Abstract
Introduction
As new treatments have become established, more frail pre-ICU patients are being admitted to intensive care units (ICUs); this is creating new challenges to provide adequate care and to ensure that resources are allocated in an ethical and economical manner. This systematic review evaluates the current standard for assessing frailty on the ICU, including methods of assessment, time point of measurements, and cut-offs.
Methods
A systematic search was conducted on MEDLINE, Clinical Trials, Cochrane Library, and Embase. Randomized and non-randomized controlled studies were included that evaluated diagnostic tools and ICU outcomes for frailty. Exclusion criteria were the following: studies without baseline assessment of frailty on ICU admission, studies in paediatric patients or pregnant women, and studies that targeted very narrow populations of ICU patients. Eligible articles were included until January 31, 2021. Methodological quality was assessed using the Newcastle-Ottawa Scale. No meta-analysis was performed, due to heterogeneity.
Results
N = 57 articles (253,376 patients) were included using 19 different methods to assess frailty or a surrogate. Frailty on ICU admission was most frequently detected using the Clinical Frailty Scale (CFS) (n = 35, 60.3%), the Frailty Index (n = 5, 8.6%), and Fried's frailty phenotype (n = 6, 10.3%). N = 22 (37.9%) studies assessed functional status. Cut-offs, time points, and manner of baseline assessment of frailty on ICU admission varied widely. Frailty on ICU admission was associated with short- and long-term mortality, functional and cognitive impairment, increased health care dependency, and impaired quality of life post-ICU discharge.
Conclusions
Frailty assessment on the ICU is heterogeneous with respect to methods, cut-offs, and time points. The CFS may best reflect frailty in the ICU. Frailty assessments should be harmonized and performed routinely in the critically ill.
Keywords: Frailty, Assessment, Decision-making, Critical care, Geriatric medicine, Outcome
Introduction
In our ageing society, there are increasing possibilities for medical treatment, especially in critical care, and growing numbers of frail pre-ICU patients are being admitted to intensive care units (ICUs) [1, 2]. Frailty in the general population has a high prevalence and affects 7–11% of persons aged 65 years and older and 25–40% of those aged 80 years and over [3, 4, 5]. Nonetheless, it is frequently overlooked since medical consultations often assess specific health or organ problems rather than assessing the global health and functional state of a patient [6]. Therefore, pre-ICU frailty should be assessed before or during the early period after admitting a patient to an ICU, in order to evaluate the extent to which burdensome intensive care treatments might be beneficial for the individual patient [7, 8]. Furthermore, in times with growing developments in intensive care, careful and ethical allocation of resources is important [7, 8]. The aim of this review was to systematically assess the current literature on frailty in critical care with regards to the standard assessment on the ICU and its impact on critical care outcomes in the ICU setting.
Frailty Definition − How Is Frailty Currently Defined?
Frailty is defined as a state of increased vulnerability, characterized by the loss of physiological and cognitive reserves [6, 7, 9]. It may be associated with functional decline across several organ systems [6, 7, 9]. Frailty is not only linked to ageing but also to chronic and severe organ diseases [10], limited mobility, loss of muscle mass [3, 10], and malnutrition [11]. Thus, it is a multimodal phenomenon depending on several dynamic interrelated factors in the physical, psychological, social, and environmental domains that affect the physiological equilibrium of a person [9]. The grade of pre-ICU frailty hence varies greatly between patients and needs to be assessed on the basis of individual patient characteristics [9, 12, 13].
Furthermore, frailty is a highly individual concept as it progresses at individual rates in different people − as shown by longitudinal analyses [14]. Frailty has been shown to be a risk factor for a broad range of adverse health outcomes, such as falls, hospitalization, loss of mobility, disability, and increased mortality [7].
Assessing Frailty: Which Tools Are Available within and outside the ICU?
Currently available frailty tools are presented in the online supplementary introduction (for all online suppl. material, see www.karger.com/doi/10.1159/000523674).
Ethical Aspects of Frailty on the ICU − What Is There to Consider?
Ethical aspects of frailty on the ICU are discussed in the online supplementary introduction.
Methods
This systematic review was conducted to assess currently used methods to diagnose and classify frailty in ICU patients. The systematic review followed the Cochrane guidelines [15] for conducting systematic reviews and in adherence with the PRISMA guidelines [16].
Eligibility
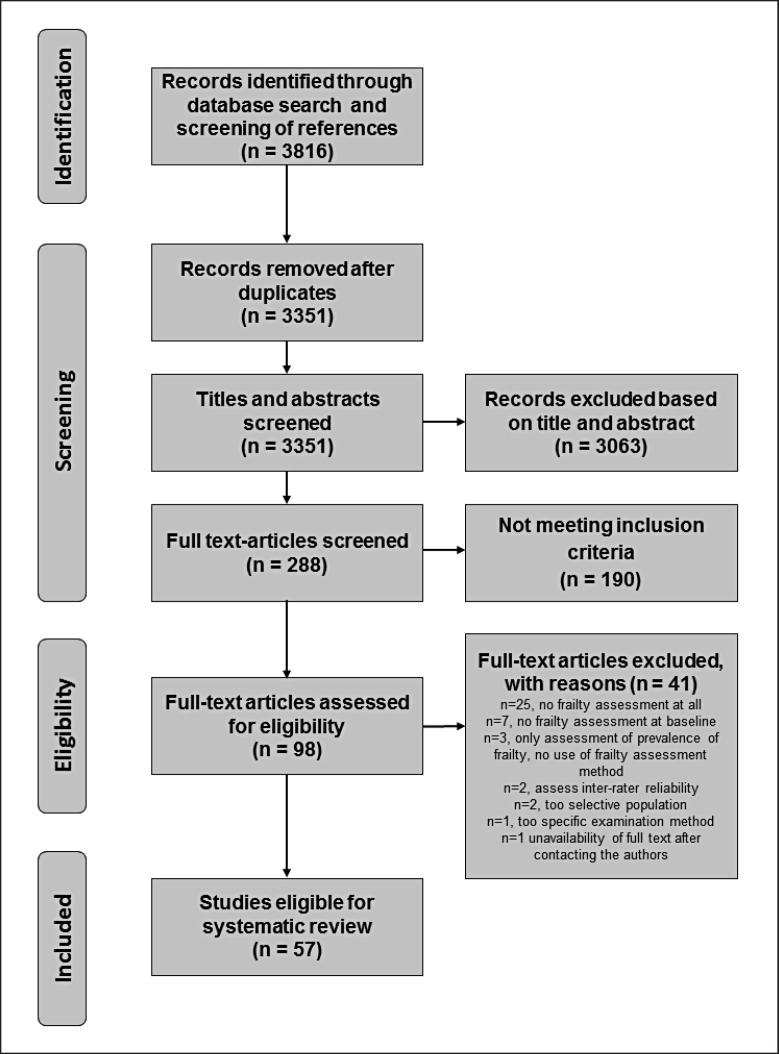
Randomized and non-randomized controlled studies on frailty were included that were within the adult ICU population and had the primary objective of evaluating diagnostic tools or ICU outcomes for frailty. The exclusion criteria were the following: studies evaluating frailty after ICU discharge without assessment on ICU admission (no baseline assessment), trials in paediatric patients and pregnant women, trials targeting very narrow populations of ICU patients, studies on inter-rater reliability, and studies exclusively investigating prevalence of frailty without assessment of diagnostic tools or outcomes. No date restriction was applied, but, we did not include any studies published after January 31, 2021. Only reports available in English or German were included. The PRISMA flowchart is shown in Figure 1.
Fig. 1.
PRISMA flowchart.
Information Sources and Search Strategy
Details on search strategy and information sources can be found in the online supplementary methods section.
Study Selection and Data Collection
All identified titles and abstracts were screened in three steps. Firstly, titles and abstracts were reviewed for the above-mentioned inclusion and exclusion criteria. Publications were excluded if a definite exclusion criterion was found. If there was insufficient information in the abstract, the full-text article was taken into account. Review articles were screened for bibliographic references. Secondly, the full text of the remaining publications was checked for inclusion and exclusion criteria, and new publications were retrieved from the citations of the screened articles. Lastly, the studies were reassessed, and data were extracted from the eligible publications. Each step was reviewed by two independent assessors. In case of discordance, a consensus was found. The PRISMA flowchart is given in Figure 1.
Study Outcomes
Details on study outcomes can be found in the online supplementary methods section.
Quality of Included Studies − Risk of Bias Assessment
Methodological quality of included studies was assessed using the Newcastle-Ottawa Scale [17]. The Newcastle-Ottawa Scale is used to assess methodological quality of cohort and case-control studies in systematic reviews. Each study is judged on eight items, categorized into three groups: the selection of the study groups, the comparability of the groups, and the ascertainment of either the exposure or outcome of interest for case-control or cohort studies, respectively. Stars are awarded for quality − up to nine stars for the highest quality. Studies were categorized as being of “high,” “fair,” “poor,” or “unknown” quality. Studies were not excluded on the basis of the Newcastle-Ottawa Scale score.
Statistical Analysis
No meta-analysis of identified studies was performed, due to the large heterogeneity of the available material.
Results
Included Studies
The search strategy identified 361 publications (PRISMA flowchart shown in Fig. 1). After removing duplicates, 283 titles and abstracts were screened for inclusion criteria, and 98 articles were retrieved for further analysis. Fifty-seven investigations, comprising a total of 253,376 patients, fulfilled the pre-specified inclusion criteria and were included in this review (see Table 1). The number of studies has increased in recent years, with 24 of the 57 included studies (42.11%) published in 2019 or later. Detailed evaluation excluded 41 studies for several reasons (shown in online suppl. Table 1). Most of the included studies (n = 40, 70.2%) were prospective cohort studies [2, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56], followed by retrospective cohort studies (n = 13, 21.1%) [10, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68].
Table 1.
Included studies
| Authors | Title | Year of publication | Patients, n | Aim of the study | Study design | Population |
|---|---|---|---|---|---|---|
| Andersen et al. [2] | Long-term outcomes after ICU admission triage in octogenarians | 2016 | 355 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | General ICU |
|
| ||||||
| Bagshaw et al. [24] | Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study | 2014 | 421 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | General ICU |
|
| ||||||
| Bagshaw et al. [28] | Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicentre cohort study | 2015 | 421 | Evaluate relation of frailty/outcome with existing tools | Prospective observational cohort study | General ICU |
|
| ||||||
| Bagshaw et al. [32] | A prospective multicentre cohort study of frailty in younger critically ill patients | 2016 | 197 | Evaluate relation of frailty/outcome with existing tools | Prospective multicentre observational cohort study | General ICU |
|
| ||||||
| Baldwin et al. [25] | The feasibility of measuring frailty to predict disability and mortality in older medical-ICU survivors | 2014 | 22 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | Patients on mechanical ventilation in medical ICU |
|
| ||||||
| Bo et al. [21] | Predictive factors of in-hospital mortality in older patients admitted to a medical ICU | 2003 | 659 | Identify prognostic factors for an adverse outcome | Prospective cohort study | Medical ICU |
|
| ||||||
| Boumendil et al. [22] | Prognosis of patients aged 80 years and over admitted in medical ICU | 2003 | 233 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | Medical ICU |
|
| ||||||
| Broslawski et al. [19] | Functional abilities of elderly survivors of intensive care | 1995 | 45 | Evaluate relation of frailty/outcome with existing tools | Prospective randomized cohort study | Medical ICU |
|
| ||||||
| Brummel et al. [36] | Frailty and subsequent disability and mortality among patients with critical illness | 2016 | 1,040 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | Patients with shock (any type) or respiratory failure |
|
| ||||||
| Bruno et al. [69] | Therapy limitation in octogenarians in German ICUs is associated with a longer LOS and increased 30 days mortality: a prospective multicentre study | 2020 | 415 | Evaluate utility of an existing tool | Prospective cohort study | General ICU |
|
| ||||||
| Chelluri et al. [18] | Long-term outcome of critically elderly patients requiring intensive care | 1993 | 97 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | General ICU |
|
| ||||||
| Darvall et al. [63] | Frailty in very old critically ill patients in Australia and New Zealand: a population-based cohort study | 2019 | 15,613 | Evaluate relation of frailty/outcome with existing tools | Retrospective cohort study | General ICU |
|
| ||||||
| Darvall et al. [44] | Contributors to frailty in critical illness: multidimensional analysis of the CFS | 2019 | 160 | Evaluate utility of an existing tool | Prospective cohort study | General ICU |
|
| ||||||
| Darvall et al. [48] | Development of an FI from routine hospital data in perioperative and critical care | 2020 | 336 | Develop a new frailty score | Prospective observational cohort study | General ICU and surgical |
|
| ||||||
| Darvall et al. [65] | Frailty and outcomes from pneumonia in critical illness: a population-based cohort study | 2020 | 5,607 | Evaluate utility of an existing tool | Retrospective cohort study | General ICU |
|
| ||||||
| Daubin et al. [23] | Predictors of mortality and short-term physical and cognitive dependence in critically ill persons 75 years and older: a prospective cohort study | 2011 | 100 | Identify prognostic factors for an adverse outcome | Prospective cohort study | Medical ICU |
|
| ||||||
| De Geer et al. [49] | Frailty predicts 30-day mortality in intensive care patients | 2020 | 872 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | General ICU |
|
| ||||||
| De Lange et al. [45] | Cumulative prognostic score predicting mortality in patients older than 80 years admitted to the ICU | 2019 | 3,730 | Develop a new frailty score | Prospective cohort study | General ICU |
|
| ||||||
| Dolera-Moreno et al. [33] | Construction and internal validation of a new mortality risk score for patients admitted to the ICU | 2015 | 1,113 | Develop a new frailty score | Prospective cohort study | General ICU |
|
| ||||||
| Fernando et al. [64] | Frailty and invasive mechanical ventilation: association with outcomes, extubation failure, and tracheostomy | 2019 | 8,110 | Evaluate relation of frailty/outcome with existing tools | Retrospective cohort study (registry data) | General ICU |
|
| ||||||
| Ferrante et al. [39] | The association of frailty with post-ICU disability, nursing home admission, and mortality | 2018 | 754 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | General ICU |
|
| ||||||
| Fisher et al. [29] | Predicting intensive care and hospital outcome with the Dalhousie CFS: a pilot assessment | 2015 | 348 | Develop a new frailty score | Prospective cohort study | General ICU |
|
| ||||||
| Flaatten et al. [91] | The impact of frailty on the ICU and 30-day mortality and the level of care in very elderly patients (≥80 years) | 2017 | 5,021 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | General ICU |
|
| ||||||
| Fronczek et al. [40] | Frailty increases mortality among patients aged ≥80 years treated in Polish ICUs | 2018 | 272 | Identify prognostic factors for an adverse outcome | Subgroup analysis of a prospective cohort study | General ICU |
|
| ||||||
| Geense et al. [50] | Changes in frailty among ICU survivors and associated factors: results of a 1-year prospective cohort study using the Dutch CFS | 2020 | 1,300 | Identify prognostic factors for an adverse outcome | Subgroup analysis of a prospective cohort study | General ICU |
|
| ||||||
| Geense et al. [51] | Physical, mental, and cognitive health status of ICU survivors before ICU admission: a cohort study | 2020 | 2,467 | Identify prognostic factors for an adverse outcome | Longitudinal prospective MONITOR-IC cohort study | General ICU |
|
| ||||||
| Guidet et al. [52] | The contribution of frailty, cognition, activity of daily life, and comorbidities on the outcome in acutely admitted patients over 80 years old in European ICUs: the VIP-2 study | 2020 | 3,920 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | General ICU |
|
| ||||||
| Hewitt et al. [66] | The FRAIL-FIT study: frailty's relationship with adverse-event incidence in the longer term, at 1 year following ICU treatment – a retrospective observational cohort study | 2019 | 400 | Evaluate relation of frailty/outcome with existing tools | Retrospective cohort study | General ICU |
|
| ||||||
| Hewitt et al. [68] | The FRAIL-FIT 30 study – factors influencing 30-day mortality in frail patients admitted to ICU: a retrospective observational cohort study | 2021 | 684 | Evaluate relation of frailty/outcome with existing tools | Retrospective observational cohort study | General ICU |
|
| ||||||
| Heyland et al. [30] | Recovery after critical illness in patients aged 80 years or older: a multicentre prospective observational cohort study | 2015 | 610 | Identify prognostic factors for an adverse outcome | Prospective observational cohort study | General ICU |
