Abstract
Purpose
To assess the association between acute disease severity and 1-year quality of life in patients discharged after hospitalisation due to coronavirus disease 2019 (COVID-19).
Methods
We conducted a prospective cohort study nested in 5 randomised clinical trials between March 2020 and March 2022 at 84 sites in Brazil. Adult post-hospitalisation COVID-19 patients were followed for 1 year. The primary outcome was the utility score of EuroQol five-dimension three-level (EQ-5D-3L). Secondary outcomes included all-cause mortality, major cardiovascular events, and new disabilities in instrumental activities of daily living. Adjusted generalised estimating equations were used to assess the association between outcomes and acute disease severity according to the highest level on a modified ordinal scale during hospital stay (2: no oxygen therapy; 3: oxygen by mask or nasal prongs; 4: high-flow nasal cannula oxygen therapy or non-invasive ventilation; 5: mechanical ventilation).
Results
1508 COVID-19 survivors were enrolled. Primary outcome data were available for 1156 participants. At 1 year, compared with severity score 2, severity score 5 was associated with lower EQ-5D-3L utility scores (0.7 vs 0.84; adjusted difference, − 0.1 [95% CI − 0.15 to − 0.06]); and worse results for all-cause mortality (7.9% vs 1.2%; adjusted difference, 7.1% [95% CI 2.5%–11.8%]), major cardiovascular events (5.6% vs 2.3%; adjusted difference, 2.6% [95% CI 0.6%–4.6%]), and new disabilities (40.4% vs 23.5%; adjusted difference, 15.5% [95% CI 8.5%–22.5]). Severity scores 3 and 4 did not differ consistently from score 2.
Conclusions
COVID-19 patients who needed mechanical ventilation during hospitalisation have lower 1-year quality of life than COVID-19 patients who did not need mechanical ventilation during hospitalisation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-022-06953-1.
Keywords: COVID-19, Post-acute COVID-19 syndrome, Respiration, Artificial, Critical care outcomes
Take-home message
| After 1 year of follow-up, patients with more severe COVID-19, defined as need for mechanical ventilation during hospitalisation, had significantly lower health-related quality of life and worse results for mortality, major cardiovascular events, re-hospitalisation, new disabilities in instrumental activities of daily living, anxiety and post-traumatic stress symptoms, and return to work or study than COVID-19 patients who did not need mechanical ventilation during hospitalisation |
Introduction
Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2 infection, has affected millions of people around the world. Brazil has been severely hit by the pandemic with the number of cases surpassing 35 million, with more than 680,000 deaths from COVID-19 by November 2022 [1]. Although a large amount of comprehensive data on acute symptoms and clinical management have been published, the long-term effects of COVID-19 remain unclear [2]. Recent studies have drawn attention to an increasing number of people experiencing prolonged symptoms following the acute phase of COVID-19 [3–7]. However, our knowledge of the long-term impact of acute COVID-19 severity on relevant outcomes, such as quality of life, cardiovascular events, new functional disabilities, and mental health symptoms, is rather limited. Notably, this evidence gap may constitute a barrier to understanding epidemiology, risk factors, and the natural history of post-COVID-19 disabilities, precluding the implementation of effective prevention and rehabilitation strategies. Accordingly, we conducted the Coalition VII prospective cohort study to investigate whether acute COVID-19 severity is associated with 1-year quality of life.
Methods
Study design and follow-up
The rationale and design of the Coalition VII (NCT04376658) have been published previously [8]. Briefly, this is a multicentre prospective cohort study nested in five randomised clinical trials originally designed to assess the effects of specific COVID-19 treatments in hospitalised adult patients in Brazil [9–13]. Survivors were followed up for 1 year by means of structured and centralised telephone interviews conducted at 3, 6, 9, and 12 months after enrolment in clinical trials that compose this study. For patients with communication difficulties, the follow-up interviews were conducted with their proxy.
All randomised clinical trials that compose the present cohort study, including their amendments for 1-year telephone follow-up, were approved by Brazil’s National Ethics Committee (Electronic Supplemental Material, ESM 1). Written informed consent was obtained from participants or their proxies at the time of enrolment during hospital stay. Participants were re-consented during the first telephone call.
Participants
This study included patients aged ≥ 18 years requiring hospitalisation for proven or suspected SARS-CoV-2 infection and meeting eligibility criteria for Coalition I (hospitalised patients with suspected or confirmed COVID-19 who were receiving either no supplemental oxygen or a maximum of 4 L/min of supplemental oxygen) [9], Coalition II (hospitalised patients with suspected or confirmed COVID-19 and at least one additional severity criteria: use of oxygen supplementation > 4 L/min; use of high-flow nasal cannula; use of non-invasive ventilation; or use of mechanical ventilation) [10], Coalition III (hospitalised patients with suspected or confirmed COVID-19 with moderate-to-severe acute respiratory distress syndrome, ARDS) [11], Coalition IV (hospitalised patients with confirmed COVID-19 and elevated serum d-dimer concentration) [12], and Coalition VI (hospitalised patients with confirmed COVID-19 who were receiving supplemental oxygen or mechanical ventilation and had abnormal levels of at least two serum biomarkers: C-reactive protein, d-dimer, lactate dehydrogenase, or ferritin) [13] randomised clinical trials. Complete information on the objectives, eligibility criteria, and period of enrolment for each trial that composes the present cohort is provided in the ESM 2. We excluded patients who died during hospitalisation, who lacked a telephone contact, or who refused or withdrew consent to participate.
Patients with a positive reverse transcription-polymerase chain reaction (RT-PCR) test for SARS-CoV-2 were considered proven cases. Suspected cases were defined according to the Brazilian Ministry of Health criteria: presence of fever and at least one respiratory sign or symptom (e.g. cough, shortness of breath, nasal congestion, difficulty swallowing, sore throat, oxygen saturation < 95%, signs of cyanosis, intercostal retraction, and dyspnoea) and patients from an endemic region, or travelling from an endemic region in the last 14 days, or in contact with a suspected or confirmed case in the last 14 days [14].
Acute disease severity
Acute disease severity was determined by the highest score on a modified six-point ordinal scale [15] during hospital stay, which consisted of the following categories: a score of 1 indicated not hospitalised; 2, hospitalised but no supplemental oxygen needed; 3, hospitalised and receiving supplemental oxygen; 4, hospitalised and receiving high-flow nasal cannula oxygen therapy or non-invasive ventilation; 5, hospitalised and receiving mechanical ventilation; and 6, death. Patients classified as score 1 or 6 were not enrolled in this study.
