Abstract
Introduction
Within 30 years, 20–50% of IgA nephropathy (IgAN) patients progress to end-stage kidney disease (ESKD). Identifying these patients can be difficult since renal function may deteriorate after being stable for years. The International IgAN Risk Prediction tool (IgAN-RPT) combines histologic lesions and clinical risk factors to predict renal outcome up to 5 or 7 years of follow-up. The clinical value beyond 7 years is unknown and microhematuria data has not been assessed.
Methods
We studied the long-term renal outcome of 95 Swedish IgAN patients from the derivation cohort for the IgAN-RPT. The median follow-up was 11.2 years. Microhematuria at baseline was defined as high-degree by microscopy measurement of >10 red blood cell/high-power field of view or urine dipstick grading of 2–3. Primary outcome was defined as a 50% decrease in estimated glomerular filtration rate or ESKD.
Results
The mean predicted 5-year risk for increasing quartiles was 0.95%, 2.57%, 5.88%, and 23.31% and the observed 5-year-outcome was 0%, 0%, 0%, and 33.33%. During continued follow-up, 0%, 4.2%, 21.7%, and 75.0% of patients reached the primary outcome. ROC curve analysis identified the 5-year risk thresholds of under 4% and over 11% for very low and very high-risk patients, respectively. High-degree microhematuria was not significantly associated with renal outcome (p = 0.14).
Conclusions
The IgAN-RPT identifies long-term high- and low-risk patients, which can guide decisions on the frequency of clinical control visits and the selection of patients for clinical trials. Patients with intermediate risk remain a clinical challenge with an urgent need for novel biomarkers and treatments. Microhematuria could be a valuable marker of inflammatory activity, but measurement needs to be standardized for implementation in risk prediction tools.
Keywords: Hematuria, IgA nephropathy, Prediction model, Prognosis, Progression
Introduction
IgA nephropathy (IgAN) is the most common glomerulonephritis in Europe and Asia, with an incidence reported between 2 and 10 biopsy-verified cases/100.000 person-years [1, 2, 3, 4]. Prognosis varies and about 30–50% of patients reach end-stage kidney disease (ESKD) within 30 years, with an impact on life-expectancy [5, 6, 7, 8]. In Sweden, where the kidney biopsy frequency is around 12–14 cases/100.000 person-years, 55% of the biopsies showed a diagnosis of glomerulonephritis and of those, 33% were specified as IgAN [9].
There are several established clinical risk factors for a worse prognosis, including decreased glomerular filtration rate (GFR) at presentation, high-degree proteinuria and hypertension, with proteinuria generally receiving the most attention and setting the guidelines for treatment [6, 10, 11, 12, 13]. Despite this, identifying those at risk of developing ESKD has proven difficult and patients may progress to ESKD after years of stable renal function [8, 14]. To improve the identification of high-risk patients, several risk prediction tools have been created utilizing known clinical and/or histological risk factors to estimate the risk of ESKD for the individual patient [15, 16, 17, 18, 19]. The most widely accepted system for classifying histologic lesions in IgAN is the Oxford classification, which includes mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), tubular atrophy/interstitial fibrosis (T) and crescents (C) [17, 18]. The recently developed International IgAN Risk Prediction tool (IGAN-RPT) has the advantage of combining the validated Oxford classification with readily available clinical parameters to estimate the risk of ESKD in an individual patient up to 7 years after diagnosis [17, 19]. The factors included in the full model with race are creatinine estimated GFR (eGFR), age, proteinuria, mean arterial blood pressure (MAP), MEST-score (excluding crescents), treatment with RAS-inhibition, treatment with immunosuppression, and patient origin [19]. The IGAN-RPT is proposed to be used clinically as well as in research, for the identification of high-risk patients for clinical trials and to test novel biomarkers against established risk factors. The IGAN-RPT has since been externally validated but not beyond 7 years of follow-up [19, 20, 21]. Data on hematuria were not collected when merging multinational cohorts for the creation of the international risk prediction model. The prognostic value of macro- and microhematuria has been controversial with conflicting study results, but recent studies demonstrate that persistent high-degree microhematuria, especially in combination with proteinuria, affects prognosis negatively and that remission of hematuria is associated with improved renal outcome [22, 23, 24, 25, 26, 27, 28].
The aim of our study was to examine the long-term outcome of IgAN patients from our department who had been included in the derivation cohort for the IgAN-RPT. We also evaluated the association between hematuria at baseline and renal outcome in our cohort.
Materials and Methods
The study was a retrospective analysis of a prospectively followed Swedish IgAN patient cohort. Ninety-five patients were included, none of which were of Japanese or Chinese origin, and all patients had a diagnostic kidney biopsy performed at Karolinska University Hospital between 1992 and 2014. They all participated in our local longitudinal follow-up study and had been selected for the VALIGA study and the Global IgAN Initiative, and thus the derivation cohort for the IgAN-RPT [17, 29]. Oxford MEST-classification was performed by one single pathologist at Karolinska University Hospital for the VALIGA- and Global collaboration. Written informed consent was obtained from all patients when recruited.
From a total of 266 prevalent and incident IgAN patients recruited consecutively since 1994 to our local research project, 171 patients had been excluded from the VALIGA- and Global IgAN collaboration according to prespecified exclusion criteria [17, 29]. Patients were excluded due to the biopsy having been performed at another hospital or otherwise being unavailable for a secondary evaluation of MEST-score by our pathologist (n = 67), when the biopsy included less than 8 assessable glomeruli (n = 5) or when less than 12 months of follow-up data were available (n = 7). Other exclusion criteria were: onset of ESKD or initiation of renal replacement therapy before inclusion in our cohort (n = 11), type 1 diabetes (n = 1), type 2 diabetes with diabetic retinopathy (n = 1), malignant- (n = 5), rheumatologic- (n = 7), inflammatory bowel disease (n = 3), concomitant renal disorder (n = 3) or symptoms of IgA-Vasculitis (n = 52). Nine patients chose to not participate or withdrew consent during follow-up.