|
| ||||||
| Heyland et al. [34] | Predicting performance status 1 year after critical illness in patients 80 years or older: development of a multivariable clinical prediction model | 2016 | 527 | Develop a new frailty score | Prospective longitudinal cohort study | General ICU |
|
| ||||||
| Hope et al. [60] | Frailty prior to critical illness and mortality for elderly medicare beneficiaries | 2015 | 47,427 | Evaluate relation of frailty/outcome with existing tools | Retrospective cohort study | General ICU |
|
| ||||||
| Hope et al. [37] | Assessing the usefulness and validity of frailty markers in critically ill adults | 2017 | 95 | Develop a new frailty score | Prospective observational cohort study | General ICU |
|
| ||||||
| Hope et al. [46] | Frailty, acute organ dysfunction, and increased disability after hospitalization in older adults who survive critical illness: a prospective cohort study | 2019 | 302 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | General ICU |
|
| ||||||
| Ibarz et al. [53] | Sepsis at ICU admission does not decrease 30-day survival in very old patients: a post hoc analysis of the VIP1 multinational cohort study | 2020 | 3,869 | Identify prognostic factors for the adverse outcome | Prospective cohort study | General ICU |
|
| ||||||
| Jankowski et al. [10] | Using a CriSTAL scoring system to identify premorbid conditions associated with a poor outcome after admission to intensive care in people aged 70 years or older | 2019 | 1,000 | Develop a new frailty score | Retrospective cohort study | General ICU |
|
| ||||||
| Kizilarslanoglu et al. [38] | Is frailty a prognostic factor for critically ill elderly patients? | 2016 | 122 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | Medical ICU |
|
| ||||||
| Kokoszka-Bargiet et al. [67] | ICU admissions during the first 3 months of the COVID-19 pandemic in Poland: a single-centre, cross-sectional study | 2020 | 67 | Evaluate relation of frailty/outcome with existing tools in the specific setting of COVID-19 | Retrospective observational cohort study | COVID-19-dedicated unit |
|
| ||||||
| Komori et al. [54] | Characteristics and outcomes of frail patients with suspected infection in ICUs: a descriptive analysis from a multicentre cohort study | 2020 | 1,302 | Identify prognostic factors for an adverse outcome | Secondary analysis of a prospective multicentre cohort study | Patients with suspected infection in a general ICU |
|
| ||||||
| Le Maguet et al. [26] | Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicentre, observational study | 2014 | 196 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | General ICU |
|
| ||||||
| Lopez Cuenca et al. [47] | Frailty in patients over 65 years of age admitted to ICUs (FRAIL-ICU) | 2019 | 132 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | General ICU |
|
| ||||||
| Mattison et al. [58] | Nursing home patients in the ICU: risk factors for mortality | 2006 | 123 | Identify prognostic factors for an adverse outcome | Retrospective cohort study | General ICU |
|
| ||||||
| Mayer-Oakes et al. [57] | Predictors of mortality in older patients following medical intensive care: the importance of functional status | 1991 | 398 | Identify prognostic factors for an adverse outcome | Retrospective cohort study | Medical ICU |
|
| ||||||
| Montuclard et al. [20] | Outcome, functional autonomy, and quality of life of elderly patients with a long-term ICU stay | 2000 | 75 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | Patients on mechanical ventilation |
|
| ||||||
| Muessig et al. [41] | CFS reliably stratifies octogenarians in German ICUs: a multicentre prospective cohort study | 2018 | 308 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | General ICU |
|
| ||||||
| Orsini et al. [35] | Assessing the utility of ICU admission for octogenarians | 2015 | 52 | Identify prognostic factors for an adverse outcome | Prospective cohort study | General ICU |
|
| ||||||
| Pasin et al. [70] | The impact of frailty on mortality in older patients admitted to an ICU | 2020 | 302 | Evaluate relation of frailty/outcome with existing tools | Unmatched case-control study | Medical ICU |
|
| ||||||
| Pietiläinen et al. [61] | Premorbid functional status as a predictor of 1-year mortality and functional status in intensive care patients aged 80 years or older | 2018 | 1,827 | Evaluate relation of frailty/outcome with existing tools | Retrospective cohort study (registry data) | General ICU |
|
| ||||||
| Roch et al. [59] | Long-term outcome in medical patients aged 80 or over following admission to an ICU | 2011 | 299 | Identify prognostic factors for an adverse outcome | Retrospective case-control study | Medical ICU |
|
| ||||||
| Sanchez et al. [71] | Frailty, delirium, and hospital mortality of older adults admitted to intensive care: the delirium (Deli) in the ICU study | 2020 | 997 | Evaluate relation of frailty/outcome with existing tools | Randomized stepped-wedge intervention trial | General ICU |
|
| ||||||
| Silva-Obregon et al. [72] | Frailty as a predictor of short- and long-term mortality in critically ill older medical patients | 2020 | 285 | Evaluate relation of frailty/outcome with existing tools | Retrospective cohort study | General ICU |
|
| ||||||
| So et al. [42] | The association of clinical frailty with outcomes of patients reviewed by rapid response teams: an international prospective observational cohort study | 2018 | 1,133 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | General ICU |
|
| ||||||
| Tipping et al. [55] | The impact of frailty in critically ill patients after trauma: a prospective observational study | 2020 | 138 | Evaluate relation of frailty/outcome with existing tools in trauma patients | Prospective observational study | Trauma ICU |
|
| ||||||
| Tripathy et al. [27] | Critically ill elderly patients in a developing world – mortality and functional outcome at 1 year: a prospective single-centre study | 2014 | 109 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | General ICU |
|
| ||||||
| Wernly et al. [56] | Sex-specific outcome disparities in very old patients admitted to intensive care medicine: a propensity matched analysis | 2020 | 7,555 | Identify prognostic factors for an adverse outcome | Secondary analysis of 2 prospective, multicentre cohort studies | General ICU |
|
| ||||||
| Zampieri et al. [62] | Association of frailty with short-term outcomes, organ support, and resource use in critically ill patients | 2018 | 129,680 | Evaluate relation of frailty/outcome with existing tools | Retrospective observational cohort study | General ICU |
|
| ||||||
| Zeng et al. [31] | Mortality in relation to frailty in patients admitted to a specialized geriatric ICU | 2015 | 155 | Evaluate relation of frailty/outcome with existing tools | Prospective cohort study | General ICU |
LOS, length of stay.
Quality of the Included Studies
The overall quality of the included studies was good. A description of the quality of included studies is shown in the online supplementary results and Tables (online suppl. Table 2).
Patient Characteristics and Study Focus of Included Studies
A description of the patient characteristics of included studies and their study focus can be found in the online supplementary results and tables.
Methods of Frailty Assessment in the Critically Ill and Cut-Offs Used
Table 2 depicts the tools used for frailty assessment in the critically ill. In the identified studies, 19 different methods were used to assess frailty or a surrogate for frailty. Most of the studies use established scores and scales from the primary care setting (Clinical Frailty Scale [CFS], Frailty Index [FI], and Fried's frailty phenotype [FFP]) to define and grade frailty (n = 46, 79.3%). Thirty-five studies (n = 35, 60.3%) used the CFS to detect frailty (shown in Table 3) [2, 10, 23, 24, 26, 28, 32, 33, 34, 35, 36, 37, 40, 41, 42, 44, 45, 46, 48, 50, 51, 52, 53, 54, 56, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72], usually defining frailty by a CFS ≥5. Six of these studies included the definition of “vulnerable” with a CFS of 4 [2, 10, 32, 37, 52, 53, 54]. Four studies did not define a “cut-off”-level for frailty but worked with graded scales [33, 34, 41, 45]. The study by Orsini et al. [35] used a simplified version of the CFS, and Darvall [65] used a modified eight category CFS.
Table 2.
Diagnosis of frailty and its assessment on the ICU
| Authors | Year of publication | Enrolment criteria | Timing of frailty assessment | Diagnostic tool and criteria/cut-off for frailty | Reliability and missing values |
|---|---|---|---|---|---|
| CFS | |||||
|
| |||||
| Bagshaw et al. [24] | 2014 | Age ≥50 years ICU admission |
Baseline: pre-admission assessment at home with the help of the patient or relatives Time point: directly before current hospital admission |
CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: none Loss to follow-up: none |
|
| |||||
| Bagshaw et al. [28] | 2015 | Age ≥50 years ICU admission |
Baseline: pre-admission assessment at home with the help of the patient or relatives Time point: directly before current hospital admission |
CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: none Loss to follow-up: 37.8% first FU at 6 months, respectively; 24.3% second FU at 12 months |
|
| |||||
| Bagshaw et al. [32] | 2016 | Age: 50–64.9 years ICU admission |
Baseline: pre-admission assessment at home with the help of the patient or relatives Time point: directly before current hospital admission |
CFS ≥5 frail, 4 vulnerable, and ≤3 fit |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided Remark: substudy of the “Bagshaw et al. [32] association between frailty and short- and long-term outcomes among critically ill patients: a multicenter prospective cohort study” |
|
| |||||
| Brummel et al. [36] | 2016 | ICU admission for respiratory failure or shock | Baseline: pre-admission assessment at home with the help of the patient or relatives (within 72 h of ICU inclusion) Time point: directly before hospital admission |
CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: none Loss to follow-up: none |
|
| |||||
| Bruno et al. [69] | 2020 | Age ≥80 years Admission to ICU |
Baseline: pre-admission assessment at home with the help of the patient or relatives Exact time point: not specified | CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided Remark: uses data from the VIP-1 and the VIP-2 study |
|
| |||||
| Darvall et al. [63] | 2019 | Age ≥80 years Admission to ICU |
Baseline: pre-admission assessment at home with the help of the patient or relatives Time point: 2 months before hospital admission |
CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: 65% excluded due to missing frailty scores Loss to follow-up: no information provided |
|
| |||||
| Darvall et al. [44] | 2019 | Age ≥50 years Admission to ICU |
Baseline: pre-admission assessment with the help of the patient or relatives Time point: prior to the onset of acute illness precipitating hospital admission |
CFS Not frail: CFS 1–3 Vulnerable: CFS 4 Mildly frail: CFS 5 Moderately frail: CFS 6, severely frail: CFS ≥7 EFS not frail: EFS 0–5 Vulnerable: EFS 6–7 Mildly frail: EFS 8–9 Moderately frail: EFS 10–11 Severely frail: EFS ≥12 |
Inter-rater reliability: no information Exclusion due to insufficient data: incomplete frailty in 28.12% (patients unable to perform the clock drawing test due to sedation or deceased consciousness) Loss to follow-up: 2.50% |
|
| |||||
| Darvall et al. [48] | 2020 | Age ≥50 years when admitted to the ICU Age ≥65 years when admitted for surgery |
Baseline: pre-admission assessment at home with the help of the patient or relatives Time point: 2 months before hospital admission |
Self-constructed an FI with 36 elements score of ≥0.25 considered frail CFS CFS ≥5 frail EFS ≥8 frail |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Darvall et al. [65] | 2020 | Adults aged ≥16 years ICU admission |
Baseline: pre-admission assessment at home with the help of the patient or relatives Time point: 2 months before hospital admission |
CFS Non-frail (CFS 1–4) Mild/moderate frailty (CFS 5–6) Severe/very severe frailty (CFS 7–8) |
Inter-rater reliability: no information provided Exclusion due to insufficient data: 35.4% excluded due to incomplete frailty data Loss to follow-up: no information provided |
|
| |||||
| De Geer et al. [49] | 2020 | Admission to the ICU (only primary admission and no readmissions) | Baseline: pre-admission assessment at home with the help of the patient or relatives Time point: 2 months before the acute illness |
CFS ≥5 frail |
Inter-rater reliability: no information provided Exclusion due to insufficient data: none Loss to follow-up: 2% |
|
| |||||
| De Lange et al. [45] | 2019 | Age >80 years Acute ICU admission |
Baseline: method not specified Time point: at ICU admission |
CFS |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Dolera-Moreno et al. [33] | 2015 | ICU admission | At ICU admission Exact timing and method not specified |
Functional status (independent, dependent, and disability) FFP – none, pre-frail, and frail FI CFS |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Fernando et al. [64] | 2019 | ICU admission Mechanical ventilation (except pts with chronic invasive ventilation at admission) |
Baseline: staff assessment utilized to retrospectively score each patient on the CFS Time point: during first 24 h of ICU stay |
CFS ≥5 defines frailty |
Inter-rater reliability: weighted kappa 0.95 Exclusion due to insufficient data: 1.1% Loss to follow-up: no information provided |
|
| |||||
| Flaatten et al. [91] | 2017 | Age ≥80 years ICU admission Classification into one of 12 admission diagnosis groups |
Baseline: pre-admission assessment at home with the help of the patient or relatives Time point: directly before hospital admission |
CFS ≥5 frail/4 pre-frail/1–3 not frail |
Inter-rater reliability: no information provided Exclusion due to insufficient data: 2.2% Loss to follow-up: no information provided |
|
| |||||
| Fronczek et al. [40] | 2018 | Age ≥80 years ICU admission |
Baseline: before the onset of acute illness Exact timing and method not specified |
CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided Remark: uses data from the VIP-1 |
|
| |||||
| Geense et al. [50] | 2020 | Age ≥16 years Admitted for at least 12 h to the ICU Expected to survive the ICU |
Baseline: pre-admission assessment with the help of the patient or relatives Time point: 1 day before ICU admission |
CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: 12.27% (incomplete questionnaire) Loss to follow-up: 38.8% |
|
| |||||
| At hospital discharge 3 and 12 months after ICU admission |
|||||
|
| |||||
| Geense et al. [51] | 2020 | Age ≥16 years Admitted for at least 12 h to the ICU |
Baseline: pre-admission assessment with the help of the patient or relatives Time point: a few days before ICU admission |
CFS ≥5 defines frailty “Fatigue”: eight-item subscale of the checklist individual strength (CIS)-20 |
Inter-rater reliability: no information provided Exclusion due to insufficient data: 14.23% Loss to follow-up: no information provided |
|
| |||||
| Guidet et al. [52] | 2020 | Age ≥80 years ICU admission between May 2018 and May 2019 |
Baseline: pre-admission assessment with the help of the patient or relatives Timing: before hospital admission and before acute illness |
CFS ≥5 defines frailty, CFS 4 “pre-frailty” Katz ADL index (with an ADL score ≤4 defining disability) |
Inter-rater reliability: weighted kappa 0.85 Exclusion due to insufficient data: missing values: CFS 0.4%, Katz 11.4%, IQCODE 24%, comorbidity and polypharmacy score 0.2%, and missing values Loss to follow-up: 0.1% (ICU vital status) and 0.6% (30-day vital status), respectively |
|
| |||||
| Hewitt et al. [66] | 2019 | ICU admission for >24 h Frailty score completed at ICU admission |
Baseline: pre-admission assessment with the help of the patient or relatives Time point: before hospital admission |
CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: 0.2% had incomplete CFS scores Loss to follow-up: no information provided |
