Abstract
Basal cell carcinoma (BCC) is the most common nonmelanoma skin cancer in Switzerland and worldwide. Most BCCs can be treated in a curative setting. However, patients can develop locally destructive and, rarely, metastatic tumors that require a different treatment approach. The clinical subtype of individual lesions provides prognostic information and influences management decisions. Surgical excision, topical therapies, and radiotherapy are highly effective in the majority of subtypes as well as in low- and high-risk diseases. For patients with low-risk diseases and superficial tumors not amenable to surgery, several nonsurgical alternatives are available. Systemic therapy is indicated for high-risk BCCs, which are not amenable to either surgery or radiotherapy. Hedgehog pathway inhibitors (HHI) are currently approved. Other therapeutic options such as immune checkpoint inhibitors show promising results in clinical trials. This first version of Swiss recommendations for diagnosis and management of BCC was prepared through extensive literature review and an advisory board consensus of expert dermatologists and oncologists in Switzerland. The present guidelines recommend therapies based on a multidisciplinary team approach and rate of recurrence for individual lesions. Based on the risk of recurrence, two distinct groups have been identified: low-risk (easy-to-treat) and high-risk (difficult-to-treat) tumors. Based on these classifications, evidence-based recommendations of available therapies are presented herein.
Keywords: Basal cell carcinoma, Locally advanced tumor, Metastatic tumor, Hedgehog pathway inhibitors, PD-1 inhibitors
Introduction
Definition and Epidemiology
Basal cell carcinoma (BCC) is a type of epithelial skin cancer, which usually follows a slow, progressive course [1]. It is the most frequently diagnosed cancer in Caucasian populations [2], accounting for about 80% of all nonmelanoma skin cancers (NMSC) [1, 3, 4]. However, this number may be greater as not every individual lesion is registered due to its high incidence. Incidence increases with age and typically peaks during the eighth decade of life [5]. In Switzerland, the prevalence of BCC is estimated at 75.1 per 10,000 population, which is one of the highest rates in Europe. This may be attributable to a better reporting system in Switzerland compared to other European countries [6] or other, not yet identified, factors. BCC rarely exhibits a propensity for metastasis, with a less than 0.6% reported incidence [7, 8, 9]. Although this type of skin cancer is typically not life-threatening, the disease can cause considerable morbidity due to local tissue destruction and disfigurement if untreated [1, 10].
Etiology
The etiology of BCC is multifactorial, involving environmental and genetic factors [1]. Solar ultraviolet radiation is by far the most significant environmental risk factor, which is why most BCCs occur on sun-exposed areas of the body, such as the head and neck [1, 11]. Second-degree sunburn, chemical carcinogens such as arsenic, ionizing radiation, male gender, increasing age, and immunosuppression are also associated with a higher risk to develop BCC [12, 13]. Genomic studies discovered that aberrant activation of various signaling pathways is linked to the development of BCCs. Somatic or inherited mutations in the Patched 1 (PTCH1), Patched 2 (PTCH2), smoothened (SMO), suppressor of fused (SUFU), and glioma-associated (GLI) genes, among others, are typically associated with BCC [1, 13, 14]. PTCH1 is a transmembrane receptor that acts negatively in the hedgehog (HH) signaling pathway [13]. The pathway is active during fetal development but is strongly regulated after birth. Loss-of-function mutations of PTCH1 have been implicated in the pathogenesis of BCC [13]. Approximately, 75% of BCCs harbor somatic PTCH1 gene mutations [15]. In contrast, alterations in SMO genes are detected in up to 20% of BCCs [15]. Moreover, BCC has the highest tumor mutational burden (TMB) among the analyzed cancer types [16].
Specific genetic syndromes are also characterized by a tendency for early or increased development of BCCs. Aberrant HH signaling activation is a known prerequisite for developing Gorlin-Goltz's syndrome (OMIM 109400), a rare inherited genetic disease characterized by multiple BCCs at a young age [13]. Multiple face, neck, and head BCCs are a hallmark of another rare genetic condition, xeroderma pigmentosum (XP). BCC is also a prominent feature of other specific genetic conditions, including Bazex-Dupré-Christol and Rombo syndromes [17, 18].
Clinical and Histological Features
Several clinical and histological subtypes of BCC have been defined. The most common clinical types are nodular (micronodular), superficial and morphoeic (infiltrative and sclerosing) [1]. Ulcerated (ulcus rodens), destructive (ulcus terebrans), pigmented, and mixed variants may also be observed [1, 19]. Nodular is the most frequent subtype, accounting for approximately 50–79% of all BCCs, followed by superficial BCC (15%) [7, 20, 21]. Nodular subtypes are well-demarcated reddish to skin-colored nodules or plaques that can be translucent and have a lowering in the middle [1]. Superficial BCCs appear as scaly erythematous patches or plaques that are also typically well-demarcated, while morpheaformic BCCs present as slightly erythematous or skin-colored, scar-looking lesions with poorly defined margins [1]. The more common histological subtypes of BCCs include nodular, superficial, sclerosing/morphoeic, pigmented, infundibulocystic, basosquamous, infiltrating, BCC with sarcomatoid differentiation, and micronodular [22]. Less common variants include adenoid, trabecular, cystic, and fibroepithelial BCCs (Pinkus tumor) [23].
Recurrence Risk and Stratification
The risk of recurrence is dependent on the clinical and histopathological subtype as well as on BCC location, tumor diameter, age, immune status, gender of the patient, and the used treatment modality (Table 1) [23, 24, 25]. Recurrence is generally low after standard treatments such as surgery, with an estimated 5-year recurrence probability of 2–8% for most primary BCCs [1, 23, 24, 26]. A small proportion (5%) of patients develop BCC with a higher risk of recurrence, which can be challenging to manage [1, 25, 27]. BCC classification based on high- and low-risk is endorsed in the National Comprehensive Cancer Network (NCCN) 2021 guidelines (Table 1) [28].
Table 1.
