Abstract
Introduction
The aim of this study is to evaluate signal alteration in the inner ear using three-dimensional (3D)-constructive interference in steady state (CISS) sequence in patients with Ménière's disease and labyrinthitis and its correlation with clinical and audiological parameters.
Methods
The medical records of the department of otorhinolaryngology were searched for patients with Ménière's disease or labyrinthitis who underwent MRI with 3D-CISS sequence. Blinded analysis of these patients and of MRI from control subjects without middle or inner ear symptoms was performed to detect any signal asymmetry of the inner ear structures. The results were correlated with clinical symptoms and results of audiological and vestibular tests.
Results
Fifty-eight patients with definite Ménière's disease and 5 patients with labyrinthitis as well as 41 control exams were included. A separate analysis was performed for patients with probable Ménière's disease (n = 68). A total of 172 3D-CISS sequences were analyzed by 2 blinded independent neuroradiologists. A CISS-hypointense signal of the inner ear structures was found in 3 patients with definite Ménière's disease (5.2%), in 4 patients with probable Ménière's disease (5.9%), and 2 patients with labyrinthitis (40%). No CISS hypointensity was found in the control group. Although no significant difference in symptoms or audiological test results was found between patients with and without this signal change, the side of hypointensity was frequently correlated with the symptomatic side and with hearing impairment.
Discussion/Conclusion
CISS hypointensity of the inner ear structures was evident in patients with clinical conditions other than vestibular schwannoma − more frequently in labyrinthitis than in Ménière's disease. This signal alteration was frequently encountered on the same symptomatic side as that of the pathological audiology tests, but it is not a predictor for hearing or vestibular impairment.
Keywords: Inner ear, Cochlea, Constructive interference in steady state, MRI, Endolymphatic hydrops
Introduction
Patients with vestibular schwannoma show an increased protein concentration in the perilymph [Silverstein, 1971], most probably due to unknown toxic substances, and this has been suggested to be the cause of hearing loss in patients with schwannoma [Lassaletta et al., 2019]. In MRI, high protein levels have also been suggested to be the explanation for hypointensity in the three-dimensional (3D) constructive interference in steady state (CISS) [Wagner et al., 2018a, b].
Histopathological studies have shown elevation in the protein content in the inner ear structures in patients with other diseases, such as Ménière's disease [Silverstein, 1971; Lin et al., 2019]. Furthermore, inflammatory proteins were found to play a role in labyrinthitis [Schraff et al., 2007]. However, no imaging studies have looked for changes in the CISS sequence similar to those seen in vestibular schwannoma.
The purpose of this study was to evaluate the presence of CISS signal changes in the cochlea and vestibule in patients with Ménière's disease and labyrinthitis and to correlate these changes with symptoms, audiological and vestibular tests. We aimed also to find out if there is a difference between probable and definite Ménière's disease.
Methods
The ethics committee of the canton of Bern approved the retrospective analysis in the study (KEK 2018-01509).
Patients
Medical records from the Department of Otorhinolaryngology, Inselspital, Bern University Hospital were searched for patients with a diagnosis of (1) Ménière's disease according to the criteria published by Lopez-Escamez et al. [2015] and (2) patients diagnosed with labyrinthitis, based on the clinical presentation of acute sustained vestibular syndrome consistent with a peripheral origin [Hotson and Baloh, 1998].
Inclusion criteria were a diagnosis of Ménière's disease or labyrinthitis and MRI including 3D-CISS sequence. Exclusion criteria were suboptimal image quality, including motion artifacts or incomplete enclosure of the inner ear structures, other conditions that may cause signal changes in the inner ear, such as vestibular schwannoma, postoperative cases, or previous radiotherapy. In addition, a control group was randomly selected by searching PACS of Inselspital, Bern University Hospital for patients with 3D-CISS sequence but no pathological conditions of the inner ear.
Clinical Assessment
Clinical data of the patients included in the study were retrospectively analyzed for clinical symptoms (symptom onset, affected side, tinnitus, hearing fluctuations, aural fullness, and vertigo episodes). The records were also searched for hearing loss (according to current procedural terminology of the American Medical Association: CPT-AMA) [AMA, 1942], auditory evoked potential (AEP), and bithermal caloric testing.