Outcomes
Primary outcome
The primary outcome was the health-related quality of life utility score measured at 1 year after enrolment with the EuroQol five-dimension three-level (EQ-5D-3L) questionnaire [16]. The EQ-5D-3L consists of a descriptive system with five dimensions of health-related quality of life (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), and each dimension has three levels (no problems, some problems, and extreme problems). The utility score derived from the descriptive system for the Brazilian population ranges from − 0.17 (where 0 is a health state equivalent to death; negative values are valued as worse than death) to 1 (best health state) [16]. The estimated minimal clinically important difference of EQ-5D-3L is 0.03 [17], and the mean value for the Brazilian population is 0.82 [18]. Patients who died during follow-up received a score of 0 on all follow-ups after the event.
Secondary outcomes
Secondary outcomes included EQ-5D-3L utility scores measured at 3, 6, and 9 months after enrolment, and all-cause mortality, major cardiovascular events (non-fatal stroke, non-fatal myocardial infarction, and cardiovascular death), re-hospitalisations, new disabilities in instrumental activities of daily living assessed by the Lawton and Brody instrumental activities of daily living scale [19] (any impairment, moving from independent to partially dependent or from partially dependent to totally dependent, in at least one of the following domains: telephone use, transportation, shopping, responsibility for own medications, and ability to handle finances) relative to 1 month before hospitalisation, dyspnoea assessed by the modified Medical Research Council dyspnoea scale [20], need for home ventilatory support (oxygen, non-invasive ventilation, or mechanical ventilation), anxiety and depression symptoms assessed by the Hospital Anxiety and Depression Scale (scores > 7 indicate possible cases of anxiety or depression) [21], post-traumatic stress symptoms assessed by the Impact of Event Scale-Revised (scores > 33 indicate possible cases of post-traumatic stress disorder) [22], and return to work or study at 3, 6, 9, and 12 months after enrolment.
Statistical analysis
The baseline characteristics and outcomes of participants were presented as median (interquartile range, IQR) or mean (standard deviation, SD) for continuous variables, and as counts and percentages for categorical variables. Generalised estimating equations adjusted for age, sex, number of comorbidities, and the trial in which the patient was enrolled were used to estimate adjusted differences and 95% confidence intervals (CIs) for the association between acute disease severity and outcomes. Variables used for covariate adjustment were selected based on their association with health-related quality of life [18, 23] and to account for the cluster effect.
Sensitivity analyses for the primary outcome considering only patients with proven COVID-19, adjusting analyses by pre-morbid functional dependency, and using mixed effects model for statistical modelling were performed to assess the consistency of the findings. Additionally, we assessed the association between acute disease severity and all-cause mortality using a frailty model (adjusted for age, sex, number of comorbidities, and the trial in which the patient was enrolled) to account to censored data. Additional post hoc analyses are described in the ESM 3. We used R version 3.6.2 (R Foundation for Statistical Computing) for all statistical analyses. All tests were two-tailed, and p values < 0.05 were considered statistically significant. We did not adjust the confidence interval widths and p values of secondary outcomes for multiple testing.
Results
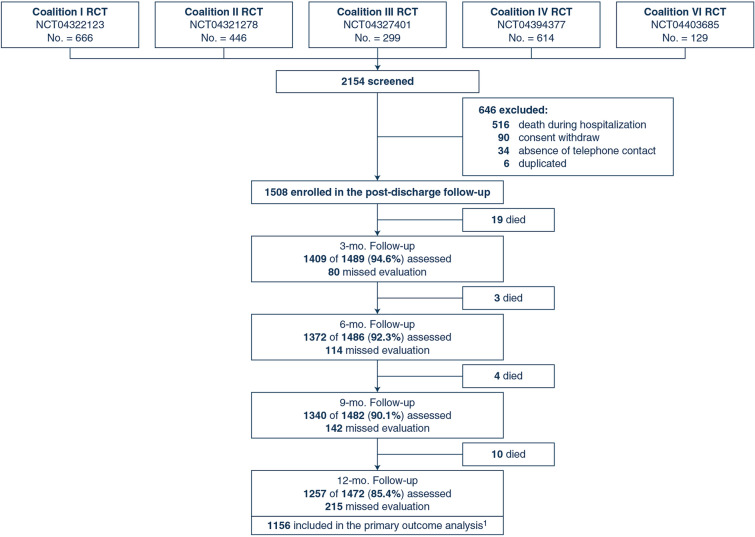
A total of 1508 patients (including 1332 patients with confirmed COVID-19) at 84 sites were enrolled between March 29, 2020, and February 26, 2021 (Fig. 1). Amongst the 1508 enrolled patients, 36 (2.4%) died before completing the 1-year follow-up. Thus, 1472 patients were available for the 1-year follow-up, which was completed on March 11, 2022. Amongst the 1472 patients eligible for the 1-year follow-up, 1257 (85.4%) were assessed. Data on the primary outcome were available for 1156 participants (1120 survivors and 36 dead patients).
Fig. 1.
Flow diagram of post-hospitalisation survivors of COVID-19. RCT randomised clinical trial. 11120 survivors and 36 dead patients
Characteristics of participants
Table 1 shows the characteristics of participants. The median age of participants was 53.2 years (IQR 42.3–64), and 917 (60.8%) were men. The most common comorbidities were hypertension (681 patients [45.2%]), obesity (451 patients [30.2%]), and diabetes (365 patients [24.2%]). Pre-morbid functional dependency was present in 615/1315 (46.8%). According to the highest score on the ordinal severity scale during hospital stay, 688 (45.6%) were categorised as score 2, 394 (26.1%) as score 3, 94 (6.2%) as score 4, and 332 (22%) as score 5. The median duration of mechanical ventilation was 10 days (IQR 6–18). The median length of hospital stay was 8 days (IQR 5–19.8). The baseline characteristics of patients assessed for the primary outcome and patients with missing values for the primary outcome were similar (ESM Table S1).
Table 1.