Data collection had been performed retrospectively at inclusion and annually based on medical reports from routine follow-up visits, the frequency of which had been decided by the responsible physician. Information was collected on creatinine values, albuminuria, microhematuria, MAP, BMI, and medications. Albuminuria values were obtained from a 24 h urine collection when available, otherwise 24 h albuminuria was estimated using morning spot urine albumin-creatinine ratio (reported in milligrams per millimole) and the Cockcroft-Gault formula in accordance to Fournier [30]. The degree of microhematuria had been measured with automated microscopy, evaluating the amount of red blood cells (RBCs) in a high-power field of view (HPF) or with urinary dipsticks. To combine these different methods in our analyses, we defined the microscopy results by the following categories, based on correlations observed in patients where both methods had been used: 0–3 RBC/HPF correspond to 0 measured with dipsticks, 4–10 RBC/HPF = 1, 10–21 RBC/HPF = 2, and >21 RBC/HPF = 3. Due to the insensitivity and variability of microhematuria measurements, microhematuria was divided into low-degree for a measurement of 0–1 and high-degree for 2–3. eGFR was calculated using creatinine and the CKD-EPI formula for patients over the age of 18 years. In patients under the age of 18, the Bedside Schwartz formula was used instead. All eGFR values over 120 mL/min were capped at 120 mL/min. An eGFR-level of over 120 mL/min may be the result of an overestimation due to hyperfiltration with causes such as nephrotic syndrome or severe sarcopenia, which would not allow us to use a 50% decrease in eGFR as a part of our studied outcome, as patients could reach this outcome with near normal renal function. Estimated GFR-slope was calculated using linear regression with the least squares method on collected eGFR measurements with a minimum of 3 measurements.
Study Endpoint
The studied outcome was defined for the IgAN-RPT as a composite outcome of a 50% decrease in eGFR from baseline or ESKD defined as an eGFR of less than 15 mL/min × 1.73 m2 or initiation of RRT (dialysis or renal transplantation).
Statistical Analysis
All statistical analyses were performed using SPSS 27 (released 2020; IBM Corp., Armonk, NY, USA) or R (R Core Team 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. URL [https://www.R-project.org/] depending on availability of functionalities). Test of normality was performed using the Shapiro-Wilk test. For comparison of means, Student's t test or Mann Whitney U test were used depending on if the variable was normally distributed. For comparing multiple groups, one-way ANOVA and Kruskal-Wallis H tests were used for normally and non-normally distributed continuous variables, respectively. Post hoc testing between risk quartiles was performed using Tukey's test and Mann Whitney U test for normally and non-normally distributed continuous variables, respectively. Comparisons of categorical variables were performed using χ2 test. Post hoc testing for categorical variables was performed using adjusted residuals as described by MacDonald et al. [31]. p values from post hoc analysis between risk quartiles were adjusted using the Bonferroni correction for multiple testing with a correction factor of 6 for comparing 4 groups. A predicted 5-year risk was calculated for each patient using the IgAN-RPT full model with race [19]. Survival analyses were performed using univariate Cox-regression and Kaplan-Meier estimation with a log-rank test. p values in tables from survival analyses comparing groups were obtained using the log-rank test. To find clinically useful risk thresholds for the IgAN-RPT, time-independent ROC curve analysis was performed using predicted 5-year risk as the discriminatory variable with the outcome recorded at 7-year, 10-year, and full-length follow-up.
Results
In our cohort, 25.3% of patients (n = 24) reached the outcome, with a median follow-up time of 11.2 years (134.1 months), as shown in Table 1. Patients who did not reach the outcome had a median follow-up time of 12.9 years. Hematuria measurements at baseline were available for 77 patients. Patients with immunosuppressive treatment, at any time during the disease course, had mainly received prednisolone (n = 21, of which 3 with additional intravenous methylprednisolone) and/or an enteral-released formula of budesonide (n = 7). One patient had also been treated with cyclophosphamide and another with plasmapheresis, rituximab, and mycophenolate mofetil. One patient received only methylprednisolone. Comparing patients diagnosed up to (n = 18) or after (n = 77) the year 1999, when the Pozzi trial on corticosteroid treatment in IgAN had been published, we found a trend towards a higher frequency of immunosuppressive treatment within 1 year of diagnosis (21% vs. 6%, p = 0.129) [32]. There also was a trend towards an increased percentage of patients being treated with RAS-inhibition at inclusion (43% vs. 22%, p = 0.106).
Table 1.