Image Acquisition
MRI studies performed at our institute as well as at external institutions were considered for analysis, as long as the inclusion criteria were met. In-house exams were performed on either a 1.5-T or 3-T imaging system (Magnetom Avanto, Aera, Verio, or Vida, Siemens Medical Solutions). The parameters for the 3D-CISS sequence were as follows: for 1.5 T, 1,400 ms; TE 186 ms; slice thickness 0.6 mm; FOV 190 × 190 mm; matrix 640 × 640; and acquisition time 4:29 min, and for 3 T, TR 1,000 ms; TE 132 ms; slice thickness 0.5 mm; FOV 82 × 160 mm; matrix 164 × 320; and acquisition time 2:37 min. External exams were also performed on either a 1.5-T or 3-T scanner. MRI on 1.5-T scanners was performed using Aera, Avanto, Espree, Symphony, or Harmony (Siemens Medical Solutions); or Signa Excite, Signa HDx, or Optima (GE Healthcare); or Panorama, Intera, or Achieva (Philips Healthcare) with TR ranging between 1,200 and 1,500 ms; TE 178–269 ms; slice thickness 0.5–1.4 mm; and acquisition time 2:16–5:25 min. MRI scans on 3-T scanners were performed using TrioTim or Skyra (Siemens Medical Solutions), Discovery or Signa Pioneer (GE Healthcare), or Ingenia (Philips Healthcare) with TR ranging between 1,000 and 2,000 ms; TE 132–210 ms; slice thickness 0.5 − 1 mm; and acquisition time 2:26–4:28 min.
3D-CISS images from all patients and the control group were anonymized and randomly arranged. The evaluation of all patients was performed using a multiplanar reformation tool that allowed symmetrical adjustment of the inner ear structure for comparison.
Qualitative Analysis
Images from patients and the control group were anonymized and randomized. Blinded imaging analysis was performed by 2 board-certified neuroradiologists with more than 10 years of experience. The qualitative analysis aimed to identify hypointensity in the cochlea and/or vestibule. The signal intensity of 4 regions of each inner ear structure (cochlea and vestibule bilaterally) was compared to each other and to the signal intensity of the CSF in the cerebellopontine angle. Disagreement between the 2 raters was resolved by consensus.
The control group and patient groups were defined according to the final diagnosis: Ménière's disease or labyrinthitis. Patients with Ménière's disease were further split according to whether they had probable or definite Ménière's disease according to the diagnostic criteria of Lopez-Escamez [Lopez-Escamez et al., 2015]. The primary objective was to analyze patients with definite Ménière's disease. However, as a matter of scientific interest, the patients with probable Ménière's were included in a separate analysis as a secondary objective.
According to the results of the qualitative analysis, patients were divided into CISS-positive, with a signal hypointensity, and CISS-negative, without signal asymmetry. The CISS-positive group was compared to the CISS-negative group in terms of clinical and audiological parameters.
Quantitative Analysis
On each 3D-CISS sequence, 5 regions of interest (ROIs) were drawn as follows: 2 in the caudal part of the basal turn of each cochlea and 2 in the vestibules. A fifth ROI was drawn in the cerebellopontine angle. The values of signal intensities obtained were used to calculate several ratios: cochlear/CSF ratio and vestibular/CSF ratio for each ear, and the difference between the two ears (i.e., cochlear/CSF ratio difference between right and left side, as well as vestibular/CSF ratio difference).
The cochlear/CSF and vestibular CSF ratio differences were compared between the 3 groups of patients and the control group. The aim was to explore the possibility of identifying a threshold that would differentiate the 3 patient groups from the control group.
Statistics
Statistical analyses were performed using SPSS (Version 27.0). For all statistical analyses, the level of significance was set at p = 0.05 (two-tailed). Due to the different and small sample sizes, nonparametric tests were used. Interrater variability was calculated according to Cohen's kappa. Quantitative group differences (n per group) were analyzed with the χ2 test. Qualitative group differences were analyzed with the Wilcoxon test for dependent variables or the Mann-Whitney U-test for independent variables.
Results
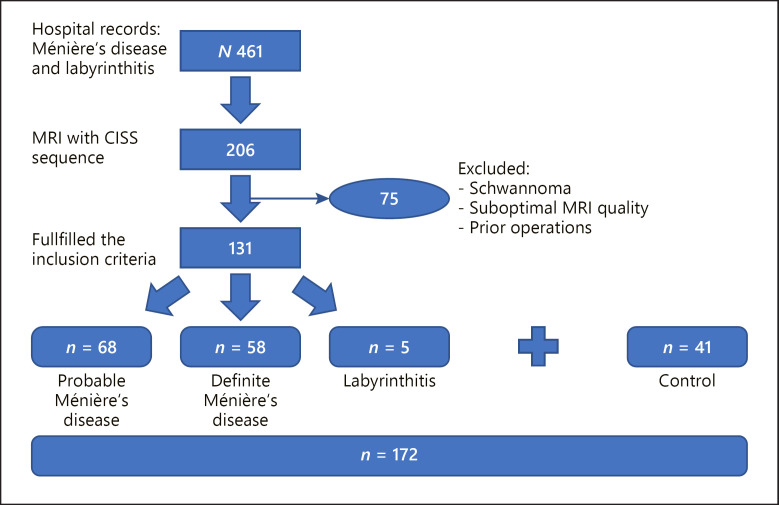
A total of 461 patients examined between 2003 and 2019 were identified. Only 131 fulfilled the inclusion criteria (68 probable Ménière's disease, 58 definite Ménière's disease, and 5 labyrinthitis). Forty-one exams were added as a control group (Fig. 1). Online supplementary Table 1 (see www.karger.com/doi/10.1159/000525419 for all online suppl. material) summarizes patients' demographics and results.