Characteristics of enrolled patients
| Characteristic | Entire cohorta (N = 1508) |
Cohort of confirmed casesb (N = 1332) |
|---|---|---|
| Age, years | 53.2 (42.3–64) | 53.1 (43–63.9) |
| Sex | ||
| Men | 917/1508 (60.8%) | 818/1332 (61.4%) |
| Women | 591/1508 (39.2%) | 514/1332 (38.6%) |
| Comorbidities | ||
| Hypertension | 681/1508 (45.2%) | 609/1332 (45.7%) |
| Obesity | 451/1494 (30.2%) | 427/1325 (32.2%) |
| Diabetes | 365/1508 (24.2%) | 333/1332 (25%) |
| Current smoking | 155/1508 (10.3%) | 124/1332 (9.3%) |
| Asthma | 80/1508 (5.3%) | 62/1332 (4.7%) |
| Cancer | 43/1508 (2.9%) | 35/1332 (2.6%) |
| Chronic obstructive pulmonary disease | 41/1508 (2.7%) | 32/1332 (2.4%) |
| Heart failure | 37/1508 (2.5%) | 30/1332 (2.3%) |
| Chronic renal disease | 31/1508 (2.1%) | 30/1332 (2.3%) |
| Others | 280/1186 (23.6%) | 243/1046 (23.2%) |
| Number of comorbidities | 1 (1–2) | 1 (1–2) |
| Pre-morbid functional dependencec | 615/1315 (46.8%) | 538/1169 (46%) |
| Time from symptom onset to enrolment, days | 8 (6–11) | 9 (7–11) |
| Highest score on six-point ordinal severity scale during hospital stay | ||
| Score 2: no oxygen therapy | 688/1508 (45.6%) | 558/1332 (41.9%) |
| Score 3: oxygen by mask or nasal prongs | 394/1508 (26.1%) | 380/1332 (28.5%) |
| Score 4: high-flow nasal cannula oxygen therapy or non-invasive ventilation | 94/1508 (6.2%) | 89/1332 (6.7%) |
| Score 5: mechanical ventilation | 332/1508 (22%) | 305/1332 (22.9%) |
| Duration of mechanical ventilationd, days | 10 (6–18) | 10 (7–19) |
| Length of hospital staye, days | 8 (5–19.8) | 9 (5–20) |
Data are median (IQR) or n/N (%). The differing denominators used indicate missing data
aSuspected COVID-19 patients were enrolled when RT-PCR tests for SARS-CoV-2 were still not readily available in some Brazilian hospitals. Patients with suspected SARS-CoV-2 infection defined according to the following Brazilian Ministry of Health criteria: presence of fever and at least one respiratory sign or symptom (e.g. cough, shortness of breath, nasal congestion, difficulty swallowing, sore throat, oxygen saturation less than 95%, signs of cyanosis, intercostal retraction, and dyspnoea) and patients from an endemic region, or travelling from an endemic region in the last 14 days, or in contact with a suspected or confirmed case in the last 14 days
bPatients with a positive polymerase chain reaction test for SARS-CoV-2
cDefined as any impairment (partially dependent or totally dependent) in at least one of the domains the Lawton & Brody instrumental activities of daily living scale (telephone use, transportation, shopping, responsibility for own medications, and ability to handle finances) 1 month before hospitalisation for COVID-19
dFor patients requiring invasive mechanical ventilation
eTime from enrolment in clinical trials that compose this study to hospital discharge
Quality of life
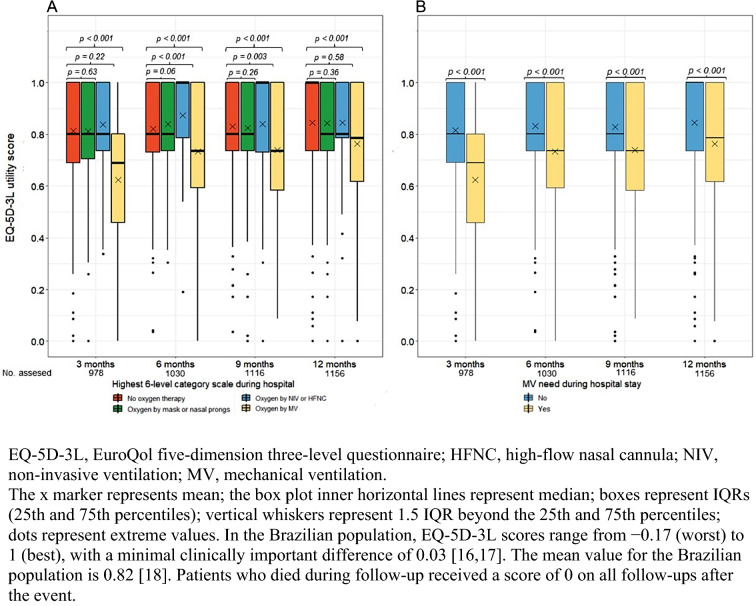
The results of quality of life are showed in Table 2. All 1156 participants with available data for the EQ5D-3L at 1 year were included in the primary outcome analysis. At 1 year, the mean EQ-5D-3L utility score for the entire cohort was 0.8 (SD, 0.24). Patients with severity score 5 had lower mean EQ-5D-3L utility scores than those with score 2 (0.7 vs 0.84; adjusted difference, − 0.1 [95% CI − 0.15 to − 0.06]). The mean EQ-5D-3L utility scores of patients with severity scores 3 and 4 did not differ significantly from those of patients with score 2. The mean EQ-5D-3L utility scores at 3, 6, and 9 months were also lower for severity score 5 vs score 2 patients (Fig. 2A). Compared to patients who did not need mechanical ventilation (severity scores 2–4), patients who needed mechanical ventilation (severity score 5) had lower mean EQ-5D-3L utility scores at 1 year (0.7 vs. 0.8; adjusted difference, − 0.07 [95% CI − 0.11 to − 0.04]; Fig. 2B).
Table 2.