Baseline and follow-up characteristics of the 95 patients included in the study
| Age, years | 36.5±13.2 |
| Gender (male:female as %) | 73:27 |
| Follow-up (median as months) | 134.1±62.7 |
| eGFR at baseline, mL/min | 77.6±27.8 |
| eGFR-slope 5 years past biopsy, mL/min/year | –1.76±5.07 |
| MAP at baseline, mm Hg | 98.9±13.9 |
| TA-MAP, mm Hg | 94.9±8.0 |
| Albuminuria at baseline, g/day | 1.57±1.89 |
| TA-Albuminuria, g/day | 0.92±1.39 |
| High-degree microhematuria (n = 77), n (%) | 61 (79.2) |
| BMI (n = 94) at baseline, kg/m2 | 26.0±4.7 |
| Treatment with ACEi or ARB at the time of biopsy, n (%) | 37 (38.9) |
| Treatment with ACEi or ARB at the time of at least one follow-up, n (%) | 81 (85.3) |
| Immunosuppression within 1 year of biopsy, n (%) | 16 (16.8) |
| Immunosuppression at any time point during or before the study, n (%) | 25 (26.3) |
| History of macrohematuria, n (%) | 39 (41.1) |
| Predicted 5-year risk, % | 8.2±10.6 |
| Reached the outcome within 5 years, n (%) | 8 (8.4) |
| Reached the outcome during follow-up, n (%) | 24 (25.3) |
| MEST-score, n (%): | |
| M1 | 32 (33.8) |
| E1 | 23 (24.2) |
| S1 | 78 (82.1) |
| T (2:1:0) | 10:27:58 |
| (10.5: 28.4: 61.1) |
Continuous variables are presented as mean ± standard deviation (SD) unless otherwise stated. When information on a variable is missing from patients, the number in the parenthesis states the number of patients with a valid measurement of the variable in question.
Most of the variables used to calculate the linear predictor in the IgAN-RPT were significantly different between the risk quartiles defined by the IgAN-RPT, as shown in Table 2. Patients in the 4th quartile had worse baseline renal function (p = 0.038), higher baseline albuminuria (p = 0.033), higher time-averaged 5-year albuminuria (p = 0.027), higher MAP (p = 0.032), more frequent M- (p = 0.019), S- (p = 0.049), T1- (p = 0.007), and T2- (p = 0.0002) histologic lesions in post hoc analysis. Compared with the 1st quartile, the 4th quartile was also treated more frequently with RAS-inhibition at biopsy (p = 0.017) and immunosuppression at any time during follow-up (p = 0.021).
Table 2.
Comparison of baseline characteristics, treatment, and outcome of risk quartiles as determined by the International IgAN Risk Prediction tool
| Quartiles based on 5-year risk of the outcome | First quartile (n = 24) | Second quartile (n = 24) | Third quartile (n = 23) | Forth quartile (n = 24) | Significance |
|---|---|---|---|---|---|
| 5-Year risk of the outcome (median & range), % | 0.88 (0.3–1.7) | 2.24 (1.8–3.9) | 5.67 (3.9–11.1) | 20.1 (11.1–48.6) | |
| Age at biopsy, years | 34.9±11.6 | 37.0±15.5 | 39.5±14.1 | 34.6±11.7 | p = 0.576 |
| Sex (male:female as %) | 62.5:37.5 | 58:42 | 78:22 | 92:8 | p = 0.038 |
| Follow-up (median as months) | 149.2±62.2 | 155.9±58.1 | 160.3±46.7 | 66.3±57.3 | p < 0.0005 |
| eGFR, mL/min | 94.5±24.0 | 89.6±23.8 | 74.5±24.5 | 51.6±16.9 | p < 0.0005 |
| eGFR-slope 5 years past biopsy, mL/min/year | –0.28±4.36 | –0.74±2.85 | –1.60±3.08 | –4.43±7.62 | p = 0.105 |
| MAP, mm Hg | 95.8±12.4 | 94.6±9.6 | 96.7±11.9 | 108.3±16.8 | p = 0.002 |
| TA-MAP 5 years past biopsy, mm Hg | 93.7±8.6 | 92.6±5.4 | 93.5±6.9 | 99.8±8.78 | p = 0.006 |
| Albuminuria, g/day | 0.20±0.22 | 1.27±1.51 | 1.38±0.77 | 3.41 ±2.47 | p < 0.0005 |
| TA-Albuminuria 5 years past biopsy, g/day | 0.20±0.19 | 0.66±0.63 | 0.73±0.51 | 2.10±2.25 | p < 0.0005 |
| High-degree microhematuria (n = 77), n (%) | 13 (68.4) (n = 19) | 13 (65) (n = 20) | 19 (100) (n = 19) | 16 (84.2) (n = 19) | p = 0.028 |
| History of macrohematuria, n (%) | 15 (62.5) | 9 (37.5) | 11 (47.8) | 4 (16.7) | p = 0.012 |
| BMI (n = 94) | 25.3±5.0 | 26.1±5.0 | 27.4±4.5 (n = 22) | 25.4±4.2 | p = 0.306 |
| Treatment with ACEi or ARB at the time of biopsy, n (%) | 4 (16.7) | 8 (33.3) | 11 (47.8) | 14 (58.3) | p = 0.02 |
| Treatment with ACEi or ARB at the time of at least one follow-up, n (%) | 14 (58.3) | 23 (95.8) | 23 (100) | 21 (87.5) | p < 0.0005 |
| Immunosuppression within 1 year of biopsy, n (%) | 1 (4.2) | 5 (20.8) | 2 (8.7) | 8 (33.3) | p = 0.032 |
| Immunosuppression at any time point during or before the study, n (%) | 2 (8.3) | 7 (29.2) | 5 (21.7) | 11 (45.8) | p = 0.028 |
| Reached the outcome within 5 years, n (%) | 0 (0) | 0 (0) | 0 (0) | 8 (33.3) | p < 0.0005 |
| Reached the outcome, n (%) | 0 (0) | 1 (4.2) | 5 (21.7) | 18 (75) | p < 0.0005 |
| M1 | 4 (16.7) | 8 (33.3) | 6 (26.1) | 14 (58.3) | p = 0.017 |
| E1 | 2 (8.3) | 10 (41.7) | 5 (21.7) | 6 (25) | p = 0.061 |
| S1 | 13 (54.2) | 19 (79.2) | 22 (95.7) | 24 (100) | p < 0.0005 |
| T 2:1:0 | 0:2:22 (0:8.3:91.7) | 0:2:22 (0:8.3:91.7) | 2:10:11 (8.7:43.5:47.8) | 8:13:3 (33.3:54.2:12.5) | p < 0.0005 |
Continuous variables are reported as mean ± standard deviation (SD) unless otherwise stated. When information on a variable is missing from patients, the number in the parenthesis states the number of patients with a valid measurement of the variable in question. Significant p values are highlighted in bold.