Fig. 1.
Flowchart showing the patient selection process.
Qualitative Signal Hypointensity
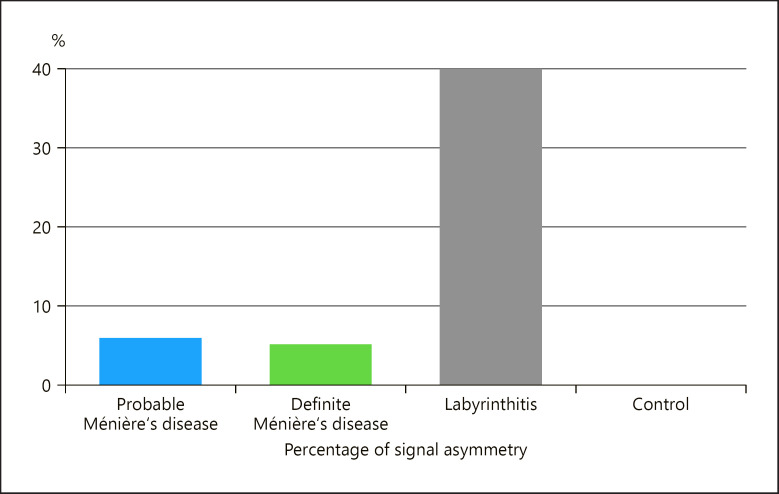
Of the 131 patients included in the study, 9 (6.9%) showed asymmetrical signal (Fig. 2), 4 patients (of 68 patients; 5.9%) with probable Ménière's disease, 3 patients (of 58 patients; 5.2%) with definite Ménière's disease, and 2 patients (of 5 patients; 40%) with labyrinthitis (Fig. 3). No asymmetry in signal was found in any patient in the control group.
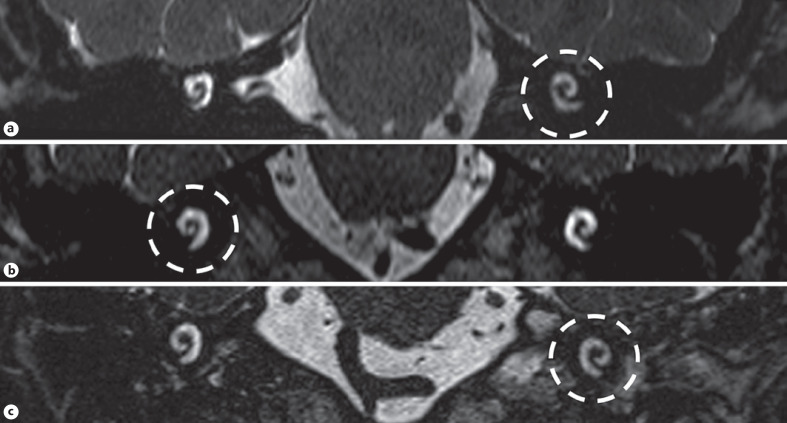
Fig. 2.
Coronal reformation of the 3D-CISS sequences from 3 different patients diagnosed with probable Ménière's disease (a), definite Ménière's disease (b), and labyrinthitis (c). There is asymmetrical signal intensity of the cochlea with a unilateral hypointense signal (circles).
Fig. 3.
Bar chart showing the percentage of signal asymmetry in each patient group. Note: no signal asymmetry was found in the control group.
Eight of the 9 patients in the CISS-positive group showed signal hypointensity in both ipsilateral cochlea and vestibule, and only 1 patient (with probable Ménière's disease) showed a hypointense signal involving only the vestibule, with no cochlear involvement. The signal intensity in the semicircular canals always followed the signal intensity of the vestibule in all positive cases.
The results from the 2 raters showed differences in rating of 3 regions in 2 patients out of the 688 regions of the 172 patients, with interrater variability of 0.43%. In the CISS-positive group, 5 of the 9 patients underwent 1.5 T and the other 4 underwent 3-T MRI. In the CISS-negative group 86 patients underwent 1.5 T and 36 patients 3-T MRI. There was no statistical difference between the 2 groups (χ2 = 0.88, p = 0.34). There was no correlation between CISS asymmetry and age or sex.
Symptoms
In the CISS-positive group, symptom onset ranged from 13 years prior to MRI to the same time period as MRI acquisition (average 3.6 years). In the CISS-negative group, symptom onset ranged from 40 years prior to MRI to 4 years after (average 4.5 years).
In the labyrinthitis group, 3 patients were scanned in the acute phase (0–2 days from symptom onset). Two of these 3 patients (66.6%) showed a unilateral hypointense signal and the other did not show an asymmetrical signal. The other 2 patients with labyrinthitis were examined in the chronic stage (2 and 18 months from symptom onset).