Health-related quality of life, mortality, cardiovascular events and re-hospitalisation among post-hospitalisation COVID-19 patients
| Outcome | Total | Highest score on six-point ordinal severity scale during hospital stay | Adjusted differencea (95%CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Score 2: no oxygen therapy | Score 3: oxygen by mask or nasal prongs | Score 4: HFNC oxygen therapy or NIV | Score 5: MV | Score 3 vs Score 2 | Score 4 vs Score 2 | Score 5 vs Score 2 | ||
| EQ-5D-3L utility scoreb at 12 months | ||||||||
| Mean (SD) |
0.8 (0.24) |
0.84 (0.21) |
0.83 (0.22) |
0.83 (0.21) |
0.7 (0.31) |
0.01 (− 0.01 to 0.04) |
0.02 (− 0.05 to 0.09) |
− 0.1 (− 0.15 to − 0.06) |
| Median (IQR) |
0.8 (0.73–1) |
1 (0.74–1) |
0.8 (0.74–1) |
0.8 (0.79–1) |
0.79 (0.58–1) |
|||
| n assessed | 1156 | 520 | 305 | 77 | 254 | |||
| All-cause mortalityc | ||||||||
| 0–3 months |
19/1428 (1.3%) |
3/663 (0.4%) |
1/367 (0.3%) |
0/92 (0%) |
15/306 (4.9%) |
− 0.2 (− 0.8 to 0.4) |
− 0.5 (− 0.7 to − 0.2) |
4.4 (1 to 7.9) |
| 0–6 months |
22/1394 (1.6%) |
3/647 (0.5%) |
1/357 (0.3%) |
0/90 (0%) |
18/300 (6%) |
− 0.2 (− 0.7 to 0.3) |
− 0.5 (− 0.6 to − 0.3) |
5.5 (2.5 to 8.6) |
| 0–9 months |
26/1366 (1.9%) |
3/633 (0.5%) |
4/347 (1.2%) |
1/88 (1.1%) |
18/298 (6.1%) |
0.4 (0.2 to 0.7) |
0.5 (− 1.4 to 2.3) |
5.7 (2.7 to 8.7) |
| 0–12 months |
36/1293 (2.8%) |
7/600 (1.2%) |
5/329 (1.5%) |
2/83 (2.4%) |
22/281 (7.9%) |
0.8 (0.3 to 1.3) |
1.3 (− 2.8 to 5.5) |
7.1 (2.5 to 11.8) |
| Major cardiovascular eventsc,d | ||||||||
| 0–3 months |
13/1202 (1.1%) |
4/577 (0.7%) |
1/317 (0.3%) |
1/77 (1.3%) |
7/231 (3%) |
− 0.9 (− 1.3 to − 0.5) |
− 0.3 (− 2.5 to 3) |
1.9 (− 0.5 to 4.3) |
| 0–6 months |
19/1092 (1.7%) |
7/511 (1.4%) |
1/292 (0.3%) |
2/72 (2.8%) |
9/217 (4.1%) |
− 1.9 (− 2.3 to − 1.5) |
− 0.4 (− 3.8 to 4.7) |
2 (− 0.2 to 4.3) |
| 0–9 months |
21/1020 (2.1%) |
8/466 (1.7%) |
2/275 (0.7%) |
2/67 (3%) |
9/212 (4.2%) |
− 1.4 (− 2.1 to − 0.7) |
0.7 (− 2.9 to 4.3) |
2 (0.1 to 4.2) |
| 0–12 months |
26/944 (2.7%) |
10/427 (2.3%) |
3/255 (1.2%) |
2/64 (3.1%) |
11/198 (5.6%) |
− 1.8 (− 3 to − 0.5) |
0.2 (− 3.2 to 3.6) |
2.6 (0.6 to 4.6) |
| Re-hospitalisationsc | ||||||||
| 0–3 months |
60/1201 (5%) |
23/576 (4%) |
15/317 (4.7%) |
4/77 (5.2%) |
18/231 (7.8%) |
0.6 (− 2 to 3.3) |
1.1 (− 3.61 to 5.82) |
3.9 (1.6 to 6.2) |
| 0–6 months |
88/1094 (8%) |
34/514 (6.6%) |
18/292 (6.2%) |
6/71 (8.4%) |
30/217 (13.8%) |
− 0.1 (− 3.8 to 3.6) |
2.2 (− 2.7 to 7.1) |
7.3 (3.9 to 10.7) |
| 0–9 months |
133/1032 (12.9%) |
60/470 (12.8%) |
24/277 (8.7%) |
8/67 (11.9%) |
41/218 (18.8%) |
− 3.3 (− 8.1 to 1.4) |
0.1 (− 8.4 to 8.6) |
6.1 (3.3 to 9) |
| 0–12 months |
179/972 (18.4%) |
86/438 (19.6%) |
33/261 (12.6%) |
9/64 (14.1%) |
51/209 (24.4%) |
− 6.3 (− 9.6 to − 2.9) |
− 4.8 (− 15.5 to 5.8) |
4.2 (1 to 7.4) |
Data are mean (SD), or median (IQR), or n/N (%). The differing denominators used indicate missing data
CI confidence interval, EQ-5D-3L EuroQol five-dimension three-level questionnaire, HFNC high-flow nasal cannula, IQR interquartile range (p25–p75), NIV non-invasive ventilation, MV mechanical ventilation, SD standard deviation
aMean difference for continuous outcomes or absolute difference for categorical outcomes adjusted for age, sex, number of comorbidities, and the trial in which the patient was enrolled (cluster effect)
bIn the Brazilian population, scores range from − 0.17 (worst) to 1 (best), with a minimal clinically important difference of 0.03 [16, 17]. The mean value for the Brazilian population is 0.82 [18]. This analysis included 1120 survivors and 36 dead patients. A total of 958 (82.9%) of 1156 assessments were performed directly with patients, whereas 198 (17.1%) of 1156 assessments were performed indirectly with proxies
cNumber of patients with new outcome events divided by the population at risk at the beginning of period except patients with missing outcome data
dComposite of non-fatal stroke, non-fatal myocardial infarction, and cardiovascular death
Fig. 2.
Effect of COVID-19 severity on EQ-5D-3L utility scores at 3, 6, 9, and 12 months
Mortality, cardiovascular events and re-hospitalisations
Patients with severity score 5 had higher 1-year incidence of all-cause mortality (7.9% vs 1.2%; adjusted difference, 7.1% [95% CI 2.5–11.8%]), major cardiovascular events (5.6% vs 2.3%; adjusted difference, 2.6% [95% CI 0.6–4.6%]), and re-hospitalisations (24.4% vs 19.6%; adjusted difference, 4.2% [95% CI 1–7.4%]) than score 2 patients (Table 2).
Regarding the 1-year incidence of components of major cardiovascular events (ESM Table S2), compared with severity score 2 patients, score 5 patients had higher incidence of non-fatal myocardial infarction (adjusted difference, 2% [95% CI 1–3%]) and cardiovascular death (adjusted difference, 1% [95% CI 0.2–2%]). The incidence of non-fatal stroke did not differ significantly between severity score groups.
New disabilities, home ventilatory support, mental health symptoms and return to work or study
Severity score 5 patients had higher 1-year incidence of new disabilities in instrumental activities of daily living (40.4% vs 23.5%; adjusted difference, 15.5% [95% CI 8.5–22.5%]), higher 1-year prevalence of home ventilatory support (3.4% vs 1.3%; adjusted difference, 2.1% [95% CI 0.6–3.6%]), anxiety symptoms (24.7% vs 17.5%; adjusted difference 6.5% [95% CI 3.1–9.8%]) and post-traumatic stress symptoms (14% vs 7.1%; adjusted difference, 6.4%, [95% CI 4.6–8.2%]), and lower 1-year incidence of return to work (88.1% vs 97.5%; adjusted difference, − 7.4% [95% CI − 11.8 to − 2.9%]) or study (88.9% vs 96.9%; adjusted difference, − 10.5% [95% CI − 16.2 to − 4.9%]) than score 2 patients (Table 3).
Table 3.