Comparing patients in the 3rd risk-quartile with an intermediate 5-year risk of 5.67% with the two bottom risk quartiles showed that patients in the 3rd risk-quartile had more frequently chronic histologic lesions (p = 0.044 for S-lesions and p = 0.0030 for T1-lesions). The 3rd risk quartile also had a higher degree of albuminuria at baseline (p < 0.0005), a higher 5-year time averaged albuminuria (p = 0.001), and were more frequently treated with RAS-inhibition during follow-up (p = 0.0029), but only compared to the 1st quartile. Treatment frequency with immunosuppression did not differ between the 3rd risk quartile and the bottom quartiles.
During 5-year follow-up, the calculated eGFR slope was not significantly different between the risk quartiles. The frequency of high-degree microhematuria was significantly different between risk quartiles but in post hoc analysis was only significantly higher in the 3rd risk quartile (p = 0.013).
Dividing patients into quartiles based on the risk as calculated by the IgAN-RPT, the mean predicted 5-year risk for increasing quartiles was 0.95%, 2.57%, 5.88%, and 23.31% and the recorded 5-year-outcome was 0%, 0%, 0%, and 33.3%. During continued follow-up, with a median of 11.2 years, 0%, 4.2%, 21.7%, and 75.0% of patients reached the outcome. As shown in Figure 1, the risk quartiles kept diverging past the 7 years the IgAN-RPT is validated for. In univariate Cox-regression, each quartile increment was associated with a HR of 7.7 (95% CI: 3.61–16.43, p < 0.0005) for the outcome.
Fig. 1.
Comparison of renal survival by risk of renal outcome. The graph illustrates the renal survival with regards to the outcome comparing patients in different quartiles of risk for the outcome. Risk quartiles were determined using the full model with race from the International Risk Prediction tool [19]. Survival curves were created using Kaplan-Meier estimation. The p value was obtained through log-rank test.
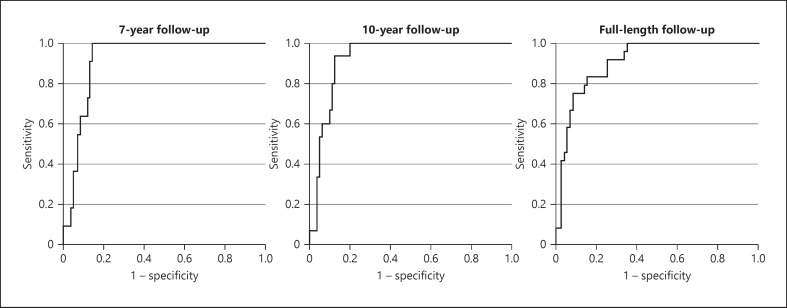
Evaluation of the IgAN-RPT using a ROC curve analysis in our cohort showed that the IgAN-RPT significantly predicts the outcome during full-length follow-up with an area under the curve value of 0.907 (p < 0.0005) as visualized in Figure 2. Using a cut-off of 3.88%, predicted 5-year risk yielded a sensitivity of 95.8% and a specificity of 66.2% for the outcome. Inversely, using a higher cut-off of 11.09% yielded a sensitivity of 75% and a specificity of 91.5%. The discriminatory ability of the ROC curve analysis was similar when the outcome was recorded at 7 and 10 years with area under the curve values of 0.920 and 0.924, respectively, visualized in Figure 2.
Fig. 2.
ROC curve for the IgAN-RPTs prediction of the renal outcome. The graph illustrates time-independent ROC curve analyses using the predicted 5-year risk as the discriminatory variable and the outcome recorded at 7-year, 10-year, and full-length follow-up as stated in respective graph.
Patients with high-degree microhematuria at the time of biopsy were more frequently male and had a higher mean predicted 5-year risk (8.84% vs. 5.68%) compared to patients with low-degree microhematuria. During continued follow-up, 26.2% in the high-degree hematuria group reached the outcome compared to 6.3% in the low-degree hematuria group, but this did not reach statistical significance. There were no statistically significant differences in baseline clinical parameters or histologic lesions between the two groups as shown in Table 3.
Table 3.