Overall, the number of patients in the CISS-positive group with hearing fluctuation was slightly higher (5 out of 9 patients; 55.6%) than in the CISS-negative group (59 out of 122; 48.4%), but there was no significant difference (χ2 = 0.17, p = 0.67). In patients with probable Ménière's disease, hearing fluctuation was a significantly more frequent finding (4 out of 4, 100%) in CISS-positive than CISS-negative (29 out of 64; 45.3%) (χ2 = 4.5, p = 0.034).
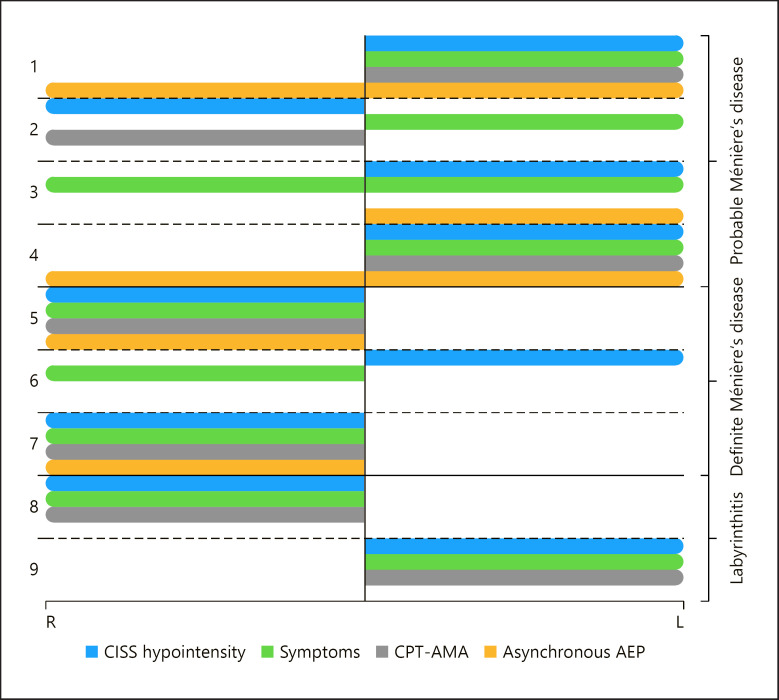
In patients with definite Ménière's disease, tinnitus was a more frequent finding (3 out of 3 patients) for those in the CISS-positive group than patients in the CISS-negative (46 out of 55 patients; 83.6%), but there was no statistically significant difference. In 7 out of the 9 patients in the CISS-positive group, the hypointense signal was ipsilateral to symptoms (Fig. 4). In the 2 patients in whom symptoms were contralateral to CISS hypointensity, symptoms started 9 years and 1 year prior to MRI.
Fig. 4.
Diagram showing the side of the CISS signal hypointensity compared to the symptomatic side, as well as the side of hearing impairment according to CPT-AMA and AEP. Patients 3 and 6 showed no hearing impairment, patients 2 and 6 did not have abnormal AEP. There was no record of AEP testing for patients 8 and 9.
Hearing Loss
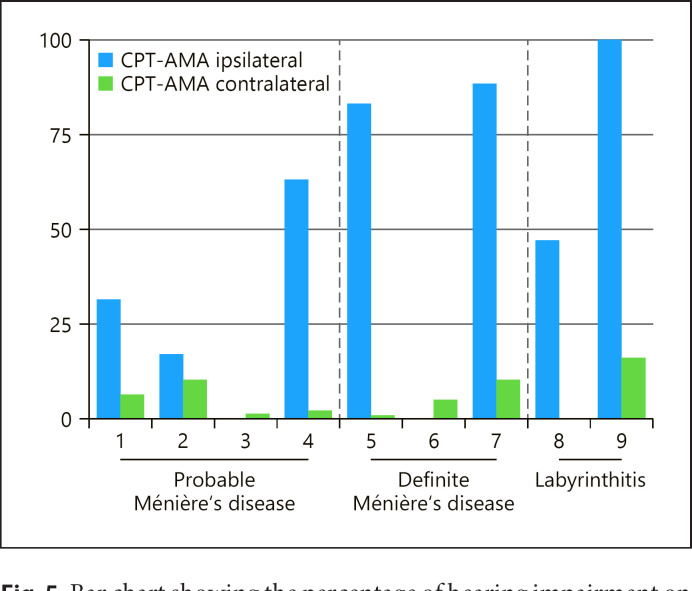
Of the 9 patients in the CISS-positive group, 7 patients (77.8%) showed CPT-AMA values >0 on the same side as the signal hypointensity. The mean hearing impairment on the ipsilateral side to the CISS hypointensity of the 9 patients was 47.8% and on the contralateral side 5.8% (Fig. 5). This difference between the ipsilateral and the contralateral side is statistically significant (Z = −2.3, p = 0.021). However, 2 patients in the CISS-positive group had no hearing impairment (CPT-AMA 0%): 1 patient with probable Ménière's disease in whom symptoms started 13 years prior to MRI, and 1 patient with definite Ménière's disease, with symptoms onset 2 years prior to MRI.