Classification of the level of recurrence riska in BCC
| Recurrence-risk group | High−/moderate-risk (H and M zones) [23] | Low-risk (L zone) [23] |
|---|---|---|
| History and physical | ||
| Location and size | Trunk, extremities ≥2 cm | Trunk, extremities <2 cm |
| Cheeks, forehead, scalp, neck, and pretibial any size “Mask areas”b of the face, genitalia, hands, and feet | ||
| Borders | Poorly defined | Well-defined |
| Primary versus recurrent | Recurrent | Primary |
| Immunosuppression | (+) | (−) |
| Site of prior RT | (+) | (−) |
| Histology | ||
| Subtype | Aggressive growth pattern: infiltrative, micronodular, morpheaform, sclerosing, and furtherd | Nonaggressive growth pattern: nodular, superficial, and othere |
| Perineural involvement | (+) | (−) |
Adapted from the NCCN 2021 guidelines [28]. CCPDMA, Complete Circumferential Peripheral and Deep Margin Assessment; H zone, high-risk zone; L zone, low-risk zone; M zone, moderate-risk zone; RT, radiotherapy.
Any high-risk factor places the patient in the high-risk category.
“Mask areas” of the face: central face, eyelids, eyebrows, periorbital, nose, lips (cutaneous and vermilion), chin, mandible, preauricular, and postauricular skin/sulci, temple, and ear.
cThis area is a high-risk location, independent of size.
Having (mixed) infiltrative, micronodular, morpheaform, basosquamous, sclerosing, or carcinosarcomatous differentiation features in any portion of the tumor. In some cases, basosquamous tumors may be prognostically similar to SCC; clinicopathologic correlation is recommended in these cases.
Low-risk histologic subtypes include nodular, superficial, and other nonaggressive growth patterns such as keratotic, infundibulocystic, and fibroepithelioma of Pinkus.
Diagnostic Workup
Diagnosis is based on clinical, dermatoscopic, and/or histological features. A biopsy may be used to confirm a diagnosis when clinical and dermoscopic-based diagnosis is not possible. Biopsy is mandatory in ambiguous lesions, large BCCs, and high-risk areas (Table 2). Other noninvasive diagnostic approaches such as confocal microscopy and optical coherence tomography can also be used if available. In addition to confirming a diagnosis, a histopathological examination should identify histopathological subtypes as low- or high-risk tumors.
Table 2.
Requirements for histopathological examination of BCC based on EADO 2019 guidelines
| Histopathology | Evidence-based recommendation |
|---|---|
| Grade of recommendation: B | Histopathological confirmation is mandatory in ambiguous lesions, large tumors, and BCCs located in high-risk areas. Histological confirmation of diagnosis must also be obtained before surgical and radiotherapy procedures |
| Level of evidence: 3 | De novo literature search (80) − strength of consensus 100% |
Adapted from Peris et al. [1] 2019. Grade of recommendation B: aided noninvasive diagnosis with dermatoscopy, reflectance confocal microscopy, and/or optical coherence tomography can improve the diagnostic accuracy in difficult-to-recognize BCCs [1]. BCC, basal cell carcinoma; EADO, European Association of Dermato-Oncology.
For suspected metastatic BCC (mBCC), diagnostic workup should include baseline imaging with ultrasonography or positron emission tomography and computed tomography or magnetic resonance imaging for suspected locally advanced BCC (laBCC).
Disease Management
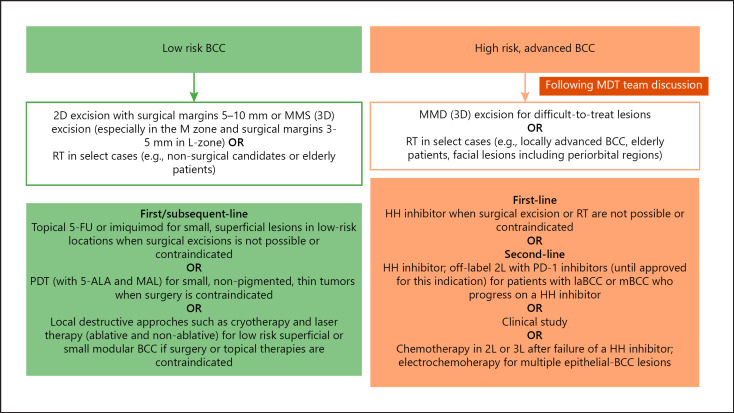
An algorithm for the treatment of primary BCC, stratified by recurrence risk, is shown in Figure 1.
Fig. 1.
Treatment algorithm for BCC. Adapted from Lang et al. [23] 2019. 5-ALA, 5-aminolevulinic acid; BCC, basal cell carcinoma; 2D, two-dimensional; 3D, three-dimensional; 5-FU, 5-fluorouracil; HH, hedgehog; laBCC, locally advanced BCC; MAL, methyl ester; mBCC, metastatic BCC; MDT, multidisciplinary tumor; MMS, Mohs micrographic surgery; PDT, photodynamic therapy.
Management of Low Recurrence Risk BCC
Surgery
The primary treatment of low-risk BCCs is complete excision by surgery, either using conventional (2D) surgery with safety margins (5–10 mm) to prevent recurrence or microscopically controlled (3D), stepwise surgery. The latter is especially appropriate for moderate-risk (M) zone (cheeks, forehead, chin, lower lip, capillitium, neck, pretibial) and low-risk (L) zone (torso, limbs) tumors [1, 23, 29]. More than 95% of all BCCs can be treated by this standard method, which concurrently allows for histological confirmation [1].
RT provides an effective alternative to surgery in select cases, e.g., for nonsurgical candidates or elderly patients. For small BCCs without high-risk features, rates of disease control are high (75–100%) in early-stage BCC [1, 30]. However, in the treatment of BCC of the face, radiotherapy (RT) is associated with inferior oncologic and cosmetic outcomes compared to surgery [31]. RT or other local less aggressive treatments described below can be considered as a nonsurgical approach for patients who refuse surgery [32].
Topical Therapies
When surgical excision or other better established treatments are not possible or contraindicated, 5% imiquimod and topical 5% 5-fluorouracil (5-FU) therapies are recommended as a treatment option for small, superficial lesions in low-risk locations [1, 33]. Five-year follow-up data demonstrated a probability of tumor-free survival of 80.5% for imiquimod (95% CI: 74.0–85.6) and 70.0% for 5-FU (95% CI: 62.9–76.0) in patients with superficial BCC [34]. For nodular or infiltrative BCCs, topical 5-FU or imiquimod are not recommended as primary monotherapy [33].