Comparison of baseline characteristics, treatment, and outcome by the degree of microhematuria (n = 77)
| Microhematuria | Low-degree, 0–1 (n = 16) | High-degree, 2–3 (n = 61) | Significance |
|---|---|---|---|
| Age at biopsy, years | 37.6±14.8 | 37.1±13.4 | p = 0.910 |
| Sex (male:female as %) | 56:44 | 80:20 | p = 0.047 |
| Follow-up time (median as months) | 132.8±52.9 | 128.3±57.0 | p = 0.631 |
| eGFR, mL/min | 86.9±27.0 | 74.3±29.2 | p = 0.121 |
| eGFR-slope 5 years past biopsy | 0.05±3.75 | –2.20±5.18 | p = 0.103 |
| MAP, mm Hg | 96.6±11.7 | 97.7±10.4 | p = 0.734 |
| TA-MAP 5 years past biopsy, mm Hg | 94.0±7.4 | 93.5±6.8 | p = 0.817 |
| Albuminuria, g/day | 1.12±1.54 | 1.75±2.11 | p = 0.132 |
| TA-Albuminuria 5 years past biopsy, g/day | 0.55±0.69 | 0.97±1.54 | p = 0.145 |
| BMI (n = 76) | 26.2±5.1 | 26.1±4.7 (n = 60) | p = 0.929 |
| Treatment with ACEi or ARB at the time of biopsy, n (%) | 8 (50.0) | 24 (39.3) | p = 0.441 |
| Treatment with ACEi or ARB at the time of at least one follow-up, n (%) | 13 (81.3) | 52 (85.2) | p = 0.695 |
| Immunosuppression within 1 year of biopsy, n (%) | 2 (12.5) | 13 (23.0) | p = 0.428 |
| Immunosuppression at any point during or before the study, n (%) | 5 (31.3) | 15 (26.2) | p = 0.589 |
| Predicted 5-year risk, % | 5.68±10.1 | 8.84±10.6 | p = 0.03 |
| Reached the outcome of –50% eGFR or ESRD, n (%) | 1 (6.3) | 16 (26.2) | p = 0.109 |
| M | 5 (31.3) | 18 (29.5) | p = 0.892 |
| E | 3 (18.8) | 17 (27.9) | p = 0.459 |
| S | 12 (75.0) | 52 (85.2) | p = 0.330 |
| T (2:1:0 as %) | 0:4:12 (0:25:75) | 9:19:33 (14.8:31.1:54.1) | p = 0.177 |
Continuous variables are reported as mean ± standard deviation (SD) unless otherwise stated. When information on a variable is missing from patients, the number in the parenthesis states the number of patients with a valid measurement of the variable in question. Significant p values are highlighted in bold.
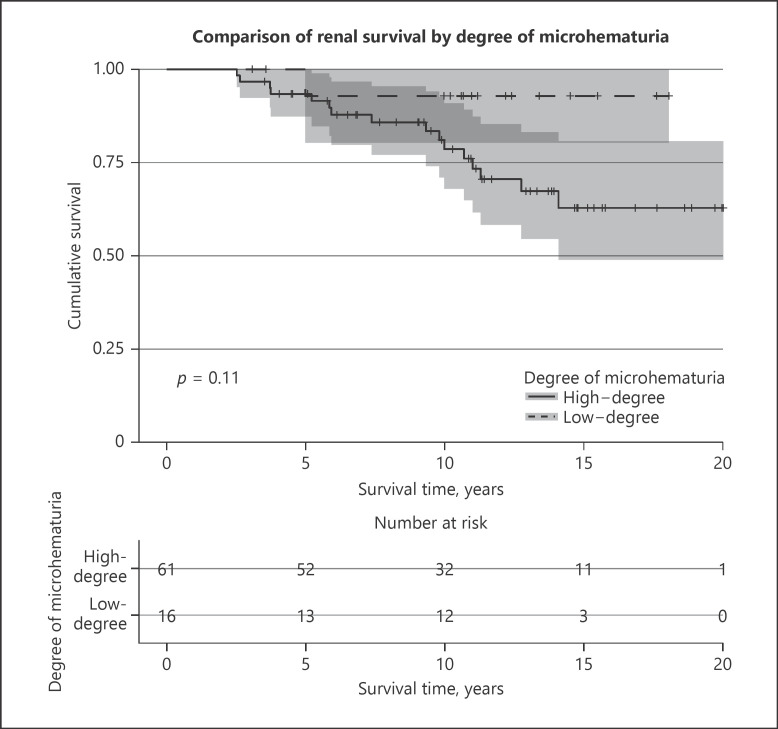
In univariate Cox-regression, high-grade microhematuria was not significantly associated with the outcome (p = 0.144). High-degree microhematuria was also nonsignificant with the log-rank test in Kaplan-Meier estimation (p = 0.109). Survival for patients with high-versus low-degree microhematuria is visualized in Figure 3.
Fig. 3.
Comparison of renal survival by degree of microhematuria. The graph illustrates the renal survival with regards to the outcome comparing patients with high-degree microhematuria and low-degree microhematuria. Survival curves were created using Kaplan-Meier estimation. The p value was obtained through log-rank test. The gray zone adjacent to the survival curves indicate the 95% confidence interval.
A history of macrohematuria was not significantly associated with renal outcome in Kaplan-Meier estimation with log-rank test (p = 0.160) as shown in Figure 4. Age did not significantly differ between the two groups with a mean age at diagnosis of 33.5 years for those with a history of macrohematuria compared to 38.5 years without (p = 0.071).
Fig. 4.
Comparison of renal survival by history of macrohematuria. The graph illustrates the renal survival with regards to the outcome comparing patients with and without a history of macrohematuria. Survival curves were created using Kaplan-Meier estimation. The p value was obtained through log-rank test. The gray zone adjacent to the survival curves indicate the 95% confidence interval.
Discussion
In our observational study of 95 patients with IgAN and a median follow-up time of 11.4 years, we were able to show that categorization in risk quartiles by the IgAN-RPT well identified patients who reached the renal outcome of a 50% decrease in eGFR or ESKD after prolonged follow-up. We propose clinically useful risk thresholds that could be applied for decision-making with regard to the frequency of follow-up visits and inclusion in clinical trials to improve future treatment. High-degree microhematuria at baseline was not significantly associated with renal outcome, but the small cohort size might have had an impact on our study results.
As our patient cohort was part of the derivation cohort for the IgAN-RPT, this study does not fill the criteria of an external validation study. Our purpose was to examine a possible clinical application of the IgAN-RPT. Categorization in risk quartiles using the IgAN-RPT and predicted 5-year risk showed that patients in the two lowest risk quartiles were very unlikely to experience deteriorating renal function up to 11 years after the time of biopsy, in contrast to the long-term vulnerability associated with a higher risk-score. For patients in the next highest and highest quartile, the percentage with 50% decreased eGFR or ESKD increased from 0% and 33.3% after 5 years to 21.70% and 75.0% after a median additional follow-up of 6 years.