Fig. 5.
Bar chart showing the percentage of hearing impairment on the ipsilateral side of the CISS hypointensity in comparison to the contralateral side in all the 9 CISS-positive cases. CPT-AMA from patients 3 and 6 was 0% (normal hearing).
Auditory Evoked Potential
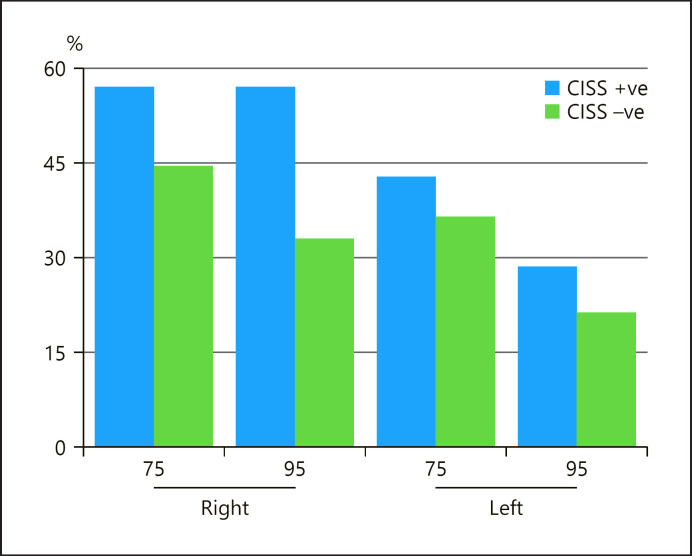
Only 112 patients in the CISS-negative and 7 patients in the CISS-positive group had records of AEP tests. Patients in the CISS-positive group showed asynchronous waves more frequently than patients in the CISS-negative group (Fig. 6), but the Mann-Whitney U-test showed no statistically significant difference: on the right-side p = 0.775 for AEP 75 and p = 0.13 for AEP 95, and on the left side p = 0.685 for AEP 75 and p = 0.869 for AEP 95.
Fig. 6.
Bar chart showing the percentage of presence of an asynchronous wave in the AEP test during 75 and 95 dB stimuli in both CISS-positive and CISS-negative patients.
Of these 7 patients, 5 patients (71.4%) showed an asynchronous wave on the same side as the cochlear signal hypointensity. Two of them had definite Ménière's disease and 3 had probable Ménière's disease. Notably, 2 of these 3 patients showed bilateral asynchronous waves.
Two patients in the CISS-positive group showed a normal wave pattern. In one of them, the AEP was performed 2 years after MRI. No data were available from patients with labyrinthitis.
Of the 7 patients with signal asymmetry, 6 (85.7%) showed hypointensity of both the cochlea and vestibule. Five of the patients in this group showed pathological results ipsilateral to hypointensity. For the patient with a normal pattern in AEP, the MRI preceded the test by 2 years.
Bithermal Caloric Test
The bithermal caloric test was performed in 116 patients in the CISS-negative group and 7 of the 9 patients in the CISS-positive group. The test was normal in 3 out of these patients, but 4 patients showed unilateral hypoexcitability (1 patient with probable Ménière's disease, 2 patients with definite Ménière's disease, and 1 patient with labyrinthitis). In 3 patients, it was on the same side as the signal hypointensity in the 3D-CISS sequence. In the patient with an abnormal test result on the contralateral side to the CISS signal hypointensity, the test was performed 2 years after the MRI.
Quantitative Analysis
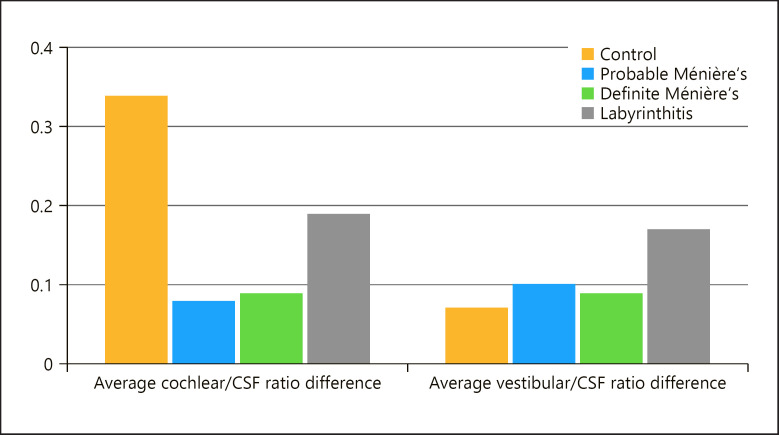
The average vestibular ratio difference between the two ears was highest in labyrinthitis (0.17), followed by probable Ménière's disease (0.10), then definite Ménière's disease (0.09), and lowest in the control group (0.07). No statistical difference was found testing our hypothesis between the 4 groups (H0[3] = 7.28, p = 0.063).