Physical Modalities
Conventional red-light photodynamic therapy (PDT) with 5-aminolevulinic acid (5-ALA) and its ester methyl aminolevulinate (MAL) may be used for small, thin (less than 2 mm) low-risk BCCs when surgery is contraindicated or unsuitable [23]. Using conventional red-light PDT, very good esthetic results can be achieved with clearance rates over 90% at 12 months in small superficial or nodular BCCs; however, recurrences are higher at 5 years than with surgery (14% vs. 4%) [23, 35, 36]. Other destructive approaches, including cryotherapy and laser therapy (ablative and non-ablative), should be considered in patients with low-risk superficial or small nodular BCC, primarily if surgery or topical therapies are contraindicated [23]. Notably, cryotherapy may be considered in patients with superficial BCC on the trunk or extremities [23].
RT provides an effective alternative to surgery in select cases, e.g., for nonsurgical candidates, elderly patients, or patients with tumors in anatomically or aesthetically important areas where surgery would lead to loss of function, such as the periocular area. After superficial RT as first-line therapy, relapses at 5-, 10-, and 15 years were 4.2%, 5.7%, and 5.7%, respectively [30, 37]. However, with recurrent BCCs, response rates are considerably lower; hence if not contraindicated, surgery should be considered [37].
Management of High-Risk BCC
Several RT methods are available, including superficial RT, external beam RT, and interstitial brachytherapy [1]. The most commonly used technique is external beam RT, which penetrates deeper tissues and can be used in the case of thick tumors [1, 19]. Superficial RT is an alternative for lesions up to approximately 6 mm in depth [1, 19]. Brachytherapy is useful for lesions arising on curved surfaces. Recommended doses of RT range between 60 and 70 Gy (using 2 Gy fractions) using megavoltage photons for larger, more advanced lesions, and 45 Gy or 51 Gy (in 10 and 17 fractions, respectively) using orthovoltage photons for smaller lesions [1]. Compared to surgery, local control rates after RT are lower (approximately, 70–90%) [38, 39].
For XP patients, and other genetic disorders, RT should be avoided because late side effects and complications have been described but are not well known. Therefore, before considering RT, knowledge of the XP complementation group and cellular sensitivity to ionizing radiation may be reasonable due to the different frequency and age of onset of cutaneous tumors [40, 41, 42, 43, 44].
HH Pathway Inhibitors
In high-risk BCC patients who are not surgical candidates or refuse surgery and where RT alone would offer insufficient control rates, alternative treatment options have been limited in the past. However, following the identification of the role of the HH signaling in the pathogenesis of BCC, a new class of systemic medications known as HH pathway inhibitors (HHIs) have been developed. In particular, vismodegib and sonidegib are now considered standard treatments in locally advanced (laBCC) and metastatic BCC (mBCC) [1]. Both HHIs are approved in Switzerland based on their clinical efficacy as demonstrated in their respective phase 2 trials, ERIVANCE [45, 46] and BOLT [47, 48, 49, 50].
In the pivotal, single-arm, 2-cohort ERIVANCE study (NCT00833417), vismodegib met its primary endpoint at 9 months, with overall response rates (ORRs) of 30% (95% CI: 16–48; p = 0.001) among 33 patients with mBCC and 43% (95% CI: 31–56; p = 0.001) among 63 patients with laBCC [46]. All 104 patients had at least one adverse event (AE), and in 12% of patients, AEs led to discontinuation of vismodegib [46]. The safety of vismodegib was confirmed in the single-arm, open-label, multicenter SafeTy Events in VIsmodEgib (STEVIE) study in 1,215 adult patients with laBCC (n = 1,119) or mBCC (n = 96) [51]. Median duration of response (DOR) was 23.0 months for laBCC and 13.9 months for mBCC, and median progression-free survival (PFS) was 23.2 and 13.1 months for laBCC and mBCC, respectively [51]. ORRs were observed in 68.5% of patients with laBCC and 36.9% of those with mBCC disease due to longer treatment duration [51]. All-grade treatment-related AEs occurred in 98% (n = 1,192) of patients [51]. The most common all-grade AEs were muscle spasm (66.4%), alopecia (61.5%), dysgeusia (54.6%), weight decrease (40.6%), and decreased appetite (24.9%). Approximately 50% of the AEs were mild to moderate [51]. Different dose reduction strategies have been suggested to reduce the severity of AEs associated with long-term vismodegib treatment [52, 53, 54]. Treatment interruptions of up to 8 weeks are recommended to manage intolerable AEs. The MIKIE study (NCT01815840) evaluated the efficacy and safety of two intermittent vismodegib dosing regimens in 229 patients with multiple BCCs [53]. This study provided evidence that intermittent dosing of vismodegib (i.e., 8 or 12 weeks of treatment followed by an 8-week interruption) is effective and tolerable in patients with multiple low-risk BCC [53]. Notably, multiple BCCs of nodular subtype should be considered as high-risk BCC and treated accordingly [1]. Evidence from a small cohort of 15 nonrecurrent BCC patients suggests that neoadjuvant vismodegib treatment for ≥3 months reduces surgical defect areas [55]. The effectiveness of vismodegib as a neoadjuvant to surgery for high-risk BCC is currently investigated in two ongoing clinical trials (NCT02667574 and NCT03035188; https://clinicaltrials.gov/).
BOLT was a randomized, double-blind, multicenter phase 2 study of sonidegib for the treatment of laBCC or mBCC (NCT01327053). Patients were randomized 1:2 to receive a daily dose of sonidegib 200 mg or 800 mg until disease progression or unacceptable toxicity. Overall, 230 patients with high-risk BCC were included in the trial. At the initial 6-month analysis, treatment with sonidegib demonstrated objective response rates by central review of 43% in patients with laBCC and 15% in patients with mBCC [47]. According to the final analysis at 42 months in the sonidegib 200-mg arm, by central review, 56% (95% CI: 43–68) and 8% (95% CI: 0.2–36) of patients with laBCC and mBCC had an objective response, respectively [50]. In the 800-mg arm, 46.1% (95% CI: 37–55) and 17% (95% CI: 5–39) of patients with laBCC and mBCC had an objective response, respectively [50]. The median DOR for responders was 26.1 months. Median PFS was 22.1 (not estimable) and 13.1 (95% CI: 5.6–33.1) months, respectively, in the 200-mg sonidegib laBCC and mBCC arms [50]. The mean time until tumor response was 4 months. Grade 3 and 4 AEs were infrequent and deemed treatment-related in 25 (32%) patients in the 200-mg sonidegib group, leading to treatment discontinuation in 11 (14%) of patients in this group. The most common AE by the preferred term was muscle spasms, reported in 43 (54%) patients in the 200-mg group [50]. With data at 42 months, the BOLT study is the longest follow-up published to date for an HHI [50].