|
| |||||
| Hewitt et al. [68] | 2021 | Adult (≥18 years) patients | Baseline: pre-admission assessment with the help of the patient or relatives at home Time point: before hospital admission/acute illness |
CFS Frail CFS ≥5 and non-frail CFS <5 |
Inter-rater reliability: no information provided Exclusion due to insufficient data: 26.00% (CFS not completed) Loss to follow-up: 0.0% |
|
| |||||
| Heyland et al. [34] | 2016 | Age ≥80 years ICU admission Excluded: elective surgery admission |
Baseline: pre-admission assessment with the patient or relatives at home, measured by CGA Time point: before hospital admission |
CFS PPS Baseline physical: SF-36 Cognitive function: IQCODE |
Inter-rater reliability: no information provided Exclusion due to insufficient data: 63.23% Loss to follow-up: 17.65% |
|
| |||||
| Follow-up: At 3, 6, 9, and 12 months | PPS | ||||
|
| |||||
| Hope et al. [37] | 2017 | ICU admission within 30 days of the emergency room admission | Baseline: pre-admission assessment with the help of the patient or relatives, completed by the critical care attending or fellow within 3 days of ICU admission Time point: before hospital admission |
CFS ADLs Questionnaire as self-defined frailty markers |
Inter-rater reliability: no information provided Exclusion due to insufficient data: 0.9% due to missing CFS Loss to follow-up: 1.8% |
|
| |||||
| Hope et al. [46] | 2019 | Age ≥50 years ICU admission for ≥24 h Except elective procedures ICU admission within 30 days of the emergency room admission |
Baseline: pre-admission assessment with the help of the patient or relatives Time point: referring before hospital admission |
CFS CFS 1–3 fit, CFS 4 vulnerable, CFS ≤5 frail IQCODE |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: 38.4% |
|
| |||||
| Telephone follow-up interviews | Modified Katz ADL score | ||||
|
| |||||
| Ibarz et al. [53] | 2019 | Age ≥80 years Acute ICU admission (11 predefined categories) |
Baseline: exact method not specified Time point: before hospital admission and not affected by the acute illness |
CFS “Fit” (CFS ≤3), “vulnerable” (CFS = 4), “frail” (CFS ≥5) |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: 2% |
|
| |||||
| Jankowski et al. [10] | 2019 | Age <70 years ICU emergency admission |
Baseline: exact method not specified Time point: prior to hospital admission |
CFS CFS 1–3 (no significant frailty), 4 (vulnerable), 5 (mildly frail), 6 (moderately frail), 7 (severely frail), 8 (very severely frail) IQCODE Modified Katz ADL score |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: 2.2% |
|
| |||||
| Follow-up (6 months) |
Self-defined new scoring system based on fifteen variables from the original model Criteria for screening and triaging to appropriate alternative care (CriSTAL) |
||||
|
| |||||
| Kokoszka-Bargiel et al. [67] | 2020 | ICU admissions due to COVID-19 infection Between 10 March and 10 June 2020 |
Baseline: retrospectively assessed based on data available in medical records Time point: on ICU admission |
CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Komori et al. [54] | 2020 | Age ≥16 years Newly suspected infection Admission from December 2017 to May 2018 |
Baseline: exact method not specified, data extracted from the SPICE database Time point: at time of inclusion |
CFS fit (score 1–3), vulnerable (score 4), and frail (score 5–9) |
Inter-rater reliability: no information provided Exclusion due to insufficient data: 0.99% (missing frailty scores) Loss to follow-up: no information provided |
|
| |||||
| Le Maguet et al. [26] | 2014 | Age ≥65 years ICU stay >24 h |
Baseline: pre-admission assessment with the help of the patient or relatives Time point: extrapolated patient's status 1 month before hospital admission |
FFP ≥3 defines frailty CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Muessig et al. [41] | 2018 | Age ≥70 years ICU admission |
Baseline: pre-admission assessment with the help of the patient or relatives Time point: before hospital admission |
CFS |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Orsini et al. [35] | 2015 | Age ≥80 years ICU admission |
Baseline obtained by clinical assessment by ICU staff at time of admission reviewing assessments in electronic medical records interviewing relatives about patients’ functional status Time point: prior to ICU admission |
Simplified CFS CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Pasin et al. [70] | 2020 | Age ≥80 years ICU admission for medical reasons |
Baseline: the CFS was derived from written information on the visual description of patients Time point: recorded in the local hospital patients’ register, before ICU |
CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Sanchez et al. [71] | 2020 | ICU admission for >24 h No delirium Assessment for delirium possible (no comatose patients, no acute, or chronic neurologic condition) |
Baseline: information obtained either directly from the patient, their family or review of any previous medical notes Time point: pre-admission assessment |
CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Silva-Obregon et al. [72] | 2020 | Aged ≥70 years Admitted to ICU ICU stay between 2009 and 2017 |
Baseline: prior to October 2013 retrospective frailty assessment by patient/proxy interviews and medical records, after October 2013: frailty stage was prospectively collected Time point: not specified |
CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data and loss to follow-up (not separately listed): 24.6% |
|
| |||||
| So et al. [42] | 2018 | All patients triggering rapid response team review | Baseline: bedside assessment on the level of patients’ frailty (based on information provided by either the patient or family members) at time of inclusion Time point: at ICU admission |
CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Tipping et al. [55] | 2020 | Critically ill trauma patients Age ≥65 years |
Baseline: pre-admission assessment with the help of the patient or relatives (trained researchers determined the level of frailty, for use in this study specifically) Time point: during 1 month preceding hospital admission |
FFP Frail: 3–5; pre-frail: 1–2, non-frail: 0 CFS CFS ≥5 defines frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: 3.6% at 6 months and 9% at 12 months |
|
| |||||
| Wernly et al. [56] | 2020 | Age ≥80 years | Baseline: data extracted from VIP 1 and VIP-2 study Time point: exact timing not specified |
CFS
ADL |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| FI | |||||
|
| |||||
| Heyland et al. [30] | 2015 | Age ≥80 years ICU admission for ≥24 h ICU admission for >24 h Frailty score completed at ICU admission |
Baseline: retrospective pre-admission assessment with the help of the patient or relatives, measured by CGA Time point: 2 weeks before hospital admission |
FI mild: >0–0.2; moderate: 0.2–0.4; severe >0.4 |
Inter-rater reliability: no information provided Exclusion due to insufficient data: 6.6% missing data in longitudinal cohort Loss to follow-up: 10.3% |
|
| |||||
| Follow-up: at 3, 6, 9, and 12 months | Physical function using the SF-36 | ||||
|
| |||||
| Kizilarslanoglu et al. [38] | 2016 | Age ≥60 years ICU admission |
Baseline: pre-admission assessment with the help of the patient or relatives, by CGA parameters Time point: before hospital admission |
FI ≤ 0.25 robust; 0.25–0.4 pre-frail; >0.4 frail |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Zampieri et al. [62] | 2018 | All ICU admissions (readmissions excluded) | Baseline: exact method of data collection not specified Time point: previous functional capacity 1 week before hospitalization |
FI (modified FI) 0 non-frail; 1–2 pre-frail; ≥3 frail |
Inter-rater reliability: no information provided Exclusion due to insufficient data: 5% excluded due to missing frailty data Loss to follow-up: no information provided |
|
| |||||
| Zeng et al. [31] | 2015 | Age ≥65 years ICU admission |
Baseline: premorbid status (mobility and dependence scores) Time point: average performance 1 month prior to admission |
FI |
Inter-rater reliability: no information provided Exclusion due to insufficient data: none Loss to follow-up: none |
|
| |||||
| FFP | |||||
|
| |||||
| Baldwin et al. [25] | 2014 | Age ≥65 years ICU admission MV (invasive or NIV) for respiratory failure |
Baseline: pre-admission assessment at home with the help of the patient or relatives Time point: within 2 weeks before current hospital admission |
FFP Robust (score of 0), intermediate-frail (score 1–2), and frail (score ≥3) |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Ferrante et al. [39] | 2018 | Age >70 years ICU admission Nondisabled in four ADLs: bathing, dressing, walking across a room, and transferring from a chair |
Pre-ICU baseline: comprehensive assessment Time point: at ICU admission |
FFP |
Inter-rater reliability: no information provided Exclusion due to insufficient data: none Loss to follow-up: none |
|
| |||||
| Follow-up Monthly assessment for disability in 13 functional activities Every 18 months comprehensive assessment for frailty |
Functional status (disability in 13 functional activities) | ||||
|
| |||||
| Others | |||||
|
| |||||
| Andersen et al. [2] | 2016 | Age ≥80 years Two groups: ICU admission versus ICU refusal |
Form filled out at time of triage for potential ICU admission Exact timing and method not specified |
Functional status (Karnofsky performance status) |
Inter-rater reliability: no information provided Exclusion due to insufficient data: 0.01% Loss to follow-up: 31.71% |
|
| |||||
| Bo et al. [21] | 2003 | Age ≥65 years Admission to the ICU directly from the first-aid unit |
Baseline: pre-admission assessment at home with the help of the patient or relatives Time point: within 2 weeks before current hospital admission |
Functional status ADLs IADLs Cognitive status: SPMSQ |
Inter-rater reliability: no information provided Exclusion due to insufficient data 3.9% Loss to follow-up: no information provided |
|
| |||||
| Boumendil et al. [22] | 2003 | Age ≥65 years Admission to MICU |
Baseline: at time of inclusion Exact time point and method not specified Follow-up: between December 2000 and February 2001, mean time between ICU discharge and the date of contact 689 days |
Baseline: Functional status Knaus classification Lawton-Brody (IADL scale) |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
|
Follow-up Lawton-Brody IADL scale |
|||||
|
| |||||
| Broslawski et al. [19] | 1995 | Age ≥65 years ICU admission with medical diagnosis (except myocardial infarction, coronary care, and post-op complication) |
Baseline: pre-admission assessment at home with the help of the patient or relatives Time point: within 1 month before current hospital admission |
Functional status
Katz ADL index Lawton-Brody IADL scale Folstein's MMS |
Inter-rater reliability: no information provided Exclusion due to insufficient data: none Loss to follow-up: none |
|
| |||||
| Chelluri et al. [18] | 1993 | Age ≥65 years (two groups: 65–74 years vs. ≥75 years) Emergency ICU admission |
Baseline: pre-admission assessment at home with the help of the patient or relatives Time point: within 1 month before hospital admission |
Functional status ADL index including 8 components (independent = all activities possible; dependent = 1 activity not possible) |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Daubin et al. [23] | 2011 | Age ≥75 years Admission to ICU Excluded: surgical patients, moribund patients, and comatose after cardiac arrest |
Baseline: pre-admission assessment at home with the help of the patient or relatives Time point: 2 months before hospital admission |
Charlson comorbidity index Katz ADL index Cognitive score (individual components of Lawton-Brody IADL scale) |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: 1% |
|
| |||||
| Fisher et al. [29] | 2015 | ICU admission All patients except palliative care and organ donation Two age groups: >65 and >85 years |
Baseline: pre-admission assessment at home with patient or relatives Time point: within 24 h of ICU admission Follow-up (at 3 months) |
DCFS (0–4 non-frail; 5–6 mild frailty; ≥7 severely frail) |
Inter-rater reliability: no information provided Exclusion due to insufficient data: 41.1% excluded due to a missing frailty scale Loss to follow-up: no information provided |
|
| |||||
| Hope et al. [60] | 2015 | Age ≥66 years ICU admission |
Baseline: frailty assessment based on data set (fee forservice claims, including hospital inpatient and outpatient, skilled nursing facility, “carrier” claims, home health agency, and durable medical equipment) Time point: during the year preceding ICU admission |
4 self-defined health categories: Robust (comparison group) Chronic organ failure Cancer Frailty |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Lopez Cuenca et al. [47] | 2019 | Age ≥65 years ICU stay >24 h |
Baseline: pre-admission assessment with the help of the patient or relatives Time point: prior to admission to the ICU |
Frail scale Morley (≥3 defining frailty) Functional status including Barthel index (BADLs) (dependency if <60) Lawton-Brody IADL scale (from 0 to 8) CDR scale: >2.5 dementia) Nutric score |
Inter-rater reliability: no information pr Exclusion due to insufficient data: no information provided Loss to follow-up: 17.4% at 1 month, 30.4% at 6 months |
|
| |||||
| Mattison et al. [58] | 2006 | Age ≥75 years Residents of nursing home |
Baseline: calculated validated scores for cognition and function using the minimum data set (MDS = quarterly resident assessment instrument mandated for all nursing home residents) Time point: last assessment before ICU admission |
Functional status including: ADL-L CPS |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Mayer-Oakes et al. [57] | 1991 | Study group: Age ≥75 years Functional limitation Control group Age 50–75 years No functional limitation |
Baseline: retrospective chart review regarding functional status Time point: before hospitalization |
Functional status (limited or not limited) |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: no information provided |
|
| |||||
| Montuclard et al. [20] | 2000 | Age ≥70 years Hospitalized for >30 days in an ICU with MV |
Baseline (by retrospective telephone interview) Time point: before hospitalization |
Katz's ADL Modified Patrick's perceived quality of life score |
Inter-rater reliability: no information provided Exclusion due to insufficient data: none Loss to follow-up: none |
|
| |||||
| For follow-up (telephone interview) | Cross-sectional study: Katz's ADL Cross-sectional study: Nottingham Health Profile |
||||
|
| |||||
| Pietiläinen et al. [61] | 2018 | Two age groups (<80 and age ≥80) Admission to ICU |
Baseline: retrieved from national registry Time point: premorbid functional status before acute illness |
Self-defined premorbid functional status Five ADLs (getting out of bed, moving indoors, dressing, climbing stairs, and walking 400 m) |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: 2% |
|
| |||||
| Roch et al. [59] | 2011 | Age ≥80 years ICU admission |
Baseline: pre-admission assessment with the help of the patient or relatives Time point: just before hospital admission |
Karnofsky performance status Knaus classification |
Inter-rater reliability: no information provided Exclusion due to insufficient data: none Loss to follow-up: none |
|
| |||||
| For follow-up in June 2009 for all patients | SF-36 questionnaire for functional status | ||||
|
| |||||
| Tripathy et al. [27] | 2014 | Age ≥65 years in two groups (65–74 years and >75 years) ICU admission |
Baseline: exact method not mentioned Time point: prior to acute illness |
ADL
MUST score |
Inter-rater reliability: no information provided Exclusion due to insufficient data: no information provided Loss to follow-up: 5.5% not contactable |
|
| |||||
| Telephonic assessment of outcome was done at 1 year | Katz ADL index | ||||
CGA, comprehensive geriatric assessment; ADL-L, activities of daily living – long form; MUST, malnutrition universal screening tool; PPS, Palliative Performance Scale; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly.
Table 3.