The average cochlear ratio difference was highest in labyrinthitis (0.19), followed by definite Ménière's disease (0.09), and lastly probable Ménière's disease (0.08). The control group showed a bigger difference than all the 3 patient groups (0.34) (Fig. 7), but the difference between the 4 groups was not statistically significant (H0[3] = 2.23, p = 0.526).
Fig. 7.
Bar chart showing the average right/left difference of the cochlear/CSF ratio and vestibular/CSF ratio in the 4 groups.
Discussion
The results of our study can be summarized as follows: (1) a hypointense CISS signal is not a finding that is specific for vestibular schwannoma but may occur in patients with labyrinthitis, specifically in the acute stage and less frequently in Ménière's disease. (2) This CISS signal alteration has often been encountered ipsilateral to the symptomatic side, but there is no direct association with audiological or clinical symptoms. (3) Hearing impairments and hypoexcitability in the bithermal caloric test were frequently found on the same side as the CISS signal hypointensity. (4) The vestibular/CSF ratio difference between the two sides is higher in patients with labyrinthitis and to a lesser extent in patients with Ménière's disease, than in control group, but the cochlear/CSF ratio difference was not helpful in differentiating patients from controls.
Recently, cochlear signal hypointensity was noted in patients with vestibular schwannoma due to complex and diverse factors [Wagner et al., 2018a, b]. One of these factors is the higher protein content. In this heavily weighted T2 sequence, physiological fluids show high signal intensity, but increasing protein content leads to shortening of T2 relaxation with a consequent reduction in signal intensity.
Protein plays an important role in the physiology of hearing. Sound waves induced by the motion of the eardrum and middle ear ossicles are transmitted to the fluids of the inner ear and stimulate the hair cells to excite the auditory cells. The endolymph contains the highest concentration of protein in the inner ear. The presence of protein causes an osmosis gradient between the endolymphatic sac lumen and the interstitial fluid, so, protein is most probably a regulator of fluid volume in the inner ear [Salt, 2001; Kim et al., 2011, 2014; Reichenbach and Hudspeth, 2014]. Consequently, any disturbance to the protein concentration may cause dysfunction of the inner ear with symptoms such as hearing loss or dizziness [Kim et al., 2011].
Different etiologies can cause labyrinthitis, resulting in the presence of inflammatory mediators, elevating protein content [Kaya et al., 2016]. Ménière's disease is considered a disease of the intralabyrinthine fluid dynamics. Although the pathomechanism is still unclear, blockage of the endolymphatic sac and duct likely lead to the accumulation of protein in the endolymph [Takeda et al., 2020].
The frequency of the hypointense signal in patients with vestibular schwannoma is around 33% [Wagner et al., 2018b]. In our cohort, a hypointense signal was found in only 5.6% of patients with Ménière's disease but was present in 40% of patients with labyrinthitis (in 66.6% if only patients in the acute phase were considered). Moreover, the protein concentration in patients with labyrinthitis is most probably higher than for Ménière's disease, as the quantitative analysis showed that the average cochlear/CSF ratio difference and vestibular/CSF ratio difference were higher in patients with labyrinthitis than Ménière's disease. The varying protein concentrations in the different pathologies may play a role here [Silverstein, 1966]. Silverstein found higher protein concentrations and more protein bands in patients with schwannoma than in patients with Ménière's disease [Silverstein, 1971]. Another possibility of the difference in frequencies between diseases is the type and number of protein fractions present, as protein fractions were found to be more numerous in patients with vestibular schwannoma than patients with Ménière's disease [Silverstein, 1971]. Another factor that might influence signal normalization in long-standing cases as the average duration between symptom onset and MRI was slightly longer in CISS-negative patients than in CISS-positive. Also, in patients with labyrinthitis, 2 out of the 3 patients in CISS-negative group underwent MRI a year after symptom onset, and 2 of the 3 patients examined in the acute phase were positive for CISS hypointensity.
Although symptoms frequently occurred on the same side as the signal hypointensity, we found no clear association between the hypointense signal and laterality of the disease. Moreover, only 77.8% of patients in the CISS-positive group showed hearing impairment on CPT-AMA, which was ipsilateral to CISS hypointensity. Therefore, factors other than protein concentration may contribute to the pathomechanism of hearing impairment. Various theories about the underlying mechanism of vertigo and deafness in patients with Ménière's disease have been suggested. These include endolymphatic hypertension [Takeda et al., 2020], rupture of the labyrinthine membrane [Lawrence and Mccabe, 1959], resulting in a fistula that is frequently observed in the distended labyrinthine membrane, physical distortion, and chemical paralysis of the sensory organ [Takeda et al., 2020].