A meta-analysis that compared the efficacy and safety of vismodegib and sonidegib reported similar ORRs but lower complete responses with sonidegib (31% vs. 3%, respectively) [56]. Similar AEs were also observed for the two HHIs [56]. Recently, 90% of a European Delphi panel consensus of 11 experts agreed that HHIs should be the first-line standard of care for high-risk BCC patients, with vismodegib being the most commonly used in Europe [57]. Moreover, 70% of the Delphi panel agreed that continuing with the same HHI is the most used therapeutic strategy for patients with stable disease after 9 months of HHI treatment, given the lack of alternatives [57]. Both HHIs are also approved in the treatment of Gorlin-Goltz patients, where they affect basal carcinoma and odontogenic keratocysts. NIELS, a noninterventional, real-world study conducted across 26 centers in Germany, confirmed the effectiveness and safety of vismodegib in 66 patients with laBCC, with an ORR of 74.2% and disease control rate of 90.9% [58].
PD-1 Inhibitors
As BCC is characterized by a high TMB, immunotherapy with checkpoint inhibitors could be a viable treatment option in patients with BCC [16]. Data on TMB of BCCs from patients with Gorlin-Goltz are limited; hence, efficacy of immunotherapy may be different in this patient population [16].
In an open-label study investigating the anti-programmed death-1 (PD-1) inhibitor cemipilimab in patients with laBCC or mBCC, patients received 350 mg cemiplimab intravenously every 3 weeks for up to 93 weeks. Among 84 patients with laBCC, cemiplimab showed a centrally confirmed ORR of 31% (95% CI: 21–42), with 6% complete responses and 25% partial responses [59]. Median DOR was not reached [59, 60]. The estimated Kaplan-Meier probability of DOR at 6 and 12 months was 91% (95% CI: 68–98) and 85% (95% CI: 61–95), respectively [59]. The median PFS was 19 months (95% CI: 9 to not estimable), with a 12-month PFS rate of 57% (95% CI: 44–67) and a 24-month overall survival rate of 80% [59]. The median overall survival was not reached [59]. No considerable differences in efficacy were observed in patients stratified by the baseline programmed death-ligand 1 (PD-L1) expression status (PD-L1 <1% vs. PD-L1 ≥1%) [61]. The safety profile was manageable and consistent with previous reports on cemiplimab and other anti-PD-1 antibodies [59, 60, 62]. Grade 3–4 treatment-emergent AEs occurred in 40 (48%) of 84 patients, leading to study discontinuation in 9 patients (11%); the most common were hypertension (5%), colitis (5%), fatigue (4%), urinary tract infection (4%), and visual impairment (4%) [59]. Immune-related AEs occurred in 21 (25%) of 84 patients, most commonly hypothyroidism (10%) and immune-related colitis (4%) [59].
A recent phase 2, open-label health-related quality-of-life study in laBCC patients treated with cemiplimab demonstrated that for the majority of patients, overall health-related quality of life and functioning improved or was maintained across the SKINDEX-16 [63] subscales (e.g., on the key symptom of pain, 31% of patients reported a clinically meaningful improvement and 44% reported maintenance) [64]. On the SKINDEX-16 emotional scale, in particular, nearly 60% of patients reported clinically meaningful improvement with cemiplimab [64].
Prespecified interim data from a pivotal phase 2 study (NCT03132636) of cemiplimab-treated patients with mBCC who progressed on or were intolerant to HHIs demonstrated clinically meaningful anti-tumor activity, including durable response (median DoR not reached) [65, 66]. Notably, the disease control rate was 67.9% (95% CI, 47.6–84.1) [65, 66]. The safety profile of cemiplimab is consistent with previous reports on other tumor types [65, 66].
Cemiplimab is approved in the USA and Europe for the treatment of adult patients with laBCC or mBCC who have progressed on or are intolerant to an HHI [67, 68]. Treatment of laBCC and mBCC with cemiplimab is off-label in Switzerland.
Chemotherapy
Chemotherapy for advanced BCC has not yet been evaluated in clinical trials, and recommendations are based on responses observed in case reports. The reported response rate with platinum-based chemotherapy is 20−30%, with a DOR of 2−3 months [69]. Given the higher efficacy and good safety profile of modern therapies, chemotherapy is usually recommended once other treatment options are exhausted.
Electrochemotherapy
Electrochemotherapy (ECT) is used for the nonspecific treatment of advanced cancers and cutaneous metastases of a wide range of primary cancer types. Epithelial tumors such as BCCs may also be treated with this method, including patients with multiple lesions [70, 71]. A randomized controlled trial compared ECT to surgery in 117 patients with primary BCC [72]. At 5 years follow-up, the recurrence rate with ECT (n = 69) was 10.4% compared to 2.5% of recurrence following surgical treatment [72].
Clinical Trials
Patients, especially those with high-risk BCC, should be evaluated for participation in a clinical trial whenever suitable. Currently, local as well as systemic therapies are being investigated for patients with laBCC and mBCC.
Prevention
All patients are recommended to protect themselves against excessive sun exposure [23]. However, there is limited evidence about whether sunscreen effectively reduces the incidence of BCC [73, 74]. Nicotinamide has been shown to offer some protection against ultraviolet-induced immunosuppression in a human skin model [75]. As immunosuppression plays a bigger role in the development of squamous cell carcinoma (SCC) than BCC, evidence from clinical studies to date indicates that nicotinamide is less effective in preventing BCC than other NMSCs (relative risk reduction approx. 20% vs. 30%, respectively) [76], with no statistical difference compared to placebo. In a phase 3 double-blinded, placebo-controlled randomized study, the rate of new NMSC was 23% lower (95% CI: 4–38) with nicotinamide than placebo (p = 0.02) [76].
Similarly, the rate of new BCC development was lower in the nicotinamide group than in the placebo group (20%, 95% CI: −6 to 39; p = 0.12) [76]. Given these data, nicotinamide may be used in patients with cutaneous carcinogenesis.