Definition of the outcome and its assessment
| Authors | Definition of “frailty relevant” outcomes and diagnostic criteria | Timing of outcome measures | Main study results regarding frailty and outcome | Key conclusions |
|---|---|---|---|---|
| CFS | ||||
|
| ||||
| Bagshaw et al. [24] | Mortality (short-term) Mortality (long-term) HRQOL at 6 + 12 months (EuroQol EQ-5D) Intensity of treatment in the ICU Health services utilization Dependence of care Major adverse events Treatment limitations |
In-hospital 6 months 12 months |
Frail patients: Higher mortality (in-hospital and at 1 year) Significantly lower quality of life Functional dependence more likely Readmission to hospital more common More major adverse events Higher APACHE score More treatment limitations No difference between frail and non-frail patients concerning SOFA scores Intensity of treatment |
Diagnosis of frailty Identifies patients at increased risk of adverse events, morbidity, and mortality Patients benefit from follow-up and intervention Could improve prognostication |
|
| ||||
| Bagshaw et al. [28] | HRQL (EuroQol EQ-5D) SF-12v2 (SF-12, physical and mental component) Functional status Comorbid conditions Prescription medications Illness severity |
6 months 12 months |
Frail patients Lower quality of life in frail patients (more pain and depression) Greater problems with mobility, self-care, and ADLs More comorbidities Higher illness severity |
Frailty in ICU survivors leads to greater impairment in health-related quality of life, functional dependence, and disability |
|
| ||||
| Bagshaw et al. [32] | Mortality (short-term) Mortality (long-term) HRQOL at 6 + 12 months (EuroQol EQ-5D) Discharge destination (dependence of care) Health service use (LOS and readmission) Dependence of care |
In ICU In-hospital 90 days 6 months 12 months 1 year |
Short-term mortality not significantly different between frail and non-frail patients (50–64.9 years) Higher rates of long-term mortality in younger frail patients Rehospitalization more frequent in frail patients Not being completely independent before hospitalization associated with frailty Frail patients less likely to be independent after hospitalization EQ-5D-VAS scores were similar for frail and non-frail patients at 6 months Greater proportion of frail patients had problems across all EQ-5D domains |
Diagnosis of frailty should also be considered in younger adults admitted to the ICU |
|
| ||||
| Brummel et al. [36] | Mortality (long-term) Functional status (IADLs [functional activities questionnaire], ADLs [Katz]) Cognition: RBANS HRQOL (SF-36 2) |
3 12 months after discharge |
Frailty was independently associated with Greater mortality Greater odds of disability in IADLs Decreased HRQOL Frailty was not associated with Disability in basic ADLSs at 3 and 12 months Deficits in cognition (RBANS) |
Independent association between frailty and the outcome (mortality and disability) No association between the CFS score and long-term cognition |
|
| ||||
| Bruno et al. [69] | Mortality (short-term and long-term) Treatment withdrawal/withhold Cognitive decline (IQCODE) LOS |
30 days |
Frail patients (CFS >4) Increased 30-day mortality Therapy limitations more frequent in patients with a higher degree of frailty Patients with any limitation of LST Significantly increased 30-day mortality Shorter LOS |
The CFS reliably predicts outcome |
|
| ||||
| Chelluri et al. [18] | Mortality (short-term) Mortality (long-term) LOS + rehospitalisation Place of residence (= dependence of care) Quality of life ADL PQOL index CES-D depression score |
1 6 12 months after discharge |
Mortality: No significant difference between age groups Influenced by severity of disease Association between functional impairment and mortality not investigated ADLs, PQOL, and CES-D No significant difference between age groups Return to prehospital functional level and independent life: more frequent in young group Place of residence: nursing home admission more frequent in older patients, relation to frailty not investigated PQOL index, CES-D depression score: relation to frailty not investigated Length of the ICU and hospital stay: relation to frailty not investigated |
Higher age does not necessarily predict long-term survival and quality of life in critically ill elderly patients but is likely to predict a higher level of dependence |
|
| ||||
| Darvall et al. [63] | Mortality (short-term) Severity of illness LOS and readmission Discharge destination |
Discharge from ICU |
Frail patients: In-hospital mortality higher More severely ill Median lengths of ICU and hospital stay: slightly longer Discharge to nursing home more frequent |
Frailty is frequent in VIPs Associated with mortality, illness severity, and dependence of care |
|
| ||||
| Darvall et al. [44] | Mortality (short-term) Mortality (long-term) LOS Severity of illness/comorbidities Readmission to ICU Place of residence Discharge destination New therapy limitations |
6 months after discharge |
Frail patients: In-hospital mortality significantly higher 6-month mortality significantly higher Readmission to ICU and hospital LOS did not vary depending on frailty status Worse health status (functional dependence, malnutrition, and prior hospital admissions) Less likely to be residing at home Higher APACHE 3 and SAPS 2 scores Higher comorbidity scores Less independence with activities of daily living Two times more therapy limitations instituted in the ICU |
Frailty in the critically ill affects mortality, functional status, and dependence of care Frailty in critically ill patients can be adequately quantified with the CFS |
|
| ||||
| Darvall et al. [48] | Mortality (short-term) Mortality (long-term) LOS Discharge destination Medical complications Treatment limitations |
6 months follow-up |
Correlation was Strong between different frailty assessment tools Frail patients 30-day mortality higher in ICU patients More likely to be discharged to an assisted living facility/rehabilitation (vs. home discharge) New treatment limitations were significantly associated with the FI More frequent unplanned re-operations and unplanned ICU admissions (complications) |
The FI can reliably be derived from hospital admission data in a cohort of critically ill and surgical patients |
|
| ||||
| Darvall et al. [65] | Mortality (short-term) Discharge disposition Organ support within the ICU ICU bed day occupancy |
Minimum 30 days | Only severe/very severe frailty scores (CFS scores ≥7) were associated with mortality Mild frailty was not associated with higher mortality Discharge to a nursing home/chronic care more frequent with higher frailty scores Frail patients: less ICU therapies (less mechanical ventilation, less vasoactives, and less ECMO) |
The allocation of critical care resources should not be based on a frailty score alone |
|
| ||||
| De Geer et al. [49] | Mortality (short-term) Mortality (long-term) LOS in the ICU |
180 days after ICU admission |
CFS ≥5 has predictive value of 30-day mortality Combining the CFS and SAPS 3 resulted in an improved discriminatory ability |
Frailty remains a strong predictor of death within 30 days |
|
| ||||
| De Lange et al. [45] | Mortality (short-term) Correlation between cumulative prognostic score and 30-day mortality |
30 days after discharge |
Independent predictors of 30-days mortality: Age Sex ICU admission diagnosis CFS SOFA score Invasive ventilation Renal replacement therapy |
Frailty is one of several independent predictors for 30-day mortality |
|
| ||||
|
| ||||
| Dolera-Moreno et al. [33] | Mortality (short-term) Functional status Type of admission Severity of illness/ICU therapy |
Dead or alive at discharge from ICU |
Factors predicting higher mortality: Functional impairment (dependent or disability) Type of admission: medical or cardiological admission and sepsis ICU therapies: mechanical ventilation and inotropic support |
Functional impairment (independent in daily live, care-dependent, and disability) can be used as part of a mortality risk prediction score |
|
| ||||
| Fernando et al. [64] | Mortality (short-term) ICU therapies (intubated patients) Discharge destination (dependence of care) Difficulties of weaning of mechanical ventilation |
Till hospital discharge or death |
Frailty in mechanically ventilated patients increased odds of Hospital mortality Discharge to long-term care Extubation failure/need for tracheostomy |
Frailty in patients requiring mechanical ventilation is associated with more complications and worse outcome |
|
| ||||
| Flaatten et al. [91] | Mortality (short-term) Severity of illness ICU therapies Treatment limitations |
30 days after discharge |
Frailty (CFS ≥5) in patients ≥80years Nearly linear relationship between mortality and increased frailty Higher SOFA score More often female More frequently therapy was withheld or withdrawn |
Frailty is one of the three most important factors for short-term mortality CFS classes are inversely associated with short-term survival |
|
| ||||
| Fronczek et al. [40] | Mortality (short-term) Severity of illness Mode of admission |
30 days after discharge |
Mortality higher if Higher SOFA score Acute mode of admission Frailty (strongly associated) |
Frailty assessment in older ICU patients can help for clinical decisions to avoid futile interventions |
|
| ||||
| Geense et al. [50] | Mechanical ventilation days ICU and hospital LOS Hospital discharge location |
The day before ICU admission At hospital discharge At 3 months 12 months after discharge |
Increase of frailty level 12 months after ICU admission 42% of the unplanned and 27% of the planned patients more frail Higher frailty level associated with Older age Longer hospital LOS Hospital discharge to care facility Lower frailty level associated with Male sex Higher education level Mechanical ventilation |
Assessment of frailty associated factors can help to identify patients at risk diagnosing frailty may help in informing patients and their family members |
|
| ||||
| Geense et al. [51] | Level of frailty (CFS) Fatigue (checklist individual strength-8) Anxiety and depression (hospital anxiety and depression scale) Cognitive functioning (cognitive failure questionnaire-14) Quality of life (SF-36) Marital status Place of residence Comorbidities Mode of admission |
In ICU (referring to time before ICU admission) | Patients with a poor pre-ICU health status (association to frailty level not examined) were more often likely: Female Older (≥65 years) Lower educated Divorced or widowed Living in a health care facility Suffering from a chronic condition Higher incidence of frailty: Unplanned admissions Factors associated with being more frail Older age Longer hospital LOS Being discharged to a revalidation centre |
Serious impairments in physical, mental, and cognitive functional status may already be present before ICU admission and should be assessed |
|
| ||||
| Guidet et al. [52] | Mortality (short-term) ICU LOS Severity of illness Organ support |
30 days after discharge | Predictors of 1-month mortality Older age ICU admission diagnosis (emergency surgery and respiratory failure) Higher severity of illness/SOFA score CFS (more frail patients) |
Frailty assessment using the CFS is able to predict short-term mortality in elderly patients admitted to ICU The CFS should be routinely collected for all elderly ICU patients in particular in connection to advance care plans and should be used in decision-making |
|
| ||||
| Hewitt et al. [66] | Mortality (short-term) Mortality (long-term) Severity of illness Healthcare use |
1 year after discharge |
Frailty is associated with Greater risks of mortality (significant) Female gender Higher sickness severity More frequent hospitalization Longer total requirements for in-hospital recovery Frailty is not associated with greater risks of discharge to dependent care living facilities |
Frailty is associated with higher age, female gender, higher sickness severity, and more healthcare use Frailty was significantly associated with mortality Frailty scoring could improve decision-making in intensive care |
|
| ||||
| Hewitt et al. [68] | Mortality (short-term) Mortality (long-term) LST use ICU use |
1 year after discharge |
Frailty significantly increased Mortality (short-term) Mortality (long-term) Days of LST Index ICU LOS Longer hospital stays after ICU discharge Frailty does not increase ICU readmissions within 1 year Proportion of discharges to dependent living facilities |
Significantly association between frailty and mortality, most pronounced in the first 30-days post-ICU admission Presence of frailty increases adverse outcomes |
|
| ||||
| Heyland et al. [34] | Functional status (PPS score) Comorbidities (Charlson comorbidity index) |
3 6 9 and - 12 months after discharge |
Association between baseline functional status (PPS) and long-term outcome (independently predictive) Associated with worse long-term outcome: Higher Charlson comorbidity index frailty (higher CFS class) |
Only 1/4 of very elderly patients have a reasonable functional outcome 1 year after admission Prediction model patients may aid in decision-making about the utility of life ICU treatment for very elderly patients |
|
| ||||
| Hope et al. [37] | Mortality (long-term) Disability (grade of assistance needed for 6 ADLs [Katz]) |
Hospital discharge At 6 months after discharge |
The presence of more frailty markers Mortality and disability higher in ICU survivors The more frailty markers present, the higher the 6-months mortality Frailty phenotype performed similarly to CSF to predict death or increased disability |
The frailty phenotype may be determined by questioning patients or surrogates about frailty markers Frailty is associated with increased risk of adverse outcomes |
|
| ||||
| Hope et al. [46] | Mortality (short-term) Functional status (modified Katz activities of daily living [ADL]) Cognitive impairment (modified version of IQCODE) Severity of disease (SOFA, APACHE) |
6 months after discharge |
Hospital survivors were Younger Less prehospital ADL disability Lower severity of illness score Post-hospital disability determined by Pre-hospital frailty Total day 1 SOFA score (weak association) Day 1 SOFA neurologic score: strong association No association with prehospital cognitive impairment |
Prehospital frailty and early acute brain dysfunction are the most important factors associated with post hospital disability |
|
| ||||
| Ibarz et al. [53] | Mortality (short-term) Sepsis versus non-sepsis ICU treatments (invasive mechanical ventilation, non-invasive ventilation, vasoactive drugs, and renal replacement therapies) Treatment limitations |
30 days after discharge | Independently associated with mortality at 30 days: Higher age Higher frailty score (CFS) Higher SOFA score/severity of illness Association between frailty and intensity of ICU therapies and treatment limitations not investigated |
Age, frailty, and illness severity were independently associated with mortality Sepsis not associated with decreased survival |
|
| ||||
| Jankowski et al. [10] | Mortality (short-term) Chronic disease variables Markers of health Documented weight loss Stay in hospital ≥5 days preceding ICU admission ICU readmission during the same hospital stay |
ICU discharge | Variables significantly associated with mortality in the ICU Myocardial infarction within 6 months Abnormal ECG Congestive cardiac failure (NYHA ≥2) Chronic pulmonary disease Chronic liver disease Metastatic cancer Stay in hospital ≥5 days preceding ICU admission Frailty (CFS ≥4) |
Incorporating frailty into an ICU outcome The model is appropriate |
|
| ||||
| Kokoszka-Bargiet et al. [67] | Mortality (short-term) Charlson comorbidity index Severity of illness (APACHE, SAPS) ICU therapies (ventilation) |
3 months after discharge | ICU-admitted patients versus non-admitted patients: Charlson comorbidity index significantly lower CFS significantly lower Hospital mortality among patients admitted to the ICU and those who were disqualified was 70% and 79%, respectively |
In frail patients with COVID-19 requiring ICU admission who had significant comorbidities, outcomes were poor and did not seem to be influenced by ICU admission |
|
| ||||
| Komori et al. [54] | Mortality (short-term) Mortality (long-term) Severity of illness Discharge destination |
3 months after discharge |
In-hospital mortality did not statistically differ among the patients according to frailty Long-term mortality higher in vulnerable and frail patients than in fit patients (not statistically significant) Rate of home discharge was lower in the frail group APACHE score higher in frail patients, no difference in the SOFA score |
Frail patients with suspected infection are at risk for poor disease outcomes No statistically significant increase in the 90-day mortality risk in this population |
|
| ||||
| Le Maguet et al. [26] | Mortality (short-term) Mortality (long-term) Severity of illness (SOFA score) |
ICU discharge Hospital discharge At 6 months after discharge |
Prevalence of frailty 41% (frailty phenotype) 23% (clinical frailty score Risk factors for ICU mortality Frailty (FP score ≥3) Risk factors for 6-month mortality CFS ≥5 Severity of illness (SOFA score ≥7) |
Frailty is independently associated with increased ICU and 6-month mortalities The CFS has better outcome prediction than the commonly used ICU illness scores |
|
| ||||
| Muessig et al. [41] | Mortality (short-term) | 30 days after discharge | More than half of the patients (53.6%) were classified as frail (CFS ≥5) Frailty (CFS) is an independent predictor of 30-day mortality |