New disabilities, home ventilatory support, mental health symptoms and return to work or study among post-hospitalisation COVID-19 patients
| Outcome | Total | Highest score on six-point ordinal severity scale during hospital stay | Adjusted differencea (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Score 2: no oxygen therapy | Score 3: oxygen by mask or nasal prongs | Score 4: HFNC oxygen therapy or NIV | Score 5: MV | Score 3 vs Score 2 | Score 4 vs Score 2 | Score 5 vs Score 2 | ||
| New disabilities in instrumental activities of daily livingb,c | ||||||||
| 0–3 months |
251/969 (25.9%) |
103/516 (20%) |
71/260 (27.3%) |
17/65 (26.2%) |
60/128 (46.9%) |
6.1 (1.5 to 10.8) |
8 (3.6 to 12.4) |
26.3 (15.7 to 37) |
| 0–6 months |
288/998 (28.8%) |
124/509 (24.4%) |
78/277 (28.2%) |
34/97 (35%) |
52/115 (42.2%) |
4.8 (1.5 to 8.2) |
11 (− 2.5 to 24.5) |
17.6 (9 to 26.1) |
| 0–9 months |
331/1081 (30.6%) |
137/500 (27.4%) |
86/307 (28%) |
31/95 (32.6%) |
77/179 (43%) |
− 0.7 (− 4.3 to 2.8) |
4.1 (− 8.7 to 16.8) |
11.9 (7.3 to 16.5) |
| 0–12 months |
305/1110 (27.5%) |
120/510 (23.5%) |
83/298 (27.8%) |
20/99 (20.2%) |
82/203 (40.4%) |
4.5 (0.3 to 8.6) |
− 2.9 (− 13.3 to 7.4) |
15.5 (8.5 to 22.5) |
| Home ventilatory supportd,e | ||||||||
| At 3 months |
39/1208 (3.2%) |
7/577 (1.2%) |
9/317 (2.8%) |
5/77 (6.5%) |
18/237 (7.6%) |
1.3 (0.3 to 2.3) |
4.7 (3.4 to 6) |
6.3 (4.4 to 8.2) |
| At 6 months |
33/1182 (2.8%) |
10/547 (1.8%) |
11/312 (3.5%) |
3/80 (3.8%) |
9/243 (3.7%) |
1.7 (− 0.5 to 4) |
1.9 (− 2.2 to 6.1) |
1.7 (0.5 to 3) |
| At 9 months |
29/1169 (2.5%) |
11/531 (2.1%) |
6/313 (1.9%) |
2/79 (2.5%) |
10/246 (4.1%) |
− 0.6 (− 1.2 to 0.1) |
0.1 (− 4.1 to 4.2) |
2 (− 0.9 to 4.8) |
| At 12 months |
27/1129 (2.4%) |
7/519 (1.3%) |
10/301 (3.3%) |
2/75 (2.7%) |
8/234 (3.4%) |
1 (0.5 to 1.6) |
0.6 (− 1.9 to 3) |
2.1 (0.6 to 3.6) |
| Anxiety symptomsd | ||||||||
| At 3 months |
177/808 (21.9%) |
103/440 (23.4%) |
48/215 (22.3%) |
10/54 (18.5%) |
16/99 (16.2%) |
− 1.4 (− 2.6 to − 0.3) |
− 1.4 (− 3.7 to 0.9) |
− 5.7 (− 9.7 to − 1.6) |
| At 6 months |
164/818 (20%) |
85/426 (20%) |
49/228 (21.5%) |
10/58 (17.2%) |
20/106 (18.9%) |
1.2 (− 2.9 to 5.3) |
− 0.8 (− 9.5 to 7.9) |
− 1.6 (− 6.6 to 3.4) |
| At 9 months |
178/847 (21%) |
81/411 (19.7%) |
50/231 (21.6%) |
10/61 (16.4%) |
37/144 (25.7%) |
3.8 (− 0.1 to 7.7) |
− 1.1 (− 5.8 to 3.7) |
5.1 (− 2.3 to 12.5) |
| At 12 months |
173/855 (20.2%) |
70/400 (17.5%) |
53/250 (21.2%) |
14/59 (23.7%) |
36/146 (24.7%) |
1.5 (0.3 to 2.7) |
9.4 (5.6 to 13.2) |
6.5 (3.1 to 9.8) |
| Depression symptomsd | ||||||||
| At 3 months |
135/808 (16.7%) |
74/440 (16.8%) |
40/215 (18.6%) |
6/54 (11.1%) |
15/99 (15.1%) |
0.7 (0.1 to 1.3) |
− 1 (− 11 to 8.9) |
− 1.2 (− 8.6 to 6.3) |
| At 6 months |
133/816 (16.3%) |
75/425 (17.6%) |
33/227 (14.5%) |
6/58 (10.3%) |
19/106 (17.9%) |
− 0.1 (− 3.2 to 3) |
− 3.5 (− 12 to 5) |
− 1.9 (− 9.9 to 6) |
| At 9 months |
154/847 (18.2%) |
69/411 (16.8%) |
47/231 (20.3%) |
7/61 (11.5%) |
31/144 (21.5%) |
− 3 (− 10 to 5) |
− 3.2 (− 7.9 to 1.6) |
3.5 (0.1 to 7) |
| At 12 months |
146/854 (17.1%) |
65/400 (16.2%) |
45/250 (18%) |
7/58 (12.1%) |
29/146 (19.9%) |
1 (− 1.7 to 3.7) |
− 2.9 (− 11.9 to 6.1) |
2.7 (− 1.6 to 7.1) |
| Post-traumatic stress symptomsd | ||||||||
| At 3 months |
88/787 (11.2%) |
49/426 (11.5%) |
15/212 (7.1%) |
7/54 (13%) |
17/95 (17.9%) |
− 2 (− 6.8 to 2.8) |
9.7 (2.6 to 16.7) |
7.9 (2.4 to 13.4) |
| At 6 months |
75/791 (9.5%) |
32/414 (7.7%) |
23/220 (10.4%) |
7/54 (13%) |
13/103 (12.6%) |
3.1 (− 1.1 to 7.3) |
8.7 (6.1 to 11.4) |
4.8 (2 to 7.5) |
| At 9 months |
84/829 (10.1%) |
27/407 (6.6%) |
22/224 (9.8%) |
7/60 (11.7%) |
28/138 (20.3%) |
2.7 (0.2 to 5.3) |
8.2 (6.9 to 9.5) |
12.9 (7.6 to 18.2) |
| At 12 months |
71/834 (8.5%) |
28/392 (7.1%) |
18/243 (7.4%) |
5/56 (8.9%) |
20/143 (14%) |
1.9 (0.1 to 3.8) |
6.9 (4.3 to 9.4) |
6.4 (4.6 to 8.2) |
| Return to workc | ||||||||
| 0–3 months |
674/808 (83.4%) |
360/403 (89.3%) |
182/209 (87.1%) |
43/52 (82.7%) |
89/144 61.8%) |
− 1 (− 8.4 to 6.4) |
− 4.4 (− 12.5 to 3.6) |
− 27 (− 31.6 to − 22.4) |
| 0–6 months |
792/867 (91.3%) |
415/439 (94.5%) |
209/223 (93.7%) |
52/55 (94.5%) |
116/150 (77.3%) |
− 0.1 (− 4 to 3.8) |
1.4 (− 3.9 to 6.6) |
− 15.5 (− 21.7 to − 9.2) |
| 0–9 months |
868/921 (94.2%) |
450/464 (97%) |
227/237 (95.8%) |
58/60 (96.7%) |
133/160 (83.1%) |
− 0.3 (− 1.5 to 0.9) |
− 1.1 (− 6.3 to 4) |
− 11.8 (− 17.1 to − 6.5) |
| 0–12 months |
911/948 (96.1%) |
469/481 (97.5%) |
240/245 (98%) |
62/63 (98.4%) |
140/159 (88.1%) |
1.4 (0.2 to 2.6) |
2.4 (− 0.7 to 5.4) |
− 7.4 (− 11.8 to − 2.9) |
| Return to studyc | ||||||||
| 0–3 months |
86/114 (75.4%) |
45/57 (78.9%) |
23/30 (76.7%) |
6/7 (85.7%) |
12/20 (60%) |
− 2.5 (− 9.3 to 4.3) |
8.1 (1 to 15.1) |
− 19 (− 42.5 to 4.4) |
| 0–6 months |
117/129 (90.7%) |
62/66 (93.9%) |
30/31 (96.8%) |
9/10 (90%) |
16/22 (72.7%) |
0.1 (− 4.8 to 4.9) |
− 7.3 (− 14.7 to 0.2) |
− 23.8 (− 41.2 to − 6.3) |
| 0–9 months |
140/149 (93.9%) |
75/78 (96.2%) |
36/37 (97.3%) |
10/11 (90.9%) |
19/23 (82.6%) |
2 (− 0.9 to 4.9) |