Follow-Up
Follow-up recommendations vary according to the risk of recurrence of the BCC observed, but along with the follow-up visits, all patients should be encouraged to perform self-checks (Table 3) [1]. For the low-risk BCCs, patients should be followed up every 6 months for the first year after complete response of BCC [23]. If no new BCCs develop during the first year of follow-up, the option for follow-up every 12 months should be considered and discussed with the patient.
Table 3.
Follow-up recommendations for basal cell carcinoma (BCC) patients
| BCC type | Follow-up recommendations (Note: All patients should be encouraged to perform self-checks) |
|---|---|
| Low-risk BCC | Every 6 months for the first 12 months after CR Every 12 months thereafter, if no BCC recurrencea |
| High−/moderate-risk BCCb | Every 6 months for the first 24 months post-excision Every 12 months thereafter, if no BCC recurrencea |
| Locally destructive and metastatic BCC | Every 3 months for the first 24 months post-excision Every 6 months thereafter, if no BCC recurrencea |
BCC, basal cell carcinoma; CR, complete response; MRI, magnetic resonance imaging; PET/CT, positron emission tomography and computed tomography.
Discussion with the patient is recommended.
Appropriate imaging may include ultrasound, PET/CT, and MRI, assessed on an individual basis.
For high-risk BCCs, there is a high probability of recurrence and development of another BCC or other form of skin cancer [77, 78]. These patients should be followed up every 6 months during the first 2 years post-excision [23]. Appropriate imaging (ultrasound, positron emission tomography and computed tomography, or magnetic resonance imaging) should be assessed on an individual basis. During the third year of follow-up, and provided no new BCCs develop in the first 2 years, follow-up options every 12 months should be considered/discussed with the patient.
During systemic treatment, patients receiving HHIs should be followed up every 4 weeks or at each treatment visit for other therapies (e.g., anti-PD-1, chemotherapy, ECT, etc.). In addition, in cases of laBCC and mBCC, appropriate imaging should be performed every 3 months to determine the extent of the tumor.
Conclusions
In order to select the most appropriate treatment and follow-up modality, it is important to identify the risk of recurrence for the individual lesion. For low-risk lesions, effective treatments may include surgical excision, RT, topical therapy (e.g., imiquimod and 5-FU), and minimally invasive procedures (e.g., PDT). High-risk BCC is primarily managed with micrographic surgical excision. In addition, promising new systemic therapies have recently been added to the BCC treatment armamentarium in Switzerland for difficult-to-treat patients, including HHIs (e.g., vismodegib and sonidegib) and PD-1 checkpoint inhibitors (e.g., off-label cemiplimab).
Key Message
Identifying recurrence risk for individual lesions enables the selection of the most effective treatment modality.
Conflict of Interest Statement
The authors declare advisory relationships with Sanofi-Aventis that could be considered a potential conflict of interest related to the submitted manuscript.
Funding Sources
This medical writing of the guideline article was financially supported by Sanofi-Aventis (Switzerland) AG. Sanofi-Aventis did not have any decision-making role in developing the manuscript and did not influence its content in any way.
Author Contributions
Egle Ramelyte and Mirjam C. Nägeli contributed equally to this work and should be considered joint first authors. Lara Valeska Maul and Reinhard Dummer contributed equally to this work and should be considered joint last authors. Robert Hunger, Rastine Merat, Olivier Gaide, Alexander A. Navarini, Lara Valeska Maul, Antonio Cozzio, Nikolaus B. Wagner, and Reinhard Dummer substantially contributed to the planning and editing of this work.
Acknowledgments
We thank Dr. Ellen Heitlinger, H + O communications Ltd., Zurich, Switzerland, for her medical writing support of the manuscript (including writing, language editing, referencing, and formatting).
Funding Statement
This medical writing of the guideline article was financially supported by Sanofi-Aventis (Switzerland) AG. Sanofi-Aventis did not have any decision-making role in developing the manuscript and did not influence its content in any way.
References
- 1.Peris K, Fargnoli MC, Garbe C, Kaufmann R, Bastholt L, Seguin NB, et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10–34. doi: 10.1016/j.ejca.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Verkouteren JAC, Ramdas KHR, Wakkee M, Nijsten T. Epidemiology of basal cell carcinoma: a scholarly review. Br J Dermatol. 2017;177((2)):359–372. doi: 10.1111/bjd.15321. [DOI] [PubMed] [Google Scholar]
- 3.Leiter U, Eigentler T, Garbe C. Epidemiology of skin cancer. Adv Exp Med Biol. 2014;810:120–140. doi: 10.1007/978-1-4939-0437-2_7. [DOI] [PubMed] [Google Scholar]
- 4.Mehta KS, Mahajan VK, Chauhan PS, Sharma AL, Sharma V, Abhinav C, et al. Metastatic basal cell carcinoma: a biological continuum of basal cell carcinoma? Case Rep Dermatol Med. 2012;2012:157187. doi: 10.1155/2012/157187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciążyńska M, Kamińska-Winciorek G, Lange D, Lewandowski B, Reich A, Sławińska M, et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci Rep. 2021;11((1)):4337. doi: 10.1038/s41598-021-83502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieberherr S, Seyed Jafari SM, Cazzaniga S, Bianchi E, Schlagenhauff B, Tscharner G, et al. Evaluation of the National Skin Cancer Campaign: a Swiss experience of Euromelanoma. Swiss Med Wkly. 2017;147:w14511. doi: 10.4414/smw.2017.14511. [DOI] [PubMed] [Google Scholar]
- 7.Apalla Z, Nashan D, Weller RB, Castellsagué X. Skin cancer: epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatol Ther. 2017;7((Suppl 1)):5–19. doi: 10.1007/s13555-016-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malone JP, Fedok FG, Belchis DA, Maloney ME. Basal cell carcinoma metastatic to the parotid: report of a new case and review of the literature. Ear Nose Throat J. 2000;79((7511–5)):511–519. [PubMed] [Google Scholar]