The CFS is valid for use in ICU for patients ≥80 years and correlates with mortality The CFS may facilitate decision-making for critically ill patients |
|
| ||||
| Orsini et al. [35] | Mortality (short-term) | At ICU discharge Hospital discharge |
In geriatric patients (mean age 85 years) Mean frailty score was similar in ICU survivors and non-survivors (no association between frailty and short-term mortality) ICU mortality strongly correlated with combination of mechanical ventilation and vasopressor therapy |
Pre-admission functional status in geriatric patients: not independently associated with unfavourable outcome |
|
| ||||
| Pasin et al. [70] | Mortality (short-term) Mortality (long-term) |
One year after discharge | Frailty Not associated with ICU mortality or 30-day mortality Significantly associated to 1-year mortality |
Frailty assessment may be helpful for ICU triage Should not be an exclusion criterion for ICU admission |
|
| ||||
| Sanchez et al. [71] | Hospital mortality (short-term) Rates of acute episodes of delirium in the ICU LOS in the ICU and hospital |
21 days |
Frail patients had significantly More episodes of delirium Higher hospital mortality Combination of delirium and frailty increases mortality (compared to non-frail patients with delirium) |
Frailty and delirium significantly increase the risk of hospital mortality Identification of frailty is important The risk of delirium in frail patients should be reduced by adequate measures |
|
| ||||
| Silva-Obregon et al. [72] | Mortality (short-term) Mortality (long-term) ICU and hospital LOS |
One year after discharge | ICU mortality Similar in frail- and non-frail patients Mortality in-hospital, at 30 days, at 3, 6, and 12 Significantly higher in frail patients |
Frailty (CFS ≥5) was independently associated with short- and long-term mortality in older medical patients in the ICU |
|
| ||||
| So et al. [42] | Mortality (short-term) Functional status |
After 24 h 30 days after discharge |
Higher frailty scores are associated with Increased mortality Increased dependence on health care |
Frailty is associated with increased mortality and dependence on care Frailty assessment should be included in discussion of goals and expectations of care on ICU triage |
|
| ||||
| Tipping et al. [55] | Mortality (short-term) Mortality (long-term) Functional status (mobility scale [IMS], MRC-SS, global functioning [Glasgow Outcome Scale-extended]) Living situation, return to employment Subjective health status (EQ-5D-5L) |
6 12 months after discharge |
Frailty was independently associated with ICU mortality and mortality at 6 and 12 months Poorer global functioning Lower subjective health status (Euro Qol 5Q-5D-5L utility score) No influence on percentage of patients living at home at 1 year |
Frailty is a useful predictor of poor outcomes in critically ill trauma patients |
|
| ||||
| Wernly et al. [56] | Mortality (short-term) Illness severity |
30 days after discharge | Association between functional impairment and mortality not investigated Male sex was associated with adverse 30-day mortality but not with ICU mortality Male VIPs were Younger Less often frail (CFS ≥4) Had higher SOFA |
Independent sex differences in outcomes of elderly ICU patients; male patients were less often frail, and 30-day mortality was higher |
|
| ||||
| FI | ||||
|
| ||||
| Heyland et al. [30] | Mortality (long-term) Physical function Quality of life (SF-36) Severity of illness |
3 6 9 and 12 months after discharge |
Association between functional impairment and mortality not investigated Predictors of functional recovery ICU diagnostic category Baseline physical function Pre-hospital functional status APACHE II scores Significantly lower physical function and physical component SF-36 scores compared to age- and sex-matched community controls 1/4 of very elderly patients returned to baseline levels of physical function 1 year after ICU |
For very old critically ill patients, routine assessment of baseline physical function, and frailty status could aid in prognostication and informed decision-making |
|
| ||||
| Kizilarslanoglu et al. [38] | Mortality (short-term) Mortality (long-term) Severity of illness (APACHE) |
ICU discharge Hospital discharge 3 6 months after discharge |
Frail group (compared to pre-frail and robust subjects) ICU mortality higher Long-term mortality significantly higher Median overall survival lower FI has an independent correlation with ICU mortality: Significant positive correlation between APACHE II and FI scores |
The FI can predict outcome of elderly patients’ clinical outcomes in ICUs |
|
| ||||
| Zampieri et al. [62] | Mortality (short-term) Discharge home without need for nursing care ICU and hospital los Utilization of ICU support organ support |
At hospital discharge |
Frailty is associated with Higher in-hospital mortality Higher hospital and ICU LOS Use of organ support Discharge to nurse-supported structures |
Frailty is associated with mortality and resource use (LOS in ICU and organ support) |
|
| ||||
| Zeng et al. [31] | Mortality (short-term) Mortality (long-term) |
30 days 300 days after discharge |
FI Strong positive correlation between the FI and 30-day mortality |
Strong association between ICU survival and the level of frailty at admission The FI based on health deficit accumulation may help improve critical care outcome prediction |
|
| ||||
| FFP | ||||
|
| ||||
| Baldwin et al. [25] | Mortality (long-term) Functional status (Katz ADL) |
4 days prior to discharge 1 month 6 months |
Bad functional status associated with increase of 6-month mortality Positive correlation between frailty score and disability |
Frailty correlates with mortality and disability in elderly ICU survivors |
|
| ||||
| Ferrante et al. [39] | Mortality (long-term) Discharge disposition (dependence of care) Functional status (BADL, IADL and 3 mobility activities, and ability to drive a car) |
6 months after ICU discharge | Linear relationship between frailty and probability of death Pre-frailty and frailty Increase disability at 6 months Frailty (3–5 Fried's frailty criteria): increase of 41% Pre-frailty (1–2 Fried's frailty criteria): increase of 28% Increased nursing home admissions |
Pre-existing frailty is associated with increased post-ICU disability, dependence of care, and disability Pre-existing frailty status may predict outcomes after a critical illness |
|
| ||||
| Others | ||||
|
| ||||
| Andersen et al. [2] | Mortality (short-term) Mortality (long-term) Discharge destination (dependence of care) Functional status (Karnofsky) HRQOL (EuroQol-5D-3L) |
1 year | Risk factors for ICU refusal in patients “too ill/old” Advanced age Low functional status Risk factors for ICU refusal in patients “too well” advanced age Male sex University hospital admission Comorbidity Low SAPS Survival (in-hospital and long-term) significantly lower for non-admitted patients considered too ill/old than for ICU-admitted patients and non-admitted patients considered too well Higher dependence of care in non-admitted patients considered too ill/old Karnofsky functional status No difference between ICU-admitted and non-admitted patients after hospitalization HRQOL after ICU stay Lower than in age-matched control group without ICU stay |
Significantly higher survival for ICU-admitted octogenarians than for refused patients due to age or pre-existing disease: benefit of ICU admission for this age group No difference in functional outcome between ICU-admitted and non-admitted patients |
|
| ||||
| Bo et al. [21] | In-hospital mortality (short-term) Functional impairment (ADL and IADL) Cognitive impairment (SPMSQ) |
Hospital discharge |
In-hospital mortality is significantly associated with Functional impairment/lack of independence (ADL and IADL) History of confinement to bed Cognitive deterioration/moderate-to-severe cognitive impairment (SPMSQ) |
Pre-existing conditions (loss of functional independence and severe and moderate cognitive impairment) relevant for prognosis after ICU stay in addition to acute disease |
|
| ||||
| Boumendil et al. [22] | Mortality (long-term) Functional outcome (Lawton IADL) Comorbidities Severity of illness |
Telephone interviews 3 years after discharge | Prognostic factors for long-term mortality Severe functional limitations Underlying fatal disease Independent factors of poor long-term prognosis Underlying fatal disease Severity of illness (initial altered consciousness, mechanical ventilation, and shock) Older age (age >85 years) |
Underlying disease and functional status relevant for long-term survival after critical illness Known factors for in-MICU survival do not influence long-term prognosis |
|
| ||||
| Broslawski et al. [19] | Mortality (long-term) Functional status (Katz-Downs ADL scale, Lawton-Brody IADL, GDS, and MMS) Severity of illness |
6 months after ICU discharge | Association between functional impairment and mortality not investigated Functional status at 6-months unrelated to Age Severity of illness Longer ICU/hospital stay predicted future decreased ADL and IADL scores Total length of hospital stay correlated negatively with the MMS score |
LOS (ICU and hospital) has the strongest correlation with functional outcome (decreased ADL and IADL scores) |
|
| ||||
| Chelluri et al. [18] | Mortality (short-term) Mortality (long-term) LOS + rehospitalisation Place of residence (= dependence of care) Quality of life ADL PQOL index CES-D depression score |
1 6 12 months after discharge |
Mortality: No significant difference between age groups Influenced by severity of disease Association between functional impairment and mortality not investigated ADLs, PQOL, and CES-D No significant difference between age groups Return to prehospital functional level and independent life: more frequent in young group Place of residence: nursing home admission more frequent in older patients, relation to frailty not investigated PQOL index CES-D depression score: relation to frailty not investigated Length of ICU and hospital stay: relation to frailty not investigated |
Higher age does not necessarily predict long-term survival and quality of life in critically ill elderly patients but is likely to predict a higher level of dependence |
|
| ||||
| Daubin et al. [23] | Mortality (long-term) Subjective health status – HRQOL (Nottingham Health Profile) Physical dependence Cognitive status |
3 months after discharge |
Predictors of mortality Charlson comorbidity index Modified IADL index Physical dependence and cognitive status had only slightly changed compared to prehospital status |
ICU stay does not have much influence on physical and cognitive dependence and subjective health status Comorbidities and severity of disease have influence on mortality |
|
| ||||
| Fisher et al. [29] | Mortality (short-term) ICU LOS Discharge destination |
ICU discharge Hospital discharge |
Frailty is Not associated with o ICU mortality Hospital mortality Discharge to rehabilitation Has a weak correlation with increased hospital LOS |
Frailty is associated with patient age and comorbidities Frailty may only predict increased hospital LOS |
|
| ||||
| Hope et al. [60] | Mortality (short-term) Mortality (long-term) |
Hospital discharge At 3 years after discharge |
Pre-ICU health categories Frailty present in 34.0% Patients with pre-ICU frailty (compared to same pre-ICU health categories without frailty): Higher hospital mortality Higher 3-year mortality |
The pre-ICU frailty level may be important for understanding risk of death during and after ICU treatment |
|
| ||||
| Lopez Cuenca et al. [47] | Mortality (short-term) Mortality (long-term) |
Hospital discharge 1 month 6 months after discharge |
Frailty prevalence: 34% in study population Frailty Significant association with short- and long-term mortality |
Frailty is associated with increased ICU mortality and increased 6-month mortality The CFS predicts outcomes more effectively than commonly used ICU illness scores |
|
| ||||
| Mattison et al. [58] | Mortality (short-term) Mortality (long-term) |
90 days after discharge | Increased functional dependency (ADL-L) before ICU admission Independently associated with increased 90-day mortality |
Impaired functional status in elderly nursing home residents surviving an ICU hospitalization is independently associated with increased 90-day mortality |
|
| ||||
| Mayer-Oakes et al. [57] | Mortality (short-term) Mortality (long-term) |
Hospital discharge 6 months after discharge |
Limited functional status pre-hospitalization Present in 42% of included patients Leads to 6× higher mortality (hospital mortality and 6-month mortality) in patients ≥75 years compared to the reference group (50–64 years without functional limitations) |
Functional status is an important predictor of outcome in older MICU patients |
|
| ||||
| Montuclard et al. [20] | Mortality (short-term) Functional status Katz's ADL 6 Nottingham Health Profile (subjective health status) HRQOL (modified Patrick's perceived QOL score) |
First week of September 1996 and 1998 |
After ICU stay of patients ≥70 years ICU survival rate 67% Hospital survival rate 47% Independence significantly reduced QOL remained good |
After ICU stay in elderly patients: survival is reasonable, independence is reduced, and QOL remained good |
|
| ||||
| Pietilàinen et al. [61] | Mortality (short-term) Mortality (long-term) Functional status (ADLs and ability to climb stairs) |
At hospital discharge 1 year after discharge |
Premorbid functional status Was poor for 43.3% of the patients Poor PFS predicted an increased risk of in-hospital and 1-year mortality In 78% of ICU survivors at 1 year functional status comparable to premorbid state |
Mortality increases with worse premorbid functional status Knowledge of pre-ICU functional status improved the prediction of 1-year mortality |
|
| ||||
| Roch et al. [59] | Mortality (short-term) Mortality (long-term) Functional status HRQOL (SF-36) at 2 years |
2-year follow-up, all patients assessed at the same time 2.5 years after last inclusion (June 2009) | Factors independently associated with high hospital and 2-year mortality Existence of fatal disease according to McCabe score HRQOL according to SF-g36 was poor in long-term survivors Not independently associated with hospital mortality and mortality at 2 years Functional status evaluated by Knaus classification or the Karnofsky index |
Severity of illness and comorbidities are associated with mortality Functional status is not associated with mortality |
|
| ||||
| Tripathy et al. [27] | Mortality (short-term) Mortality (long-term) Functional status (Katz ADLs) |
28 days 3, 6 and, 12 months after ICU discharge |
Functional (Katz ADL) status prior to acute illness Is one of the risk factors for short-term mortality No significant association with long-term survival 72% of ICU survivors have favourable functional status |
Pre-ICU functional impairment is associated with short-term mortality ICU survivors had a good functional outcome |
RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; PQOL, Perceived Quality of Life Scale; LOS, length of stay; SOFA, Sequential Organ Failure Assessment; LST, life-sustaining therapy; PPS, Palliative Performance Scale; MRC-SS, Medical Research Council, Manual Muscle Test Sum Score; GDS, Geriatric Depression Scale; MMS, Mini-Mental State; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly.
Five studies (n = 5, 8.6%) used the FI [30, 31, 33, 38, 48, 62], usually by classifying patients as robust with an FI <0.2 or 0.25, pre-frail with an FI between 0.2 or 0.25 and 0.4, and frail with an FI >0.4. The method of obtaining the necessary information to construct the index was however not consistent: Zeng et al. [31] used information extracted from patients' existing charts and documents, Heyland et al. [34] conducted the comprehensive geriatric assessment questionnaire in-person with a family member, Kizilarslanoglu et al. [38] conducted a geriatric assessment, evaluating the presence or absence of 40 predefined deficits. Zampieri et al. [62] used a modified and shortened version of the original index. Darvall et al. [48] aimed to modify the existing FI to acute care.
Another six studies (n = 6, 10.3%) evaluated frailty according to the FFP [25, 26, 29, 33, 39, 55], grading patients as robust (score of 0), intermediate-frail (score 1–2), and frail (score ≥3). Some studies used less frequently described instruments to measure frailty: Fisher et al. [29] used the Dalhousie Frailty Scale (DCFS) and Darvall the Edmonton Frailty Scale (EFS) in two studies [44, 48] and Lopez Cuenca et al. [47], the Morley Frailty Scale [73].
A few studies (n = 4, 6.9%) worked with more than one scale. Dolera-Moreno et al. [33] compared three different frailty scales (FI, FP, and CFS) in order to construct and validate a new mortality risk score; Hope et al. [37] used two scales (FP and FI) to examine the validity of frailty markers in critically ill adults. Le Maguet et al. [26] and Tipping [55] used the CFS and the FFP, and Darvall et al. [44, 48] worked with a combination of the CFS and EFS in two studies. The latter [48] examined the correlation between this newly constructed and existing frailty tools.
Twenty-two (n = 22, 37.9%) studies assessed functional status as a surrogate for frailty − using scales assessing the patient's ability to perform activities of daily living (ADL) and/or instrumental ADL. Twelve (n = 12, 20.6) studies exclusively used this approach [2, 18, 19, 20, 21, 22, 23, 27, 58, 59, 60, 61], without using any additional frailty score. Ten studies [10, 30, 33, 34, 37, 39, 46, 47, 52, 56] only assessed functional status for follow-up, after assessing frailty at the time of hospitalization.
