough sensitivity and health status in patients with chronic cough. Respir Med. 2006;100(6):1105–1109. doi: 10.1016/j.rmed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 43.Faruqi S., Thompson R., Wright C., Sheedy W., Morice A.H. Quantifying chronic cough: objective versus subjective measurements. Respirology. 2011;16(2):314–320. doi: 10.1111/j.1440-1843.2010.01893.x. [DOI] [PubMed] [Google Scholar]
- 44.Decalmer S.C., Webster D., Kelsall A.A., McGuinness K., Woodcock A.A., Smith J.A. Chronic cough: how do cough reflex sensitivity and subjective assessments correlate with objective cough counts during ambulatory monitoring? Thorax. 2007;62(4):329–334. doi: 10.1136/thx.2006.067413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berkhof F.F., Boom L.N., ten Hertog N.E., Uil S.M., Kerstjens H.A., van den Berg J.W. The validity and precision of the Leicester Cough Questionnaire in COPD patients with chronic cough. Health Qual Life Outcomes. 2012;10:4. doi: 10.1186/1477-7525-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray M.P., Turnbull K., MacQuarrie S., Pentland J.L., Hill A.T. Validation of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis. Eur Respir J. 2009;34(1):125–131. doi: 10.1183/09031936.00160508. [DOI] [PubMed] [Google Scholar]
- 47.Takao S., Tabusadani M., Yamane K., et al. Is the Leicester Cough Questionnaire useful for nontuberculous mycobacterial lung disease? Respir Invest. 2021;59(1):120–125. doi: 10.1016/j.resinv.2020.06.005. [DOI] [PubMed] [Google Scholar]