- 9.von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. Report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10((6)):1043–1060. doi: 10.1016/s0190-9622(84)80334-5. [DOI] [PubMed] [Google Scholar]
- 10.McDaniel B, Badri T, Steele RB. In: StatPearls (Internet) Treasure Island, FL: StatPearls Publishing; 2021. Basal cell carcinoma. [PubMed] [Google Scholar]
- 11.Cameron MC, Lee E, Hibler BP, Barker CA, Mori S, Cordova M, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80((2)):303–317. doi: 10.1016/j.jaad.2018.03.060. [DOI] [PubMed] [Google Scholar]
- 12.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146((Suppl 61)):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 13.Pellegrini C, Maturo MG, Di Nardo L, Ciciarelli V, Gutiérrez García-Rodrigo C, Fargnoli MC. Understanding the molecular genetics of basal cell carcinoma. Int J Mol Sci. 2017;18((11)):2485. doi: 10.3390/ijms18112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sternfeld A, Rosenwasser-Weiss S, Ben-Yehuda G, Shefer HK, Friedman-Gohas M, Yassur I, et al. Gene-related response of basal cell carcinoma to biologic treatment with vismodegib. Sci Rep. 2020;10((1)):1244. doi: 10.1038/s41598-020-58117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dika E, Scarfì F, Ferracin M, Broseghini E, Marcelli E, Bortolani B, et al. Basal cell carcinoma: a comprehensive review. Int J Mol Sci. 2020;21((15)):E5572. doi: 10.3390/ijms21155572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonilla X, Parmentier L, King B, Bezrukov F, Kaya G, Zoete V, et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 2016;48((4)):398–406. doi: 10.1038/ng.3525. [DOI] [PubMed] [Google Scholar]
- 17.Castori M, Morrone A, Kanitakis J, Grammatico P. Genetic skin diseases predisposing to basal cell carcinoma. Eur J Dermatol. 2012;22((3)):299–309. doi: 10.1684/ejd.2011.1633. [DOI] [PubMed] [Google Scholar]
- 18.Rubatto M, Merli M, Avallone G, Agostini A, Mastorino L, Caliendo V, et al. Immunotherapy in Xeroderma Pigmentosum: a case of advanced cutaneous squamous cell carcinoma treated with cemiplimab and a literature review. Oncotarget. 2021;12((11)):1116–1121. doi: 10.18632/oncotarget.27966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Telfer NR, Colver GB, Morton CA, British Association of Dermatologists Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159((1)):35–48. doi: 10.1111/j.1365-2133.2008.08666.x. [DOI] [PubMed] [Google Scholar]
- 20.Ghanadan A, Abdollahi P, Rabet M, Naraghi Z, Abbasi MA, Moslehi H, et al. Different anatomical distribution of basal cell carcinoma subtypes in Iranian population: association between site and subtype. Ann Dermatol. 2014;26((5)):559–563. doi: 10.5021/ad.2014.26.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88((2)):167–179. [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization (WHO) WHO Classification of skin tumors. In: Elder DE, Massi D, Scolyer RA, Willenze R, editors. WHO Classification of Tumours. 4th ed. Vol. 11. [Google Scholar]
- 23.Lang BM, Balermpas P, Bauer A, Blum A, Brölsch GF, Dirschka T, et al. S2k guidelines for cutaneous basal cell carcinoma − part 2: treatment, prevention and follow-up. J Dtsch Dermatol Ges. 2019;17((2)):214–230. doi: 10.1111/ddg.13755. [DOI] [PubMed] [Google Scholar]
- 24.Chren MM, Linos E, Torres JS, Stuart SE, Parvataneni R, Boscardin WJ. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133((5)):1188–1196. doi: 10.1038/jid.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trakatelli M, Morton C, Nagore E, Ulrich C, Del Marmol V, Peris K, et al. Update of the European guidelines for basal cell carcinoma management. Eur J Dermatol. 2014;24((3)):312–329. doi: 10.1684/ejd.2014.2271. [DOI] [PubMed] [Google Scholar]
- 26.Kuiper EM, van den Berge BA, Spoo JR, Kuiper J, Terra JB. Low recurrence rate of head and neck basal cell carcinoma treated with Mohs micrographic surgery: a retrospective study of 1,021 cases. Clin Otolaryngol. 2018;43((5)):1321–1327. doi: 10.1111/coa.13176. [DOI] [PubMed] [Google Scholar]
- 27.Campione E, Di Prete M, Del Principe I, Diluvio L, Citarella L, Orlandi A, et al. Lack of efficacy of imiquimod in patients with basal cell carcinoma previously treated with rituximab for B cell lymphoma: two case reports. J Med Case Reports. 2016;10((1)):57. doi: 10.1186/s13256-016-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Network (NCCN) 2021. Basal cell skin cancer V2. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1416. [Google Scholar]
- 29.Weesie F, Naus NC, Vasilic D, Hollestein LM, van den Bos RR, Wakkee M. Recurrence of periocular basal cell carcinoma and squamous cell carcinoma after Mohs micrographic surgery: a retrospective cohort study. Br J Dermatol. 2019;180((5)):1176–1182. doi: 10.1111/bjd.17516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fahradyan A, Howell AC, Wolfswinkel EM, Tsuha M, Sheth P, Wong AK. Updates on the management of Non-Melanoma Skin Cancer (NMSC) Healthcare. 2017;5((4)):82. doi: 10.3390/healthcare5040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avril MF, Auperin A, Margulis A, Gerbaulet A, Duvillard P, Benhamou E, et al. Basal cell carcinoma of the face: surgery or radiotherapy? Results of a randomized study. Br J Cancer. 1997;76((1)):100–106. doi: 10.1038/bjc.1997.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puig S, Berrocal A. Management of high-risk and advanced basal cell carcinoma. Clin Transl Oncol. 2015;17((7)):497–503. doi: 10.1007/s12094-014-1272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Love WE, Bernhard JD, Bordeaux JS. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: a systematic review. Arch Dermatol. 2009;145((12)):1431–1438. doi: 10.1001/archdermatol.2009.291. [DOI] [PubMed] [Google Scholar]