In 7 studies, the Katz et al. [74] index was used for this purpose [10, 19, 20, 23, 27, 46, 52] but two of these studies employed a modified version [10, 46]. Five studies [19, 21, 22, 23, 47] used the Lawton-Brody instrumental activity of daily living (IADL) scale [75], and 4 of these [19, 22, 23, 47] also used the Katz index. Two studies use the Karnofsky [76] status [2, 59]. Ten (n = 10, 17.2%) studies created their own functional status [18, 27, 33, 37, 39, 56, 57, 58, 60, 61]. Three studies (n = 3, 5.3%) used the Short Form (SF)-36 [77] in addition to frailty assessment instruments [30, 34, 59]. Two studies (n = 2, 3.5%) used the “Palliative Performance Scale (PPS),” but mainly for follow-up [34, 60]
The Karnofsky Performance Status was also used by Andersen et al. [2] as a criterion for enrolment as well as for the outcome assessment. For this purpose, Boumendil et al. [22] used the Knauss classification [78] as based on physiological parameters.
Some studies included cognition in their functional assessment, by using the Short Portable Mental Status Questionnaire (SPMSQ) [21] or Folstein's MMS [19], the cognitive score as component of Lawton-Brody IADL scale [23], the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) [10, 34, 46], the Clinical dementia rating scale (CDR) [47], or the Cognitive Performance Scale (CPS) [58]. Two studies added the nutritional status [27, 47].
Eight studies (17%) established a frailty diagnosis based on their own criteria [10, 21, 33, 45, 59, 60, 61, 79] − 4 of them exclusively [21, 60, 61, 79] − but the others in combination with established scores. The most common criterion was a combination of decreased cognitive function and functional status and disability in daily life, with the exception of Ball et al. [79] who only referred to physiological parameters. Another approach to establish a frailty diagnosis on the ICU was to define “frailty” by the presence of pre-existing diseases: the Charlson comorbidity index [80] was used in 3 studies [23, 81, 82] as a diagnostic criterion and the Elixhauser Comorbidity Score [83] by another study [84]. In addition, the MacCabe classification for life expectancy [85] served to define frailty in 3 studies [20, 22, 59].
Timing of Frailty Assessment
Table 2 indicates the timing of pre-ICU frailty assessment. The exact time point and the method of obtaining baseline information for pre-ICU frailty varied widely. In the majority of studies, this baseline level was obtained by questioning the patient and/or his relatives [2, 18, 19, 21, 23, 24, 25, 26, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 41, 42, 44, 46, 47, 48, 50, 51, 55, 59, 62, 63, 66, 68, 69, 71, 86]. Some of these studies state precisely that this baseline refers to a time period of 2 months [48, 49, 63, 65], 1 month [18, 19, 26, 31, 55], 2 weeks [21, 25], or 1 day [50] before hospital admission. Others define a time period of “a few days before hospitalization” [51], “directly” [24, 28, 32] or “just before hospitalization” [59]. The remaining studies do not give the exact timing.
In some studies, the pre-ICU frailty assessment was retrospectively assessed as based on data which were routinely documented for other purposes and not specifically collected for frailty measurement [56, 57, 58, 60, 61, 64, 67, 70, 72]. In these studies, the pre-ICU frailty assessment had either been reconstructed from the staff notes from the clinic where the patients were hospitalized [57, 64, 70] or was based on external datasets containing medical records of inpatients and outpatients, skilled nursing facilities, home health agencies, nursing homes, and permanent medical equipment [58, 60, 67, 72], In one study [61], pre-ICU frailty status was adopted from a national registry, and in two cases [54, 56], it was extracted from another study. In the remaining studies, a pre-ICU frailty or functional performance assessment was carried out at the unit where the patient was hospitalized previous to ICU admission, but without specifying exactly the method at the time of triage [2], time of inclusion [22, 42], or at time of ICU admission [33, 39, 45].
Frailty and Outcome in the Critically Ill
The impact of frailty on short- and long-term mortality, post-ICU physical status, ICU, cognition and health-related quality of life, post-ICU health service use, and health care dependency is shown in Table 3 and summarized in the online supplementary results and Tables.
Discussion
Frailty assessment on the ICU is still heterogeneous with respect to assessment methods, cut-offs, and exact time point of baseline assessment. The current mostly used and validated tool for critically ill patients is the CFS. The current literature indicates that frailty assessment is of prognostic value [5].
As there are increasing possibilities for the treatment of elderly and multi-morbid patients, it would be desirable to make sensible decisions about the health care status of the individual patient, bearing in mind his/her personal beliefs and ethics. Frailty assessment may serve as one amongst several basic tools for individual discussion and informed decision-making in such situations.
Unfortunately, there is currently no unified standard for assessing frailty in the ICU or any defined frailty cut-off, time point, or manner of baseline frailty assessment pre-ICU. Some authors argue that the frailty assessment tools designed for the general patient population may have limited feasibility and reliability for frailty assessment in the ICU setting [87, 88]. It is argued that the critical condition of an acutely ill patient evaluated for ICU admission excludes measurements such as gait speed or grip strength as mandatory, for example, in the FFP [3] and the EFS [89] or simply the lack of ability to retrieve any information from the patient or his relatives in the critical care setting. Therefore, the usefulness of many frailty assessment tools as a diagnostic and decision-making tool in the acute care setting may be hampered [90]. However, a recent prospective large multicentre study [90] conducted on more than 120 ICUs around the world indicated that the CFS is a reliable tool for frailty assessment on the ICU in the acute care setting. The CSF not only had a high inter-assessor reliability in the acute care setting (weighted kappa 0.86) but also showed a higher compliance by health care professionals than other scores (ADL and IQCODE) [90]. In the VIP-2 study by the same group conducted in 1,924 patients, inter-rater reliability for the CFS was also excellent (weighed kappa 0.85) [52]. A further study on 202 frailty assessments in 101 patients also found a good inter-rater variability (weighted kappa 0.74) when the CFS was used, but this study also identified differences in at least one category in almost 50% of the patients [91]. Of available tools, the CFS seems to be the best validated tool for frailty assessment in the critically ill and should be considered as standard.
A further problem of many current assessment practises in the critically ill relates to the fact that they are based on information which needs to be actively collected, most frequently by interviews with patients, families, or caregivers [29, 31, 60]. However, the time pressure under which a decision needs to be taken in the acute setting often does not permit extensive family questioning, as requested, for example, by the FI [92]. Furthermore, frequent after-hours consultations preclude a scoring system which is based on primary care health records and would necessitate contacting the patient's general practitioner. In consequence, the information necessary for frailty evaluation is often not available when a decision on ICU admission must be made. The very recent study by Flaatten et al. [90] impressively showed that CSFs obtained by interviews of the patient's relatives and by hospital chart reviews as the primary source of information were nearly identical, while the CFS obtained by patient interview were worse. A further study aiming to assess inter-rater reliability for the CFS when a retrospective record review was performed instead of patient/relative interviews and showed good reliability when medical charts were used for frailty assessment [63]. Thus, the CFS has been shown to be a promising frailty assessment tool in this regard as well.
A further issue is that many currently available scores are too time consuming to obtain in the acute setting. Attempts − though scarce − have been undertaken to establish so called “acute care frailty factors” [63]. A retrospective cohort study [63] tested a CFS score based on clinical in-hospital records. The investigators assessed the inter-rater reliability of frailty, which was found to be good. Patients were classified as “frail” according to the scoring system based on multivariate analysis considering age, Charlson Comorbidity Score, dependence with ADL, and limitation of medical treatment. However, the results of this scoring system were not validated against any established scale for frailty measurement. The same group proposed a study that helps to develop an ICU-adapted FI and to compare its performance against existing frailty measurement and risk stratification tools [93]. Results of this study are currently pending.
“Acute Care Frailty Factors” Adaptation of Existing Tools − a Potential Way to Move Forward?
In general, factors that are used in construction of frailty scores should be associated with frailty but should not or only in part be associated with the underlying acute disease of the patient [10, 94]. Furthermore, in order to be useful in the acute care setting, information on these factors should be usually available on admission to the emergency department (ED) and be frequently assessed. If they are to be useful in the acute care setting and to be good “acute care frailty factors,” parameters included in construction of an acute care frailty score must both indicate frailty and/or underlying (chronic) disease and/or disease severity and usually be readily available at the ED or at ICU admission.
Laboratory markers are frequently disturbed due to the acute disease. There are some biomarkers that indicate frailty and have been evaluated for this purpose [95]. Most of those laboratory markers such as proADM, copeptin [96], and various cytokines [97], have the disadvantage that they are not routinely measured − which precludes utilization for construction of an acute care frailty score. A subset of these markers might also be useful in the acute care setting [13, 97, 98], if they achieve sufficient specificity, although this still warrants investigation.
Previous investigations have evaluated and validated the use of the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision coding system for frailty risk assessment in hospitalized elderly patients [99, 100]. Evaluation shows that several types of disease which are often known in the ED at ICU admission are associated with frailty and functional impairment. Examples are malignant solid neoplasm [101], haematological malignancies [102], chronic anaemia [103] chronic infections [104, 105, 106, 107], immunodeficiency [104], malnutrition and nutritional deficiency [11, 108, 109] [110], cognitive impairment of any type [109], advanced chronic heart disease [13, 111, 112, 113, 114], advanced chronic pulmonary disease [115, 116], chronic liver disease [117], chronic pancreatitis [118], chronic renal insufficiency [119], and the need for renal replacement therapy [120]. Furthermore, pressure ulcers grade 3 and 4 [121], musculoskeletal diseases such as osteoarthritis [122], rheumatoid arthritis [123, 124], fibromyalgia [125], sarcopenia [126], osteoporosis [127], impairment of sensory organs (blindness/deafness) [128], and being organ-transplanted (solid/stem cells) [128, 129] are also associated with frailty.
Taken together, current evidence on frailty factors identifies a considerable body of available potential frailty factors that could be included in an acute care frailty score. In addition, further acute care specific factors such as the type of admission (surgical or medical), location of residence before acute disease (private home or retirement home and extended care facility), and the type category of acute disease (sepsis, cardiovascular, neurological, etc.) might imply prolonged recovery, worse long-term functional outcome, or death and hence could be of value in construction of an acute care frailty score. However, their value must first be evaluated. For this purpose, big databases and health care registries could be a major source of help for identifying factors relevant to acute, care via modern data scientific evaluation techniques and for construction of a preliminary acute care frailty score. Evaluation of these factors in regards to frailty is highly warranted, and the development of a “frail phenotype” for triage/extended care decisions on the ICU would be of major importance.
Limitations
This systematic review of the current literature on frailty has several important limitations. Firstly, we did not meta-analyze data due to the high heterogeneity of available studies. Secondly, all of the trials included in this review are of observational nature, thus confounding factors such as disease severity may have influenced reported outcomes. Furthermore, the time point of assessments varies widely between studies, as well as the retrievable information. Moreover, there has been a surge of studies on frailty in the past 3 years, with various aims and assessment methods. A further limitation to this review is that due to the global COVID-19 pandemic many ICUs experienced considerable limitations in available resources and the utility of frailty as a triage tool may have been hampered due to the “new disease COVID-19.” However, a recently published large multicentre study revealed that frailty assessment by the CFS is also reliable for patients with COVID-19 [5]. For many studies, no stratification has been performed on how frailty was assessed or the cut-off value used for the CFS across studies. This hampers all the qualitative conclusions that these authors have drawn. For instance, combination of studies using functional status and those with the Fried's phenotypes contradicts the basic assumption of these studies. Furthermore, the association between “frailty” and mortality or functional impairment (or dependency) in each study must be viewed critically as some of these studies are self-fulfilling prophecies − as frailty was sometimes a reason for withdrawal of life-sustaining therapies.
Conclusion
In recent years, an increasing number of publications have assessed frailty in the ICU. Frailty assessment in the ICU is still heterogeneous with respect to assessment methods, cut-offs, and exact time points of baseline assessment. Although a variety of approaches have been suggested, the CFS may currently be considered the most reliable approach in ICU patients. As frailty prior to critical illness has a negative impact on several short- and long-term clinical outcomes, it is important that assessments are harmonized and performed routinely in the critically ill. Frailty levels should be integrated into the individual treatment plans. Further research should focus on standardizing frailty assessment and its adaptation to the acute care setting.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Funding Sources
No funding.
Author Contributions
D.B. and M.S. performed the literature search and selected eligible trials. D.B. and M.S. carried out the data extraction on all trials selected for the quantitative analysis, and J.W. and C.A.P. revised the data. D.B. and C.A.P. performed the risk of bias assessment, and J.W. revised it. D.B., C.D., and C.A.P. drafted the manuscript, with all other authors co-drafting and revising the manuscript for important intellectual content. All the authors approved the final version of the manuscript and agreed to submission.
Data Availability Statement
The datasets used and/or analyzed during the current study can be made available from the corresponding author on reasonable non-commercial request.
Supplementary Material
Supplementary data
Funding Statement
No funding.