Accurately estimating the severity of disease and long-term prognosis of patients with IgAN is difficult due to the heterogeneity of the disease and the lack of validated biomarkers that can predict the response to a specific treatment. However, the recently generated risk prediction tool, which is based on established clinical risk factors at diagnosis, facilitates early identification of a patient's risk profile with regard to disease progression. Application of risk thresholds for predicted risk would allow for the conversion of risk percentage into more practically useful clinical information. According to the ROC curve analysis in our cohort, utilization of the IgAN-RPT at diagnosis, with cut-offs for predicted 5-year risk of 4% and 11%, respectively, identifies patients who are very unlikely or very likely to reach the outcome long-term. These risk thresholds could enable decision making on the frequency of necessary follow-up visits and the need for additional therapeutic alternatives. Defining such thresholds in larger prospective studies could allow for clinical guidelines based on comprehensive individual risk predictions.
Patients with intermediate risk, defined as the 3rd risk quartile, have the most uncertain long-term prognosis. Knoop et al. [33] have previously reported that even for patients with benign features of IgAN at diagnosis, such as less than 1 g/day of albuminuria, the long-term prognosis might not be so favorable. In the 3rd risk quartile in our cohort, those who reached the outcome did so after 10 years or later.
The principal differences in baseline characteristics between patients in the 3rd risk quartile and patients in the two bottom risk quartiles were a higher frequency of chronic histologic lesions. The 3rd risk quartile had a similar degree of albuminuria compared with the 2nd risk quartile both at baseline (1.36 g/day vs. 1.27 g/day) and over the following 5 years (0.73 g/day vs. 0.66 g/day). Despite this, more patients in the 3rd risk quartile reached the outcome during prolonged follow-up (21.7% vs. 4.2%), highlighting the importance of chronic histologic lesions in CKD progression and the need for novel biomarkers to identify patients with worse prognosis in the intermediate risk group. Continuous risk assessment during follow-up including the evaluation of eGFR-slope and urinary findings remains necessary to guide the care of the patients.
The percentage of patients treated with immunosuppressive agents did not differ between the 3rd risk quartile and the two bottom risk quartiles. This might indicate better responders in the lower risk groups but even more probably unnecessary exposure to potentially harmful drugs in a number of patients at low risk. The role of immunosuppressive agents (mainly corticosteroids) in IgAN has not been fully elucidated with studies such as STOP-IGAN showing that most patients benefit foremost from meticulously applied conservative CKD treatment with RAS-inhibition and restriction of salt and protein intake [34]. The decision to initiate immunosuppressive therapy in this patient group had been made at the discretion of the individual clinician and was in general based on proliferative histologic lesions such as crescents or persistent high-degree proteinuria despite adequate RAS-inhibition, which is in line with earlier and the latest KDIGO-guidelines [35, 36]. A recent analysis by Barbour et al. [37] has shown that using the IgAN-RPT instead of the degree of albuminuria for decisions on immunosuppressive therapy might reduce the frequency of overtreatment in nonprogressive disease, highlighting a possible benefit of using the IgAN-RPT. Our retrospective analysis is not equipped to evaluate the effect of immunosuppressive therapy in groups with differing risks of renal deterioration; however, this is an important area for future RCTs to identify which patients receive the most benefit from current and future therapies against pathogenic mechanisms.
Recruiting patients for clinical trials based on the risk prediction tool is attractive as the likelihood of recruiting patients with progressive disease is higher than with conventional criteria based primarily on the degree of albuminuria. Using risk prediction tools does however run the risk of including patients with advanced fibrosis of viable tissue, which may be less responsive to treatment or experience more adverse events. Available immunosuppressive treatments have serious side effects, mainly infections and diabetes (primarily associated with corticosteroids), and patients with advanced CKD may be more vulnerable. This highlights the need for new treatments with low toxicity focused on pathogenic mechanisms, which can be used in patients with smoldering inflammatory activity in advanced CKD [38, 39].
In recent years, hematuria has received increasing attention as a modifiable risk factor in IgAN, with Sevillano et al. [22] showing that persistent hematuria is a risk factor for ESKD and that remission of hematuria is associated with improved renal outcome. Because hematuria was previously not regarded as a strong risk factor, it was not systematically measured in our cohort but rather at the discretion of the individual clinician, which resulted in missing values. Previous studies show that the degree of hematuria decreases over time for most patients, making a single measurement at the time of biopsy less valuable as several of these patients will have remission of hematuria with an improved prognosis [22, 26, 27]. In our study with 77 patients having available measurements of baseline microhematuria, those with high-degree microhematuria at the time of biopsy did not have a significantly worse renal outcome. Due to the retrospective design of the study and the relatively small patient cohort, we could not adjust for possible treatment effects. Another weakness of our study is that the degree of microhematuria was measured using two different methods (urine dipstick and automated microscopy). Whether urine dipstick measurements can be converted to corresponding microscopy measurements has not been validated, and errors can occur with both methods. For future studies, urine should be assessed in a standardized way, preferably using an international standard. This is essential if microhematuria were to be included in risk prediction tools. When the IGAN-RPT was conceived, the inclusion of predictor variables was decided based on a backward elimination process of predictor variables with a p value <0.20. In our study, the p value for high-degree hematuria in univariate Cox-regression was below this value, indicating that this variable might have reached criteria for inclusion in the IgAN-RPT if measurements had been available.
Previous studies evaluating the impact of macrohematuria on renal outcome in IgAN have either shown no association or a protective association [25, 28]. However, patients with macrohematuria were generally younger than the comparative group, making it difficult to draw conclusions about long-term prognosis [25]. In our study, macrohematuria was not significantly associated with renal outcome, a trend for patients experiencing macrohematuria being younger was observed but did not reach statistical significance.