− 9.8 (− 14.3 to − 5.2) |
− 15.5 (− 27.8 to − 3.3) |
| 0–12 months |
174/182 (95.6%) |
94/97 (96.9%) |
46/47 (97.8%) |
10/11 (90.9%) |
24/27 (88.9%) |
2.9 (0.8 to 5.2) |
− 11.9 (− 16.6 to − 7.3) |
− 10.5 (− 16.2 to − 4.9) |
Data are n/N (%). The differing denominators used indicate missing data
CI confidence interval, HFNC high-flow nasal cannula, NIV non-invasive ventilation, MV mechanical ventilation
aAbsolute difference adjusted for age, sex, number of comorbidities, and the trial in which the patient was enrolled (cluster effect)
bDefined as any impairment (moving from independent to partially dependent or from partially dependent to totally dependent) in at least one Lawton & Brody instrumental activities of daily living scale domain (telephone use, transportation, shopping, responsibility for own medications, and ability to handle finances) relative to 1 month before hospitalisation for COVID-19
cNumber of patients with new outcome events divided by the population at risk at the beginning of period except patients with missing outcome data
dNumber of patients with the outcome divided by the total number of patients at the indicated point in time except patients with missing outcome data
eOxygen, non-invasive ventilation, or mechanical ventilation
Regarding the 1-year prevalence of components of home ventilatory support (ESM Table S3), oxygen use was higher for severity score 5 vs score 2 patients (4.8% vs 0.3%; adjusted difference, 4.5% [95% CI 3.6–5.5%]); the prevalence of non-invasive ventilation and mechanical ventilation did not differ significantly between severity score groups.
Additional analyses
The results of the sensitivity analyses for primary outcome were consistent with the results of main analysis (ESM Tables S4–S6). The effects of acute disease severity on the hazard of 1-year all-cause mortality, as assessed by a frailty model, were also consistent with the findings of the main analysis (ESM Table S7).
The 1-year prevalence of dyspnoea was higher for severity score 5 vs score 2 patients (ESM Table S8). Dyspnoea was more severe in patients with severity score 5 than in those with score 2 at 3, 6, 9 and 12 months (ESM Fig S1). Patients with severity score 5 scored worse in the EQ-5D-3L domains of mobility, usual activities, pain/discomfort, and anxiety/depression than did those with severity scores 2 at 1 year (ESM Fig. 2). No association was observed between duration of mechanical ventilation and EQ-5D-3L utility scores at 1 year (ESM Fig. 3 and Table S9). Major cardiovascular events, re-hospitalisations, new disabilities in instrumental activities of daily living, dyspnoea, home ventilatory support, anxiety, depression and post-traumatic stress symptoms were associated with lower EQ-5D-3L utility scores at 1 year (ESM Table S10).
The results of comparison between patients who did not need mechanical ventilation and those who needed mechanical ventilation on secondary outcomes were consistent with main analyses (ESM Table S11).
Discussion
In this cohort study, we observed that, after 1 year of follow-up, patients with more severe COVID-19, defined as need for mechanical ventilation during hospitalisation, had lower health-related quality-of-life utility scores and worse results for mortality, major cardiovascular events, re-hospitalisation, new disabilities in instrumental activities of daily living, dyspnoea, anxiety and post-traumatic stress symptoms, and returning to work or study.
We found that post-hospitalisation COVID-19 patients who had received mechanical ventilation had clinically meaningful reductions in health-related quality of life utility scores at 3, 6, 9, and 12 months compared with those not requiring mechanical ventilation. Although these scores improved during the 1-year follow-up period, they were still below the mean value for the Brazilian population at 1-year. This is consistent with data from previous ARDS and long-term intensive care unit (ICU) follow-up studies. For example, Herridge et al. [24] demonstrated that, although survivors of ARDS improved their quality-of-life scores during the long-term follow-up, the mean score on the physical component of the 36-item Short-Form Health Survey at 5 years remained approximately 1 SD below the mean score for an age-matched and sex-matched control population. Similarly, Hofhuis et al. [25] showed that the health-related quality of life of medical–surgical ICU survivors remained impaired compared with their pre-admission values and with an age-matched reference population after 5 years, suggesting that the post-ICU COVID-19 and non-COVID-19 patients might have the same rehabilitation needs at the long-term. Notably, oxygen delivered by mask or nasal prongs or by non-invasive ventilation and use of high-flow nasal cannula oxygen therapy were not associated with a significant reduction in health-related quality-of-life utility scores in our study, suggesting that acute strategies aimed to prevent mechanical ventilation among patients with COVID-19 might be associated with improved long-term outcomes.