References
- 1.Ihra GC, Lehberger J, Hochrieser H, Bauer P, Schmutz R, Metnitz B, et al. Development of demographics and outcome of very old critically ill patients admitted to intensive care units. Intensive Care Med. 2012 Apr;38((4)):620–626. doi: 10.1007/s00134-012-2474-7. [DOI] [PubMed] [Google Scholar]
- 2.Andersen FH, Flaatten H, Klepstad P, Follestad T, Strand K, Kruger AJ, et al. Long-term outcomes after ICU admission triage in octogenarians. Crit Care Med. 2017 Apr;45((4)):e363–71. doi: 10.1097/CCM.0000000000002098. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56((3)):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4.Haas LEM, Boumendil A, Flaatten H, Guidet B, Ibarz M, Jung C, et al. Frailty is associated with long-term outcome in patients with sepsis who are over 80 years old: results from an observational study in 241 European ICUs. Age Ageing. 2021;50((5)):1719–1727. doi: 10.1093/ageing/afab036. [DOI] [PubMed] [Google Scholar]
- 5.Jung C, Flaatten H, Fjølner J, Bruno RR, Wernly B, Artigas A, et al. The impact of frailty on survival in elderly intensive care patients with COVID-19: the COVIP study. Crit Care. 2021 Apr 19;25((1)):149. doi: 10.1186/s13054-021-03551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee L, Patel T, Hillier LM, Maulkhan N, Slonim K, Costa A. Identifying frailty in primary care: a systematic review. Geriatr Gerontol Int. 2017 Oct;17((10)):1358–1377. doi: 10.1111/ggi.12955. [DOI] [PubMed] [Google Scholar]
- 7.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008 Sep 30;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leblanc G, Boumendil A, Guidet B. Ten things to know about critically ill elderly patients. Intensive Care Med. 2017 Feb;43((2)):217–219. doi: 10.1007/s00134-016-4477-2. [DOI] [PubMed] [Google Scholar]
- 9.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005 Aug 30;173((5)):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jankowski K, Bryden DC. Using a CriSTAL scoring system to identify pre-morbid conditions associated with a poor outcome after admission to intensive care in people 70 years or older. J Intensive Care Soc. 2019 Aug;20((3)):231–236. doi: 10.1177/1751143718804678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei K, Nyunt MS, Gao Q, Wee SL, Yap KB, Ng TP. Association of frailty and malnutrition with long-term functional and mortality outcomes among community-dwelling older adults: results from the Singapore Longitudinal Aging Study 1. JAMA Netw Open. 2018 Jul 6;1((3)):e180650. doi: 10.1001/jamanetworkopen.2018.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagshaw SM, Muscedere J. Is this intensive care unit patient frail? Unraveling the complex interplay between frailty and critical illness. Am J Respir Crit Care Med. 2017 Jul 1;196((1)):4–5. doi: 10.1164/rccm.201612-2538ED. [DOI] [PubMed] [Google Scholar]
- 13.Blodgett JM, Theou O, Howlett SE, Rockwood K. A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. GeroScience. 2017 Aug;39((4)):447–455. doi: 10.1007/s11357-017-9993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchman AS, Wilson RS, Bienias JL, Bennett DA. Change in frailty and risk of death in older persons. Exp Aging Res. 2009 Jan–Mar;35((1)):61–82. doi: 10.1080/03610730802545051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019 Oct 3;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009 Jul 21;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartling L, Milne A, Hamm MP, Vandermeer B, Ansari M, Tsertsvadze A, et al. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J Clin Epidemiol. 2013 Sep;66((9)):982–993. doi: 10.1016/j.jclinepi.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Chelluri L, Pinsky MR, Donahoe MP, Grenvik A. Long-term outcome of critically ill elderly patients requiring intensive care. JAMA. 1993 Jun 23–30;269((24)):3119–3123. [PubMed] [Google Scholar]
- 19.Broslawski GE, Elkins M, Algus M. Functional abilities of elderly survivors of intensive care. J Am Osteopath Assoc. 1995 Dec;95((12)):712–717. [PubMed] [Google Scholar]
- 20.Montuclard L, Garrouste-Orgeas M, Timsit JF, Misset B, De Jonghe B, Carlet J. Outcome, functional autonomy, and quality of life of elderly patients with a long-term intensive care unit stay. Crit Care Med. 2000 Oct;28((10)):3389–3395. doi: 10.1097/00003246-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Bo M, Massaia M, Raspo S, Bosco F, Cena P, Molaschi M, et al. Predictive factors of in-hospital mortality in older patients admitted to a medical intensive care unit. J Am Geriatr Soc. 2003 Apr;51((4)):529–533. doi: 10.1046/j.1532-5415.2003.51163.x. [DOI] [PubMed] [Google Scholar]
- 22.Boumendil A, Maury E, Reinhard I, Luquel L, Offenstadt G, Guidet B. Prognosis of patients aged 80 years and over admitted in medical intensive care unit. Intensive Care Med. 2004 Apr;30((4)):647–654. doi: 10.1007/s00134-003-2150-z. [DOI] [PubMed] [Google Scholar]
- 23.Daubin C, Chevalier S, Seguin A, Gaillard C, Valette X, Prevost F, et al. Predictors of mortality and short-term physical and cognitive dependence in critically ill persons 75 years and older: a prospective cohort study. Health Qual Life Outcomes. 2011 May 16;9:35. doi: 10.1186/1477-7525-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagshaw SM, Stelfox HT, McDermid RC, Rolfson DB, Tsuyuki RT, Baig N, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014 Feb 4;186((2)):E95–102. doi: 10.1503/cmaj.130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baldwin MR, Reid MC, Westlake AA, Rowe JW, Granieri EC, Wunsch H, et al. The feasibility of measuring frailty to predict disability and mortality in older medical intensive care unit survivors. J Crit Care. 2014 Jun;29((3)):401–408. doi: 10.1016/j.jcrc.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Maguet P, Roquilly A, Lasocki S, Asehnoune K, Carise E, Saint Martin M, et al. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med. 2014 May;40((5)):674–682. doi: 10.1007/s00134-014-3253-4. [DOI] [PubMed] [Google Scholar]
- 27.Tripathy S, Mishra JC, Dash SC. Critically ill elderly patients in a developing world–mortality and functional outcome at 1 year: a prospective single-center study. J Crit Care. 2014 Jun;29((3474)):e7–13. doi: 10.1016/j.jcrc.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Bagshaw SM, Stelfox HT, Johnson JA, McDermid RC, Rolfson DB, Tsuyuki RT, et al. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med. 2015 May;43((5)):973–982. doi: 10.1097/CCM.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 29.Fisher C, Karalapillai DK, Bailey M, Glassford NG, Bellomo R, Jones D. Predicting intensive care and hospital outcome with the Dalhousie Clinical Frailty Scale: a pilot assessment. Anaesth Intensive Care. 2015 May;43((3)):361–368. doi: 10.1177/0310057X1504300313. [DOI] [PubMed] [Google Scholar]
- 30.Heyland DK, Garland A, Bagshaw SM, Cook D, Rockwood K, Stelfox HT, et al. Recovery after critical illness in patients aged 80 years or older: a multi-center prospective observational cohort study. Intensive Care Med. 2015 Nov;41((11)):1911–1920. doi: 10.1007/s00134-015-4028-2. [DOI] [PubMed] [Google Scholar]
- 31.Zeng A, Song X, Dong J, Mitnitski A, Liu J, Guo Z, et al. Mortality in relation to frailty in patients admitted to a specialized geriatric intensive care unit. J Gerontol A Biol Sci Med Sci. 2015 Dec;70((12)):1586–1594. doi: 10.1093/gerona/glv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagshaw M, Majumdar SR, Rolfson DB, Ibrahim Q, McDermid RC, Stelfox HT. A prospective multicenter cohort study of frailty in younger critically ill patients. Crit Care. 2016 Jun 6;20((1)):175. doi: 10.1186/s13054-016-1338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolera-Moreno C, Palazon-Bru A, Colomina-Climent F, Gil-Guillen VF. Construction and internal validation of a new mortality risk score for patients admitted to the intensive care unit. Int J Clin Pract. 2016 Nov;70((11)):916–922. doi: 10.1111/ijcp.12851. [DOI] [PubMed] [Google Scholar]
- 34.Heyland DK, Stelfox HT, Garland A, Cook D, Dodek P, Kutsogiannis J, et al. Predicting performance status 1 year after critical illness in patients 80 years or older: development of a multivariable clinical prediction model. Crit Care Med. 2016 Sep;44((9)):1718–1726. doi: 10.1097/CCM.0000000000001762. [DOI] [PubMed] [Google Scholar]
- 35.Orsini J, Blaak C, Shamian B, Fonseca X, Salem A, Chen YL. Assessing the utility of ICU admission for octogenarians. Aging Clin Exp Res. 2016 Aug;28((4)):745–751. doi: 10.1007/s40520-015-0462-9. [DOI] [PubMed] [Google Scholar]
- 36.Brummel NE, Bell SP, Girard TD, Pandharipande PP, Jackson JC, Morandi A, et al. Frailty and subsequent disability and mortality among patients with critical illness. Am J Respir Crit Care Med. 2017 Jul 1;196((1)):64–72. doi: 10.1164/rccm.201605-0939OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hope AA, Hsieh SJ, Petti A, Hurtado-Sbordoni M, Verghese J, Gong MN. Assessing the usefulness and validity of frailty markers in critically ill adults. Ann Am Thorac Soc. 2017 Jun;14((6)):952–959. doi: 10.1513/AnnalsATS.201607-538OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kizilarslanoglu MC, Civelek R, Kilic MK, Sumer F, Varan HD, Kara O, et al. Is frailty a prognostic factor for critically ill elderly patients? Aging Clin Exp Res. 2017 Apr;29((2)):247–255. doi: 10.1007/s40520-016-0557-y. [DOI] [PubMed] [Google Scholar]
- 39.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. The association of frailty with post-ICU disability, nursing home admission, and mortality: a Longitudinal Study. Chest. 2018 Jun;153((6)):1378–1386. doi: 10.1016/j.chest.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fronczek J, Polok KJ, Nowak-Kozka I, Wludarczyk A, Gorka J, Czuczwar M, et al. Frailty is associated with an increased mortality among patients ≥80 years old treated in Polish ICUs. Anaesthesiol Intensive Ther. 2018;50((4)):245–251. doi: 10.5603/AIT.a2018.0032. [DOI] [PubMed] [Google Scholar]
- 41.Muessig JM, Nia AM, Masyuk M, Lauten A, Sacher AL, Brenner T, et al. Clinical frailty scale (CFS) reliably stratifies octogenarians in German ICUs: a multicentre prospective cohort study. BMC Geriatr. 2018 Jul 13;18((1)):162. doi: 10.1186/s12877-018-0847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.So RKL, Bannard-Smith J, Subbe CP, Jones DA, van Rosmalen J, Lighthall GK, et al. The association of clinical frailty with outcomes of patients reviewed by rapid response teams: an international prospective observational cohort study. Crit Care. 2018 Sep 22;22((1)):227. doi: 10.1186/s13054-018-2136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruno RR, Wernly B, Flaatten H, Scholzel F, Kelm M, Jung C. The hospital frailty risk score is of limited value in intensive care unit patients. Crit Care. 2019 Jul 2;23((1)):239. doi: 10.1186/s13054-019-2520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darvall JN, Greentree K, Braat MS, Story DA, Lim WK. Contributors to frailty in critical illness: multi-dimensional analysis of the Clinical Frailty Scale. J Crit Care. 2019 Aug;52:193–199. doi: 10.1016/j.jcrc.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 45.de Lange DW, Brinkman S, Flaatten H, Boumendil A, Morandi A, Andersen FH, et al. Cumulative prognostic score predicting mortality in patients older than 80 years admitted to the ICU. J Am Geriatr Soc. 2019 Jun;67((6)):1263–1267. doi: 10.1111/jgs.15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hope AA, Law J, Nair R, Kim M, Verghese J, Gong MN. Frailty, acute organ dysfunction, and increased disability after hospitalization in older adults who survive critical illness: a Prospective Cohort Study. J Intensive Care Med. 2019 Jun;35((12)):1505–1512. doi: 10.1177/0885066619881115. [DOI] [PubMed] [Google Scholar]
- 47.Lopez Cuenca S, Oteiza Lopez L, Lazaro Martin N, Irazabal Jaimes MM, Ibarz Villamayor M, Artigas A, et al. Frailty in patients over 65 years of age admitted to intensive care units (FRAIL-ICU) Med intensiva. 2019 Oct;43((7)):395–401. doi: 10.1016/j.medin.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Darvall JN, Greentree K, Loth J, Bose T, De Silva A, Braat S, et al. Development of a frailty index from routine hospital data in perioperative and critical care. J Am Geriatr Soc. 2020 Dec;68((12)):2831–2838. doi: 10.1111/jgs.16788. [DOI] [PubMed] [Google Scholar]
- 49.De Geer L, Fredrikson M, Tibblin AO. Frailty predicts 30-day mortality in intensive care patients: a prospective prediction study. Eur J Anaesthesiol. 2020 Nov;37((11)):1058–1065. doi: 10.1097/EJA.0000000000001156. [DOI] [PubMed] [Google Scholar]
- 50.Geense W, Zegers M, Dieperink P, Vermeulen H, van der Hoeven J, van den Boogaard M. Changes in frailty among ICU survivors and associated factors: results of a one-year prospective cohort study using the Dutch Clinical Frailty Scale. J Crit Care. 2020 Feb;55:184–193. doi: 10.1016/j.jcrc.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 51.Geense WW, van den Boogaard M, Peters MAA, Simons KS, Ewalds E, Vermeulen H, et al. Physical, mental, and cognitive health status of ICU survivors before ICU admission: a Cohort Study. Crit Care Med. 2020 Sep;48((9)):1271–1279. doi: 10.1097/CCM.0000000000004443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guidet B, de Lange DW, Boumendil A, Leaver S, Watson X, Boulanger C, et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med. 2020 Jan;46((1)):57–69. doi: 10.1007/s00134-019-05853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ibarz M, Boumendil A, Haas LEM, Irazabal M, Flaatten H, de Lange DW, et al. Sepsis at ICU admission does not decrease 30-day survival in very old patients: a post-hoc analysis of the VIP1 multinational cohort study. Ann Intensive Care. 2020 May 13;10((1)):56. doi: 10.1186/s13613-020-00672-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Komori A, Abe T, Yamakawa K, Ogura H, Kushimoto S, Saitoh D, et al. Characteristics and outcomes of frail patients with suspected infection in intensive care units: a descriptive analysis from a multicenter cohort study. BMC Geriatr. 2020 Nov 20;20((1)):485. doi: 10.1186/s12877-020-01893-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tipping CJ, Bilish E, Harrold M, Holland AE, Chan T, Hodgson CL. The impact of frailty in critically ill patients after trauma: a prospective observational study. Aust Crit Care. 2020 May;33((3)):228–235. doi: 10.1016/j.aucc.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Wernly B, Bruno RR, Kelm M, Boumendil A, Morandi A, Andersen FH, et al. Sex-specific outcome disparities in very old patients admitted to intensive care medicine: a propensity matched analysis. Sci Rep. 2020 Oct 29;10((1)):18671. doi: 10.1038/s41598-020-74910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayer-Oakes SA, Oye RK, Leake B. Predictors of mortality in older patients following medical intensive care: the importance of functional status. J Am Geriatr Soc. 1991 Sep;39((9)):862–868. doi: 10.1111/j.1532-5415.1991.tb04452.x. [DOI] [PubMed] [Google Scholar]
- 58.Mattison ML, Rudolph JL, Kiely DK, Marcantonio ER. Nursing home patients in the intensive care unit: risk factors for mortality. Crit Care Med. 2006 Oct;34((10)):2583–2587. doi: 10.1097/01.CCM.0000239112.49567.BD. [DOI] [PubMed] [Google Scholar]
- 59.Roch A, Wiramus S, Pauly V, Forel JM, Guervilly C, Gainnier M, et al. Long-term outcome in medical patients aged 80 or over following admission to an intensive care unit. Crit Care. 2011;15((1)):R36. doi: 10.1186/cc9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hope AA, Gong MN, Guerra C, Wunsch H. Frailty before critical illness and mortality for elderly medicare beneficiaries. J Am Geriatr Soc. 2015 Jun;63((6)):1121–1128. doi: 10.1111/jgs.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pietilainen L, Hastbacka J, Backlund M, Parviainen I, Pettila V, Reinikainen M. Premorbid functional status as a predictor of 1-year mortality and functional status in intensive care patients aged 80 years or older. Intensive Care Med. 2018 Aug;44((8)):1221–1229. doi: 10.1007/s00134-018-5273-y. [DOI] [PubMed] [Google Scholar]
- 62.Zampieri FG, Iwashyna TJ, Viglianti EM, Taniguchi LU, Viana WN, Costa R, et al. Association of frailty with short-term outcomes, organ support and resource use in critically ill patients. Intensive Care Med. 2018 Sep;44((9)):1512–1520. doi: 10.1007/s00134-018-5342-2. [DOI] [PubMed] [Google Scholar]
- 63.Darvall JN, Boonstra T, Norman J, Murphy D, Bailey M, Iwashyna TJ, et al. Retrospective frailty determination in critical illness from a review of the intensive care unit clinical record. Anaesth Intensive Care. 2019 Jul;47((4)):343–348. doi: 10.1177/0310057X19856895. [DOI] [PubMed] [Google Scholar]