Conclusion
The IGAN-RPT effectively identifies patients that are very likely or unlikely to lose 50% GFR or reach ESKD during long-term follow-up. Using cut-offs of 11% and 4%, predicted 5-year risk could with high certainty discriminate between patients with progressive and nonprogressive disease. This could be used to guide decisions on the frequency of follow-up visits and aid selection of patients for clinical trials, but these thresholds need to be validated externally in larger cohorts. Patients with intermediate risk remain a clinical challenge with an urgent need for novel biomarkers to identify patients with progressive disease as well as novel therapy options with low toxicity targeting pathogenic mechanisms. The assessment of microhematuria could be a valuable addition to the IgAN-RPT as a marker of acute or smoldering inflammatory activity, but the method of measurement needs to be standardized for future studies before incorporation in risk prediction tools.
Statement of Ethics
The study protocol was reviewed and approved by the Regional Medical Ethics Review Board in Stockholm, Sweden, approval numbers Dnr 94-322, 04-400T, 2010/213-32, and 2016/1472-32/3. Written informed consent was obtained from all subjects at recruitment. The study was conducted in accordance with the Declaration of Helsinki.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
Funding for this study was received from the Swedish Society of Medicine and Eric & Ebba Jonssons foundation. The funders were not involved in the preparation of data or the manuscript.
Author Contributions
Robin Ebbestad, Mazdak Sanaei Nurmi, and Sigrid Lundberg contributed to the design of the study. Data acquisition was done by Robin Ebbestad and Sigrid Lundberg. Statistical analysis was done by Robin Ebbestad with interpretation done by Robin Ebbestad, Mazdak Sanaei Nurmi, and Sigrid Lundberg. Robin Ebbestad wrote the first draft with subsequent revisions being done by Robin Ebbestad, Mazdak Sanaei Nurmi, and Sigrid Lundberg. The final version was approved by all the authors. Sigrid Lundberg is the guarantor of the manuscript.
Data Availability Statement
The data underlying this article cannot be shared publicly for the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
Acknowledgments
We would like to thank our statistician Fredrik Johansson at Karolinska Institutet for his assistance in conducting the statistical analyses of this study.
Funding Statement
Funding for this study was received from the Swedish Society of Medicine and Eric & Ebba Jonssons foundation. The funders were not involved in the preparation of data or the manuscript.
References
- 1.McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26((2)):414–30. doi: 10.1093/ndt/gfq665. [DOI] [PubMed] [Google Scholar]
- 2.O'Shaughnessy MM, Hogan SL, Thompson BD, Coppo R, Fogo AB, Jennette JC. Glomerular disease frequencies by race, sex and region: results from the International Kidney Biopsy Survey. Nephrol Dial Transplant. 2018;33((4)):661–9. doi: 10.1093/ndt/gfx189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347((10)):738–48. doi: 10.1056/NEJMra020109. [DOI] [PubMed] [Google Scholar]
- 4.Simon P, Ramee MP, Boulahrouz R, Stanescu C, Charasse C, Ang KS, et al. Epidemiologic data of primary glomerular diseases in western France. Kidney Int. 2004;66((3)):905–8. doi: 10.1111/j.1523-1755.2004.00834.x. [DOI] [PubMed] [Google Scholar]
- 5.Jarrick S, Lundberg S, Welander A, Carrero JJ, Hoijer J, Bottai M, et al. Mortality in IgA nephropathy: a nationwide population-based cohort study. J Am Soc Nephrol. 2019;30((5)):866–76. doi: 10.1681/ASN.2018101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriyama T, Tanaka K, Iwasaki C, Oshima Y, Ochi A, Kataoka H, et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One. 2014;9((3)):e91756. doi: 10.1371/journal.pone.0091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee H, Kim DK, Oh KH, Joo KW, Kim YS, Chae DW, et al. Mortality of IgA nephropathy patients: a single center experience over 30 years. PLoS One. 2012;7((12)):e51225. doi: 10.1371/journal.pone.0051225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knoop T, Vikse BE, Svarstad E, Leh S, Reisæter AV, Bjørneklett R. Mortality in patients with IgA nephropathy. Am J Kidney Dis. 2013;62((5)):883–90. doi: 10.1053/j.ajkd.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Segelmark M. Njurbiopsi. Svenskt Njurregister Årsrapport. [Renal biopsy. Annual Report Swedish Renal Registry] Jönköping. 2020. Available from: https://www.medscinet.net/snr/rapporterdocs/Svenskt%20Njurregister%20%C3%85rsrapport%202020.pdf.