- 34.Jansen MHE, Mosterd K, Arits AHMM, Roozeboom MH, Sommer A, Essers BAB, et al. Five-year results of a randomized controlled trial comparing effectiveness of photodynamic therapy, topical imiquimod, and topical 5-fluorouracil in patients with superficial basal cell carcinoma. J Invest Dermatol. 2018;138((3)):527–533. doi: 10.1016/j.jid.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Szeimies RM, Ibbotson S, Murrell DF, Rubel D, Frambach Y, de Berker D, et al. A clinical study comparing methyl aminolevulinate photodynamic therapy and surgery in small superficial basal cell carcinoma (8-20 mm), with a 12-month follow-up. J Eur Acad Dermatol Venereol. 2008;22((11)):1302–1311. doi: 10.1111/j.1468-3083.2008.02803.x. [DOI] [PubMed] [Google Scholar]
- 36.Rhodes LE, de Rie MA, Leifsdottir R, Yu RC, Bachmann I, Goulden V, et al. Five-year follow-up of a randomized, prospective trial of topical methyl aminolevulinate photodynamic therapy vs surgery for nodular basal cell carcinoma. Arch Dermatol. 2007;143((9)):1131–1136. doi: 10.1001/archderm.143.9.1131. [DOI] [PubMed] [Google Scholar]
- 37.Schulte KW, Lippold A, Auras C, Bramkamp G, Breitkopf C, Elsmann HJ, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53((6)):993–1001. doi: 10.1016/j.jaad.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 38.Kwan W, Wilson D, Moravan V. Radiotherapy for locally advanced basal cell and squamous cell carcinomas of the skin. Int J Radiat Oncol Biol Phys. 2004;60((2)):406–411. doi: 10.1016/j.ijrobp.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Locke J, Karimpour S, Young G, Lockett MA, Perez CA. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol Biol Phys. 2001;51((3)):748–755. doi: 10.1016/s0360-3016(01)01656-x. [DOI] [PubMed] [Google Scholar]
- 40.Sakata K, Aoki Y, Kumakura Y, Karasawa K, Nakagawa K, Muta N, et al. Radiation therapy for patients with xeroderma pigmentosum. Radiat Med. 1996;14((2)):87–90. [PubMed] [Google Scholar]
- 41.Schaffer JV, Orlow SJ. Radiation therapy for high-risk squamous cell carcinomas in patients with xeroderma pigmentosum: report of two cases and review of the literature. Dermatology. 2011;223((2)):97–103. doi: 10.1159/000324509. [DOI] [PubMed] [Google Scholar]
- 42.Ben Salah H, Bahri M, Turki H, Abdelmoula M, Frikha M, Daoud J. Radiotherapy for cutaneous cancers with xeroderma pigmentosum. Cancer Radiother. 2011;15((5)):400–403. doi: 10.1016/j.canrad.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Kaloga M, Dioussé P, Diatta BA, Bammo M, Kourouma S, Diabate A, et al. Squamous cell carcinoma in African children with xeroderma pigmentosum: three case reports. Case Rep Dermatol. 2016;8((3)):311–318. doi: 10.1159/000452438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lasso JM, Yordanov YP, Pinilla C, Shef A. Invasive basal cell carcinoma in a xeroderma pigmentosum patient: facing secondary and tertiary aggressive recurrences. J Craniofac Surg. 2014;25((4)):e336–8. doi: 10.1097/SCS.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 45.Sekulic A, Migden MR, Lewis K, Hainsworth JD, Solomon JA, Yoo S, et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol. 2015;72((6)):1021–6.e8. doi: 10.1016/j.jaad.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366((23)):2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16((6)):716–728. doi: 10.1016/S1470-2045(15)70100-2. [DOI] [PubMed] [Google Scholar]
- 48.Dummer R, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, et al. The 12-month analysis from basal cell carcinoma outcomes with LDE225 treatment (BOLT): a phase II, randomized, double-blind study of sonidegib in patients with advanced basal cell carcinoma. J Am Acad Dermatol. 2016;75((1)):113–25.e5. doi: 10.1016/j.jaad.2016.02.1226. [DOI] [PubMed] [Google Scholar]
- 49.Lear JT, Migden MR, Lewis KD, Chang ALS, Guminski A, Gutzmer R, et al. Long-term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J Eur Acad Dermatol Venereol. 2018;32((3)):372–381. doi: 10.1111/jdv.14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dummer R, Guminksi A, Gutzmer R, Lear JT, Lewis KD, Chang ALS, et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br J Dermatol. 2020;182((6)):1369–1378. doi: 10.1111/bjd.18552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basset-Séguin N, Hauschild A, Kunstfeld R, Grob J, Dréno B, Mortier L, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334–348. doi: 10.1016/j.ejca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 52.Woltsche N, Pichler N, Wolf I, Di Meo N, Zalaudek I. Managing adverse effects by dose reduction during routine treatment of locally advanced basal cell carcinoma with the hedgehog inhibitor vismodegib: a single centre experience. J Eur Acad Dermatol Venereol. 2019;33((4)):e144–5. doi: 10.1111/jdv.15367. [DOI] [PubMed] [Google Scholar]
- 53.Dréno B, Kunstfeld R, Hauschild A, Fosko S, Zloty D, Labeille B, et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18((3)):404–412. doi: 10.1016/S1470-2045(17)30072-4. [DOI] [PubMed] [Google Scholar]
- 54.Chanu P, Musib L, Wang X, Cheeti S, Girish S, Bruno R, et al. Vismodegib efficacy in advanced basal cell carcinoma maintained with 8-week dose interruptions: a model-based evaluation. J Invest Dermatol. 2021;141((4)):930–933. doi: 10.1016/j.jid.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 55.Ally MS, Aasi S, Wysong A, Teng C, Anderson E, Bailey-Healy I, et al. An investigator-initiated open-label clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal cell carcinoma. J Am Acad Dermatol. 2014;71((5)):904–11.e1. doi: 10.1016/j.jaad.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 56.Xie P, Lefrançois P. Efficacy, safety, and comparison of sonic hedgehog inhibitors in basal cell carcinomas: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79((6)):1089–0.e17. doi: 10.1016/j.jaad.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Basset-Seguin N, Martin-Algarra S, Rabanel S, LaFontaine P, Lewis M, Blackney M, et al. Using a modified Delphi panel to understand epidemiology and treatment patterns in Europe for advanced basal cell carcinoma patients in the post-hedgehog pathway inhibitor. ASCO Virtual. 2021 Jun;:4–8. Poster presentation P-023. [Google Scholar]