Concerning physical and mental disabilities, the findings of the present study showed a higher occurrence of new functional disabilities, dyspnoea and of anxiety and post-traumatic stress symptoms in patients requiring mechanical ventilation during hospitalisation. For example, mechanical ventilation patients had twice the prevalence of post-traumatic stress symptoms than the general population in Brazil [26]. We hypothesise that these results might have contributed to the worse quality of life among patients with more severe COVID-19, which is underlined by the association between physical and mental health outcomes and reduced 1-year quality of life in our study. Accordingly, new physical and mental disabilities have been associated with reduced quality of life among survivors of critical illness [27, 28].
The association between COVID-19 severity and higher occurrence of major cardiovascular events in this study is consistent with the population-based cohort study conducted by Xie et al., [29] who showed that, beyond the first 30 days after infection, individuals with COVID-19 are at increased risk of incident cardiovascular disease, and with the literature on long-term sepsis outcomes, which shows an excess hazard of late cardiovascular events among sepsis survivors which may persist for at least 5 years following hospital discharge [30].
The higher impact of COVID-19 severity on all-cause mortality and re-hospitalisations is also a reason for concern. These data reinforce that the attributable impact of COVID-19 on hospitalisations and mortality is even higher than that associated with acute illness.
Potential explanations were considered for the association between need for mechanical ventilation and poor long-term outcomes among survivors of COVID-19. First, need for mechanical ventilation can be interpreted as a proxy for disease severity, which may lead to persistent organ dysfunction after acute SARS-CoV-2 infection, thus contributing to long-term disabilities. Accordingly, studies have shown that patients with COVID-19 requiring mechanical ventilation are more likely to have elevated inflammatory markers, more extensive lung involvement, multiple organ dysfunction, and higher in-hospital mortality [31–33]. Second, the supportive care required by mechanically ventilated patients with COVID-19 and mechanical ventilation-related complications might have contributed to a higher occurrence of physical and mental disabilities among survivors of COVID-19. Studies of survivors of critical illness have found an association between mechanical ventilation-related factors (such as profound sedation, neuromuscular blocking agents, corticosteroids, immobilisation, and ventilator-associated pneumonia) and worse long-term outcomes (such as ICU-acquired weakness, post-traumatic stress, post-discharge mortality, and reduced quality of life) [34–37]. Third, the unprecedented critical care capacity strain caused by the COVID-19 pandemic might have been associated with lower adherence to interventions aimed at preventing long-term disabilities among mechanically ventilated patients, such as minimising sedation and use of neuromuscular blocking agents, pain control, early mobilisation, and family presence [38].
Strengths of our study include its prospective design, large sample size, 1-year follow-up, and the assessment of patient-centred outcomes. However, this study has limitations. Although the study recruited from many hospitals, the sample was limited to one middle-income country. COVID-19 may have different effects on long-term outcomes across distinct contexts in terms of post-discharge access to rehabilitation services. We did not evaluate the pre-COVID-19 values of EQ-5D-3L, precluding the assessment of utility score variations in comparison to the pre-morbid period. We did not evaluate potentially relevant variables that could modify the association between acute COVID-19 severity and long-term outcomes, such as vaccination, infection with different SARS-CoV-2 variants, and specific treatments. The number of missing assessments for 1-year outcomes was relevant. Finally, we did not include a control group of patients without COVID-19, precluding the differentiation between specific COVID-19-mediated and critical illness-mediated effects on long-term outcomes.
Conclusions
COVID-19 patients who needed mechanical ventilation during hospitalisation have lower 1-year quality of life than COVID-19 patients who did not need mechanical ventilation during hospitalisation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to all the supporting staff at all sites who helped to recruit and enrol the participants.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CI
Confidence interval
- COVID-19
Coronavirus disease 2019
- EQ-5D-3L
EuroQol five-dimension three-level questionnaire
- ESM
Electronic Supplemental Material
- ICU
Intensive care unit
- IQR
Interquartile range
- RT-PCR
Reverse transcription-polymerase chain reaction
- SD
Standard deviation
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
Author contributions
RGR, ABC, LCPA, VCV, AA, FRM, OB, RDL, and MF conceived and designed the study and take responsibility for the integrity of the data and the accuracy of the data analysis. RGR, DdeS, RRMS, RFCS, DS, CCR, TAH, VELP, LGB, APS, LSC, BMB, MPP, JG, NSS, APAD, JMN, SSS, BPG, and VBS were responsible for the follow-up of participants. RGR, GSR, GPME, and MF performed the statistical analysis. RGR drafted the first version of the manuscript. ABC, LCPA, VCV, DdeS, RRMS, RFCS, GSR, GT, DS, CCR, TAH, VELP, LGB, APS, LSC, BMB, MPP, JG, NSS, APAD, JMN, SSS, BPG, VBS, GPME, CMP, AAP, LKD, BMT, TCL, CT, FGZ, APZ, BJG, AA, FRM, OB, RDL, and MF critically revised the manuscript for important intellectual content and gave final approval for the version to be published.
Funding
The Coalition VII was an investigator-initiated study. Pfizer provided partial financial support for this study (Grant number 68065723). Amongst trials that compose the present nested cohort, Coalition I and Coalition II have received partial support from EMS; Coalition III, from Laboratórios Farmacêuticos; Coalition IV, from Bayer; and Coalition VI, from Fleury Laboratory and Instituto Votorantim. The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to publish these results.
Availability of data and material
The authors encourage interested parties to contact the corresponding author with data sharing requests, including for access to dataset and additional unpublished data.
Code availability
The analytical code generated during this study is available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
RGR reports research grants from Pfizer related to this submitted work, and research grants from Pfizer and Brazilian Ministry of Health and lectures fees from Novartis outside of this submitted work. CAP declares research grants from National Institute for Health Technology Assessment, FAPERGS, CNPq, and Brazilian Ministry of Health (PROADI-SUS), and consultant and lecture fees from Novartis, Roche, Bayer, Bristol-Meyers-Squibb, Amgen, Pfizer, Astrazeneca outside of this submitted work. LKD reports research grants from Brazilian Ministry of Health (PROADI-SUS), Boehringer Ingelheim, Bristol-Myers-Squibb and consulting fees from Lilly, Roche and Gilead outside of this submitted work. FGZ reports research grants from Ionis Pharmaceuticals and Bactiguard and consultant from Bactiguard. APZ reports research grants from Pfizer and consultant from fees Spero Therapeutics outside of this submitted work. OB reports research grants from AstraZeneca, Pfizer, Bayer, Boehringer Ingelheim, Servier, and Amgen outside of this submitted work. RDL reports research grants from BMS, Glaxo Smith Kline, Medtronic, Portola, Bayer, Pfizer, Sanofi, Daiichi Sankyo, Merck and Boehringer Ingleheim, and consulting fees from Bayer, BMS, Glaxo Smith Kline, Portola, Merck, Boehringer Ingleheim, Daiichi Sankyo, Medtronic, Sanofi and Pfizer outside of this submitted work. The other authors have no conflict to declare.