Signal changes in the 3D-CISS sequence are usually difficult to identify. Nevertheless, the interrater variability was very low, as both raters were trained to identify this sign in patients with vestibular schwannoma. Comparing the signal intensity from both sides to detect signal asymmetry is the best approach. However, in patients with bilateral pathologies, a signal reduction could be missed and tiny changes in protein content may not cause enough variations to be detected in the qualitative analysis. For these reasons, finding a threshold that could be applied in quantitative analysis would have been helpful. However, owing to difficulties in drawing ROIs inside the small structures of the inner ear, this solution is not realistic in daily practice. In addition, the cochlear/CSF ratio difference did not show relevant variance between the control group and patient groups due to different reasons; the presence of CSF artifacts in the cerebellopontine angle, and the presence of the spiral lamina that contribute to this hypointensity in both patient and control groups. Because of the absence of this structure in vestibules, the vestibular/CSF ratio difference was higher in the patient groups than the control groups.
Significance
Post-contrast FLAIR and inversion recovery are helpful sequences for the diagnosis of Ménière's disease. Our results are not aimed for the diagnosis Ménière's disease but to raise the awareness that CISS hypointensity may be found in Ménière's disease.
Regarding labyrinthitis, early detection is important before developing labyrinthitis ossificans [Aschendorff et al., 2005]. MRI may show enhancement [Lee et al., 2019]; however, it may be negative in early stages [Campion et al., 2019; Benson et al., 2020], or contrast agent may not be administered. Here, the CISS hypointensity may help in the detection. However, due to the small number of patients in our study, further research is needed to investigate the sensitivity of this sequence in early stage of labyrinthitis. With labyrinthitis progression, hypointensity may be seen as well due to fibrosis and ossification [Campion et al., 2019].
Limitations
Our study is a retrospective analysis that included patients in whom MRI was clinically indicated. The overall number of patients studied was relatively small (especially those with labyrinthitis), limiting statistical analysis and correlation with the different clinical and audiological tests. The robustness of evaluation is particularly affected in the labyrinthitis group, due to the very small number of patients included (n = 5), and only 3 in the acute phase. Another limitation is the nonhomogeneous data, with regard to the availability of clinical and audiological tests and variable quality of 3D-CISS sequences. Finally, MRI exams were not always undertaken at the same time of clinical onset, with symptoms occurring from 40 years before imaging to 4 years after. Further prospective studies that overcome these limitations will be helpful in acquiring knowledge of the clinical and imaging course of these diseases.
Conclusion
CISS-hypointense signal of the inner ear structures is not specific for vestibular schwannoma and can also be seen in patients with labyrinthitis and Ménière's disease. This signal alteration was frequently encountered ipsilateral to the pathological audiology tests. However, symptoms and audiological tests were not always pathological in patients with signal alteration, indicating a multifactorial process. The CISS-hypointense signal may serve as a supportive finding in the detection of labyrinthitis before the development of fibrosis and ossification.
Statement of Ethics
This study protocol was reviewed and approved by the Ethics Committee of the canton of Bern, approval number KEK 2018-01509. Written informed consent has been granted an exemption by the Ethical Committee of the canton of Bern. The study was conducted in accordance with the World Medical Association Declaration of Helsinki.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research did not receive grant or funding.
Author Contributions
Arsany Hakim designed the study, performed the imaging analysis, and drafted the first version of the manuscript. Sara-Lynn Hool provided the clinical data, the audiological and vestibular tests, and helped in drafting the clinical section in the manuscript. Nabil Yassa performed literature review and helped in drafting the manuscript. Philipe Sebastian Breiding performed imaging analysis and reviewed the manuscript. Manuela Pastore-Wapp carried out the statistical analysis. Marco Caversaccio provided advice and validated the results. Lukas Anschuetz provided the clinical data and clinical correlation of results and reviewed the manuscript. Franca Wagner designed the study, applied for the ethical approval, performed the imaging analysis, and reviewed the manuscript.
Data Availability Statement
The data generated during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Funding Statement
This research did not receive grant or funding.