- 10.Radford MG, Jr, Donadio JV, Jr, Bergstralh EJ, Grande JP. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. 1997;8((2)):199–207. doi: 10.1681/ASN.V82199. [DOI] [PubMed] [Google Scholar]
- 11.Coppo R, Lofaro D, Camilla RR, Bellur S, Cattran D, Cook HT, et al. Risk factors for progression in children and young adults with IgA nephropathy: an analysis of 261 cases from the VALIGA European cohort. Pediatr Nephrol. 2017;32((1)):139–50. doi: 10.1007/s00467-016-3469-3. [DOI] [PubMed] [Google Scholar]
- 12.Knoop T, Vagane AM, Vikse BE, Svarstad E, Magnusdottir BT, Leh S, et al. Addition of eGFR and age improves the prognostic absolute renal risk-model in 1,134 Norwegian patients with IgA nephropathy. Am J Nephrol. 2015;41((3)):210–9. doi: 10.1159/000381403. [DOI] [PubMed] [Google Scholar]
- 13.Reich HN, Troyanov S, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18((12)):3177–83. doi: 10.1681/ASN.2007050526. [DOI] [PubMed] [Google Scholar]
- 14.Lee H, Hwang JH, Paik JH, Ryu HJ, Kim DK, Chin HJ, et al. Long-term prognosis of clinically early IgA nephropathy is not always favorable. BMC Nephrol. 2014;15:94. doi: 10.1186/1471-2369-15-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SM, Rao VM, Franklin WA, Schiffer MS, Aronson AJ, Spargo BH, et al. IgA nephropathy: morphologic predictors of progressive renal disease. Hum Pathol. 1982;13((4)):314–22. doi: 10.1016/s0046-8177(82)80221-9. [DOI] [PubMed] [Google Scholar]
- 16.Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29((6)):829–42. doi: 10.1016/s0272-6386(97)90456-x. [DOI] [PubMed] [Google Scholar]
- 17.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76((5)):534–45. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 18.Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91((5)):1014–21. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Barbour SJ, Coppo R, Zhang H, Liu ZH, Suzuki Y, Matsuzaki K, et al. Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med. 2019;179((7)):942–52. doi: 10.1001/jamainternmed.2019.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Guo L, Wang Z, Wang J, Er L, Barbour SJ, et al. External validation of international risk-prediction models of IgA nephropathy in an Asian-Caucasian Cohort. Kidney Int Rep. 2020;5((10)):1753–63. doi: 10.1016/j.ekir.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Huang B, Liu Z, Wang X, Xie M, Guo R, et al. External validation of the international IgA nephropathy prediction tool. Clin J Am Soc Nephrol. 2020;15((8)):1112–20. doi: 10.2215/CJN.16021219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sevillano AM, Gutierrez E, Yuste C, Cavero T, Merida E, Rodriguez P, et al. Remission of hematuria improves renal survival in IgA nephropathy. J Am Soc Nephrol. 2017;28((10)):3089–99. doi: 10.1681/ASN.2017010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu GZ, Guo L, Dong JF, Shi SF, Liu LJ, Wang JW, et al. Persistent hematuria and kidney disease progression in IgA nephropathy: a Cohort Study. Am J Kidney Dis. 2020;76((1)):90–9. doi: 10.1053/j.ajkd.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Yoshikawa N, Ito H, Iijima K, Nakahara C, Maehara K, Hasegawa O, et al. Macroscopic hematuria in childhood IgA nephropathy. Clin Nephrol. 1987;28((5)):217–21. [PubMed] [Google Scholar]
- 25.Le W, Liang S, Chen H, Wang S, Zhang W, Wang X, et al. Long-term outcome of IgA nephropathy patients with recurrent macroscopic hematuria. Am J Nephrol. 2014;40((1)):43–50. doi: 10.1159/000364954. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki C, Moriyama T, Tanaka K, Takei T, Nitta K. Effect of hematuria on the outcome of immunoglobulin A nephropathy with proteinuria. J Nephropathol. 2016;5((2)):72–8. doi: 10.15171/jnp.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka K, Moriyama T, Iwasaki C, Takei T, Nitta K. Effect of hematuria on the outcome of IgA nephropathy with mild proteinuria. Clin Exp Nephrol. 2015;19((5)):815–21. doi: 10.1007/s10157-014-1068-9. [DOI] [PubMed] [Google Scholar]
- 28.D'Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol. 2004;24((3)):179–96. doi: 10.1016/j.semnephrol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014;86((4)):828–36. doi: 10.1038/ki.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fournier A, Achard JM. Mnemotechnical note on the use of Cockcroft creatinine clearance formula for the validation of a 24-h urine collection. Nephrol Dial Transplant. 2000;15((10)):1677–8. doi: 10.1093/ndt/15.10.1677. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald PL, Gardner RC. Type I error rate comparisons of post hoc procedures for I j chi-square tables. Educ Psychol Meas. 2000;60((5)):735–54. [Google Scholar]
- 32.Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, et al. Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet. 1999;353((9156)):883–7. doi: 10.1016/s0140-6736(98)03563-6. [DOI] [PubMed] [Google Scholar]
- 33.Knoop T, Vikse BE, Mwakimonga A, Leh S, Bjørneklett R. Long-term outcome in 145 patients with assumed benign immunoglobulin A nephropathy. Nephrol Dial Transplant. 2017;32((11)):1841–50. doi: 10.1093/ndt/gfx242. [DOI] [PubMed] [Google Scholar]
- 34.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, et al. Intensive Supportive Care plus Immunosuppression in IgA Nephropathy. N Engl J Med. 2015;373((23)):2225–36. doi: 10.1056/NEJMoa1415463. [DOI] [PubMed] [Google Scholar]
- 35.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. 2012;2:139–274. [Google Scholar]
- 36.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100((4S)):S1–276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 37.Barbour SJ, Canney M, Coppo R, Zhang H, Liu ZH, Suzuki Y, et al. Improving treatment decisions using personalized risk assessment from the International IgA Nephropathy Prediction Tool. Kidney Int. 2020;98((4)):1009–19. doi: 10.1016/j.kint.2020.04.042. [DOI] [PubMed] [Google Scholar]
- 38.Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, et al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA. 2017;318((5)):432–42. doi: 10.1001/jama.2017.9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarcina C, Tinelli C, Ferrario F, Pani A, De Silvestri A, Scaini P, et al. Changes in proteinuria and side effects of corticosteroids alone or in combination with azathioprine at different stages of IgA nephropathy. Clin J Am Soc Nephrol. 2016;11((6)):973–81. doi: 10.2215/CJN.02300215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared publicly for the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.