Ethics approval
All five randomised clinical trials that compose the present cohort study, including their amendments for 1-year telephone follow-up, were approved by Brazil’s National Ethics Committee. Written informed consent was obtained from all participants or their proxies at the time of enrolment during hospital stay. Participants were re-consented during the first telephone call.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Johns Hopkins University & Medicine (2022) Coronavirus resource center. https://coronavirus.jhu.edu/map.html
- 2.Munblit D, Nicholson TR, Needham DM, et al. Studying the post-COVID-19 condition: research challenges, strategies, and importance of Core Outcome Set development. BMC Med. 2022;20:50. doi: 10.1186/s12916-021-02222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blomberg B, Mohn KG, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9:1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heesakkers H, van der Hoeven JG, Corsten S, et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA. 2022;327:559–565. doi: 10.1001/jama.2022.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosa RG, Robinson CC, Veiga VC, et al. Quality of life and long-term outcomes after hospitalization for COVID-19: protocol for a prospective cohort study (Coalition VII) Rev Bras Ter Intensiva. 2021;33:31–37. doi: 10.5935/0103-507X.20210003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. N Engl J Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furtado RHM, Berwanger O, Fonseca HA, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396:959–967. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopes RD, de Barros ESPGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veiga VC, Prats J, Farias DLC, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:n84. doi: 10.1136/bmj.n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministério da Saúde do Brasil (2020) Diretrizes para diagnóstico e tratamento da COVID-19. https://saude.rs.gov.br/upload/arquivos/202004/14140600-2-ms-diretrizes-covid-v2-9-4.pdf
- 15.Working Group on the Clinical Characterisation and Management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos M, Cintra MA, Monteiro AL, et al. Brazilian Valuation of EQ-5D-3L Health States: results from a saturation study. Med Decis Making. 2016;36:253–263. doi: 10.1177/0272989X15613521. [DOI] [PubMed] [Google Scholar]
- 17.Coretti S, Ruggeri M, McNamee P. The minimum clinically important difference for EQ-5D index: a critical review. Expert Rev Pharmacoecon Outcomes Res. 2014;14:221–233. doi: 10.1586/14737167.2014.894462. [DOI] [PubMed] [Google Scholar]
- 18.Santos M, Monteiro AL, Santos B. EQ-5D Brazilian population norms. Health Qual Life Outcomes. 2021;19:162. doi: 10.1186/s12955-021-01671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dos Santos RL, Virtuoso JS., Jr Reliability of the Brazilian version of the Scale of Instrumental Activities of Daily Living. Rev Bras Prom Saúde. 2008;21:290–296. doi: 10.5020/575. [DOI] [Google Scholar]
- 20.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 22.Caiuby AV, Lacerda SS, Quintana MI, Torii TS, Andreoli SB. Cross-cultural adaptation of the Brazilian version of the Impact of Events Scale-Revised (IES-R) Cad Saude Publica. 2012;28:597–603. doi: 10.1590/s0102-311x2012000300019. [DOI] [PubMed] [Google Scholar]
- 23.Fortin M, Bravo G, Hudon C, et al. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual Life Res. 2006;15:83–91. doi: 10.1007/s11136-005-8661-z. [DOI] [PubMed] [Google Scholar]
- 24.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 25.Hofhuis JG, van Stel HF, Schrijvers AJ, Rommes JH, Spronk PE. ICU survivors show no decline in health-related quality of life after 5 years. Intensive Care Med. 2015;41:495–504. doi: 10.1007/s00134-015-3669-5. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro WS, de Mari JJ, Quintana MI, et al. The impact of epidemic violence on the prevalence of psychiatric disorders in Sao Paulo and Rio de Janeiro, Brazil. PLoS ONE. 2013;8(5):e63545. doi: 10.1371/journal.pone.0063545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodgson CL, Udy AA, Bailey M, et al. The impact of disability in survivors of critical illness. Intensive Care Med. 2017;43:992–1001. doi: 10.1007/s00134-017-4830-0. [DOI] [PubMed] [Google Scholar]
- 28.Teixeira C, Rosa RG, Sganzerla D, et al. The burden of mental illness among survivors of critical care-risk factors and impact on quality of life: a multicenter prospective cohort study. Chest. 2021;160:157–164. doi: 10.1016/j.chest.2021.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosyakovsky LB, Angriman F, Katz E, et al. Association between sepsis survivorship and long-term cardiovascular outcomes in adults: a systematic review and meta-analysis. Intensive Care Med. 2021;47(9):931–942. doi: 10.1007/s00134-021-06479-y. [DOI] [PubMed] [Google Scholar]
- 31.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholson CJ, Wooster L, Sigurslid HH, et al. Estimating risk of mechanical ventilation and in-hospital mortality among adult COVID-19 patients admitted to Mass General Brigham: the VICE and DICE scores. EClinicalMedicine. 2021;33:100765. doi: 10.1016/j.eclinm.2021.100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranzani OT, Bastos LSL, Gelli JGM, et al. Characterisation of the first 250,000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021;9:407–418. doi: 10.1016/S2213-2600(20)30560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;370:1626–1635. doi: 10.1056/NEJMra1209390. [DOI] [PubMed] [Google Scholar]
- 35.Girard TD, Shintani AK, Jackson JC, et al. Risk factors for post-traumatic stress disorder symptoms following critical illness requiring mechanical ventilation: a prospective cohort study. Crit Care. 2007;11:R28. doi: 10.1186/cc5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39:371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 37.Rosa RG, Falavigna M, Robinson CC, et al. Early and late mortality following discharge from the ICU: a multicenter prospective cohort study. Crit Care Med. 2020;48:64–72. doi: 10.1097/CCM.0000000000004024. [DOI] [PubMed] [Google Scholar]
- 38.Wilde H, Mellan T, Hawryluk I, et al. The association between mechanical ventilator compatible bed occupancy and mortality risk in intensive care patients with COVID-19: a national retrospective cohort study. BMC Med. 2021;19:213. doi: 10.1186/s12916-021-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors encourage interested parties to contact the corresponding author with data sharing requests, including for access to dataset and additional unpublished data.
The analytical code generated during this study is available from the corresponding author on reasonable request.