References
- 1.AMA Counsil on physical therapy. JAMA. 1942;120:372–3. [Google Scholar]
- 2.Aschendorff A, Klenzner T, Laszig R. Deafness after bacterial meningitis: an emergency for early imaging and cochlear implant surgery. Otolaryngol Head Neck Surg. 2005;133:995–6. doi: 10.1016/j.otohns.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 3.Benson JC, Carlson ML, Lane JI. MRI of the internal auditory canal, labyrinth, and middle ear: how we do it. Radiology. 2020;297:252–65. doi: 10.1148/radiol.2020201767. [DOI] [PubMed] [Google Scholar]
- 4.Campion T, Taranath A, Pinelli L, Ugga L, Nash R, Talenti G, et al. Imaging of temporal bone inflammations in children: a pictorial review. Neuroradiology. 2019;61:959–70. doi: 10.1007/s00234-019-02258-1. [DOI] [PubMed] [Google Scholar]
- 5.Hotson JR, Baloh RW. Acute vestibular syndrome. N Engl J Med. 1998 doi: 10.1056/NEJM199809033391007. [DOI] [PubMed] [Google Scholar]
- 6.Kaya S, Tsuprun V, Hizli Ö, Schachern PA, Paparella MM, Cureoglu S. Cochlear changes in serous labyrinthitis associated with silent otitis media: a human temporal bone study. Am J Otolaryngol. 2016;37((2)):83–8. doi: 10.1016/j.amjoto.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim DY, Lee JH, Goh MJ, Sung YS, Choi YJ, Yoon RG, et al. Clinical significance of an increased cochlear 3d fluid-attenuated inversion recovery signal intensity on an mr imaging examination in patients with acoustic neuroma. AJNR Am J Neuroradiol. 2014;35:1825–9. doi: 10.3174/ajnr.A3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SH, Kim UK, Lee WS, Bok J, Song JW, Seong JK, et al. Albumin-like protein is the major protein constituent of luminal fluid in the human endolymphatic Sac. PLoS One. 2011;6:e21656. doi: 10.1371/journal.pone.0021656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lassaletta L, Calvino M, Morales-Puebla JM, Lapunzina P, Rodriguez-de la Rosa L, Varela-Nieto I, et al. Biomarkers in vestibular Schwannoma-associated hearing loss. Front Neurol. 2019;10:978. doi: 10.3389/fneur.2019.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence M, Mccabe BF. Inner-ear mechanics and deafness: special consideration of meniere's syndrome. J Am Med Assoc. 1959;171:1927–32. doi: 10.1001/jama.1959.03010320017005. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Lee SA, Kim BG, Hong HS, Lee JY, Lee JD. Feasibility of magnetic resonance imaging in the differential diagnosis of isolated acute audiovestibular loss. J Vestib Res. 2019;28:385–91. doi: 10.3233/VES-190649. [DOI] [PubMed] [Google Scholar]
- 12.Lin HC, Ren Y, Lysaght AC, Kao SY, Stankovic KM. Proteome of normal human perilymph and perilymph from people with disabling vertigo. PLoS One. 2019;14:e0218292. doi: 10.1371/journal.pone.0218292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandalà M, et al. Diagnostic criteria for Menière's disease. J Vestib Res. 2015;25((1)):1–7. doi: 10.3233/VES-150549. [DOI] [PubMed] [Google Scholar]
- 14.Reichenbach T, Hudspeth AJ. The physics of hearing: fluid mechanics and the active process of the inner ear. Rep Prog Phys. 2014;77:076601. doi: 10.1088/0034-4885/77/7/076601. [DOI] [PubMed] [Google Scholar]
- 15.Salt AN. In: Annals of the New York academy of sciences. 2001. Regulation of endolymphatic fluid volume; pp. p. 306–12. [DOI] [PubMed] [Google Scholar]
- 16.Schraff SA, Schleiss MR, Brown DK, Meinzen-Derr J, Choi KY, Greinwald JH, et al. Macrophage inflammatory proteins in cytomegalovirus-related inner ear injury. Otolaryngol Head Neck Surg. 2007;137:612–8. doi: 10.1016/j.otohns.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 17.Silverstein H, Schuknecht HF. Biochemical studies of inner ear fluid in man. Changes in otosclerosis, Meniere's disease, and acoustic neuroma. Arch Otolaryngol. 1966;84:395–402. doi: 10.1001/archotol.1966.00760030397003. [DOI] [PubMed] [Google Scholar]
- 18.Silverstein H. Inner ear fluid proteins in acoustic neuroma, Menière's disease and otosclerosis. Ann Otol Rhinol Laryngol. 1971;80:27–35. doi: 10.1177/000348947108000105. [DOI] [PubMed] [Google Scholar]
- 19.Takeda T, Takeda S, Kakigi A. A possible mechanism of the formation of endolymphatic hydrops and its associated inner ear disorders. Auris Nasus Larynx. 2020;47((1)):25–41. doi: 10.1016/j.anl.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Wagner F, Gandalini M, Hakim A, Ermis E, Leiser D, Zbinden M, et al. Radiosurgery of vestibular schwannoma: prognostic factors for hearing outcome using 3D-constructive interference in steady state (3D-CISS) Strahlenther Onkol. 2018a;194:1132–43. doi: 10.1007/s00066-018-1361-8. [DOI] [PubMed] [Google Scholar]
- 21.Wagner F, Herrmann E, Wiest R, Raabe A, Bernasconi C, Caversaccio M, et al. 3D-constructive interference into steady state (3D-CISS) labyrinth signal alteration in patients with vestibular schwannoma. Auris Nasus Larynx. 2018b;45:702–10. doi: 10.1016/j.anl.2017.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The data generated during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.