- 58.Gutzmer R, Schulze HJ, Hauschild A, Leiter U, Meier F, Haferkamp S, et al. Effectiveness, safety and utilization of vismodegib in locally advanced basal cell carcinoma under real-world conditions in Germany − the non-interventional study NIELS. J Eur Acad Dermatol Venereol. 2021;35((8)):1678–1685. doi: 10.1111/jdv.17332. [DOI] [PubMed] [Google Scholar]
- 59.Stratigos AJ, Sekulic A, Peris K, Bechter O, Prey S, Kaatz M, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22((6)):848–857. doi: 10.1016/S1470-2045(21)00126-1. [DOI] [PubMed] [Google Scholar]
- 60.Stratigos AJ, Sekulic A, Peris K, Bechter O, Prey S, Kaatz M, et al. Primary analysis of phase 2 results for cemiplimab in patients with locally advanced basal cell carcinoma (laBCC) who progress on or are intolerant to hedgehog inhibitors (HHIs) ESMO Virtual. 2020;31:S1175–S1176. Oral presentation LBA47. [Google Scholar]
- 61.Stratigos AJ, Sekulic A, Peris K, Bechter O, Prey S, Kaatz M, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22((6)):848–857. doi: 10.1016/S1470-2045(21)00126-1. [DOI] [PubMed] [Google Scholar]
- 62.Lewis K, Peris K, Sekulic A, Stratigos A, Dunn L, Eroglu Z, et al. Interim analysis of phase 2 results for cemiplimab in patients with metastatic basal cell carcinoma (mBCC) who progressed on or are intolerant to Hedgehog inhibitors (HHIs) Society for Immunotherapy of Cancer (SITC) Virtual Congress. 2021 Jan 1; Poster presentation 428. [Google Scholar]
- 63.Chren MM, Lasek RJ, Sahay AP, Sands LP. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5((2)):105–110. doi: 10.1007/BF02737863. [DOI] [PubMed] [Google Scholar]
- 64.Stratigos A, Chen C, Ivanescu C, Lewis K, Peris K, Bechter O, et al. Health-related quality of life (HRQoL) in patients (pts) with locally advanced basal cell carcinoma (laBCC) treated with cemiplimab: analysis of a phase II, open-label clinical trial. ASCO Virtual. 2021 Jun;:4–8. Abstract-9566, Poster presentation 95662021. [Google Scholar]
- 65.Lewis KD, Peris K, Sekulic A, Stratigos AJ, Dunn L, Eroglu Z, et al. Interim analysis of phase 2 results for cemiplimab in patients with metastatic basal cell carcinoma (mBCC) who progressed on or are intolerant to hedgehog inhibitors (HHIs) Poster 428. Presented at the Society for Immunotherapy of Cancer (SITC) Virtual Congress. 2020 Nov;:9–14. [Google Scholar]
- 66.Lewis KD, Peris K, Sekulic A, Stratigos AJ, Dunn L, Eroglu Z, et al. Interim analysis of phase 2 results for cemiplimab in patients with metastatic basal cell carcinoma who progressed on or are intolerant to hedgehog inhibitors. Poster P-009. Presented at the 10th World Congress of Melanoma in conjunction with 17th European Association of Dermato Oncology Virtual Congress. 2021 Apr;:15–17. [Google Scholar]
- 67.European Medicines Agency (EMA) Libtayo Product Information 2021. 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/libtayo.
- 68.U.S. Food and Drug Administration (FDA) Libtayo prescribing information. 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761097s007lbl.pdf.
- 69.McCusker M, Basset-Seguin N, Dummer R, Lewis K, Schadendorf D, Sekulic A, et al. Metastatic basal cell carcinoma: prognosis dependent on anatomic site and spread of disease. Eur J Cancer. 2014;50((4)):774–783. doi: 10.1016/j.ejca.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 70.Campana LG, Testori A, Curatolo P, Quaglino P, Mocellin S, Framarini M, et al. Treatment efficacy with electrochemotherapy: a multi-institutional prospective observational study on 376 patients with superficial tumors. Eur J Surg Oncol. 2016;42((12)):1914–1923. doi: 10.1016/j.ejso.2016.06.399. [DOI] [PubMed] [Google Scholar]
- 71.Kis E, Baltás E, Kinyó A, Varga E, Nagy N, Gyulai R, et al. Successful treatment of multiple basaliomas with bleomycin-based electrochemotherapy: a case series of three patients with Gorlin-Goltz syndrome. Acta Derm Venereol. 2012;92((6)):648–651. doi: 10.2340/00015555-1361. [DOI] [PubMed] [Google Scholar]
- 72.Clover AJP, Salwa SP, Bourke MG, McKiernan J, Forde PF, O'Sullivan ST, et al. Electrochemotherapy for the treatment of primary basal cell carcinoma; a randomised control trial comparing electrochemotherapy and surgery with five year follow up. Eur J Surg Oncol. 2020;46((5)):847–854. doi: 10.1016/j.ejso.2019.11.509. [DOI] [PubMed] [Google Scholar]
- 73.Sánchez G, Nova J, Rodriguez-Hernandez AE, Medina RD, Solorzano-Restrepo C, Gonzalez J, et al. Sun protection for preventing basal cell and squamous cell skin cancers. Cochrane Database Syst Rev. 2016;7((7)):Cd011161. doi: 10.1002/14651858.CD011161.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Green A, Williams G, Neale R, Hart V, Leslie D, Parsons P, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet. 1999;354((9180)):723–729. doi: 10.1016/S0140-6736(98)12168-2. [DOI] [PubMed] [Google Scholar]
- 75.Yiasemides E, Sivapirabu G, Halliday GM, Park J, Damian DL. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30((1)):101–105. doi: 10.1093/carcin/bgn248. [DOI] [PubMed] [Google Scholar]
- 76.Chen AC, Martin AJ, Choy B, Fernández-Peñas P, Dalziell RA, McKenzie CA, et al. A Phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373((17)):1618–1626. doi: 10.1056/NEJMoa1506197. [DOI] [PubMed] [Google Scholar]
- 77.van Egmond S, Wakkee M, Droger M, Bastiaens MT, van Rengen A, de Roos KP, et al. Needs and preferences of patients regarding basal cell carcinoma and cutaneous squamous cell carcinoma care: a qualitative focus group study. Br J Dermatol. 2019;180((1)):122–129. doi: 10.1111/bjd.16900. [DOI] [PubMed] [Google Scholar]
- 78.van der Leest RJT, Hollestein LM, Liu L, Nijsten T, de Vries E. Risks of different skin tumour combinations after a first melanoma, squamous cell carcinoma and basal cell carcinoma in Dutch population-based cohorts: 1989–2009. J Eur Acad Dermatol Venereol. 2018;32((3)):382–389. doi: 10.1111/jdv.14587. [DOI] [PubMed] [Google Scholar]