- 64.Fernando SM, McIsaac DI, Rochwerg B, Bagshaw SM, Muscedere J, Munshi L, et al. Frailty and invasive mechanical ventilation: association with outcomes, extubation failure, and tracheostomy. Intensive Care Med. 2019 Dec;45((12)):1742–1752. doi: 10.1007/s00134-019-05795-8. [DOI] [PubMed] [Google Scholar]
- 65.Darvall JN, Bellomo R, Bailey M, Paul E, Young PJ, Rockwood K, et al. Frailty and outcomes from pneumonia in critical illness: a population-based cohort study. Br J Anaesth. 2020 Nov;125((5)):730–738. doi: 10.1016/j.bja.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hewitt D, Booth MG. The FRAIL-FIT study: frailtyʼs relationship with adverse-event incidence in the longer term, at one year following intensive care unit treatment − a retrospective observational cohort study. J Intensive Care Soc. 2020 May;21((2)):124–133. doi: 10.1177/1751143719838212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kokoszka-Bargiel I, Cyprys P, Rutkowska K, Madowicz J, Knapik P. Intensive care unit admissions during the first 3 months of the covid-19 pandemic in Poland: a Single-Center, Cross-Sectional Study. Med Sci Monit. 2020 Sep 26;26:e926974. doi: 10.12659/MSM.926974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hewitt D, Ratcliffe M, Booth MG. The FRAIL-FIT 30 Study − factors influencing 30-day mortality in frail patients admitted to ICU: a retrospective observational cohort study. J Intensive Care Soc. 2021:1751143720985164. doi: 10.1177/1751143720985164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bruno RR, Wernly B, Beil M, Muessig JM, Rahmel T, Graf T, et al. Therapy limitation in octogenarians in German intensive care units is associated with a longer length of stay and increased 30 days mortality: a prospective multicenter study. J Crit Care. 2020 Dec;60:58–63. doi: 10.1016/j.jcrc.2020.07.024. [DOI] [PubMed] [Google Scholar]
- 70.Pasin L, Boraso S, Golino G, Fakhr BS, Tiberio I, Trevisan C. The impact of frailty on mortality in older patients admitted to an intensive care unit. Med Intensiva. 2020 Jul 9;S0210–5691:30191–1. doi: 10.1016/j.medin.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 71.Sanchez D, Brennan K, Al Sayfe M, Shunker SA, Bogdanoski T, Hedges S, et al. Frailty, delirium and hospital mortality of older adults admitted to intensive care: the Delirium (Deli) in ICU study. Crit Care. 2020 Oct 15;24((1)):609. doi: 10.1186/s13054-020-03318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silva-Obregon JA, Quintana-Diaz M, Saboya-Sanchez S, Marian-Crespo C, Romera-Ortega MA, Chamorro-Jambrina C, et al. Frailty as a predictor of short- and long-term mortality in critically ill older medical patients. J Crit Care. 2020 Feb;55:79–85. doi: 10.1016/j.jcrc.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 73.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012 Jul;16((7)):601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of Adl: a standardized measure of biological and psychosocial function. JAMA. 1963 Sep 21;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 75.Gold DA. An examination of instrumental activities of daily living assessment in older adults and mild cognitive impairment. J Clin Exp Neuropsychol. 2012;34((1)):11–34. doi: 10.1080/13803395.2011.614598. [DOI] [PubMed] [Google Scholar]
- 76.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984 Mar;2((3)):187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 77.Reed PJ, Moore DD. SF-36 as a predictor of health states. Value Health. 2000 May–Jun;3((3)):202–207. doi: 10.1046/j.1524-4733.2000.33005.x. [DOI] [PubMed] [Google Scholar]
- 78.Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981 Aug;9((8)):591–597. doi: 10.1097/00003246-198108000-00008. [DOI] [PubMed] [Google Scholar]
- 79.Ball IM, Bagshaw SM, Burns KE, Cook DJ, Day AG, Dodek PM, et al. A clinical prediction tool for hospital mortality in critically ill elderly patients. J Crit Care. 2016 Oct;35:206–212. doi: 10.1016/j.jcrc.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 80.DʼHoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med. 1993 Nov;32((5)):382–387. [PubMed] [Google Scholar]
- 81.Poses RM, McClish DK, Smith WR, Bekes C, Scott WE. Prediction of survival of critically ill patients by admission comorbidity. J Clin Epidemiol. 1996 Jul;49((7)):743–747. doi: 10.1016/0895-4356(96)00021-2. [DOI] [PubMed] [Google Scholar]
- 82.Barnato AE, Albert SM, Angus DC, Lave JR, Degenholtz HB. Disability among elderly survivors of mechanical ventilation. Am J Respir Crit Care Med. 2011 Apr 15;183((8)):1037–1042. doi: 10.1164/rccm.201002-0301OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998 Jan;36((1)):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 84.Fernando SM, Reardon PM, McIsaac DI, Eagles D, Murphy K, Tanuseputro P, et al. Outcomes of older hospitalized patients requiring rapid response team activation for acute deterioration. Crit Care Med. 2018 Dec;46((12)):1953–1960. doi: 10.1097/CCM.0000000000003442. [DOI] [PubMed] [Google Scholar]
- 85.Reilly JS, Coignard B, Price L, Godwin J, Cairns S, Hopkins S, et al. The reliability of the McCabe score as a marker of co-morbidity in healthcare-associated infection point prevalence studies. J Inf Prev. 2016 May;17((3)):127–129. doi: 10.1177/1757177415617245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Geer L, Fredrikson M, Tibblin AO. Frailty predicts 30-day mortality in intensive care patients: a prospective prediction study. Eur J Anaesthesiol. 2020;37((11)):1058–1065. doi: 10.1097/EJA.0000000000001156. [DOI] [PubMed] [Google Scholar]
- 87.Pugh RJ, Ellison A, Pye K, Subbe CP, Thorpe CM, Lone NI, et al. Feasibility and reliability of frailty assessment in the critically ill: a systematic review. Crit Care. 2018 Feb 26;22((1)):49. doi: 10.1186/s13054-018-1953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Falvey JR, Ferrante LE. Frailty assessment in the ICU: translation to “real-worldˮ clinical practice. Anaesthesia. 2019 Jun;74((6)):700–703. doi: 10.1111/anae.14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006 Sep;35((5)):526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Flaatten H, Guidet B, Andersen FH, Artigas A, Cecconi M, Boumendil A, et al. Reliability of the Clinical Frailty Scale in very elderly ICU patients: a prospective European study. Ann Intensive Care. 2021 Feb 3;11((1)):22. doi: 10.1186/s13613-021-00815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pugh RJ, Battle CE, Thorpe C, Lynch C, Williams JP, Campbell A, et al. Reliability of frailty assessment in the critically ill: a multicentre prospective observational study. Anaesthesia. 2019 Jun;74((6)):758–764. doi: 10.1111/anae.14596. [DOI] [PubMed] [Google Scholar]
- 92.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001 Aug 8;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Darvall JN, Braat S, Story DA, Greentree K, Bose T, Loth J, et al. Protocol for a prospective observational study to develop a frailty index for use in perioperative and critical care. BMJ Open. 2019 Jan 9;9((1)):e024682. doi: 10.1136/bmjopen-2018-024682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ranzani OT, Besen B, Herridge MS. Focus on the frail and elderly: who should have a trial of ICU treatment? Intensive Care Med. 2020 May;46((5)):1030–1032. doi: 10.1007/s00134-020-05963-1. [DOI] [PubMed] [Google Scholar]
- 95.Saedi AA, Feehan J, Phu S, Duque G. Current and emerging biomarkers of frailty in the elderly. Clin interv Aging. 2019;14:389–398. doi: 10.2147/CIA.S168687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schuetz P, Hausfater P, Amin D, Amin A, Haubitz S, Faessler L, et al. Biomarkers from distinct biological pathways improve early risk stratification in medical emergency patients: the multinational, prospective, observational TRIAGE study. Crit Care. 2015 Oct 29;19:377. doi: 10.1186/s13054-015-1098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blodgett JM, Theou O, Howlett SE, Wu FC, Rockwood K. A frailty index based on laboratory deficits in community-dwelling men predicted their risk of adverse health outcomes. Age Ageing. 2016 Jul;45((4)):463–468. doi: 10.1093/ageing/afw054. [DOI] [PubMed] [Google Scholar]
- 98.Ellis HL, Wan B, Yeung M, Rather A, Mannan I, Bond C, et al. Complementing chronic frailty assessment at hospital admission with an electronic frailty index (FI-Laboratory) comprising routine blood test results. CMAJ. 2020 Jan 6;192((1)):E3–8. doi: 10.1503/cmaj.190952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018 May 5;391((10132)):1775–1782. doi: 10.1016/S0140-6736(18)30668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eckart A, Hauser SI, Haubitz S, Struja T, Kutz A, Koch D, et al. Validation of the hospital frailty risk score in a tertiary care hospital in Switzerland: results of a prospective, observational study. BMJ Open. 2019 Jan 15;9((1)):e026923. doi: 10.1136/bmjopen-2018-026923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McCarthy AL, Peel NM, Gillespie KM, Berry R, Walpole E, Yates P, et al. Validation of a frailty index in older cancer patients with solid tumours. BMC Cancer. 2018 Sep 14;18((1)):892. doi: 10.1186/s12885-018-4807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Atakul E, Akyar I. Frailty prevalence and characteristics in older adults with hematologic cancer: a Descriptive Study. Asia Pac J Oncol Nurs. 2019 Jan–Mar;6((1)):43–9. doi: 10.4103/apjon.apjon_35_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zulfiqar AA, Sui Seng X, Gillibert A, Kadri N, Doucet J. Anemia and frailty in the elderly hospitalized in an acute unit: preliminary results. Euro J Intern Med. 2017 Mar;38:e8–9. doi: 10.1016/j.ejim.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 104.Li H, Manwani B, Leng SX. Frailty, inflammation, and immunity. Aging Dis. 2011 Dec;2((6)):466–473. [PMC free article] [PubMed] [Google Scholar]
- 105.Jang ES, Kim YS, Kim KA, Lee YJ, Chung WJ, Kim IH, et al. Factors associated with health-related quality of life in Korean patients with chronic hepatitis C infection using the SF-36 and EQ-5D. Gut Liver. 2018 Jul 15;12((4)):440–448. doi: 10.5009/gnl17322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.OʼBrien KK, Hanna S, Solomon P, Worthington C, Ibanez-Carrasco F, Chan Carusone S, et al. Characterizing the disability experience among adults living with HIV: a structural equation model using the HIV disability questionnaire (HDQ) within the HIV, health and rehabilitation survey. BMC Inf Dis. 2019 Jul 8;19((1)):594. doi: 10.1186/s12879-019-4203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Si J, Yu C, Guo Y, Bian Z, Meng R, Yang L, et al. Chronic hepatitis B virus infection and total and cause-specific mortality: a prospective cohort study of 0.5 million people. BMJ Open. 2019 Apr 9;9((4)):e027696. doi: 10.1136/bmjopen-2018-027696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clegg A, Hassan-Smith Z. Frailty and the endocrine system. Lancet Diabetes Endocrinol. 2018 Sep;6((9)):743–752. doi: 10.1016/S2213-8587(18)30110-4. [DOI] [PubMed] [Google Scholar]
- 109.Picca A, Coelho-Junior HJ, Cesari M, Marini F, Miccheli A, Gervasoni J, et al. The metabolomics side of frailty: toward personalized medicine for the aged. Exp Gerontol. 2019 Oct 15;126:110692. doi: 10.1016/j.exger.2019.110692. [DOI] [PubMed] [Google Scholar]
- 110.Ju SY, Lee JY, Kim DH. Low 25-hydroxyvitamin D levels and the risk of frailty syndrome: a systematic review and dose-response meta-analysis. BMC Geriatr. 2018 Sep 4;18((1)):206. doi: 10.1186/s12877-018-0904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001 Mar;56((3)):M158–66. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 112.Chang YW, Chen WL, Lin FG, Fang WH, Yen MY, Hsieh CC, et al. Frailty and its impact on health-related quality of life: a cross-sectional study on elder community-dwelling preventive health service users. PloS One. 2012;7((5)):e38079. doi: 10.1371/journal.pone.0038079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cooper LB, Hammill BG, Allen LA, Lindenfeld J, Mentz RJ, Rogers JG, et al. Assessing frailty in patients undergoing destination therapy left ventricular assist device: observations from interagency registry for mechanically assisted circulatory support. ASAIO J. 2018 Jan/Feb;64((1)):16–23. doi: 10.1097/MAT.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 114.Kleipool EE, Hoogendijk EO, Trappenburg MC, Handoko ML, Huisman M, Peters MJ, et al. Frailty in older adults with cardiovascular disease: cause, effect or both? Aging Dis. 2018 Jun;9((3)):489–497. doi: 10.14336/AD.2017.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bone AE, Hepgul N, Kon S, Maddocks M. Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis. 2017 Feb;14((1)):85–99. doi: 10.1177/1479972316679664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kennedy CC, Novotny PJ, LeBrasseur NK, Wise RA, Sciurba FC, Benzo RP. Frailty and clinical outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2019 Feb;16((2)):217–224. doi: 10.1513/AnnalsATS.201803-175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang CW, Lebsack A, Chau S, Lai JC. The range and reproducibility of the liver frailty index. Liver Transplant. 2019 Jun;25((6)):841–847. doi: 10.1002/lt.25449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Machicado JD, Amann ST, Anderson MA, Abberbock J, Sherman S, Conwell DL, et al. Quality of life in chronic pancreatitis is determined by constant pain, disability/unemployment, current smoking, and associated co-morbidities. Am J Gastroenterol. 2017 Apr;112((4)):633–642. doi: 10.1038/ajg.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nixon AC, Bampouras TM, Pendleton N, Woywodt A, Mitra S, Dhaygude A. Frailty and chronic kidney disease: current evidence and continuing uncertainties. Clin Kidney J. 2018 Apr;11((2)):236–245. doi: 10.1093/ckj/sfx134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nitta K, Hanafusa N, Tsuchiya K. Role of frailty on outcomes of dialysis patients. Contrib Nephrol. 2018;195:102–109. doi: 10.1159/000486940. [DOI] [PubMed] [Google Scholar]
- 121.Landi F, Onder G, Russo A, Bernabei R. Pressure ulcer and mortality in frail elderly people living in community. Arch Gerontol Geriatr. 2007;44(Suppl 1):217–223. doi: 10.1016/j.archger.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 122.Castell MV, van der Pas S, Otero A, Siviero P, Dennison E, Denkinger M, et al. Osteoarthritis and frailty in elderly individuals across six European countries: results from the European Project on OSteoArthritis (EPOSA) BMC Musculoskelet Disord. 2015 Nov 17;16:359. doi: 10.1186/s12891-015-0807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kapetanovic MC, Lindqvist E, Nilsson JA, Geborek P, Saxne T, Eberhardt K. Development of functional impairment and disability in rheumatoid arthritis patients followed for 20 years: relation to disease activity, joint damage, and comorbidity. Arthritis Care Res. 2015 Mar;67((3)):340–348. doi: 10.1002/acr.22458. [DOI] [PubMed] [Google Scholar]
- 124.Haider S, Grabovac I, Berner C, Lamprecht T, Fenzl KH, Erlacher L, et al. Frailty in seropositive rheumatoid arthritis patients of working age: a cross-sectional study. Clin Exp Rheumatol. 2019 Jul–Aug;37((4)):585–592. [PubMed] [Google Scholar]
- 125.Costa ID, Gamundi A, Miranda JG, Franca LG, De Santana CN, Montoya P. Altered functional performance in patients with fibromyalgia. Front Hum Neurosci. 2017;11:14. doi: 10.3389/fnhum.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gingrich A, Volkert D, Kiesswetter E, Thomanek M, Bach S, Sieber CC, et al. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. 2019 Apr 27;19((1)):120. doi: 10.1186/s12877-019-1115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Greco EA, Pietschmann P, Migliaccio S. Osteoporosis and sarcopenia increase frailty syndrome in the elderly. Front Endocrinol. 2019;10:255. doi: 10.3389/fendo.2019.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.A T J, Dias A, Philp I, Beard J, Patel V, Prince M. Identifying common impairments in frail and dependent older people: validation of the COPE assessment for non-specialised health workers in low resource primary health care settings. BMC Geriatr. 2015 Oct 14;15:123. doi: 10.1186/s12877-015-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lai JC, Segev DL, McCulloch CE, Covinsky KE, Dodge JL, Feng S. Physical frailty after liver transplantation. Am J Transplant. 2018 Aug;18((8)):1986–1994. doi: 10.1111/ajt.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The datasets used and/or analyzed during the current study can be made available from the corresponding author on reasonable non-commercial request.